Abstract
During nutrient starvation, Escherichia coli elicits a stringent response involving the ribosome-associated protein RelA. Activation of RelA results in a global change in the cellular metabolism including enhanced expression of the stationary-phase sigma factor RpoS. In the human pathogen Pseudomonas aeruginosa, a complex quorum-sensing circuitry, linked to RpoS expression, is required for cell density-dependent production of many secreted virulence factors, including LasB elastase. Quorum sensing relies on the activation of specific transcriptional regulators (LasR and RhlR) by their corresponding autoinducers (3-oxo-C12-homoserine lactone [HSL] and C4-HSL), which function as intercellular signals. We found that overexpression of relA activated the expression of rpoS in P. aeruginosa and led to premature, cell density-independent LasB elastase production. We therefore investigated the effects of the stringent response on quorum sensing. Both lasR and rhlR gene expression and autoinducer synthesis were prematurely activated during the stringent response induced by overexpression of relA. Premature expression of lasR and rhlR was also observed when relA was overexpressed in a PAO1 rpoS mutant. The stringent response induced by the amino acid analogue serine hydroxamate (SHX) also led to premature production of the 3-oxo-C12-HSL autoinducer. This response to SHX was absent in a PAO1 relA mutant. These findings suggest that the stringent response can activate the two quorum-sensing systems of P. aeruginosa independently of cell density.
In their natural environment, microorganisms must frequently cope with nutrient limitations and have evolved specialized metabolic states that allow survival during prolonged periods of starvation. In gram-negative bacteria, the adaptation to starvation involves a series of global physiological changes. Whereas the bulk of protein synthesis decreases, specific sets of genes are induced, leading to increased stress resistance and maintenance of viability under adverse conditions (23). One important physiological response of Escherichia coli to nutritional stress is the so-called stringent response, which primarily results in inhibition of stable RNA synthesis (5). The effector of the stringent control is the nucleotide guanosine 3′,5′-bisdiphosphate (ppGpp), synthesized by the ribosome-associated RelA protein. During amino acid starvation, the ppGpp synthetase activity of RelA is triggered by the ribosome binding of uncharged tRNA, so that the ppGpp level acts as an intracellular signal that allows cells to perceive their own inability to produce aminoacyl-tRNA. In E. coli, an additional pathway for ppGpp synthesis exists, which relies on the activity of the bifunctional SpoT protein that shares both ppGpp synthetase and ppGpp hydrolase activities (16).
The effect of the stringent response is not limited to the quick arrest of stable RNA synthesis but also includes inhibition of other processes related to growth (46), as well as the positive regulation of amino acid biosynthetic and transport system operons (5), cell division (21), and antibiotic production pathways (6). Moreover, in E. coli the stringent response activates the expression of another key regulatory gene, rpoS, which encodes an alternative sigma factor (17). RpoS is responsible for the transcription of a variety of genes expressed after cells enter stationary phase or during starvation and stress conditions (28). Expression of RpoS itself increases during entry into stationary phase (25). Cellular levels of inorganic polyphosphate (polyP) accumulated during the stringent response also modulate the induction of rpoS in E. coli (24, 42). Furthermore, expression of rpoS during starvation conditions is prevented in E. coli cells lacking polyP (42). The stringent response thereby contributes through multiple and interrelated pathways to triggering changes in the pattern of gene expression that allow transition from exponential to stationary phase in response to nutritional deficiencies.
Homologs of RpoS and RelA have been identified in Pseudomonas aeruginosa (18, 48). The rpoS gene plays a role in general stress tolerance, as well as in the production of several virulence factors by this opportunistic pathogen (20, 45). As observed in other gram-negative bacteria, expression of P. aeruginosa rpoS increases upon entry into stationary phase (12). Although little is known about the mechanisms of RpoS induction in this organism, it has been reported to involve quorum-sensing regulation (26), and this control was not observed in another recent study (52). Quorum sensing relies on the bacterial production of autoinducer molecules (N-acylhomoserine lactones [AHLs]) that accumulate in the surrounding medium and allow individual cells to sense the population density (13). P. aeruginosa has two quorum-sensing systems, las (lasR-lasI) and rhl (rhlR-rhlI, also termed vsmR-vsmI), that are connected through a regulatory cascade (26, 36). LasR and RhlR are transcriptional regulators (27, 32, 33) which are activated by N-(3-oxododecanoyl)-l-homoserine lactone (3-oxo-C12-HSL) and N-butanoyl-l-homoserine lactone (C4-HSL), respectively. The synthesis of 3-oxo-C12-HSL is directed by LasI (34), while RhlI is responsible for the synthesis of C4-HSL (53). At high cell density, AHLs reach a threshold concentration and activate their cognate regulator. The las and rhl systems control the expression of several extracellular virulence factors including LasB elastase (51), as well as components of the Xcp type II protein secretion system (7).
Since induction of the stringent response entails pleiotropic effects in E. coli, leading to cellular adaptation to nutrient depletion, we wondered whether it could be connected to RpoS expression in P. aeruginosa. We found that overexpression of RelA prematurely activates rpoS transcription early during the growth phase. As rpoS might be regulated by quorum sensing (26), we investigated the effects of the stringent response on the components of the quorum-sensing systems, las and rhl. We show that the stringent response can prematurely activate the two layers of the quorum-sensing circuitry, leading to the production of extracellular virulence factors such as LasB elastase at low cell density.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Bacterial strains and plasmids used in this work are listed in Table 1. E. coli TG1 was used as a host for cloning experiments. To construct pMRL4, the 2.5-kb EcoRI-HindIII fragment from pSM10 that contains the E. coli relA structural gene was subcloned by standard procedures (29) into the broad-host-range vector pMMB67. Plasmid pLFR2 for complementation in the PAΩR3 strain was constructed as follows. A 2.7-kb fragment of PAO1 genomic DNA (positions 1022782 to 1025472; see http://www.pseudomonas.com for sequence data) containing the P. aeruginosa relA gene (encoding a protein with 46% identity and 65% similarity to the E. coli RelA) was amplified by PCR using two oligonucleotides (5′-GGCGGTACGCGAAATGAGTTCT-3′ and 5′-ATCCCAGGGGCAGCCGAATTC-3′), cloned into the SmaI site of pUC19, and then removed as an EcoRI-HindIII fragment. This fragment was cloned into the corresponding sites of pLAFR3 to yield pLFR2. The conjugative properties of pRK2013 were used to transfer plasmids from E. coli to P. aeruginosa by triparental mating. Unless noted otherwise, bacterial cells were grown at 37°C in Luria-Bertani broth (LB). Antibiotics used were tetracycline (100 μg/ml) and carbenicillin (300 μg/ml) for P. aeruginosa and tetracycline (20 μg/ml) and ampicillin (50 μg/ml) for E. coli. Proteolytic enzyme production was tested on tryptic soy agar plates containing either 1.5% skim milk (Difco) or elastin (Sigma).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| E. coli strains | ||
| TG1 | supE Δ(lac-proAB) thi hsdRΔ5 (F′ traD36 proA+B+lacIq ZΔM15) | 29 |
| E. coli [λI14] | Δ(argF-lac) λ::lasIp-lacZ | 41 |
| P. aeruginosa strains | ||
| PAO1 | Wild type, prototroph | B. W. Holloway |
| PAO-R1 | PAO1 ΔlasR::tet Smr | 15 |
| PAO-JP2 | ΔlasI ΔrhlI Tcr Hgr | 35 |
| PAΩR3 | PAO1 relA::pKoRΔ3 | This work |
| SS24 | PAO1 rpoS101::aacCI Gmr | 45 |
| Plasmids | ||
| pMMB67 | IncQ tacp lacIq Ap/Cbr | 14 |
| pLAFR3 | pLAFR1 derivative, IncP1 oriT cos Tcr | 11 |
| pSM10 | E. coli relA+ | 40 |
| pMRL4 | pMMB67 with E. coli relA under tacp | This work |
| pLFR2 | pLAFR3 with P. aeruginosa relA gene | This work |
| pMAL.S | IncP TcrP. aeruginosa rpoS′-lacZ fusion | 26 |
| pMAL.R | IncP TcrlasR′-lacZ fusion | 26 |
| pMAL.V | IncP TcrrhlR′-lacZ fusion | 26 |
| pRK2013 | ColE1 Tra+ Mob+ (pRK2) Kmr | 10 |
| pECP61.5 | ColE1 oriP. aeruginosa rhlAp-lacZ fusion tacp-rhlR+ Apr | 35 |
| pPCS1 | ColE1 lasR+ Apr | 41 |
| pKoRΔ3 | pUC19 with an 0.48-kb internal fragment of P. aeruginosa relA Ap/Cbr | This work |
Apr, ampicillin resistant; Cbr, carbenicillin resistant; Gmr, gentamicin resistant; Hgr, mercury resistant; Kmr, kanamycin resistant, Smr, streptomycin resistant; Tcr, tetracycline resistant.
Growth conditions for induction of the stringent response by serine hydroxamate (SHX) in P. aeruginosa were as previously described (22) with the following changes. Overnight LB cultures were washed, and bacteria were inoculated into morpholinepropanesulfonic acid (MOPS) glucose medium (31). When the cultures reached a turbidity of 0.1 at 600 nm, cells were washed and resuspended at a turbidity of 0.08 at 600 nm in low-phosphate (0.4 mM) MOPS medium supplemented with either 200 or 500 μM SHX (Sigma). Cells were grown with vigorous shaking, and culture supernatants were collected at regular intervals for determination of 3-oxo-C12-HSL concentrations. Experiments with the relA mutant PAΩR3 were performed in the presence of carbenicillin in order to maintain the insertional inactivation. To confirm that carbenicillin did not affect the experimental conditions, control experiments were performed using the wild-type strain PAO1 containing the pMMB67 vector. The premature induction of 3-oxo-C12-HSL provoked by the addition of SHX was similar in the presence and the absence of carbenicillin.
Construction of a P. aeruginosa relA mutant.
To generate a null allele of the P. aeruginosa relA gene, we amplified by PCR, using the PAO1 genome as a template and two oligonucleotides (5′-GATCAGCGCCAGCCTCAATCC-3′ and 5′-CAGTGCGCGCAGACTGGGAGCT-3′), a 726-bp internal fragment of the relA coding sequence corresponding to amino acids 125 to 365 of the P. aeruginosa RelA protein (747 residues). The PCR product was blunt ended and cloned into the SmaI site of pUC19. As it has been shown that the N-terminal segment of RelA may possess synthetic activity (40), a deletion of the sequences located downstream of the BglII unique site in relA (nucleotide position 848 relative to the start codon of relA) was subsequently made in the resulting construct, in order to shorten the promoter-proximal relA domain that remains after allelic disruption. The resulting plasmid (pKoRΔ3) carrying a 477-bp relA fragment was electroporated into PAO1, and potential relA mutants were selected as having carbenicillin resistance (encoded by the suicide plasmid). Insertional inactivation of relA, resulting from homologous recombination between pKoRΔ3 and the chromosomal relA locus, was checked by PCR analysis by using oligonucleotides (5′-GGCGGTACGCGAAATGAGTTCT-3′ and 5′-ATCCCAGGGGCAGCCGAATTC-3′) that hybridized to the P. aeruginosa relA flanking sequences. The chromosomal structure was further checked using primers that hybridized to vector sequences in combination with primers corresponding to the chromosomal flanking sequences. We identified a mutant, PAΩR3, containing relA::pKoRΔ3 in place of relA.
SDS-PAGE and immunoblot analysis.
Cellular proteins were dissolved by heating for 5 min at 95°C in sample buffer (2% sodium dodecyl sulfate [SDS], 0.75 M β-mercaptoethanol, 10% glycerol, 60 mM Tris-HCl [pH 6.8], 0.02% bromophenol blue) prior to SDS-polyacrylamide gel electrophoresis (PAGE) on 11% acrylamide gels. Extracellular proteins in the culture medium were precipitated with 10% (wt/vol) trichloroacetic acid and resuspended in sample buffer for gel analysis. Proteins were transferred onto nitrocellulose membranes as previously described (3) and incubated with antisera directed against elastase or RpoS. Immunoblots were developed by chemiluminescence (Pierce) using secondary antibodies conjugated to horseradish peroxidase.
β-Gal assays.
Overnight cultures of P. aeruginosa harboring lacZ transcriptional fusion plasmids were diluted to a turbidity of 0.01 at 600 nm in LB containing the appropriate antibiotics. Samples were harvested at intervals for determination of turbidity at 600 nm, quantification of cellular proteins (Bio-Rad protein assay), and β-galactosidase (β-Gal) assay as described elsewhere (7). All experiments were performed at least three times, and values from a representative experiment are presented. β-Gal activity is reported as micromoles of o-nitrophenol released per minute per milligram of protein.
Determination of autoinducer concentrations.
Culture supernatants were extracted with ethyl acetate, and autoinducer concentrations were determined in bioassays as previously described, using E. coli [λI14](pPCS1) for 3-oxo-C12-HSL (41) and PAO-JP2(pECP61.5) for C4-HSL (35). It has been shown that the 3-oxo-C12-HSL autoinducer can block the binding of C4-HSL to RhlR in E. coli, thereby inhibiting the activation of the rhlA′-lacZ fusion in pECP61.5 (36). To ascertain the absence of interference by coextracted 3-oxo-C12-HSL in the C4-HSL bioassay in P. aeruginosa PAO-JP2(pECP61.5), a competitive assay was performed in the presence of 1, 5, or 10 μM synthetic C4-HSL, together with increasing concentrations of synthetic 3-oxo-C12-HSL (0.1 to 25 μM). No differences in β-Gal activity could be detected for each given C4-HSL concentration in the absence or the presence of even high concentrations of 3-oxo-C12-HSL (data not shown), indicating that 3-oxo-C12-HSL does not interfere with our C4-HSL bioassay in P. aeruginosa.
RESULTS
Overexpression of the relA gene increases RpoS levels in P. aeruginosa
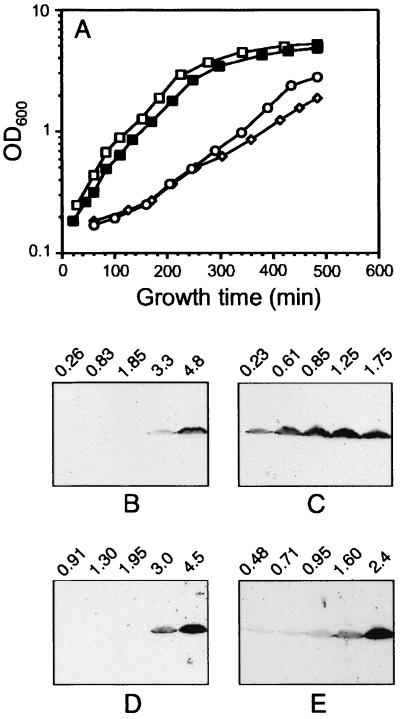
It has been shown elsewhere for E. coli that relA overexpression elicits the stringent response under constant nutritional abundance, so that cellular physiology is minimally disturbed (9, 40). Induction of the stringent response and ppGpp synthesis have also been demonstrated in Pseudomonas putida by overexpression of the E. coli relA gene (47). In order to test the effect of stringent control on rpoS expression in P. aeruginosa, we first examined the level of RpoS in the presence of the relA plasmid pMRL4 compared to the vector control pMMB67. When grown in LB medium, PAO1(pMRL4) showed a significantly altered growth rate compared to PAO1(pMMB67), with a twofold increase in doubling time (Fig. 1A). Similar growth rate reduction was previously observed upon relA overexpression in E. coli and P. putida (40, 47). The repression of the tacp promoter on pMRL4 is incomplete in P. aeruginosa, and the basal level of relA expression appears sufficient to induce stringent control. Addition of increasing concentrations of isopropyl-β-d-thiogalactopyranoside resulted in progressive inhibition of growth (data not shown). Culture samples of PAO1(pMMB67) and PAO1(pMRL4) were withdrawn throughout the growth cycle, and changes in the cellular content of RpoS were monitored by SDS-PAGE and immunoblotting. As shown in Fig. 1B and C, the pattern of RpoS accumulation was strongly modified in the presence of the relA plasmid. In the control strain PAO1(pMMB67), RpoS was detected only when the cultures had reached the onset of stationary phase (turbidity of 3.5 to 4.5 at 600 nm) (Fig. 1B), in agreement with previous observations (48). In contrast, in PAO1(pMRL4), significant amounts of RpoS were detected already in early growth phase (turbidity of 0.23 at 600 nm) and increased rapidly thereafter (Fig. 1C).
FIG. 1.
Growth and RpoS levels in the presence of relA multicopy in P. aeruginosa. (A) Growth curves of PAO1(pMMB67) (closed squares), PAO-R1(pMMB67) (open squares), PAO1(pMRL4) (diamonds), and PAO-R1(pMRL4) (circles) in LB medium. (B) Cellular RpoS levels during the growth of PAO1(pMMB67). Cells were grown in LB medium, and samples were taken at the turbidity at 600 nm (OD600) indicated above each lane. Immunoblot analysis was performed with 50 μg of total cellular protein applied per lane. (C) Same experiment as in panel B with the PAO1(pMRL4) strain. (D) PAO-R1(pMMB67) strain. (E) PAO-R1(pMRL4) strain.
While the expression of rpoS has been shown to be reduced in the lasR-minus strain PAO-R1 (26), it is currently controversial whether rpoS is regulated by quorum sensing (52). To investigate a possible link between the effect observed above and the quorum-sensing regulation, we analyzed RpoS expression in the lasR mutant strain PAO-R1 containing pMMB67 or pMRL4 (Fig. 1D and E). The overexpression of relA had a lesser effect on RpoS synthesis in the lasR mutant PAO-R1 than in wild-type PAO1. Indeed, during the early log phase of growth (up to a turbidity of 1 at 600 nm), the amount of RpoS was lower in strain PAO-R1(pMRL4) (Fig. 1E) than in strain PAO1(pMRL4) (Fig. 1C). However, when cell density exceeded a turbidity of 1 at 600 nm, RpoS accumulation was observed in PAO-R1(pMRL4) (Fig. 1E), in contrast to PAO-R1(pMMB67), in which RpoS was not detected until the culture reached a turbidity of 3 at 600 nm (Fig. 1D). These results indicate that the absence of a functional las quorum-sensing system in the PAO-R1 mutant does not preclude rpoS expression but has a partial effect on cellular levels produced under stringent response conditions. It was also noticeable that a similar growth rate decrease was caused by relA expression in PAO1 and in PAO-R1 (Fig. 1A), suggesting that the global effects of RelA on cell metabolism are likely not mediated by quorum sensing or RpoS.
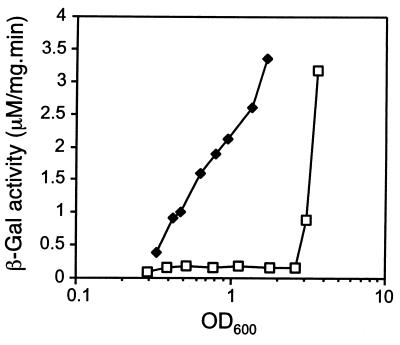
In order to investigate whether RelA affects the transcriptional control of rpoS in P. aeruginosa, we tested the expression of an rpoS′-lacZ fusion in strains PAO1(pMMB67, pMAL.S) and PAO1(pMRL4, pMAL.S). The induction pattern of rpoS was significantly changed by the presence of pMRL4 (Fig. 2). In accordance with previous reports, rpoS transcription remained at basal levels until the onset of stationary phase in the control strain (12, 26). In contrast, PAO1(pMRL4, pMAL.S) demonstrated high levels of rpoS expression already during early growth phase, indicating that relA positively affects the transcription of the P. aeruginosa rpoS gene.
FIG. 2.
Effect of relA multicopy on transcription of rpoS in P. aeruginosa. The expression of the rpoS′-lacZ transcriptional fusion in PAO1(pMMB67, pMAL.S) (squares) and PAO1(pMRL4, pMAL.S) (diamonds) grown in LB medium was monitored with β-Gal activity assays as described in Materials and Methods.
Influence of the stringent response on cell density-regulated proteins.
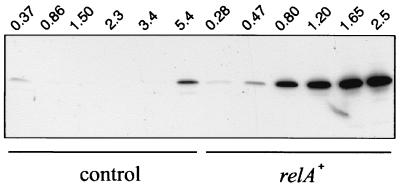
In view of the above results, we wondered whether the stringent response would also be able to activate the production of quorum-sensing-dependent virulence factors. To test this hypothesis, we investigated the proteolytic activity of PAO1(pMRL4). The synthesis of the LasB elastase, the major secreted protease of P. aeruginosa, is positively regulated by LasR and 3-oxo-C12-HSL (33) as well as by RhlR and C4-HSL (4). Using casein- and elastin-agar plate assays, it was apparent that PAO1(pMRL4) produced higher amounts of proteases than PAO1(pMMB67) (data not shown). To ascertain that elastin-degrading activity was correlated with an increased LasB synthesis, we analyzed culture supernatants of PAO1(pMMB67) and PAO1(pMRL4) by anti-LasB immunoblotting (Fig. 3). As expected, LasB elastase became detectable in PAO1(pMMB67) supernatants only when the cultures reached the onset of stationary phase (turbidity of 5.4 at 600 nm). In contrast, we observed a significant accumulation of LasB during early growth (turbidity of 0.47 at 600 nm) in PAO1(pMRL4) supernatants. As a control, immunodetection of plasmid-encoded β-lactamase did not reveal differences between the protein levels observed in PAO1(pMMB67) and PAO1(pMRL4) (data not shown). Therefore, it appeared from these experiments that relA overexpression is able to induce the synthesis of LasB elastase at low cell density.
FIG. 3.
Effects of relA overexpression on elastase production. The production of extracellular elastase in PAO1(pMMB67) (control) and PAO1(pMRL4) (relA+) was analyzed. Proteins from aliquots of culture supernatant corresponding to a constant mass of cells were used for immunoblot analysis with antibodies against LasB elastase. Samples were taken during growth in LB medium at the indicated turbidities at 600 nm.
Additionally, we found that the XcpY type II secretion factor, which is regulated by the quorum-sensing circuitry (7), was also produced at a lower cell density in strain PAO1(pMRL4) than in strain PAO1(pMMB67) (data not shown). Thus, in the presence of overexpressed relA, two proteins normally synthesized at high cell density under the control of the quorum-sensing circuitry were produced during the early phases of growth. These results suggested that the stringent response might be able to activate the quorum-sensing circuitry independently of the cell density.
Effect of relA overexpression on regulatory components of quorum sensing.
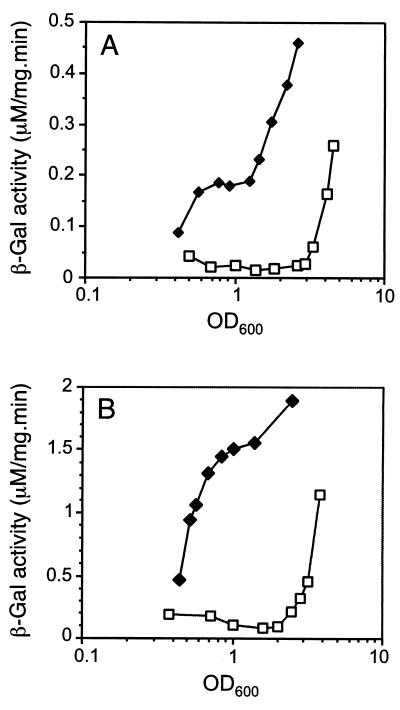
To determine how the stringent response could prematurely activate the quorum-sensing circuitry, we assayed the activity of a lasR′-lacZ fusion (pMAL.R) in the presence of pMRL4 (Fig. 4A). The lasR transcription profile in the control strain PAO1(pMMB67, pMAL.R) was consistent with previous reports showing that lasR is expressed in a cell density-dependent manner (1, 35). In contrast, lasR was already transcribed during early growth phase (turbidity of 0.5 at 600 nm) in strain PAO1(pMRL4, pMAL.R). Therefore, the stringent response seemed to activate lasR transcription prematurely. Since lasR regulates the expression of rhlR (26), we examined whether the presence of pMRL4 also affects rhlR transcription using the reporter fusion pMAL.V (rhlR′-lacZ) (Fig. 4B). Activation of rhlR in the control strain PAO1(pMMB67, pMAL.V) was very similar to that of lasR in strain PAO1(pMMB67, pMAL.R) and was cell density dependent as previously observed (36). In contrast, in strain PAO1(pMRL4, pMAL.V), rhlR was expressed early in the growth phase and independently of cell density. We concluded that relA overexpression has a positive effect on the transcription of both the lasR and the rhlR regulatory genes.
FIG. 4.
Effect of relA on the P. aeruginosa quorum-sensing regulators lasR and rhlR. (A) Expression of the lasR′-lacZ fusion in PAO1(pMMB67, pMAL.R) (squares) and PAO1(pMRL4, pMAL.R) (diamonds) during growth in LB medium. (B) Expression of the rhlR′-lacZ fusion in PAO1(pMMB67, pMAL.V) (squares) and PAO1(pMRL4, pMAL.V) (diamonds) in LB medium.
Previous studies with E. coli have shown that RelA can act indirectly by increasing the synthesis of RpoS, which in turn stimulates the expression of target genes (9). To determine whether the premature induction of lasR and rhlR in the presence of the multicopy relA is mediated by rpoS, we examined the effect of pMRL4 on the transcription of lasR′-lacZ and rhlR′-lacZ fusions in P. aeruginosa strain SS24, in which the rpoS gene is inactivated (45). Both lasR and rhlR fusions were transcribed in the control strain SS24(pMMB67), and the kinetics of their expression were similar to those observed in the wild-type strain PAO1 (data not shown). The overexpression of relA caused an increased expression of both quorum-sensing regulatory genes early during the growth of the rpoS mutant (2.6-fold increase of lasR′-lacZ activity and 3.2-fold increase of rhlR′-lacZ activity at a turbidity of 0.5 at 600 nm; 2.4-fold increase of lasR′-lacZ activity and 3.4-fold increase of rhlR′-lacZ activity at a turbidity of 1 at 600 nm; means of three independent experiments). Thus, rpoS is not required for the premature activation of the lasR and rhlR gene transcription by the stringent response.
In order to ascertain that the premature synthesis of elastase observed above is related to the activation of the quorum-sensing circuitry, we investigated the effect of relA overexpression on the extracellular elastase activity in the PAO-R1 background. Measurements of elastin Congo red hydrolysis performed as previously described (15) showed that culture supernatants of PAO-R1(pMMB67) and PAO-R1(pMRL4) did not contain elastin-degrading activity, while the PAO1(pMMB67) control strain produced elastolytic activity under the same growth conditions (data not shown). This confirmed that the effect of the stringent response on elastase production is mediated through the premature activation of the quorum-sensing circuitry.
Production of autoinducers during relA overexpression.
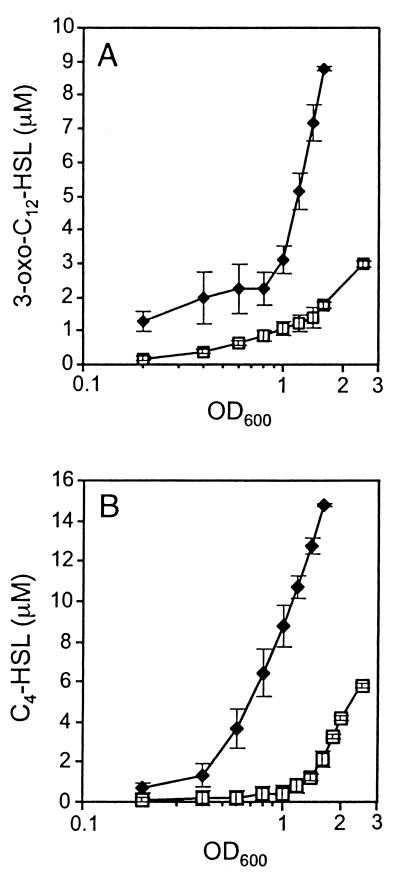
To further examine the effect of the stringent response on the quorum-sensing systems, we determined the effects of relA overexpression on the production of AHL signaling molecules in culture supernatants. The expression of the lasI′-lacZ fusion in E. coli [λI14](pPCS1) was monitored to investigate differences in 3-oxo-C12-HSL concentrations in culture supernatants of strains PAO1(pMMB67) and PAO1(pMRL4) (see Materials and Methods). As shown in Fig. 5A, accumulation of the 3-oxo-C12-HSL autoinducer was higher in early-growth-phase supernatants obtained from the pMRL4-containing strain. The difference was even more pronounced during the second half of growth, where the 3-oxo-C12-HSL concentration increased by a factor of 4 in one generation in strain PAO1(pMRL4) (turbidity of 0.8 to 1.6 at 600 nm), to reach about 9 μM. At the same growth stage (turbidity of 1.6 at 600 nm), the 3-oxo-C12-HSL concentration in culture fluid of strain PAO1(pMMB67) was only 1.8 μM.
FIG. 5.
Effect of relA overexpression on the production of 3-oxo-C12-HSL and C4-HSL. The concentrations of autoinducers were determined in bacterial supernatants of either strain PAO1(pMMB67) (squares) or PAO1(pMRL4) (diamonds). Overnight cell cultures were washed and resuspended in LB medium at an initial turbidity at 600 nm of 0.05. Supernatants were collected at regular intervals, extracted with ethyl acetate, and assayed for the presence of both autoinducer molecules using specific bioassays as described in Materials and Methods. Shown are the means (± SDs) of results from three independent experiments performed in triplicate. (A) Bioassay for 3-oxo-C12-HSL. (B) Bioassay for C4-HSL.
To investigate the effect on the autoinducer of the rhl system, C4-HSL accumulation was measured by monitoring the activity of an rhlA reporter fusion in PAO-JP2(pECP61.5) as described in Materials and Methods. Results in Fig. 5B show that strain PAO1(pMRL4) yielded detectable C4-HSL concentrations already from the beginning of growth (turbidity of 0.2 at 600 nm). In contrast, the C4-HSL was detected in the supernatants of the control strain PAO1(pMMB67) only when cell density reached a turbidity of 1.2 to 1.5 at 600 nm. Together, the analysis of lasR and rhlR gene expression described above and the determination of autoinducer concentrations in culture supernatants suggested that the overexpression of relA leads to premature, cell density-independent activation of both the las and the rhl quorum-sensing systems.
Production of 3-oxo-C12-HSL upon induction of the stringent response by SHX.
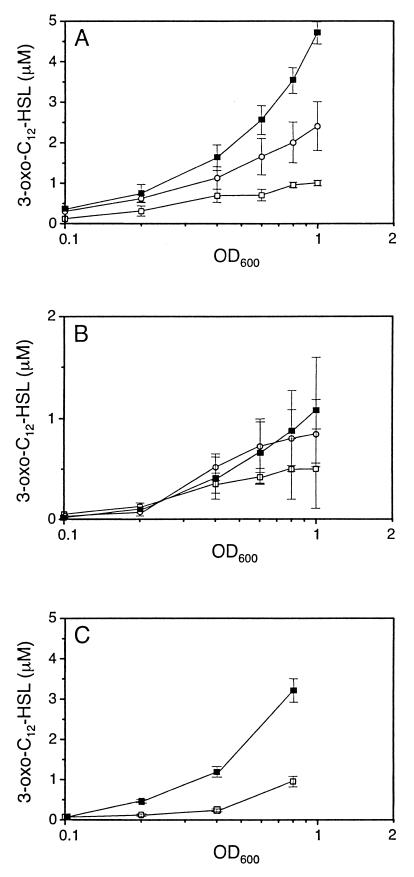
In order to confirm that the production of autoinducers can be activated at low cell density, we decided to induce the stringent response by a second mechanism that does not involve manipulation of RelA levels through relA overexpression. It has been previously demonstrated that the amino acid analogue SHX, a competitive inhibitor of seryl-tRNA synthetase (50), can induce the stringent response and ppGpp accumulation in E. coli as well as in P. aeruginosa (5, 22). We therefore measured the levels of the 3-oxo-C12-HSL autoinducer in PAO1 supernatants upon addition of SHX during growth in MOPS minimal medium as previously described (22). Curves in Fig. 6A show that 3-oxo-C12-HSL accumulation occurred at lower cell density in the presence of either 200 or 500 μM SHX. Whereas the 3-oxo-C12-HSL concentrations rose only slowly in the control culture supernatant, to reach about 1 μM at a turbidity of 1.0 at 600 nm, a similar level of autoinducer was already detected at a turbidity of 0.2 at 600 nm in the presence of 500 μM SHX. At a turbidity of 1.0 at 600 nm, the 3-oxo-C12-HSL concentration had reached 2.45 and 4.75 μM in the presence of 200 and 500 μM SHX, respectively. This demonstrated that inducing the stringent response by addition of SHX or by relA overexpression provokes a similar effect on premature autoinducer production.
FIG. 6.
Effect of the amino acid stringent response on the production of 3-oxo-C12-HSL. Bioassays for 3-oxo-C12-HSL extracted from the culture supernatants of the wild-type strain PAO1 (A), the relA mutant strain PAΩR3 (B), and the complemented strain PAΩR3(pLFR2) (C) were performed. Strains were grown in MOPS medium either in the absence (open squares) or in the presence of 200 (open circles) or 500 (closed squares) μM SHX as described in Materials and Methods. Shown are the means (± SDs) of three (A) and two (B and C) independent experiments performed in triplicate.
Autoinducer production by a P. aeruginosa relA mutant.
The recent annotation of the PAO1 genome sequence identified a gene coding for a homolog of RelA, located between bp 1023053 and 1025472 on the PAO1 chromosome (44; see also http://www.pseudomonas.com). In order to confirm that premature autoinducer production can be attributed to the induction of the relA-dependent stringent response, we constructed a P. aeruginosa relA mutant (PAΩR3) by insertional inactivation. Mutant PAΩR3 was still able to produce elastase (as determined by elastin-agar plates) and both autoinducers in LB medium (3-oxo-C12-HSL, 2.4 ± 0.1 μM; C4-HSL, 3.4 ± 0.2 μM; mean of three independent experiments ± standard deviation [SD], measured at a turbidity of 3.2 to 3.5 at 600 nm). The growth of the PAΩR3 strain in MOPS medium was similar to that of the wild-type strain PAO1 (data not shown). The production of the 3-oxo-C12-HSL autoinducer was then measured in the absence or in the presence of SHX. In the absence of SHX, the production of 3-oxo-C12-HSL was slightly delayed and reduced in amount compared to the wild-type strain (Fig. 6B). In contrast to the effects observed with the wild-type strain (Fig. 6A), neither 200 nor 500 μM SHX induced accumulation of high levels of 3-oxo-C12-HSL in the supernatants of the relA mutant strain PAΩR3 (Fig. 6B; 3-oxo-C12-HSL concentrations measured at a turbidity of 1 at 600 nm: 0.55 ± 0.32 and 1.05 ± 0.5 μM in the absence or presence of 500 μM SHX, respectively). As an additional control for relA-mediated accumulation of 3-oxo-C12-HSL, we monitored autoinducer concentrations in supernatants of PAΩR3 carrying pLFR2, which contains the P. aeruginosa relA gene (see Materials and Methods for details of construction). Complementation of the relA mutant with pLFR2 restored the accumulation of 3-oxo-C12-HSL during growth in the presence of 500 μM SHX (Fig. 6C). These results indicate that the P. aeruginosa relA gene is required for the premature production of the 3-oxo-C12-HSL autoinducer upon induction of the stringent response by SHX.
DISCUSSION
Studies with E. coli have revealed that relA overexpression induces the stringent response and activates the transcription of the stationary-phase sigma factor RpoS. Exploring the effects of stringent control on the expression of rpoS in P. aeruginosa, we found that relA overexpression induces premature rpoS transcription during early phases of growth. This effect was decreased when RelA was overproduced in a ΔlasR mutant strain, suggesting that rpoS induction by the stringent response is partially dependent on an intact quorum-sensing circuitry. We demonstrated that the stringent response activates the two quorum-sensing regulators lasR and rhlR at low cell density. Consistently, the accumulation of the two signaling molecules 3-oxo-C12-HSL and C4-HSL was considerably increased during early growth phases in the presence of multiple copies of relA. Using the amino acid analogue SHX to elicit the stringent response, we also observed a premature accumulation of 3-oxo-C12-HSL in the wild-type strain PAO1 but not in the relA mutant PAΩR3. Therefore, it seems that the stringent response can activate the quorum-sensing circuitry independently of cell density by changing the transcription pattern of regulatory genes and the kinetics of autoinducer production. Importantly, we determined that the P. aeruginosa relA mutant was still able to produce elastase and to accumulate 3-oxo-C12-HSL and C4-HSL when grown in rich media. This implies that the function of the stringent response in modulating quorum sensing is likely to be restricted to cellular adaptation during nutrient deprivation, rather than being a permanent, superimposed control level.
The link between rpoS and quorum sensing has recently given rise to controversy. Whereas a previous report had described the regulation of rpoS transcription by quorum sensing (26), this effect was not observed in a more recent study (52), which suggested that rpoS negatively regulates rhlI. The reason for this discrepancy remains unknown. From our studies, it appears that, under some conditions (i.e., when provoking the stringent response by relA expression), rpoS expression is affected by the absence of a functional lasR gene, but it must be stressed that RpoS is still produced in such a mutant background and that rpoS is not required for the premature activation by the stringent response of the quorum-sensing circuitry. The apparent contradiction in the experiments reported by Latifi et al. (26) and Whiteley et al. (52) could therefore reflect differences in growth conditions and/or nutritional status of bacterial strains. Clearly, more work is needed to explain these differences and to determine the precise connection between rpoS and quorum sensing. Interestingly, studies with E. coli have suggested that nonacylated HSL generated as a consequence of ppGpp accumulation in response to nutrient starvation might be the signal for inducing transcription of rpoS regardless of bacterial density (19, 43).
Much has been learned recently about AHL-dependent gene regulation, and it is now apparent that quorum-sensing systems in P. aeruginosa are subject to additional levels of control (1, 39). A third intercellular signal, the Pseudomonas quinolone signal, may play a role in the response to cellular stress provoked by late-growth-phase conditions (30). The Pseudomonas quinolone signal is not involved in sensing population density, but it is related to the quorum-sensing hierarchy and upregulates the rhl system. A gene coding for a homolog of LasR and RhlR, qscR, was recently identified (8). A mutation in qscR leads to early production of 3-oxo-C12-HSL and C4-HSL, as well as overexpression of quorum-sensing-controlled virulence factors. Our data indicate that, under adverse nutritional conditions, the stringent response might also control the activation of the quorum-sensing circuitry at low population density.
It has been shown that both production of autoinducer and production of extracellular virulence factors by P. aeruginosa are dependent on polyphosphate kinase, the enzyme responsible for the synthesis of polyP (38). PolyP is also essential for nutritional stress adaptation and survival in stationary phase in E. coli (37, 42). Given that polyP accumulation in E. coli depends on multiple mechanisms including relA expression (2), it is possible that the mechanisms by which the stringent response influences quorum sensing could be related in part to the regulatory role of polyP. The stringent response might thus act in an adaptive response pathway leading to polyP accumulation, activation of the quorum-sensing systems, and adjustments of gene expression by modulation of promoter selectivity of RNA polymerase (42). The synthesis of the biofilm polysaccharide alginate, which helps P. aeruginosa to survive in nutrient-poor environments (49), is coregulated with polyP in mucoid strains. It has been shown that regulation of alginate production involves the enzyme nucleoside diphosphate kinase (Ndk), which enhances formation of GTP, ppGpp, and polyP during starvation conditions (22). Thus, polyP accumulation is correlated with the activation of a complex network contributing to cell adaptation to stressful conditions as well as expression of virulence traits. Further investigation of a link between the stringent response and polyP synthesis in P. aeruginosa, their respective effects on quorum sensing, and the analysis of the effects of alginate regulators on rpoS expression will be required to clarify the means by which the metabolic response to nutritional deficiencies takes place in P. aeruginosa.
When grown under laboratory conditions, bacteria typically encounter nutrient limitation only at high cell density. However, free-living bacteria are likely to be exposed to exhaustion of carbon or amino acid sources independently of their own cell density. During establishment of host infection, P. aeruginosa could therefore be exposed to nutritional stress before a critical cell density has been reached. In this situation, the stringent response might prematurely activate the production of quorum-sensing-regulated virulence factors. These factors would then provide the bacteria with new nutrients through their enzymatic activity or allow them to disseminate to more favorable niches. Under these circumstances, the quorum-sensing circuit may help P. aeruginosa to rapidly adapt to nutritional deficiencies.
ACKNOWLEDGMENTS
We are grateful to M. Foglino and A. Latifi for their interest and support throughout the course of this work and for supplying lacZ fusions used in this paper. We thank A. Lazdunski for laboratory facilities at the LISM that allowed the completion of a part of this work and G. Ball for excellent technical assistance. We gratefully acknowledge B. H. Iglewski for strains and for providing us with purified autoinducers. We also thank K. Tanaka for the generous gift of anti-RpoS antibodies and D. E. Ohman for providing the P. aeruginosa rpoS mutant strain.
This work was supported by Swiss National Research Foundation grants 3231-051940.97 and 3200-052189.97 to C.V.D.
REFERENCES
- 1.Albus A M, Pesci E C, Runyen-Janecky L J, West S E H, Iglewski B H. Vfr controls quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3928–3935. doi: 10.1128/jb.179.12.3928-3935.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ault-Riché D, Fraley C D, Tzeng C-M, Kornberg A. Novel assay reveals multiple pathways regulating stress-induced accumulation of inorganic polyphosphate in Escherichia coli. J Bacteriol. 1998;180:1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ball G, Chapon-Hervé V, Bleves S, Michel G, Bally M. Assembly of XcpR in the cytoplasmic membrane is required for extracellular protein secretion in Pseudomonas aeruginosa. J Bacteriol. 1999;181:382–388. doi: 10.1128/jb.181.2.382-388.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brint J M, Ohman D E. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177:7155–7163. doi: 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cashel M, Gentry D R, Hernandez V J, Vinella D. The stringent response. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1458–1496. [Google Scholar]
- 6.Chakraburtty R, Bibb M. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J Bacteriol. 1997;179:5854–5861. doi: 10.1128/jb.179.18.5854-5861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapon-Hervé V, Akrim M, Latifi A, Williams P, Lazdunski A, Bally M. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol Microbiol. 1997;24:1169–1178. doi: 10.1046/j.1365-2958.1997.4271794.x. [DOI] [PubMed] [Google Scholar]
- 8.Chugani S A, Whiteley M, Lee K M, D'Argenio D, Manoil C, Greenberg E P. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2001;98:2752–2757. doi: 10.1073/pnas.051624298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eichel J, Chang Y Y, Riesenberg D, Cronan J E., Jr Effect of ppGpp on Escherichia coli cyclopropane fatty acid synthesis is mediated through the RpoS sigma factor (ςS) J Bacteriol. 1999;181:572–576. doi: 10.1128/jb.181.2.572-576.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;79:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman A M, Long S R, Brown S E, Buikema W J, Ausubel F M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982;18:289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- 12.Fujita M, Tanaka K, Takahashi H, Amemura A. Transcription of the principal sigma-factor genes, rpoD and rpoS, in Pseudomonas aeruginosa is controlled according to the growth phase. Mol Microbiol. 1994;13:1071–1077. doi: 10.1111/j.1365-2958.1994.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 13.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 14.Fürste J P, Pansegrau W, Frank R, Blöcker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 15.Gambello M J, Iglewski B H. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentry D R, Cashel M. Mutational analysis of the Escherichia coli spoT gene identifies distinct but overlapping regions involved in ppGpp synthesis and degradation. Mol Microbiol. 1996;19:1373–1384. doi: 10.1111/j.1365-2958.1996.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 17.Gentry D R, Hernandez V J, Nguyen L H, Jensen D B, Cashel M. Synthesis of the stationary-phase sigma factor ςS is positively regulated by ppGpp. J Bacteriol. 1993;175:7982–7989. doi: 10.1128/jb.175.24.7982-7989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenway D L, England R R. ppGpp accumulation in Pseudomonas aeruginosa and Pseudomonas fluorescens subjected to nutrient limitation and biocide exposure. Lett Appl Microbiol. 1999;29:298–302. doi: 10.1046/j.1365-2672.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 19.Huisman G W, Kolter R. Sensing starvation: a homoserine lactone-dependent signalling pathway in Escherichia coli. Science. 1994;265:537–539. doi: 10.1126/science.7545940. [DOI] [PubMed] [Google Scholar]
- 20.Jørgensen F, Bally M, Chapon-Hervé V, Michel G, Lazdunski A, Williams P, Stewart G S A B. RpoS-dependent stress tolerance in Pseudomonas aeruginosa. Microbiology. 1999;145:835–844. doi: 10.1099/13500872-145-4-835. [DOI] [PubMed] [Google Scholar]
- 21.Joseleau-Petit D, Vinella D, D'Ari R. Metabolic alarms and cell division in Escherichia coli. J Bacteriol. 1999;181:9–14. doi: 10.1128/jb.181.1.9-14.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H Y, Schlictman D, Shankar S, Xie Z, Chakrabarty A M, Kornberg A. Alginate, inorganic polyphosphate, GTP and ppGpp synthesis co-regulated in Pseudomonas aeruginosa: implications for stationary phase survival and synthesis of RNA/DNA precursors. Mol Microbiol. 1998;27:717–725. doi: 10.1046/j.1365-2958.1998.00702.x. [DOI] [PubMed] [Google Scholar]
- 23.Kolter R, Siegele D A, Tormo A. The stationary phase of the bacterial life cycle. Annu Rev Microbiol. 1993;47:855–874. doi: 10.1146/annurev.mi.47.100193.004231. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda A, Murphy H, Cashel M, Kornberg A. Guanosine tetra- and pentaphosphate promote accumulation of inorganic polyphosphate in Escherichia coli. J Biol Chem. 1997;272:21240–21243. doi: 10.1074/jbc.272.34.21240. [DOI] [PubMed] [Google Scholar]
- 25.Lange R, Hengge-Aronis R. The cellular concentration of the ςS subunit of RNA polymerase in Escherichia coli is controlled at the level of transcription, translation, and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 26.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 27.Latifi A, Winson M K, Foglino M, Bycroft B W, Stewart G S A B, Lazdunski A, Williams P. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol. 1995;17:333–343. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]
- 28.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςS (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 29.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 30.McKnight S L, Iglewski B H, Pesci E C. The Pseudomonas quinolone signal regulates quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 2000;182:2702–2708. doi: 10.1128/jb.182.10.2702-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neidhardt F C, Bloch P L, Smith D F. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochsner U A, Reiser J. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:6424–6428. doi: 10.1073/pnas.92.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 34.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearson J P, Pesci E C, Iglewski B H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pesci E C, Pearson J P, Seed P C, Iglewski B H. Regulation of las and rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 1997;179:3127–3132. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao N N, Kornberg A. Inorganic polyphosphate supports resistance and survival of stationary-phase Escherichia coli. J Bacteriol. 1996;178:1394–1400. doi: 10.1128/jb.178.5.1394-1400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rashid M H, Rumbaugh K, Passador L, Davies D G, Hamood A N, Iglewski B H, Kornberg A. Polyphosphate kinase is essential for biofilm development, quorum sensing, and virulence of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2000;97:9636–9641. doi: 10.1073/pnas.170283397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reimmann C, Beyeler M, Latifi A, Winteler H, Foglino M, Lazdunski A, Haas D. The global activator GacA of Pseudomonas aeruginosa PAO positively controls the production of the autoinducer N-butyryl-homoserine lactone and the formation of the virulence factors pyocyanin, cyanide, and lipase. Mol Microbiol. 1997;24:309–319. doi: 10.1046/j.1365-2958.1997.3291701.x. [DOI] [PubMed] [Google Scholar]
- 40.Schreiber G, Metzger S, Aizenman E, Roza S, Cashel M, Glaser G. Overexpression of the relA gene in Escherichia coli. J Biol Chem. 1991;266:3760–3767. [PubMed] [Google Scholar]
- 41.Seed P C, Passador L, Iglewski B H. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J Bacteriol. 1995;177:654–659. doi: 10.1128/jb.177.3.654-659.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiba T, Tsutsumi K, Yano H, Ihara Y, Kameda A, Tanaka K, Takahashi H, Munekata M, Rao N N, Kornberg A. Inorganic polyphosphate and the induction of rpoS expression. Proc Natl Acad Sci USA. 1997;94:11210–11215. doi: 10.1073/pnas.94.21.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sitnikov D M, Schineller J B, Baldwin T O. Control of cell division in Escherichia coli: regulation of transcription of ftsQA involves both rpoS and SdiA-mediated autoinduction. Proc Natl Acad Sci USA. 1996;93:336–341. doi: 10.1073/pnas.93.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stover C K, Pham X Q, Erwin A L, Mizoguchi S D, Warrener P, Hickey M J, Brinkman F S L, Hufnagle W O, Kowalik D J, Lagrou M, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 45.Suh S J, Silo-Suh L, Woods D E, Hassett D J, West S E, Ohman D E. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3890–3897. doi: 10.1128/jb.181.13.3890-3897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svitil A L, Cashel M, Zyskind J W. Guanosine tetraphosphate inhibits protein synthesis in vivo. A possible protective mechanism for starvation stress in Escherichia coli. J Biol Chem. 1993;268:2307–2311. [PubMed] [Google Scholar]
- 47.Sze C C, Shingler V. The alarmone (p)ppGpp mediates physiological-responsive control at the sigma 54-dependent Po promoter. Mol Microbiol. 1999;31:1217–1228. doi: 10.1046/j.1365-2958.1999.01264.x. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka K, Takahashi H. Cloning, analysis and expression of an rpoS homologue gene from Pseudomonas aeruginosa PAO1. Gene. 1994;150:81–85. doi: 10.1016/0378-1119(94)90862-1. [DOI] [PubMed] [Google Scholar]
- 49.Terry J M, Piña S E, Mattingly S J. Role of energy metabolism in conversion of nonmucoid Pseudomonas aeruginosa to the mucoid phenotype. Infect Immun. 1992;60:1329–1335. doi: 10.1128/iai.60.4.1329-1335.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tosa T, Pizer L I. Biochemical bases for the antimetabolite action of l-serine hydroxamate. J Bacteriol. 1971;106:972–982. doi: 10.1128/jb.106.3.972-982.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Delden C, Iglewski B H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whiteley M, Parsek M R, Greenberg E P. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J Bacteriol. 2000;182:4356–4360. doi: 10.1128/jb.182.15.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winson M K, Camara M, Latifi A, Foglino M, Chhabra S R, Daykin M, Bally M, Chapon V, Salmond G P, Bycroft B W, Lazdunski A, Stewart G S A B, Williams P. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]