Abstract
Objectives
Transurethral resection of bladder tumor with photodynamic diagnosis has been reported to result in lower residual tumor and intravesical recurrence rates in non‐muscle invasive bladder cancer. We aimed to evaluate the usefulness of photodynamic diagnosis‐transurethral resection of bladder tumor combined with oral 5‐aminolevulinic acid hydrochloride for high‐risk non‐muscle invasive bladder cancer.
Methods
High‐risk non‐muscle invasive bladder cancer patients with an initial photodynamic diagnosis‐transurethral resection of bladder tumor (photodynamic diagnosis group) were prospectively registered between 2018 to 2020. High‐risk non‐muscle invasive bladder cancer cases with a history of initial white‐light transurethral resection of bladder tumor (white‐light group) were retrospectively registered. Propensity score‐matching analysis was used to compare residual tumor rates, and factors that could predict residual tumors at the first transurethral resection of bladder tumor were evaluated.
Results
Analyses were conducted with 177 and 306 cases in the photodynamic diagnosis and white‐light groups, respectively. The residual tumor rates in the photodynamic diagnosis and white‐light groups were 25.7% and 47.3%, respectively. Factor analysis for predicting residual tumors in the photodynamic diagnosis group showed that the residual tumor rate was significantly higher in cases with a current/past smoking history, multiple tumors, and pT1/pTis. When each factor was set as a risk level of 1, cases with a total risk score ≤1 showed a significantly lower residual tumor rate than cases with a total risk score ≥2 (8.3% vs 33.3%, odds ratio 5.46 [1.81–22.28]).
Conclusions
In high‐risk non‐muscle invasive bladder cancer cases, the odds of a residual tumor after initial photodynamic diagnosis‐transurethral resection of bladder tumor were 0.39‐fold that of the odds of those after initial white‐light transurethral resection of bladder tumor. A risk stratification model could be used to omit the second transurethral resection of bladder tumor in 27% of the cases.
Keywords: 5‐aminolevulinic acid, bladder cancer, high‐risk NMIBC, photodynamic diagnosis, second TURBT
Abbreviations & Acronyms
- 5‐ALA
5‐aminolevulinic acid
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CIS
cancer in situ
- HAL
hexylaminolevulinate
- NMIBC
non‐muscle invasive bladder cancer
- OR
odds ratio
- PDD
photodynamic diagnosis
- PDT
photodynamic therapy
- PPIX
protoporphyrin IX
- ROS
reactive oxygen species
- TURBT
transurethral resection of the bladder tumor
- UC
urothelial carcinoma
- UTUC
upper tract urothelial carcinoma
- WL
white light
Introduction
Bladder cancer is associated with very high morbidity and mortality rates, with 430 000 new cases and 1.65 million deaths worldwide every year. 1 The standard therapy for NMIBC, which accounts for approximately 70% of bladder cancers, is TURBT. 2 TURBT is performed for complete tumor resection. However, patients with high‐risk NMIBC, defined as pT1 cancer invading the lamina propria, high‐grade UC, or CIS, show residual tumor detection rates of 33–78% during the second TURBT, 3 , 4 with the majority of residual tumors appearing in the first TURBT lesion area. 4 Therefore, a second TURBT is strongly recommended 3–8 weeks after the first procedure. 2 , 5 , 6
PDD involves the administration of the photosensitizers 5‐ALA and HAL in either an oral (5‐ALA) or intravesical (5‐ALA, HAL) manner. PPIX is also administered, which selectively accumulates in tumor cells and is excited by blue light (400–410 nm) using a fluorescent cystoscope, and lesions that show red fluorescence (approximately 635 nm) are detected. PDD can improve the detection rate of CIS, 7 , 8 and TURBT combined with PDD (PDD‐TURBT) can reduce the residual tumor and intravesical recurrence rates. 9 , 10 , 11 The residual tumor rate in patients treated with PDD‐TURBT, including high‐risk cases, was 10.3%, which was significantly lower than that associated with normal TUR (25.4%). 12 However, the residual rate for high‐risk cases defined according to the 8th edition of the Union of International Cancer Control has not been reported. In Japan, treatment with oral 5‐ALA hydrochloride has been covered by health insurance since 2017, and PDD‐TURBT is now included in the list of insured medical treatments. 13 , 14 , 15
This study aimed to compare the residual tumor rate after initial PDD‐TURBT for high‐risk cases with those after the second TURBT for high‐risk NMIBC cases previously treated with WL. We also investigated the usefulness of PDD‐TURBT and created a risk‐stratification model to identify cases that may not require a second TURBT. To our knowledge, this is the first multicenter prospective study to investigate the residual tumor rate after PDD‐TURBT using oral 5‐ALA hydrochloride for high‐risk NMIBC cases to our knowledge (UMIN ID: UMIN000035712).
Methods
Research design
We conducted a single‐arm, multicenter, prospective study using propensity score‐matching analysis with retrospective historical control.
Sample size determination
The target sample size was set by assuming 40% and 20% residual tumor rates at the second TURBT after the first TURBT using WL and PDD, respectively, for high‐risk cases. 10 Thus, the sample size with a significance level of 0.05 and power of 0.9 was 118 cases. However, a prospective sample size of 200 cases was set to account for the reductions caused by propensity score‐matching analysis (assuming a matching rate of 60%). Based on historical data, it was assumed that 60% of the cases could be matched, and a target sample size of 300 cases was set.
Endpoints
The primary endpoint was the residual tumor rate at the time of the second TURBT, which was evaluated by histopathological analysis of the tissue around the tumor and the bottom of the tumor using a WL source. The secondary endpoint was recurrence‐free survival, evaluated at 3‐month intervals up to 2 years after the second TURBT. Exploratory endpoints included the predicted residual tumor and recurrence‐free survival rates by UroVysion for cases with concurrent CIS observed at the first TURBT.
Prospective study
PDD‐TURBT was conducted with radical curative intent at participating study institutions from December 2018 to December 2020. Patients aged ≥20 and <90 years with a histopathological diagnosis of high‐risk NMIBC (high‐grade UC, pT1, or concurrent CIS) who wished to participate in the study were included. In principle, all patients with suspected high‐risk NMIBC were designated to be consecutively treated with PDD‐TURBT during the enrollment period. Cases involving incomplete resection or extensive CIS involving more than 10% of the bladder at the time of the first PDD‐TURBT were excluded.
Historical data
We included patients who underwent TURBT under WL and were diagnosed with high‐risk NMIBC, followed by a second TURBT within 2 months after the first visit were retrospectively included as participants from January 2006 to November 2016. The recruitment period of the historical cohort was set to be the period of time necessary to recruit 300 cases for statistical calculations and to follow the progress of the cohort for 2 years.
PDD‐TURBT
(i) Patients diagnosed with NMIBC who consented to the implementation of PDD‐TURBT were orally administered 5‐ALA hydrochloride (20 mg/kg) dissolved in distilled water 3 h before the insertion of the cystoscope (range 2–4 h). (ii) Intravesical observations were performed under PDD before TURBT, areas around red fluorescence sites were marked, and TURBT was performed as usual under WL. (iii) After completing the resection procedures, intravesical observations were performed again under PDD, the absence of residual red mucosa was confirmed, and the procedure was completed.
Equipment used
The primary equipment used in this study was a D‐LIGHT system (KARL STORZ Japan, Tokyo, Japan). A charge‐coupled device camera was attached to a dedicated telescope, and images were observed on a monitor screen. Some institutions used Aladuck LS‐DLED (SBI Pharma, Tokyo, Japan) as the light source and VISERA ELITE II (Olympus, Tokyo, Japan) as the image monitor with an ordinary telescope equipped with a dedicated cut filter. The observation times under PDD were set to 5–10 min to prevent photobleaching.
Procedure standardization
Before study initiation, the PDD procedure was standardized among institutions by distributing and viewing typical PDD‐TURBT video images.
Second TURBT and subsequent management
Generally, the second TURBT under WL was performed within 2 months of the first TURBT. The second TURBT involved resectioning the bottom of the tumor and the surrounding mucosa, centering on the previously resected area. Subsequently, intravesical recurrence was evaluated over 2 years of regular medical examinations (urinary cytology and cystoscopy every 3 months) performed as a daily clinical practice. Postoperative adjuvant therapy was mainly intravesical bacillus Calmette‐Guerin infusion therapy, per the bladder cancer clinical practice guidelines.
Complications
Adverse events were investigated for elevated AST or ALT levels, skin photosensitivity, gastrointestinal disorders, and severe hypotension were investigated based on the Common Terminology Criteria for Adverse Events ver. 5.0.
Data collection and analysis
Patient data from each institution were collected and analyzed by electronic data capture using the REDCap system. All endpoints were analyzed with a per‐protocol set, which excluded ineligible cases, cases without endpoint data, and cases that deviated from the research protocol.
The primary endpoint and patient background data were locked in February 2021 and analyzed. Propensity score matching analysis was conducted for the primary endpoints. Propensity scores were calculated for the initial PDD‐TURBT group and the initial WL‐TURBT group (historical data group) by using the background factors of age, sex, tumor diameter, history of bladder cancer, and the number of tumors. Pair matching was performed by setting the caliper to 0.2, and the primary endpoints were compared between the postmatching groups using Fisher’s exact test. Logistic regression analysis was also performed to identify the factors that affected the primary endpoint. The free software EZR ver. 1.54 was used for the statistical analysis. The significance level of the tests was 5% on both sides, and the confidence interval estimation was 0.95.
Results
Summary of the study
Among the 188 cases registered in the PDD group, 177 were included in the analyses after excluding 11 ineligible cases. For historical data evaluation, 306 of the 313 cases registered in the WL group were analyzed after excluding seven ineligible cases (Fig. 1). The patient backgrounds of each group are shown in Table S1. Multivariate analysis using logistic regression was performed for the factors predicting residual tumors at the second TURBT in all 483 cases. Significant predictive factors included the presence of PDD use during the initial TURBT (P < 0.001; OR 0.46 [0.3–0.71]), number of tumors (P < 0.001; OR 2.58 [1.66–4.0]), and the presence of concurrent CIS during the initial TURBT (P = 0.003; OR 1.96 [1.24–3.11]) (Table S2).
Fig. 1.
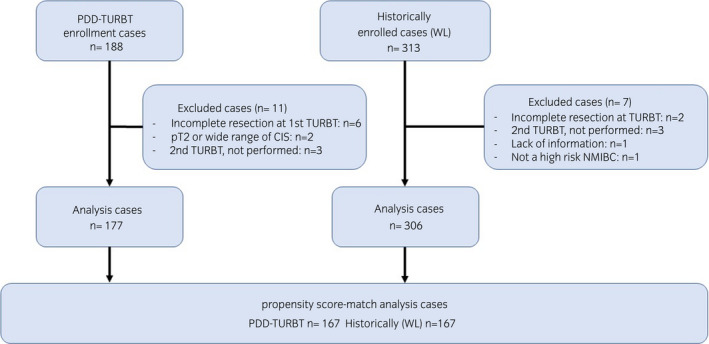
Diagram of registered research cases.
Propensity score‐matching analysis
The two groups were compared after propensity score matching (167 cases in each group). The two groups showed no significant differences in clinical background factors (Table 1). The residual tumor rates at the second TURBT, which was the primary endpoint, were 25.7% in the PDD group and 47.3% in the WL group, indicating significant differences between the two groups (P < 0.001; OR 0.39 [0.24–0.63]; Fig. 2). Upstaging to muscle‐invasive bladder cancer at the second TURBT was observed in four cases (3.0%) in the WL group but not in the PDD group (data not shown).
Table 1.
Patient characteristics of WL group and PDD group after propensity score‐match analysis
| WL (%) | PDD (%) | P‐value† | |
|---|---|---|---|
| (n = 167) | (n = 167) | ||
| Median age (interquartile range) | 72 (64, 77) | 72 (66, 77) | 0.789 |
| Sex | |||
| Female | 25 (15.0) | 24 (14.4) | 1 |
| Male | 142 (85.0) | 143 (85.6) | |
| Previous NMIBC | |||
| No | 152 (91.0) | 152 (91.0) | 1 |
| Yes | 15 (9.0) | 15 (9.0) | |
| History of UTUC | |||
| No | 130 (77.8) | 124 (74.3) | 0.818 |
| Yes | 37 (22.2) | 43 (25.7) | |
| Tumor size | |||
| <3 cm | 61 (36.5) | 58 (34.7) | 0.522 |
| ≥3 cm | 106 (63.5) | 109 (65.3) | |
| Number of tumors | |||
| Single | 157 (94.6) | 156 (93.4) | 0.819 |
| Multiple | 9 (5.4) | 11 (6.6) | |
| Residual tumor | |||
| Absence | 88 (52.7) | 124 (74.3) | <0.001 |
| Presence | 79 (47.3) | 43 (25.7) | |
Statistical test performed: Fisher's exact test.
Fig. 2.
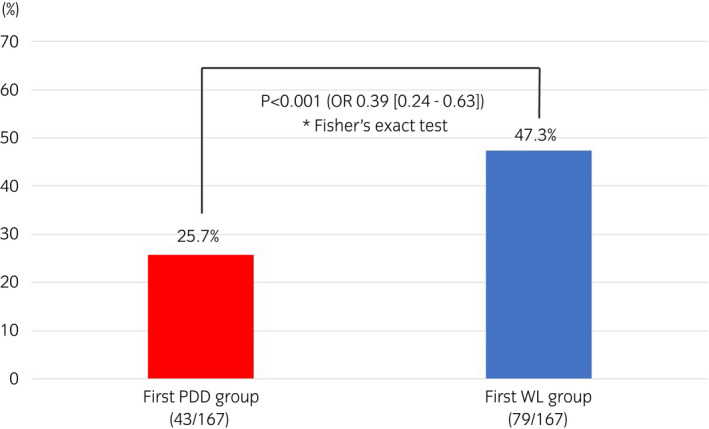
Residual tumor rates in the first PDD‐TURBT group (n = 167) and first WL‐TURBT group (n = 167). A significant difference test was conducted between the two groups using Fisher’s exact test. *Number in parentheses; number of residual tumor/number of patients.
Prediction of residual tumor rate at the second TURBT after PDD‐TURBT
Multivariate analysis using logistic regression was performed for the factors predicting residual tumors at the second TURBT after PDD‐TURBT, using the data for the PDD group (177 cases) as participants. The significant predictive factors included the presence or absence of smoking (P = 0.034; OR 3.88 [1.11–13.6]), number of tumors (P = 0.042; OR 2.52 [1.04–6.13]), and pathological stage (P = 0.036; OR 2.52 [1.06–5.98]) (Table 2). The residual tumor rates at the second TURBT within the PDD group for each factor are shown in Table S3. In stratification, using the risk scores based on current/past smoking history, multiple tumors, and pTis/pT1 were set as 1, with the OR for the residual tumor risk of each variable. The residual tumor rates were 8.3% for cases with a risk score of 1 or less (n = 48) and 33.3% for those with a risk score of 2 or more (n = 129), and significant differences were found between the two groups (P < 0.001; OR 5.46 [1.81–22.28]; Fig. 3).
Table 2.
Multivariable logistic regression analysis for residual tumor at second TUR in PDD group (n = 177)
| OR | 95% CI | P‐value | |
|---|---|---|---|
| Age | 0.98 | 0.94–1.03 | 0.578 |
| Sex | |||
| Female versus male | 0.76 | 0.23–2.55 | 0.664 |
| Smoking status | |||
| No smoking versus past smoking | 1.96 | 0.74–5.24 | 0.177 |
| No smoking versus current smoking | 3.88 | 1.11–13.6 | 0.034 |
| ECOG performance status | |||
| 0 versus 1 | 0.45 | 0.05–4.58 | 0.504 |
| Previous NMIBC | |||
| No versus yes | 1.03 | 0.34–3.11 | 0.957 |
| History of UTUC | |||
| No versus yes | 0.90 | 0.21–3.91 | 0.889 |
| Tumor size | |||
| <3 cm versus ≥3 cm | 1.30 | 0.58–2.94 | 0.528 |
| Number of tumors | |||
| Single versus multiple | 2.52 | 1.04–6.13 | 0.042 |
| Concurrent CIS | |||
| No versus yes | 1.29 | 0.54–3.09 | 0.564 |
| Pathological stage | |||
| pTa versus pTis | 5.67 | 0.29–113 | 0.255 |
| pTa versus pT1 | 2.52 | 1.06–5.98 | 0.036 |
| Tumor grade | |||
| Low versus high | 0.62 | 0.09–4.06 | 0.616 |
Fig. 3.
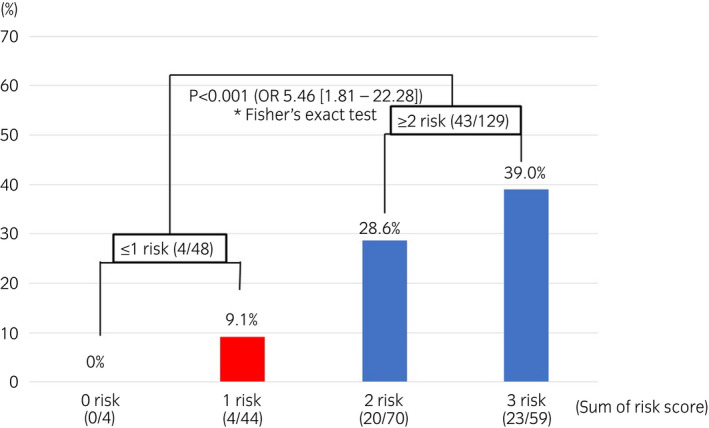
Residual tumor rates of the sum of risk (smoking, pathological stage, number of tumors) due to clinical pathological factors at the time of the first PDD‐TURBT. The risks of current/past smoking history and pathological stage of pT1 or pTis at the time of the first PDD‐TURBT. Multiple tumors were each set to 1; the residual tumor rates of the group with a sum of risk score of ≤1 (n = 48), and the group with a sum of risk score >2 (n = 129) was analyzed with a significant difference test using Fisher’s exact test. *Number in parentheses; number of residual tumor/number of patients.
Adverse drug reactions
Adverse reactions at the time of the PDD‐TURBT were as follows: elevated AST or ALT levels, 12 cases (6.3%); gastrointestinal symptoms, five cases (2.6%) (all grade 1); and severe hypotension, seven cases (3.1%) (grade 3). However, no severe, life‐threatening adverse reactions were observed.
Discussion
The present prospective study showed that an initial PDD‐TURBT using oral 5‐ALA hydrochloride for high‐risk NMIBC could significantly reduce the residual tumor rate compared to conventional WL‐TURBT. Smoking history, the number of tumors, and pathological stage were also identified as factors that influenced the occurrence of residual tumors at the second TURBT, in 48 cases (27%) with a risk score ≤1, calculated based on these factors, the residual tumor rate was <10%. Thus, the risk score model proposed in this study could be used to omit the second TURBT in approximately one‐fourth of high‐risk NMIBC cases.
A previous PDD‐TURBT prospective trial using 5‐ALA showed that the residual tumor rate in the PDD group was 4.5–32.7%, whereas that in the WL group was 25.2–53.1%, with an OR of 0.14–0.43. 10 However, there were some differences between this trial and the present study. For example, in that trial, 5‐ALA was administered via intravesical injection, and the participants included patients other than those with high‐risk NMIBC. However, in our study, the OR of the PDD group to the WL group was 0.46, which is consistent with previously reported findings.
Geavlete et al. performed a randomized controlled trial of PDD‐TURBT and WL‐TURBT using HAL in 446 high‐risk NMIBC cases. 9 Their results showed that the residual tumor rate at the time of the second TURBT was 31.2% for WL‐TURBT and 11.1% for PDD‐TURBT, indicating a significantly favorable result for the latter. 9 However, their definition of “high‐risk” was based on the 1973 World Health Organization classification (grades 1–3); 16 therefore, it is difficult to compare their findings with the current results for high‐risk NMIBC, which are based on the 2016 World Health Organization classification (low‐ and high‐grade). 17
In our study, the number of tumors, pathological stage, and the presence or absence of concurrent CIS at the time of the first TURBT were prognostic factors for predicting residual tumors at the second TURBT across all cases. These have already been established as risk factors for NMIBC recurrence and progression. 18 Our results are consistent with existing literature. Smoking history has conventionally been reported as an essential risk factor for the development of bladder cancer. 19 Still, in our study, smoking was also identified as a risk factor for residual tumors. Residual tumor rates proportionally increased with the extent of smoking, ranging from 18.8% for nonsmokers to 28.5% for those with a history of smoking and 34.6% for current smokers. In evaluations of the association between smoking and prognosis, cases with current or previous smoking history have been reported to show a significantly shorter recurrence‐free survival time compared to nonsmoking cases. 20 Because the presence of residual tumors almost certainly reduces the recurrence‐free rate, these reports indicate the validity of smoking as a predictive factor for residual tumors. Association between smoking and residual tumor has not been reported and is a subject for future study.
The major adverse reactions reported to date for 5‐ALA hydrochloride include liver dysfunction, photosensitivity, and gastrointestinal symptoms (e.g. nausea and vomiting). However, hypotension has recently been reported as a new adverse severe reaction. 21 In this study, elevated AST or ALT levels, gastrointestinal symptoms, and severe hypotension were reported in 6.3%, 2.6%, and 3.1% of cases, respectively. Adverse reactions of TURBT by oral 5‐ALA administration, but no life‐threatening severe adverse reactions, were also reported. A randomized controlled trial reported that the incidences of elevated AST levels, elevated ALT levels, gastrointestinal symptoms, and severe hypotension were 24.6%, 11.4%, 4.9%, and 1.6%, respectively, 15 which were similar to those in the present study. These adverse reactions have not been observed in studies from Western countries that have evaluated intravesical injections. However, after considering the patients’ physical distress and medical complexity associated with preanesthesia urethral catheterization, this may be a comparable adverse reaction.
As a PDD, 5‐ALA hydrochloride has been used not only for NMIBC but also for malignant glioma, but its usefulness in PDT has also been reported. The administration of 5‐ALA, a precursor of PPIX, has been suggested to induce tumor cell‐specific accumulation of PPIX in the mitochondria and promote the production of ROS upon irradiation with a specific wavelength of light. ROS accumulation has been reported to induce apoptosis and necrosis in tumor cells. 22 , 23 The efficacy of 5‐ALA hydrochloride in PDD against high‐risk NMIBC has been shown in this study, but this has also been reported for other cancer types; 22 , 24 the combined use of oral 5‐ALA in PDT against NMIBC is expected in the future.
This study had several limitations. First, this was not a randomized controlled trial, and the WL group consisted of retrospectively collected historical data. However, propensity score‐matching is thought to facilitate pseudorandomization. Moreover, the residual tumor rate varied at each institution in this multicenter study (data not shown). The residual tumor rate after PDD‐TURBT at institutions that enrolled more than 10 patients in this study ranged from 5.1% to 41.6%. However, many participating institutions introduced PDD‐TURBT from an early stage following approval for 5‐ALA hydrochloride coverage under insurance. Standardization of the technology was achieved to the extent possible by having all institutions watch representative PDD‐TURBT video images before the start of this study. The differences between institutions were also thought to reflect real‐world differences in the data. In this study, smoking status in the historical group was not investigated. This is a limitation of the study. It is unknown whether the risk factors in this PDD‐TURBT group may be adapted in WL‐TURBT. In the planning phase of this study, the goal was to prospectively include 200 patients with PDD‐TURBT. The final analysis was performed on 188 cases, which were deemed statistically comparable to the historical group. As for the secondary and exploratory endpoints, we are currently in the observation period for recurrence, and the results were excluded from this article.
The odds of residual tumors after the first PDD‐TURBT for high‐risk NMIBC cases were 0.39 times that after WL‐TURBT. Thus, the risk model proposed in this study could be used to omit the second TURBT in approximately a quarter of high‐risk NMIBC cases. By performing PDD‐TURBT, second‐TUR may be omitted for high risk NMIBC without risk factors, which may contribute to reducing patient burden and medical costs. This may also contribute the limited use of operation theaters, which is a great advantage under the influence of the COVID‐19 pandemic. We believe statistical decrease of the residual tumor rate should decrease the recurrence rate. Ongoing endpoint of 2‐year recurrence‐free survival will address the eventual advantage of PDD‐TUR as compared with WL‐TUR.
Author contributions
Keita Kobayashi: Data curation; Writing – original draft; Writing – review & editing. Hideyasu Matsuyama: Conceptualization; Project administration; Writing – original draft; Writing – review & editing. Taketo Kawai: Data curation. Atsushi Ikeda: Data curation. Makito Miyake: Data curation. Koshiro Nishimoto: Data curation. Yuto Matsushita: Data curation. Kazumasa Komura: Data curation. Takashige Abe: Data curation. Haruki Kume: Supervision. Hiroyuki Nishiyama: Supervision. Kiyohide Fujimoto: Supervision. Masafumi Oyama: Supervision. Hideaki Miyake: Supervision. Keiji Inoue: Supervision. Takahiko Mitsui: Supervision. Mutsushi Kawakita: Supervision. Chikara Ohyama: Supervision. Atsushi Mizokami: Supervision. Hajime Kuroiwa: Formal analysis; Methodology.
Conflict of interest
H. Matsuyama reports personal fees as meeting lecture and grants from SBI Pharmaceuticals Co., Ltd. and Chugai Pharmaceuticals Co., Ltd. H. Nishiyama reports personal fees as consultant and grants from Chugai Pharmaceuticals Co., Ltd. The funding for this study was provided by Chugai Pharmaceutical Co., Ltd. and SBI Pharmaceuticals Co., Ltd. The funding source had no role in the design, practice, or analysis of this study.
Approval of the research protocol by an Institutional Reviewer Board
This study was approved by Ethics Committee of Yamaguchi University (H30‐122).
Informed consent
Written informed consent was obtained from all participants.
Registry and the Registration No. of the study/trial
Registration number of UMIN is 000035712.
Animal studies
N/A.
Supporting information
Table S1. Patients and tumor characteristics of WL group and PDD group.
Table S2. Multivariable logistic regression analysis for residual tumor at second TUR in all cases (n = 479).
Table S3. Residual tumor rates for smoking status, tumor multiplicity, and pathological stage.
Acknowledgments
The following investigators also participated in this study, but are not listed as co‐authors: Hideo Fukuhara (Department of Urology, Kochi Medical School, Nangoku, Japan), Takanori Mochizuki (Department of Urology, University of Yamanashi Graduate School of Medical Sciences, Chuo, Japan), Takahiro Yoneyama (Department of Urology, Hirosaki University Graduate School of Medicine, Hirosaki, Japan), Takahiro Nohara (Department of Integrative Cancer Therapy and Urology, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan), Hidetoshi Kokubun (Department of Urology, Kobe City Medical Center General Hospital, Kobe, Japan), Seiji Yano (Department of Urology, Graduate School of Medicine, Yamaguchi University, Ube, Japan), Haruto Azuma (Department of Urology, Osaka Medical and Pharmaceutical University Faculty of Medicine, Takatsuki, Japan), Nobuo Shinohara (Department of Renal and Genitourinary Surgery, Graduate School of Medicine, Hokkaido University, Sapporo, Japan), Yoichi M. Ito (Data Science Center, Promotion Unit, Institute of Health Science Innovation for Medical Care, Hokkaido University Hospital, Sapporo, Japan). The funding for this study was provided by Chugai Pharmaceutical Co., Ltd. and SBI Pharmaceuticals Co., Ltd. This work was supported by the Center for Clinical Research, Yamaguchi University Hospital. We thank Editage (www.editage.com) for English language editing.
References
- 1. Antoni S, Ferlay J, Soerjomataram I et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur. Urol. 2017; 71: 96–108. [DOI] [PubMed] [Google Scholar]
- 2. Matsumoto H, Shiraishi K, Azuma H et al. Clinical practice guidelines for bladder cancer 2019 update by the Japanese Urological Association: summary of the revision. Int. J. Urol. 2020; 27: 702–9. [DOI] [PubMed] [Google Scholar]
- 3. Miladi M, Peyromaure M, Zerbib M et al. The value of a second transurethral resection in evaluating patients with bladder tumours. Eur. Urol. 2003; 43: 241–5. [DOI] [PubMed] [Google Scholar]
- 4. Cumberbatch MGK, Foerster B, Catto JWF et al. Repeat transurethral resection in non–muscle‐invasive bladder cancer: a systematic review. Eur. Urol. 2018; 73: 925–33. [DOI] [PubMed] [Google Scholar]
- 5. Kunieda F, Kitamura H, Niwakawa M et al. Watchful waiting versus intravesical BCG therapy for high‐grade pT1 bladder cancer with pT0 histology after second transurethral resection: Japan Clinical Oncology Group Study JCOG1019. Jpn. J. Clin. Oncol. 2012; 42: 1094–8. [DOI] [PubMed] [Google Scholar]
- 6. Chang SS, Boorjian SA, Chou R et al. Diagnosis and treatment of non‐muscle invasive bladder cancer: AUA/SUO guideline. J. Urol. 2016; 196: 1021–9. [DOI] [PubMed] [Google Scholar]
- 7. Denzinger S, Wieland WF, Otto W et al. Does photodynamic transurethral resection of bladder tumour improve the outcome of initial T1 high‐grade bladder cancer? A long‐term follow‐up of a randomized study. BJU Int. 2008; 101: 566–9. [DOI] [PubMed] [Google Scholar]
- 8. Inoue K, Fukuhara H, Shimamoto T et al. Comparison between intravesical and oral administration of 5‐aminolevulinic acid in the clinical benefit of photodynamic diagnosis for nonmuscle invasive bladder cancer. Cancer 2012; 118: 1062–74. [DOI] [PubMed] [Google Scholar]
- 9. Geavlete B, Jecu M, Multescu R et al. HAL blue‐light cystoscopy in high‐risk nonmuscle‐invasive bladder cancer–re‐TURBT recurrence rates in a prospective, randomized study. Urology 2010; 76: 664–9. [DOI] [PubMed] [Google Scholar]
- 10. Kausch I, Sommerauer M, Montorsi F et al. Photodynamic diagnosis in non‐muscle‐invasive bladder cancer: a systematic review and cumulative analysis of prospective studies. Eur. Urol. 2010; 57: 595–606. [DOI] [PubMed] [Google Scholar]
- 11. Rink M, Babjuk M, Catto JWF et al. Hexyl aminolevulinate‐guided fluorescence cystoscopy in the diagnosis and follow‐up of patients with non‐muscle‐invasive bladder cancer: a critical review of the current literature. Eur. Urol. 2013; 64: 624–38. [DOI] [PubMed] [Google Scholar]
- 12. Shen P, Yang J, Wei W et al. Effects of fluorescent light‐guided transurethral resection on non‐muscle‐invasive bladder cancer: a systematic review and meta‐analysis. BJU Int. 2012; 110: E209–15. [DOI] [PubMed] [Google Scholar]
- 13. Inoue K, Anai S, Fujimoto K et al. Oral 5‐aminolevulinic acid mediated photodynamic diagnosis using fluorescence cystoscopy for non‐muscle‐invasive bladder cancer: a randomized, double‐blind, multicentre phase II/III study. Photodiagnosis Photodyn. Ther. 2015; 12: 193–200. [DOI] [PubMed] [Google Scholar]
- 14. Inoue K, Matsuyama H, Fujimoto K et al. The clinical trial on the safety and effectiveness of the photodynamic diagnosis of non‐muscle‐invasive bladder cancer using fluorescent light‐guided cystoscopy after oral administration of 5‐aminolevulinic acid (5‐ALA). Photodiagnosis Photodyn. Ther. 2016; 13: 91–6. [DOI] [PubMed] [Google Scholar]
- 15. Nakai Y, Inoue K, Tsuzuki T et al. Oral 5‐aminolevulinic acid‐mediated photodynamic diagnosis using fluorescence cystoscopy for non‐muscle‐invasive bladder cancer: a multicenter phase III study. Int. J. Urol. 2018; 25: 723–9. [DOI] [PubMed] [Google Scholar]
- 16. Mostofi FK, Sobin LH, Torloni H. Histological typing of urinary bladder tumours. World Health Organization, Geneva, 1973. [Google Scholar]
- 17. Moch H, Cubilla AL, Humphrey PA et al. The 2016 WHO classification of tumours of the urinary system and male genital organs‐part A: renal, penile, and testicular tumours. Eur. Urol. 2016; 70: 93–105. [DOI] [PubMed] [Google Scholar]
- 18. Babjuk M, Burger M, Compérat EM et al. European association of urology guidelines on non‐muscle‐invasive bladder cancer (TaT1 and carcinoma in situ)‐2019 update. Eur. Urol. 2019; 76: 639–57. [DOI] [PubMed] [Google Scholar]
- 19. Freedman ND, Silverman DT, Hollenbeck AR et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011; 306: 737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lammers RJM, Witjes WPJ, Hendricksen K et al. Smoking status is a risk factor for recurrence after transurethral resection of non‐muscle‐invasive bladder cancer. Eur. Urol. 2011; 60: 713–20. [DOI] [PubMed] [Google Scholar]
- 21. Nohara T, Kato Y, Nakano T et al. Intraoperative hypotension caused by oral administration of 5‐aminolevulinic acid for photodynamic diagnosis in patients with bladder cancer. Int. J. Urol. 2019; 26: 1064–8. [DOI] [PubMed] [Google Scholar]
- 22. Nokes B, Apel M, Jones C et al. Aminolevulinic acid (ALA): photodynamic detection and potential therapeutic applications. J. Surg. Res. 2013; 181: 262–71. [DOI] [PubMed] [Google Scholar]
- 23. Inoue K, Fukuhara H, Kurabayashi A et al. Photodynamic therapy involves an antiangiogenic mechanism and is enhanced by ferrochelatase inhibitor in urothelial carcinoma. Cancer Sci. 2013; 104: 765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casas A. Clinical uses of 5‐aminolaevulinic acid in photodynamic treatment and photodetection of cancer: a review. Cancer Lett. 2020; 490: 165–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patients and tumor characteristics of WL group and PDD group.
Table S2. Multivariable logistic regression analysis for residual tumor at second TUR in all cases (n = 479).
Table S3. Residual tumor rates for smoking status, tumor multiplicity, and pathological stage.


