Abstract
Background
Extracorporeal photopheresis (ECP) is a blood‐based therapeutic procedure increasingly used for modulation of immune dysregulation in various underlying disease settings. The aim of this study was to compare the procedure times and blood collection efficiencies between the two approaches currently utilized in European centers: the integrated versus the multistep nonintegrated procedures.
Methods
A retrospective data analysis was conducted, comparing treatment data from patients who received ECP therapy at the Central Institute for Blood Transfusion & Department of Immunology (ZIB) of the Tirol Kliniken GmbH, where the integrated and multistep nonintegrated procedures are routinely used in an approximated setup.
Results
During the observation period, a total of 15 patients who were treated with alternating systems on 2 consecutive days were identified. This allowed treatment pair comparisons with minimal interpatient variabilities, similar to a cross‐over design even though analyzed retrospectively. Total average procedure times with the integrated system were 99.3 vs 122.0 minutes with the multistep nonintegrated procedures, respectively. Significant differences were observed for all steps of the ECP procedure: (a) time for buffy coat collection, 66.5 vs 74.7; (b) handling/transfer, 2.8 vs 18.7; (c) irradiation, 20.3 vs 11.7; and (d) reinfusion/handling time, 9.6 vs 16.3 minutes. The calculated collection throughput was 7.79 mL/min for the integrated and 7.84 mL/min for the multistep nonintegrated procedures, and with a white blood cell (WBC) collection efficiency of 34.2% and 21.0%, respectively.
Conclusion
The data presented in this study show a significant shorter overall procedure time and higher WBC collection efficiency for the integrated ECP system.
Keywords: collection efficiency, collection throughput, ECP, procedure time, time steps, WBC cell collection
1. INTRODUCTION
Extracorporeal photopheresis (ECP) is a therapeutic approach in which the white blood cells (WBC) of the patient are ex vivo exposed to 8‐methoxypsoralen (8‐MOP) and ultraviolet A (UV‐A) light and subsequently reinfused back to the patient. First approved by the US Food and Drug Administration (FDA) in 1988 for cutaneous T‐cell lymphoma (CTCL), its clinical utility has been expanded over the past decades to graft‐versus‐host disease (GvHD), solid‐organ transplant rejection, and autoimmune diseases. 1 , 2 , 3
There are two methods currently being used to provide ECP in European centers. One of them relies on the use of integrated ECP systems in which blood collection, cell separation, irradiation, and reinfusion stages are fully integrated and automated, whereas another approach combines the use of different devices for blood separation and photoactivation also known as multistep nonintegrated ECP systems.
Recently published guidelines 2 , 3 provide expert recommendations for the use of ECP in varying therapeutic settings; however, there is no universally accepted consensus with regard to preferences between the systems.
A variety of attempts have been made trying to compare both therapeutic systems in terms of procedure time, 4 , 5 , 6 number and types of cells collected during the process, 7 collection efficiency (CE), 7 and costs. 8 , 9 , 10 However, the results of these studies are driven by a variety of factors, including but not limited to interpatient variabilities, venous access used, processed blood volume, devices used in the process as well as device settings. 2
The aim of this retrospective observational data analysis was to assess and compare the procedure times as performed at the Tirol Kliniken in Innsbruck, Austria with the integrated (closed) system (THERAKOS CELLEX Photopheresis System) to a multistep nonintegrated (open) procedures (Spectra OPTIA Apheresis System and MacoGenic G2) and thereby contribute to comparative data, in terms of overall procedure time, treated cells and CE while minimizing interpatient driven treatment variables.
2. MATERIALS AND METHODS
2.1. Study design and objectives
A retrospective observational data analysis, comparing treatment data from adults who received ECP treatments between August 1, 2016 and December 31, 2016 in the Central Institute for Blood Transfusion & Department of Immunology (ZIB) of the Tirol Kliniken GmbH, was conducted for this study. The ZIB maintains paper medical records and charts, as well as computerized systems for the measurement and documentation of ECP procedure times and CE. The protocol for this study was approved by the local ethics committee. All patients undergoing ECP have given written informed consent.
The primary objective of the study was to compare the two photopheresis systems (integrated vs nonintegrated, multistep procedure system) by assessing total procedure time as well as each specific procedural step where applicable. The secondary objectives of the study were to compare cell collection efficiencies and collection throughput while providing a descriptive analysis of the buffy coat compositions.
2.2. Study population
Treatment data for adult patients who received ECP therapy and who fulfilled the following inclusion criteria were extracted from medical records and evaluated: (a) patient treatments with available data from 2 consecutive days, (b) with both systems (integrated and multistep nonintegrated) in an unspecified order, and (c) double‐needle treatments.
2.3. Instrument settings
The devices used in the ZIB center are the Therakos CELLEX Photopheresis System, Software version 5.1 (Therakos; Mallinckrodt Pharmaceuticals) for the integrated ECP procedure on the one hand and for the multistep nonintegrated procedure the combination of the Spectra OPTIA apheresis device Software version 11.3 on Continuous Mononuclear Cell Collection (cMNC) program (Terumo BCT), the Macogenic G2 system (Macopharma) for buffy coat irradiation, and additional devices, such as a mobile sealing device, stood welder, weight scale, transfusion set or other equipment required for handling procedures on the other hand. Blood samples were measured for a CBC (complete blood count) on Sysmex XS‐1000i (Sysmex Austria GmbH). Blood samples were extracted from the injection port of the treatment bag in the integrated system; blood samples for the multistep nonintegrated system were taken during handling procedures. All photopheresis‐related parts, independent of which procedure being performed, are all located in the same room, potentially minimizing the transfer times between procedural steps.
The ECP procedures with noticeably different process flows were run during the observation period as follows:
(a) The integrated system was set up to process 1.5 L of blood (default setting).
(b) The multistep nonintegrated procedure was operated to get a target buffy coat volume of 100 mL after handling procedure. Therefore, the Spectra OPTIA was set to collect 110 mL volume of buffy coat, as about 10 mL volume loss may be expected caused by handling steps like sealing, blood sampling, and so forth. In this setup, the Spectra OPTIA was run with a collection rate of 2 mL/min on cMNC program. The integrated system was primed with 10 000 IU heparin/500 mL saline followed by 4% citrate for anticoagulation during collection of the buffy coat. Citrate 4% was the applied anticoagulant for the multistep nonintegrated procedure. For the integrated system, the software calculates the 8‐MOP dose using the following formula: Uvadex = treatment volume × 0.017. For 300 mL volume in the multistep nonintegrated procedure (= 100 mL buffy coat + 200 mL saline), 3 mL of 8‐MOP were added through the injection port of the irradiation bag.
2.4. Statistical analysis
The relevant data from paired (back‐to‐back) patient treatments on consecutive days on the two available system options (integrated vs nonintegrated, multistep) using double‐needle procedures were extracted and analyzed including demographic data and information of underlying conditions, as well as information from hemograms before treatment and the composition of the collected buffy coats.
Assessed parameters comprised procedure times in minutes (min) with minimum and maximum values, WBC (giga per liter [G/L]), harvested WBC (G/L and in absolute counts), and the processed blood volume in mL. Mean differences of results were tested for statistical significance by paired t test and Wilcoxon signed‐rank test. In this study, the standardized CE and collection throughput adapted from Brosig et al 4 was calculated, while we only used the WBC concentration before the procedure instead of an average of WBC before and after the procedure to minimize measurement distortions caused by dilution effects.
where BC, buffy coat; CE, collection efficiency; CT, collection throughput; and WBC, white blood cells.
Data sets with missing time points where no data imputation methods could be applied were excluded. No carry‐over effects were expected and the sequence of the used ECP systems was considered irrelevant.
3. RESULTS
3.1. Treatment and patient data
During the observation period, 304 ECP treatments were conducted on 40 patients. Of the overall sample, 15 patients (5 females, 10 males) fulfilling the inclusion criteria received ECP therapy with both processes in alternating order and with double‐needle treatments. All these 30 paired ECP treatments (= 60 treatments in total) on 2 consecutive days were hence eligible and included in the analysis. The underlying conditions in the patients (n = 15) included in the analysis were GvHD (n = 6), scleroderma (n = 3) and heart TX (n = 1), lung TX (n = 1), cutaneous T‐cell lymphoma (CTCL; n = 1), dermatitis (n = 1), morphea (n = 1), and Shulman syndrome (n = 1).
3.2. Procedure times
In order to determine the total treatment procedure time, the time of each of the following procedural specific steps, buffy coat collection, transfer/handling time, irradiation, reinfusion, and handling time post‐treatment was collected. The average total treatment time of the integrated and multistep nonintegrated ECP procedures amounted to 99.27 minutes (ranging from 75 to 133 minutes) and 122.03 minutes (ranging from 108 to 147 minutes), respectively, P < .0001 (Figure 1). Figure 2 shows the duration of each step of the ECP procedure per system used.
FIGURE 1.
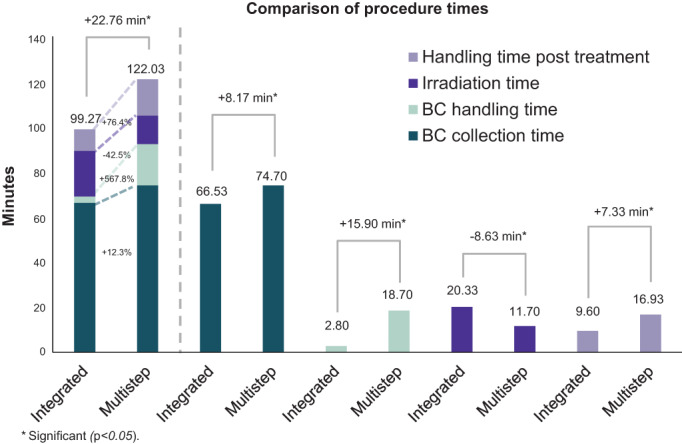
Comparison of procedure times. BC, buffy coat; Multistep refers to multistep nonintegrated procedures
FIGURE 2.
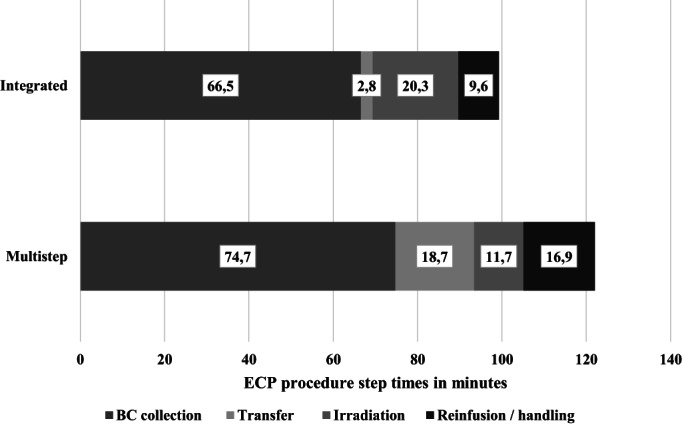
ECP procedure step times in minutes. BC, buffy coat; Multistep refers to multistep, nonintegrated procedure
A significant difference between both ECP treatment systems was determined for the time of buffy coat handling. In the integrated system, time is needed to add photosensitizer to the buffy coat, whereas additional steps (sealing/handling, buffy coat transfer to the irradiation bag, adding photosensitizer) are necessary for the multistep procedure, leading to an additional time of 15.90 minutes (range 10‐25 minutes; P < .0001) between the end of buffy coat collection and the start of irradiation). On average, handling of the buffy coat required 2.80 minutes for the integrated system and 18.70 minutes for the multistep procedure; P < .0001 (Figures 1 and 2).
Irradiation of the buffy coat was found to take longer for the integrated system 20.33 minutes (range 13‐40 minutes) versus 11.70 minutes (range 10‐13 minutes) for the multistep nonintegrated procedure; P < .0001.
During the study period, no serious adverse events or treatment discontinuations were observed with either system in the observation cohort.
3.3. Cell counts and collection efficiency
To evaluate the CE of both systems, mean total WBCs were extracted from the charts. Collected mean total WBCs with the integrated system were 3.37 × 109 and for the multistep procedure 3.89 × 109, respectively (Table 1). The mean of the standardized WBC counts per kilogram of body weight was 4.69 × 107/kg for the integrated system procedures compared to 5.27 × 107/kg for the treatments with the multistep nonintegrated procedures (Table 1).
TABLE 1.
Hemogram before treatment, processed blood volume (BV) and buffy coat (BC) volume, BC composition, collection efficiency (CE), and throughput (CT)
| Integrated | Multistep nonintegrated | Integrated | Multistep nonintegrated | |
|---|---|---|---|---|
| Mean (SD) | Median (range) | |||
| Hemogram before treatment | ||||
| WBC G/L | 7.18 (2.76) | 7.10 (2.60) | 6.66 (2.11‐13.23) | 6.92 (1.57‐12.19) |
| HCT% | 35.98 (4.55) †† | 37.19 (5.02) †† | 36.75 (28.00‐46.90) | 37.20 (29.00‐46.90) |
| Total BV (L) | 4.82 (0.67) | 4.82 (0.67) | 4.79 (3.617‐5.95) | 4.79 (3.617‐5.95) |
| BV volume and BC volume | ||||
| Processed BV (L) | 1.53 (0.02) | 2.87 (0.55) | 1.54 (1.50‐1.57) | 2.85 (1.56‐4.42) |
| BC volume (mL) | 131.50 (14.89) a , b | 100.00 (‐) ** | 129.00 (110.00‐181.00) b | 100.00 (‐) |
| Number of cells collected | ||||
| WBC × 109 | 3.37 (1.91) | 3.89 (2.38) | 2.63 (1.42‐11.50) | 3.48 (1.52‐10.50) |
| WBC × 107/kg | 4.69 (2.45) | 5.27 (2.95) | 3.97 (2.09‐13.69) | 4.46 (2.37‐14.90) |
| BC composition (imputed values for lymphocytes and monocytes) | ||||
| HCT% | 3.36 (1.52) | 2.91 (1.09) | 2.75 (1.80‐8.30) | 2.80 (1.30‐5.70) |
| WBC G/L | 26.26 (16.10) ** | 38.90 (23.80) ** | 20.40 (8.47‐95.39) | 34.72 (15.20‐104.45) |
| Lym G/L b | 10.42 (6.13)* | 17.43 (8.54)† | 7.96 (4.48‐22.35) | 17.28 (7.28‐36.65) |
| Mono G/L b | 6.76 (3.26) | 8.97 (3.60) | 5.44 (3.56‐13.84) | 8.68 (4.23‐17.58) |
| Lym % b | 45.56 (18.53) | 50.74 (15.66) | 43.10 (16.10‐69.20) | 50.00 (23.20‐71.00) |
| Mono % b | 28.45 (5.88)† | 26.98 (8.36)† | 28.00 (19.40‐40.50) | 27.70 (13.80‐44.80) |
| Purity b | 74.01 (17.76) | 77.72 (18.14) | 69.70 (43.00‐96.00) | 81.40 (37.00‐98.70) |
| Collection efficiency (CE) and throughput (CT) | ||||
| CE (%) | 34.23 (19.57) ** | 21.03 (11.82) ** | 28.78 (8.98‐105.31) | 17.98 (7.81‐64.00) |
| CT (mL/min) | 7.79 (3.63) | 7.84 (3.82) | 7.59 (2.98‐20.40) | 7.82 (2.82‐18.03) |
Abbreviations: BC, Buffy coat; BV, blood volume; CE, collection efficiency; CT, collection throughput; HCT, hematocrit; Lym, lymphocytes; Mono, monocytes; SD, standard deviation; WBC, white blood cell.
P = .0066.
P = .0088.
P = .0084.
P < .0001.
Prefilled with 50 mL saline in the bag.
Treatment pairs with missing data for MNC counts were excluded (included pairs = 11).
No statistical significance was observed in the cellular distribution of the buffy coat, with regard to cell counts of total WBCs and MNC %.
The calculated collection efficiencies (calculated as described in the formula under the methods section) were significantly higher for the integrated system with 34.23% (range 8.98%‐105.31%) based on a processed blood volume of 1.53 L (P < .0001) vs a 21.03% (range 7.81%‐64.00%) for the multistep nonintegrated procedure and a processed blood volume of 2.87 L (Table 1, Figure 3).
FIGURE 3.
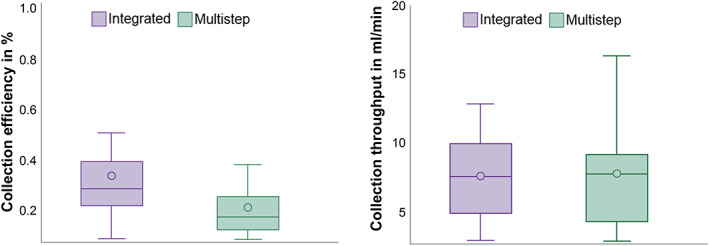
Boxplots for collection efficiency and collection throughput. Multistep refers to multistep, nonintegrated system
4. COLLECTION RATE
The collection throughput (calculated as described in the formula under the methods section) was comparable between the two systems with 7.79 (SD 3.63) mL/min for the integrated system and 7.84 (SD 3.82) mL/min for the multistep nonintegrated procedure (Table 1, Figure 3).
5. DISCUSSION
In this study, procedural times and CE of an integrated ECP system (Therakos CELLEX) compared to the multistep nonintegrated procedures (Terumo BCT Spectra OPTIA and Macopharma Macogenic G2) were analyzed, retrospectively.
Overall procedure times were significantly shorter for the integrated system (−22.8 minutes), although the irradiation time was longer compared to the multistep nonintegrated procedure (+8.6 minutes). This longer irradiation time with higher HCT for the integrated system is influenced by three factors namely buffy coat volume, hematocrit, and UV‐A lamps, whereas the buffy coat in the multistep nonintegrated system is diluted with saline and results in a drop of the hematocrit (HCT) of ≤2% and the irradiation time will be about 11 to 12 minutes, almost a fixed irradiation time. The prolonged duration of the multistep nonintegrated procedure was mainly caused by the additional time needed for transfer and dilution of the collected buffy coat prior to irradiation (+24.1 minutes), as well as for the reinfusion and related handling time (+7.3 minutes).
The results of our study are in line with recently published results by other researchers. Bueno et al 6 reported that the overall procedure time for ECP treatment was lower for the integrated system using the Therakos CELLEX than for the multistep nonintegrated procedure (106 vs 272 minutes) using Terumo BCT, Spectra OPTIA and Macopharma Macogenic G2. Adorno et al 11 reported 115 minutes vs 192 minutes for the integrated and the multistep nonintegrated procedure, respectively. Brosig et al 4 reported significantly lower median procedure times for the multistep nonintegrated system approach using an older generation integrated system device, namely UVAR XTS in single needle mode, and the time for handling, irradiation, and reinfusion in the multistep nonintegrated procedure was not included in the overall treatment time calculation. Finally, Piccirillo et al reported a significantly shorter runtime too with the “inline” system (92 [86–108] minutes versus “offline” system (140 [124–160] minutes, P = .0001. 12
In order to compare the two different procedural systems, with noticeably different process flows, we minimized interpatient and treatment variables by comparing data from back‐to‐back treatment procedures in the same patient on two consecutive days with alternating system solutions and double‐needle performance.
Considering the paired back‐to‐back comparison, the protocol ensured similar patient cohorts with regard to general condition, medication and dosing, and similar peripheral vascular access status. The flow speed was adjusted to the venous access status. In 30 paired procedures 15 × CELLEX and 15 × OPTIA were performed on day 1.
Main differences in determining or comparing the procedural efficiencies in previous attempts of both systems were (i) processed target volume, 4 , 6 , 7 (ii) buffy coat volume, and (iii) overall procedure time. 6 , 7 , 12
In terms of cell collections efficiency in our study an average of 3.37 × 109 and 3.89 × 109 WBCs were collected with the integrated system and the multistep nonintegrated procedure, respectively. Bueno et al 6 reported a total of 2.97 × 109 and 9.32 × 109 leukocytes with the integrated and multistep nonintegrated procedures, respectively. The median cells obtained by Brosig et al 4 were a total of 3.0 × 109 and 6.3 × 109 WBCs for the integrated and the multistep nonintegrated procedure, respectively. In both studies approximately 7.5 L of blood were processed with the multistep nonintegrated procedure. In terms of CE, we found that the integrated system was significantly more efficient in gathering leukocytes than in the multistep nonintegrated approach, with a WBC CE of 34.23% versus 21.03% (P < .0001) in the chosen approximated setup. A possible reason for the higher CE in the integrated system is mainly caused by the lower processed blood volume because this is a denominator in the CE calculation formula. CE for both systems for a paired comparison has not been previously been reported. Brosig et al 4 published a median WBC CE for the Spectra OPTIA (21%; range 4–38%), which is comparable with the result in this study (Table 1).
CE for WBCs is logically lower than for MNCs. CE for MNCs would of course be higher as a result of an additional enrichment of these WBC subgroup cells in the buffy coat, but this determination for MNC was not the focus of our study.
Also, collection throughput times were comparable for the integrated and multistep nonintegrated approach of 7.79 mL/min (range 2.98–20.34 mL/min) compared to 7.84 mL/min (range 2.82–18.04 mL/min) in this study (Table 1).
It is worth mentioning for both compared systems that the CELLEX can be used in a single or double‐needle mode, changeable during the collection procedure, and for irradiation and retransfusion of treated cells, but can only be used for ECP treatments, whereas Spectra OPTIA can be used for a wide range of apheresis treatments and can process large volumes of blood.
Up to today, it is still not yet clear if and how the number of treated cells affects ECP treatment outcomes in individual patients and disease states, nor has it yet been established how the individual buffy coat composition contributes to reported clinical effects. However, to date, no clear relationship between the type and number of treated cells, as well as the treatment outcome has been established. Various attempts to shed light on this subject in varying different disease states have been made and might suggest a potential threshold of cells that need to be treated 13 , 14 , 15 , 16 , 17 while a definite proof is still lacking. Most of these aforementioned studies have focused on the collection of lymphocytes and monocytes only, while very recent data might also suggest a potential role for neutrophils. 4 , 18 , 19
It was therefore decided to use the number and concentration of leukocytes to compare the cell collection of both approaches and not to limit the current analysis to mononuclear cells. However, it should be noted that in this study both methods were comparable in terms of MNC purity (integrated 74.01 vs multistep nonintegrated 77.72%). Further research is required to improve the understanding of the relevance and dose‐response relationship of different cell types in specific disease states.
There are several factors that may have contributed to the differences between our results and those previously reported. First and foremost, the total number of cells collected in the procedure is a function of the processed blood volume. It is therefore not surprising, that the larger blood volumes processed by other centers within the multistep nonintegrated approach led to higher numbers of total leukocytes collected. Furthermore, at the ZIB, the Spectra OPTIA Apheresis System was operated with a collection rate of 2 mL/min for multistep nonintegrated photopheresis in an approximated setup. This has reduced our procedure times and potentially may have impacted the composition of the buffy coat. From our data, it may be concluded that the collection time of the multistep nonintegrated ECP procedure can potentially be shortened and still achieve a comparable number of treated WBCs.
Moreover, it has to be emphasized that this setting needs increased attention during the collection phase by the operator to prevent the collection of more unwanted cells. In addition, all ECP treatment devices at the ZIB are in one room, which reduced the handling time of the apheresate in the multistep nonintegrated approach. A strength of this study is that we used the latest technical iterations of both systems. Also, by analyzing paired treatments of the same patient on 2 consecutive days and only including procedures that were performed in double‐needle mode, we reduced the impact of patient and procedural variability on the results.
Limitations of this study are the small number of patients and the influence of center‐specific treatment practices. Another limitation is the retrospective nature of our study. Retrospective studies are prone to various forms of bias. However, by applying strict inclusion criteria, we may have been able to limit the impact of nonprocedure‐related factors on the results. Another effect of the retrospective design is that the assessment of the cell types collected and treated is limited to those routinely monitored on clinical practice.
6. CONCLUSION
A cross‐over paired analysis of the two most common photopheresis systems in their latest technical iterations, including only patients with peripheral venous access treated in double needle mode, was conducted. In our study, the integrated system showed a shorter overall procedure time and a significantly higher WBC CE compared to the multistep nonintegrated system in the respective approximated setup. In terms of the number of collected and treated cells, both approaches were comparable.
More than 50% of the time savings were caused by the BC handling time, the time needed to manually dilute and transfer the collected buffy coat before irradiation when using the multistep nonintegrated procedure. However, differences in the clinical parameters showed a broad range and were mainly driven by the individual patient.
CONFLICT OF INTEREST
Two authors, Susanne Behlke and Antonis Kontekakis are employees of Therakos Ltd. and therefore state a conflict of interest. However, both could not influence the data collection or actual data, as this was a retrospective study. All other authors declared no conflicts of interest for this research article.
Mayer W, Kontekakis A, Maas C, Kuchenbecker U, Behlke S, Schennach H. Comparison of procedure times and collection efficiencies using integrated and multistep nonintegrated procedures for extracorporeal photopheresis. J Clin Apher. 2022;37(4):332-339. doi: 10.1002/jca.21974
Funding informationNo author was paid or received funding. The Tirol‐Kliniken in Innsbruck received a funding amount for data collection. Paid third party biostats support was provided by Xcenda GmbH, Lange Laube 31, 30159 Hannover, Germany, and was performed by the two authors Dr Ulrike Kuchenbecker and Christopher Maas.
DATA AVAILABILITY STATEMENT
Pseudonymized data (following the European Union's General Data Protection Regulation (GDPR)) from the medical records of patients undergoing ECP between August 1st, 2016 and December 31st, 2016 were made available to the researchers of this study.
REFERENCES
- 1. Knobler R, Berlin G, Calzavara‐Pinton P, et al. Guidelines on the use of extracorporeal photopheresis. J Eur Acad Dermatol Venereol. 2014;28 Suppl 1(Suppl 1):1‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Knobler R, Arenberger P, Arun A, et al. European dermatology forum—updated guidelines on the use of extracorporeal photopheresis 2020—part 1. J Eur Acad Dermatol Venereol. 2020;34(12):2693‐2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knobler R, Arenberger P, Arun A, et al. European dermatology forum: updated guidelines on the use of extracorporeal photopheresis 2020—part 2. J Eur Acad Dermatol Venereol. 2021;35(1):27‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brosig A, Hähnel V, Orsó E, Wolff D, Holler E, Ahrens N. Technical comparison of four different extracorporeal photopheresis systems. Transfusion. 2016;56(10):2510‐2519. [DOI] [PubMed] [Google Scholar]
- 5. Cho A, Jantschitsch C, Knobler R. Extracorporeal Photopheresis—an overview. Front Med. 2018;5:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bueno JL, Alonso R, Gonzalez‐Santillana C, et al. A paired trial comparing mononuclear cell collection in two machines for further inactivation through an inline or offline extracorporeal photopheresis procedure. Transfusion. 2019;59(1):340‐346. [DOI] [PubMed] [Google Scholar]
- 7. Piccirillo N, Putzulu R, Massini G, et al. Inline extracorporeal photopheresis: evaluation of cell collection efficiency. Transfusion. 2019;59(12):3714‐3720. [DOI] [PubMed] [Google Scholar]
- 8. Azar N, Leblond V, Ouzegdouh M, Button P. A transition from using multi‐step procedures to a fully integrated system for performing extracorporeal photopheresis: a comparison of costs and efficiencies. J Clin Apher. 2017;32(6):474‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yalniz FF, Murad MH, Lee SJ, et al. Steroid refractory chronic graft‐versus‐host disease: cost‐effectiveness analysis. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl. 2018;24(9):1920‐1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Waure C, Capri S, Veneziano MA, et al. Extracorporeal Photopheresis for second‐line treatment of chronic graft‐versus‐host diseases: results from a health technology assessment in Italy. Value Health. 2015;18(4):457‐466. [DOI] [PubMed] [Google Scholar]
- 11. Adorno G LA FEea. An efficiency study comparing an open system and Therakos CELLEX for extracorporeal photopheresis procedure. EBMT Annual Meeting April 7‐10; London; 2013.
- 12. Piccirillo N, Putzulu R, Massini G, et al. Inline and offline extracorporeal photopheresis: device performance, cell yields and clinical response. J Clin Apher. 2021;36(1):118‐126. [DOI] [PubMed] [Google Scholar]
- 13. Bertani G, Santoleri L, Ferri U, et al. Response of steroid‐refractory chronic graft‐versus‐host disease to extracorporeal photopheresis correlates with the dose of CD3+ lymphocytes harvested during early treatment cycles. Transfusion. 2016;56(2):505‐510. [DOI] [PubMed] [Google Scholar]
- 14. Hackstein H, Amoros JJ, Bein G, Woessmann W. Successful use of miniphotopheresis for the treatment of graft‐versus‐host disease. Transfusion. 2014;54(8):2022‐2027. [DOI] [PubMed] [Google Scholar]
- 15. Liu C, Shah K, Dynis M, Eby CS, Grossman BJ. Linear relationship between lymphocyte counts in peripheral blood and buffy coat collected during extracorporeal photopheresis. Transfusion. 2013;53(11):2635‐2643. [DOI] [PubMed] [Google Scholar]
- 16. Perseghin P, Galimberti S, Balduzzi A, et al. Extracorporeal photochemotherapy for the treatment of chronic graft‐versus‐host disease: trend for a possible cell dose‐related effect? Ther Apher Dial. 2007;11(2):85‐93. [DOI] [PubMed] [Google Scholar]
- 17. Perseghin P, Incontri A. Mononuclear cell collection in patients treated with extracorporeal photochemotherapy by using the off‐line method: a comparison between COBE spectra AutoPbsc version 6.1 and amicus cell separators. J Clin Apher. 2010;25(6):310‐314. [DOI] [PubMed] [Google Scholar]
- 18. Franklin C, Cesko E, Hillen U, Schilling B, Brandau S. Modulation and apoptosis of neutrophil granulocytes by extracorporeal Photopheresis in the treatment of chronic graft‐versus‐host disease. PLoS One. 2015;10(8):e0134518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han P, Hanlon D, Arshad N, et al. Platelet P‐selectin initiates cross‐presentation and dendritic cell differentiation in blood monocytes. Sci Adv. 2020;6(11):eaaz1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Pseudonymized data (following the European Union's General Data Protection Regulation (GDPR)) from the medical records of patients undergoing ECP between August 1st, 2016 and December 31st, 2016 were made available to the researchers of this study.


