Abstract
Patients with heart failure with preserved ejection fraction (HFpEF) universally complain of exercise intolerance and dyspnoea as key clinical correlates. Cardiac as well as extracardiac components play a role for the limited exercise capacity, including an impaired cardiac and peripheral vascular reserve, a limitation in mechanical ventilation and/or gas exchange with reduced pulmonary vascular reserve, skeletal muscle dysfunction and iron deficiency/anaemia. Although most of these components can be differentiated and quantified through gas exchange analysis by cardiopulmonary exercise testing (CPET), the information provided by objective measures of exercise performance has not been systematically considered in the recent algorithms/scores for HFpEF diagnosis, by neither European nor US groups. The current clinical consensus statement by the Heart Failure Association (HFA) and European Association of Preventive Cardiology (EAPC) of the European Society of Cardiology (ESC) aims at outlining the role of exercise testing and its pathophysiological, clinical and prognostic insights, addressing the implications of a thorough functional evaluation from the diagnostic algorithm to the pathophysiology and treatment perspectives of HFpEF. Along with these goals, we provide a specific analysis of the evidence that CPET is the standard for assessing, quantifying, and differentiating the origin of dyspnoea and exercise impairment and even more so when combined with echocardiography and/or invasive haemodynamic evaluation. This will lead to improved quality of diagnosis when applying the proposed scores and may also help to implement the progressive characterization of the specific HFpEF phenotypes, a critical step toward the delivery of phenotype‐specific treatments.
Keywords: HFpEF, Exercise, Functional limitation, Gas exchange analysis
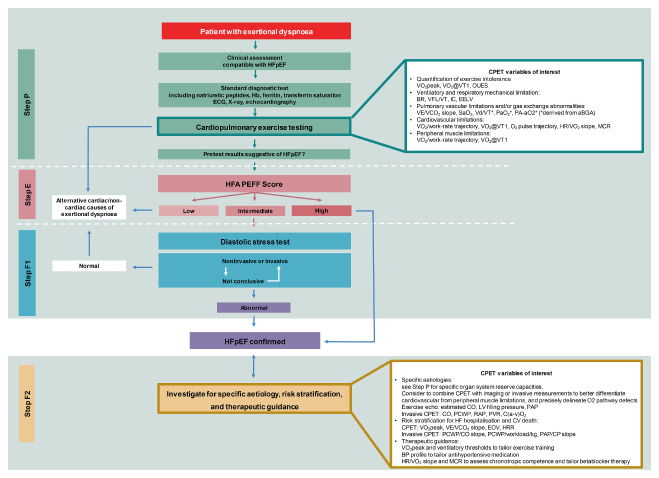
Prioritizing cardiopulmonary exercise testing (CPET) within the HFA‐PEFF diagnostic algorithm. Modified HFA‐PEFF diagnostic algorithm including CPET in Step 1 (P) pre‐assessment, and Step 4 (F) final aetiology. See text for details. Abbreviations: aBGA, arterial blood gas analysis; BP, blood pressure; BR, breathing reserve, ratio of VE/maximum volunatry ventilation; C(a–v)O2, difference in O2 content in arterial and mixed venous blood; CO, cardiac output; CV, cardiovascular; EELV, end‐expiratory lung volume; HR, heart rate; HRR, heart rate recovery; IC, inspiratory capacity; LV, left ventricular; MCR, metabolic‐chronotropic relationship; OUES, oxygen uptake efficiency slope; PA‐aO2, alveolar‐arterial oxygen gradient; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RER, respiratory exchange ratio; SaO2, arterial oxygen saturation; VCO2, carbon dioxide output; VD/VT, ratio of dead‐space ventilation to tidal ventilation; VE, ventilation; VFL/VT, percent of the tidal breath that expiratory air flow exceeds the maximal flow/volume envelope; VO2, oxygen consumption; VT, ventilatory threshold.
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) is a common and costly clinical condition primarily affecting older adults with multiple comorbid disorders and risk factors of the metabolic syndrome, e.g. hypertension, obesity, and insulin resistance. 1 The diagnosis of HFpEF is challenging, and two influential diagnostic scores have recently been introduced into clinical practice as tools to help establish the diagnosis: the HFA‐PEFF score of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) 1 and a composite score (H2FPEF) designed by the Mayo Clinic group. 2
Patients with HFpEF may present with typical signs and symptoms of HF, with or without increased levels of N‐terminal pro‐B‐type natriuretic peptide, 3 left ventricular (LV) diastolic impairment, and limited contractile reserve. Most individuals also complain dyspnoea on exertion as dominant manifestation. Remarkably, in the PARAGON‐HF (Prospective Comparison of ARNI With ARB Global Outcomes in HFpEF) trial, 50% of the HFpEF population was enrolled based on the evidence of exercise limitation, and exercise‐induced dyspnoea occurred in the 98% of cases. 4 The degree of exercise intolerance observed in HFpEF is similar to that seen in patients with HF with reduced ejection fraction (HFrEF), with impairments in the oxygen uptake (VO2) cascade and in the physiological response of multiple organ systems. 5 The relative cardiac and extracardiac contributions to exercise limitation require precise recognition and objective measurements.
Gas exchange analysis by cardiopulmonary exercise testing (CPET) provides the gold standard for a non‐invasive functional capacity evaluation 6 and offers a unique opportunity to investigate the role of lung mechanics and cardiopulmonary interactions with muscle weakness. In HFrEF, CPET is most frequently used to assess cardiac reserve and guide timing for advanced cardiac replacement therapies. In HFpEF, CPET may also play an important and distinct role to differentiate HFpEF from non‐cardiac causes of dyspnoea. Indeed, one such main aetiology that confounds evaluation is offered by chronic obstructive pulmonary disease (COPD), a main trigger of incident HFpEF 7 and a frequent comorbidity of HFpEF. 8 A subanalysis of TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist Trial) has identified a phenogroup with normal LV geometry, low arterial stiffness, and low natriuretic peptides with a favourable prognosis, despite non‐responsiveness to spironolactone. 9 This phenogroup exhibited a COPD pattern as the main driver of dyspnoea, raising the question that HFpEF was not the true cause of symptoms in at least some of these patients. 8 Even more in the PARAGON‐HF trial, one in seven patients was diagnosed with COPD and this subset presented with worse outcome. 10
Furthermore, community‐based cohort studies demonstrated a high prevalence of transthyretin amyloid cardiomyopathy in HFpEF patients with ventricular wall thickening, particularly in older men. 11 Patients with transthyretin amyloid cardiomyopathy often present with a severely reduced ventilatory efficiency and peak VO2 compared to HFpEF. 12 While myocardial dysfunction is often cited as the predominant mechanism of gas exchange impairment in patients with cardiac amyloidosis, growing evidence points on abnormal lung function and a restrictive spirometry pattern as responsible for exercise limitation. 12
Thus, documentation of the specific gas exchange response may lead investigators to think about underlying aetiologies, such as COPD or amyloidosis, early in the diagnostic process, which may prompt different treatments.
The algorithms proposed for the diagnosis of HFpEF within the H2FPEF score do not explicitly include consideration of CPET as part of the probability estimation scheme, whereas the HFA‐PEFF score algorithm suggests an initial gas exchange analysis approach only for the ruling out of non‐cardiac‐related impairment. 1
Given that exercise impairment is the central clinical expression of HFpEF, a focused appraisal of the role of exercise functional evaluation in the diagnostic process, pathophysiological insights, and evaluation of therapeutic interventions in HFpEF is warranted. While non‐invasive CPET in isolation may be insufficient to discriminate HFpEF from non‐cardiac dyspnoea in some patients without adding invasive testing, its role as an early stage investigation to exclude pulmonary disease, and its potential role in more advanced phenotyping or to gauge treatment response, may be important emerging uses. The purposes of this HFA/European Association of Preventive Cardiology (EAPC) clinical consensus statement are to provide an updated document focusing on: (i) the sources of exercise limitation and its pathophysiology in HFpEF phenotypes; (ii) the role of CPET in differentiating pulmonary from cardiac mechanisms for unexplained exertional dyspnoea from the early diagnostic process to the advanced stages, highlighting its value for risk stratification and therapeutic tailoring; and (iii) the interventions that may improve exercise performance in HFpEF effectively targeting the multiple limiting steps of oxygen (O2) kinetics.
Literature search and document approval
The writing group reviewed the exercise literature regarding HFpEF in its different phenotypes and clinical presentation highlighting the role of exercise intolerance, its pathophysiology, the diagnostic algorithms, the clinical presentation and the exercise correlates for therapies and interventions. The present HFA/EAPC clinical consensus statement has been approved and endorsed by the Clinical Practice Guidelines Committee of the ESC.
Bases of exercise limitation and symptoms in heart failure with preserved ejection fraction
The initial view that exercise limitation and symptoms are entirely due to an inadequate ventricular filling due to increased ventricular stiffness 13 has long ago been refuted. Patients with HFpEF exhibit a limited cardiac reserve on exercise 14 due to a multitude of factors including chronotropic incompetence, 15 impaired contractility, 16 atrial dysfunction, 17 atrial rhythm disorders (atrial fibrillation), 18 atrial functional mitral regurgitation 19 inducible ischaemia, 20 , 21 and unfavourable right ventricle to left ventricle interaction. 22 , 23
The overarching hallmark of HFpEF‐related exercise limitation has been expanded over the last decade by the accumulating evidence that a constellation of extracardiac pathways play a role in the impaired physiologic reserve capacity, including a limitation in gas exchange with reduced pulmonary vascular reserve, 24 impaired central and peripheral vascular reserve, 25 skeletal muscle dysfunction 26 and iron deficiency/anaemia. 27 HFpEF patients exhibit simultaneous impairment of several pathways, although one mechanism may predominate in a single patient. Historically, a comprehensive evaluation during exercise by CPET can assist in the identification and relative importance of the individual defective mechanisms 28 and the amount of information can be now further implemented by the use of combined imaging and/or invasive haemodynamic measurements.
The Fick principle states that VO2 is equal to cardiac output (CO) multiplied by the difference in O2 content in the arterial and mixed venous blood difference (a–v O2), which is determined by O2 delivery, uptake and extraction at the cellular level. The physiological concepts behind these processes are quite complex as well as the adaptive/maladaptive response in the O2 chain transport and utilization. Especially, O2 delivery needs to be viewed in a broader perspective in HFpEF considering its dependency not only on the limited cardiac reserve and impaired CO distribution but also on the potential underlying conditions that facilitate a low O2 content and limited O2 dissociation from haemoglobin (Hb). O2 content is actually determined by the ml of O2 carried by 1g of Hb (1.34) times O2 saturation and Hb concentration and decreases in hypoxia and anaemia. 6 An intriguing modality to precisely define the relative contribution of Fick principle determinants during exercise in clinical practice is to plot the relationship between CO (y‐axis) and a–v O2 difference (x‐axis) trough isopleths curves of VO2 as shown in Figure 1 , which depicts the normal response (adequate O2 delivery and extraction) versus the changes typically encountered in HFpEF (limited O2 delivery and extraction), COPD (quite preserved CO response and impaired O2 extraction) and anaemia (impaired O2 delivery and extraction with increased compensatory CO) conditions.
Figure 1.
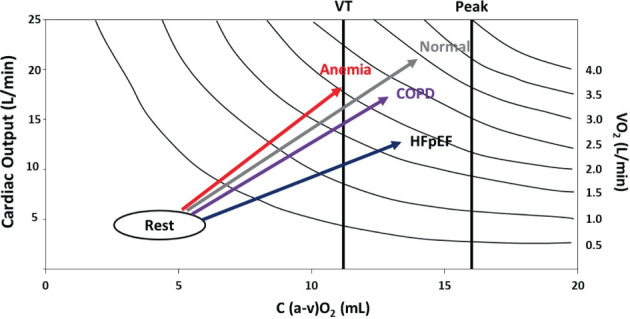
Plot of the Fick principle relating cardiac output to artero‐venous oxygen (a–vO2) difference and isoplets curves of oxygen uptake (VO2). The graph describes the expected relationship of heart failure with normal control pattern along with chronic obstructive pulmonary disease (COPD) and anaemia conditions as the most common comorbidities that affect oxygen (O2) content and delivery and may add on heart failure with preserved ejection fraction (HFpEF) haemodynamic, i.e. cardiac output, limitation. VT, ventilatory threshold.
Oxygen uptake kinetics and determinants
Physical performance and VO2 kinetics are dependent on the integrated interaction between the following processes: (i) the O2 content in inspired air; (ii) the exchange of O2 and carbon dioxide (CO2) through adequate alveolar ventilation (VA) and lung diffusion (DL); (iii) the O2 delivery activity by cardiac reserve (CO), blood Hb and vascular system to supply oxygenated blood to meet the increased O2 demand of working skeletal muscles; and (iv) the O2 diffusion (DM) process from capillaries to cells and mitochondrial respiration capacity in skeletal muscle.
In HFpEF, the O2 cascade can be limited at several levels and to a varied extent (Figure 2 ) as pointed out by many studies. 24 , 29 , 30 , 31 In an innovative HFpEF study performed with invasive CPET, i.e. by expired gas analysis and arterial and mixed venous blood sampling with key parameters directly measured (VA and CO) and others estimated (DL and DM), the individual limiting factors in the O2 cascade during peak exercise were systematically evaluated by a phenomapping approach. 5 Intriguingly, the O2 pathways causing exercise intolerance were ranked through a computational system analysis to gauge insights on the functional significance of each O2 pathway defects, examining factors that influence the magnitude of the O2 pathway defect and how this may impact on peak VO2.
Figure 2.
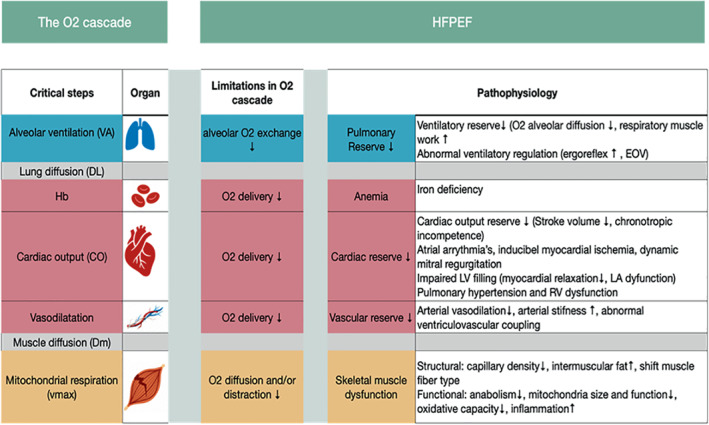
The oxygen (O2) cascade during exercise. The organ systems and pathways (from air to mitochondria) involved in exercise performance are depicted along with the limiting steps and pathophysiology behind exercise limitation in heart failure with preserved ejection fraction (HFpEF). EOV, exercise oscillatory ventilation; LA, left atrial; RV, right ventricular.
The vast majority of patients harboured compound mechanisms of exercise intolerance, defined as two or more defective steps in the O2 cascade, and a wide variability of putative mechanisms was observed. These data confirm the complexity of HFpEF as a heterogeneous entity at tissue, cellular and molecular level. 32 A typical example of different defective pathways altering the O2 chain of utilization are two subjects with the same CO increase, but distinct sets of accessory O2 pathway defects with one showing a predominant impairment in alveolar diffusion and the other presenting with a reduced delivery of O2 due to concomitant anaemia and/or insufficient O2 extraction due to impaired mitochondrial oxidative capacity.
This reasoning intriguingly advocates a profiling of the O2 cascade limitations in every patient, which then could be targeted accordingly.
Exertional limitation in HFpEF is not just related to abnormalities in the O2 cascade (forward convective and diffusive O2 properties), as increases in pulmonary capillary pressure also cause ‘backward’ induction of lung congestion resulting in changes in pulmonary mechanics, gas diffusion, and ventilation–perfusion matching. 33 These changes may occur in tandem with or independent of abnormalities in O2 delivery. In addition, symptoms of effort intolerance in HFpEF may relate to afferent signals originating in the heart, great vessels, and skeletal muscle receptors sensitive to muscle contraction and metabolic byproducts of cellular respiration (ergoreflex). 34
Ultimately, patients could be assigned to a specific exercise phenotype based on the profile displayed, and these groups may be amenable to specific and targeted therapies. This approach appears a promising avenue to be further explored and validated with precision medicine.
Cardiac contributions to impaired exercise oxygen uptake
Although stroke volume is often preserved at rest, limitations in cardiac reserve are multifold and represent the main triggers for an exercise defective exercise O2 pathway in patients with HFpEF. 35
Impaired myocardial performance and cardiac energetics
Landmark studies have documented a role for a biventricular myocyte stiffening as a major determinant of impaired LV relaxation and tension. 36 Ultrastructural and functional changes in the cardiac myocyte combined with fibrotic changes in the myocardium challenge effective ventricular performance, impairing coronary perfusion and ultimately yielding a cardiac energetic deficit. 37 The exercise‐induced failure in LV reserve is typically driven by a progressive elevation in pulmonary capillary wedge pressure (PCWP) and CO reduction and most recent observations directly link the cardiac energetic impairment (low phosphocreatinine/ATP ratio) to the elevation in PCWP and the development of pulmonary hypertension (PH). These findings have led to the intriguing new concept of a energy‐based pathway for pulmonary congestion as supported by a raised lung water content even at low workloads. 37
Chronotropic incompetence
The exercise chronotropic response account for large part of CO increase in healthy subjects and its relative proportional contribution becomes even more relevant in HFpEF. 38 Chronotropic incompetence (CI) is usually defined as failure to attain >80% of the heart rate (HR) reserve but a more objective method to define the relationship between HR and VO2 during exercise is the metabolic chronotropic relationship (MCR), calculated as the ratio of HR reserve to metabolic reserve during submaximal exercise. 39 MCR adjusts for age, physical fitness, and functional capacity and is unaffected by the exercise testing mode or protocol. A MCR ≤0.80 is indicative of CI. 40 CI is cardinal feature of physically untrained and deconditioned HFpEF patients 38 and basically contributes to a restricted maximal exercise performance as counter proven by the improvement in peak VO2 after beta‐blocker withdrawal. 41 Although very common, the aetiology of CI remains poorly defined with the most solid evidence pointing to an intrinsic electric conduction defect 15 and sino‐atrial node dysfunction. 42
Left atrial myopathy and atrial functional mitral regurgitation
The pathophysiological role of left atrial (LA) dysfunction in its different dynamic phases has progressively gained attention as a trigger for symptom generation and exercise limitation. 17 , 43 , 44 The information has rapidly evolved thanks to studies performed with speckle tracking suggesting a strong association of LA reservoir impairment measured by LA strain, with peak VO2 and an elevated ventilation (VE) to carbon dioxide (VCO2) slope. The active role of the left atrium in CO increase during exercise has recently been addressed with studies of LA dynamics showing how an altered LA reservoir and booster function limits CO increase through combined forward and backward unfavourable haemodynamics. 45
The vast majority of abnormalities in LA dynamics coexists with the burden of atrial functional mitral regurgitation and atrial fibrillation. 19 Indeed, LA remodelling and atrio‐ventricular asynchrony favoured by atrial fibrillation contribute to a low grade mitral regurgitation development that exacerbates biventricular filling impairment and pulmonary vascular dysfunction. 19
Comorbidities and extracardiac contributors to impaired exercise oxygen uptake impairment
Patients with HFpEF exhibit a high burden of comorbid conditions with an average of five or more coexisting comorbidities at the same time, primarily contributing to adverse outcome and critically impairing exercise capacity. 46 Peak VO2 is impressively restrained by comorbid conditions, explaining up to 50% of the predicted increase in functional capacity after exercise training (ET) programmes. 47 Nonetheless, a precise dissection on the role of any single comorbid and extracardiac factor may be limited by the coexistence of mixed phenotypes and wide heterogeneity of O2 pathway derangements.
Systemic arterial and venous system abnormalities
The arterial vascular system plays a central role in modulating compliance and resistances, ventriculo–vascular coupling and blood flow redistribution to the working muscles. In elderly hypertensive subjects, an impaired vascular compliance reduces the wave transit time from the LV to peripheral sites of vascular reflection and back to the aorta, generating a late systolic load which contributes to ventriculo–vascular uncoupling, increased LV filling pressures and high afterload. 48 Despite the fact that vascular stiffening is a well‐known hallmark feature of the hypertensive state, only recently have the implications of this been scrutinized under maximal exercise evaluation with gas exchange measurements by applying invasive or estimated measures of arterial functional response and load pulsatility. Exercise central aortic stiffness, assessed by converting radial artery pressure waveforms to central ones, tightly correlates with peak VO2. 25 Also non‐invasive assessment of exercise blood pressure pulsatility by proportionate pulse pressure (pulse pressure/systolic blood pressure) has shown a high ratio as typical of hypertensive and obese HFpEF phenotype, correlating with peak VO2. 49
The microvascular peripheral circulation has an important role in O2 delivery and utilization. Its dilator reserve is abnormal in HFpEF, due to vessel rarefaction, endothelial dysfunction and blunted response to muscle tissue hypoxic vasodilatation, all contributing to exercise limitation. 50 Recently, reports have focused also on the pathogenetic role for the venous system as a balancer in the circulating blood volume distribution. The circulating blood volume is functionally defined as the unstressed volume, which fills the vascular tree and the stressed blood volume which generates wall tension and intravascular pressure. An impairment in the venous capacitance critically shifts blood volume to the stressed blood volume pool, and exercise may further sustain this unphysiological redistribution. The obese phenotype typically exhibits an impaired venous capacitance and overload that abnormally increases the even minimal physiologic pulsatile loading imposed by the venous system, increases the systemic afterload, and may further impedes O2 delivery and extraction. 51
Abnormal lung mechanics, pulmonary hypertension and vascular disease
The detrimental role of PCWP elevation is key to effort‐induced dyspnoea and generates a cascade of haemodynamic and functional consequences contributing to two main mechanisms common to any HFpEF phenotype, i.e. vascular dysfunction and impaired pulmonary mechanics. Both of these contribute, ultimately, to VE inefficiency and effort‐induced dyspnoea 28 (Figure 3 ). Although lung dysfunction may result from concomitant lung disease, fluid swelling due to the alveolar capillary stress failure promotes a typical restrictive lung pattern responsible for a maladaptive heart–lung interaction. Lung interstitial fluid activates the inflammatory and cytokine cascade and leads to pulmonary vascular disease (PVD) in around 30% of HFpEF subjects. 33 Interestingly, PVD may progress independently of the hydrostatic‐induced wall breaks pressure‐injury based on the local activation of inflammatory and oxidative stress pathways as typically observed in the metabolic syndrome. 52 Pulmonary vascular remodelling involves both the venous and arterial sides of the pulmonary vasculature and critically impacts on the gas exchange process (ventilation/perfusion mismatching) and the right heart dynamics (including increased resistive load and right ventricular [RV] to pulmonary circulation [Pc] uncoupling). PVD detection relies on a thoughtful interpretation of pulmonary haemodynamics and gas exchange with analyses performed at rest and especially during exercise. An increase in resting pulmonary vascular resistance reflects PVD, a condition that can be unmasked in the earlier stages by a pulmonary vascular resistance rise >3 WU during exercise. 53 PVD can be further documented by a leftward shift of the mean pulmonary artery pressure (mPAP) versus CO relationship as a consequence of a dynamic increase in the pulmonary resistive load. 53 The right ventricle becomes stiff and is challenged in its filling and contractile properties, and uncoupling with the Pc ensues. 54
Figure 3.
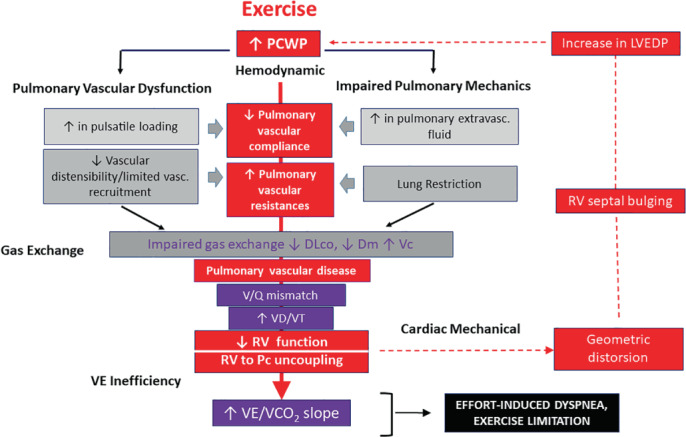
Cascade of the cardiac, haemodynamic and pulmonary maladaptive response under the effects of pulmonary capillary wedge pressure (PCWP) increase. DLco, exercise diffusing lung capacity for carbon monoxide; Dm, membrane diffusion; LVEDP, left ventricular end‐diastolic pressure; Pc, pulmonary circulation; RV, right ventricular; Vc, capillary volume; VD/VT, dead space to tidal volume ratio; VE, minute ventilation; VE/VCO2, minute ventilation to carbon dioxide output. V/Q, ventilation/perfusion
Although technically challenging, the assessment of the exercise diffusing lung capacity for carbon monoxide (DLco) and its subcomponents, i.e. membrane diffusion (Dm) and capillary volume (Vc) in parallel with CO changes, is explanatory of the altered pulmonary perfusion pattern occurring in HFpEF, 24 definitively resulting in an increased dead space to tidal volume (VD/VT) ratio and inefficient VE. 55 Exercise‐induced dynamic congestion often overlaps as an additive reason for impaired gas exchange and vascular distensibility. 56 , 57 The elevated afterload and RV dysfunction sustains further impairment in lung perfusion and challenges cardiac dynamics through an unfavourable RV to LV diastolic interaction. This may be observed under maximal exercise quite early and even in HFpEF patients who are otherwise asymptomatic at rest. 22 Specifically, in recent years the primary role of the right heart in the limited exercise performance due to the progressive increased load and PVD has been described under a continuum of sequential steps with initial geometrical changes and impairment in RV filling and diastolic stiffness, elongation in the free wall to septum tricuspid valve diameter, tricuspid regurgitation (TR) development and progressive unfavourable diastolic and systolic mechanical RV to LV interaction (Figure 4 ). 54
Figure 4.
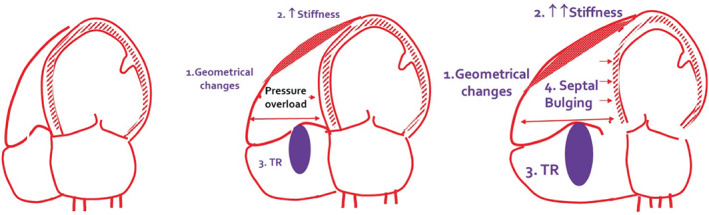
Continuous of mechanisms involved in right ventricular maladaptive response to increased load and pulmonary vascular disease, affecting cardiac output and exercise performance in heart failure with preserved ejection fraction. TR, tricuspid regurgitation.
Muscle and mitochondrial pathology
There is a clear impairment in skeletal muscle architecture and loss in mass that contribute to an impairment in O2 transport capacity. An association between peak VO2 and lean mass has been observed in skeletal muscle biopsy studies showing a change in fibre type distribution with reduction in fibres type I and impaired capillary to fibre ratio. 58 Older HFpEF patients (compared to age‐matched healthy subjects) exhibit an altered skeletal oxidative capacity and reduced mitochondrial content. 59
Anaemia and iron deficiency
Anaemia and iron deficiency are common in HFpEF and importantly contribute to worsening of symptoms and exercise intolerance by reducing O2 delivery and muscular storage (via myoglobin) in peripheral tissues. 60 Interestingly, the relative contribution of anaemia to functional capacity impairment can be calculated defining the proportion of peak VO2 loss due to anaemic condition. As each Hb gram carries 1.34 ml of O2, and as at peak exercise Hb desaturation is ∼70%, each gram of Hb delivers to the muscle about 1 ml of O2. In normal conditions, Hb is 15 g/dl, and, for a given peak CO (dl/min), one can easily estimate the amount of missing VO2 attributable to anaemia at peak effort. As an example, if peak CO is 7.5 L/min, that is 75 dl/min, and Hb is 10 g/dl, the amount of VO2 lacking because of anaemia is 15 (normal Hb) – 10 (observed Hb) × 75 = 375 ml/min. This calculation is reliable in normoxic patients with no cardiac shunt and significant O2 desaturation. Anaemia may be compensated for by an increase in stroke volume, a mechanism which may be at work in HFpEF but still results in poor O2 delivery. 60 Iron deficiency maintains an independent clinical role in HFpEF irrespective of anaemia, but its isolated contribution to exercise impairment has recently been questioned and overshadowed. 61 , 62
Obesity
Almost half of HFpEF patients are obese or show increased visceral adiposity due to senescence and/or dysmetabolic conditions. Compared to non‐obese counterpart, the HFpEF obese phenotype shows a reduced relative peak VO2. This depends on both central and peripheral mechanisms such as LV filling pressure, lung vasculopathy and increased pulmonary pressures, which rapidly evolve to haemodynamic manifestation of RV dysfunction and superimposed pericardial constraint. 23 A causative role of impaired haemodynamics has recently been recognized in a direct pericardial fat inflammatory activity to impaired filling and increased PCWP. 63 Studies have identified an obese phenotype specific source of impaired myocardial energetics source in the abnormal ATP handling of the mitochondrial creatinine kinase shuttle. 64 Another typical defect observed in obesity is the so‐called myosteatosis or excess adipose accumulation in muscle tissue, which correlates with muscle weakness, mitochondrial pathway disruption and impaired exercise performance. 65
Diabetes mellitus
The diabetic phenotype of HFpEF combines with a higher burden of comorbidities. 66 Diabetes and worse glycaemic control is associated with higher degrees of myocardial fibrosis and myocyte dysfunction. Diabetic patients manifest exercise intolerance because of HR incompetence due to the commonly impaired sympathovagal balance, higher prevalence of anaemia, microvascular disease, endothelial dysfunction, vasoconstriction and impaired mitochondrial function. 66
Methodology and clinical implications of exercise testing
Exercise limitation in HFpEF patients is primarily assessed with two modalities of exercise testing, the 6‐min walk test (6MWT) and the CPET, also combined or not with non‐invasive or invasive measurements.
Six‐minute walk test
The 6MWT offers the advantage of low cost and ease of use in daily practice. However, exercise cardiac index and filling variables show only a modest correlation with 6MWT distance, indicating that 6MWT distance is influenced by extracardiac factors reducing its value in some indications for diagnostic purposes. 67 Though 6MWT performance may still provide prognostic insights 68 and it has been used for serial therapeutic evaluations, 69 , 70 , 71 a significant learning effect in older HFpEF patients has to be accounted for, as well as non‐cardiopulmonary factors that contribute to limitation, such as orthopaedic or neurologic problems. CPET‐derived variables are superior for the quantification of exercise capacity and risk stratification. 72
Cardiopulmonary exercise test
The CPET in tandem with measurement of CO is the gold standard technique to measure aerobic capacity, and allows for interrogation of the principle organ system(s) involved in exercise limitation, 28 differentiating cardiac from pulmonary aetiologies, with the potential to enhance clinical decision‐making process and objectively determine the targets for therapies. To these aims, a comprehensive lung function evaluation by spirometry and lung diffusion capacity at rest should precede CPET.
A remarkable additive value of CPET is the well‐established capacity to predict outcomes across the various HF phenotypes. 6 Prerequisites for a correct test execution are a stable clinical condition in the previous 4 weeks, 28 and a test duration tailored to reach a 8–12 min maximal duration and a respiratory exchange ratio ≥1.10 73 to cope with the linear increase of gas exchange variables (peak VO2, HR and work rate [WR]) 74 and accurate detection of ventilatory thresholds and slopes (VO2 to WR relation, VE/VCO2 slope, oxygen uptake efficiency slope). For these goals a cycle test with a linear workload increase is preferable to treadmill testing with a less gradual increase in workload. 74 In the obese HFpEF phenotype, the excessive metabolic requirement will result in a lower expected maximal workload with equal or higher peak VO2 than in non‐obese counterparts. 23 Other factors to be considered for the test protocol selection include sex, age, cardiovascular risk factors, physical activity levels and comorbidities.
In HFpEF, peak VO2 reduction is sensitive but not specific and it can discriminate HFpEF from non‐cardiac causes of dyspnoea reliably only at very high and very low values. 75 Namely, with a peak VO2 <14 ml/kg/min, HFpEF is very likely, whereas with a peak VO2 >20 ml/kg/min HFpEF is very unlikely, and in the range of 14–20 ml/kg/min further testing with stress echo or exercise cath is required. Nevertheless, extending the analysis to the whole array of CPET‐derived variables enables more robust delineation of cardiac versus pulmonary or other non‐cardiac causes of dyspnoea. 76
For example, COPD patients with significant limitation in exercise capacity will display a reduction in breathing reserve, i.e. the relative difference between the maximal voluntary ventilation (MVV) and peak exercise VE (<15% reserve indicating a mechanical ventilatory limitation); these patients will further display spirometric abnormalities such as reduced forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ratio.
However, the study of lung mechanics by inspiratory manoeuvres offers the most sensitive and specific diagnostic tool. The combination of operating lung volumes as measured by serial inspiratory capacity manoeuvres and breathing pattern helps to detect important inspiratory mechanical constraints, relevant to dyspnoea and exercise limitation. 77 This analysis is more sensitive than traditional assessments of breathing reserve (VE/MVV), especially in milder forms of obstructive and restrictive disorders or other cardiorespiratory conditions such as pulmonary arterial hypertension. 78
Additionally, qualitative assessment of inspiratory and expiratory flow reserves is provided by tidal versus maximal flow–volume loops throughout exercise. 28 , 77 In COPD, the inefficient VE during exercise is signalled by the VE Y‐intercept that increases with greater disease severity. 79 COPD patients have a higher V̇E‐Y intercept than HFrEF patients 80 and presumably this is also true compared to HFpEF.
A significant elevation in VE/VCO2 slope without an alternative explanation should prompt further diagnostic testing toward a pulmonary vascular involvement getting into a differential diagnosis of HFpEF with coexisting pre‐capillary PH versus pulmonary arterial hypertension or chronic thromboembolic PH. The well‐established prognostic role of VE/VCO2 slope in HFpEF 81 seems to be similar to HFrEF, though its clinical interpretation should take into account age dependency and gender‐related differences. 73
Attention should also be paid to interstitial lung diseases, which may be suggested by gas exchange abnormalities including a low O2 saturation (<95% at rest or >5% drop during exercise), increased VD/VT increased alveolar–arterial (A–a) O2 pressure difference, balanced reductions in FEV1 and FVC with normal ratio, and decrease in DLco. Patients with HFpEF may display a mild restrictive defect on spirometry, as well as reduction in DLco, so this can be difficult to distinguish, and sometimes relies upon chest computed tomography to evaluate the lung parenchyma, 82 or invasive haemodynamic testing to exclude HFpEF. 83
However, many more patients can be distinguished without this requirement, and the ability to distinguish predominant pulmonary diseases from HFpEF is a major strength of CPET 84 and crucial to separate and objectively assess how much of the limited performance is pulmonary rather cardiac‐related and in case is pulmonary to further detail the cause. Overall, considering that some patients may have both lung disease limitation and HFpEF, invasive CPET may be considered even though non‐invasive CPET points to a primary pulmonary limitation. Comorbid chronic lung diseases are common in clinical trials of HFpEF, but the severity is not well‐defined even though quite advanced in some cases and this may be a significant unmet need in the correct phenotyping of patients' enrolment in trials and treatment process.
The typical cardiac reserve limitation is signalled by a reduced O2 pulse, a downward shift in the VO2 to WR relationship with or without a true VO2 flattening pattern 85 and chronotropic incompetence 76 as outlined in the 9‐plot graphical representation of Figure 5 reporting a case of a hypertensive and diabetic old lady with a HFA‐PEFF score 6 points and an H2FPEF score 0.6, which is definitively diagnostic and explanatory in terms of pathophysiology and organ system limitation. Exercise data allow phenotyping and show a typical CI (heart rate reserve (HRR) = 58% of predicted), a limited O2 pulse (7 ml/beats) and a change in VO2 kinetics under flattening pattern (defined as an inflection in VO2 linearity as a function of WR in the second part of the exercise >35% compared to the first linear slope, with a duration >30 s). These CPET manifestations are typical of a cardiogenic limitation. A moderate to severe VE inefficiency was also documented by a VE/VCO2 slope of 37.
Figure 5.
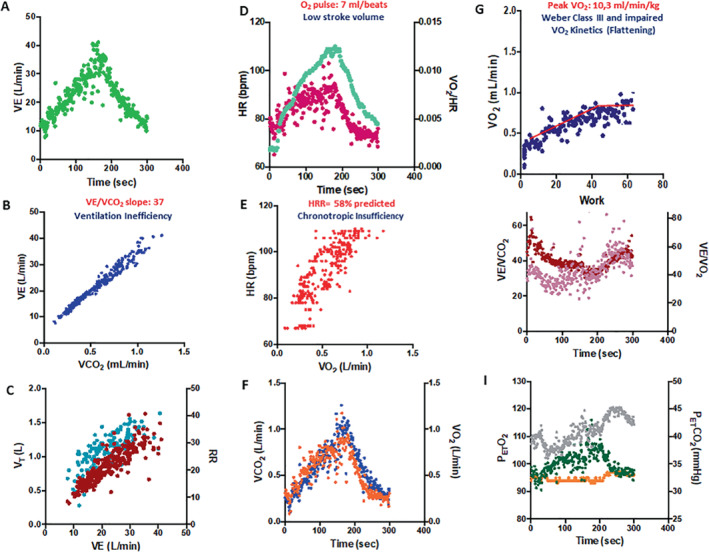
Nine‐plot analysis (A–I) of a typical cardiopulmonary exercise test response of an old hypertensive female patient with exertional dyspnoea. See text for explanation. HR, heart rate; PetCO2, end‐tidal carbon dioxide tension; PetO2, end‐tidal oxygen tension; VCO2, carbon dioxide output; VE, minute ventilation; VE/VCO2, minute ventilation to carbon dioxide output; VO2, oxygen uptake; VT, ventilatory threshold.
When abnormalities in peak VO2, O2 pulse, VO2/WR slope and CI combine with an elevated VE/VCO2 slope, the coexistence of a right heart phenotype with PH, elevated pulmonary vascular resistances 81 and RV to pulmonary circulation uncoupling 86 is very likely. A few reports have also shown that exercise oscillatory ventilation may be part of the picture of the gas exchange response during maximal exercise. 87 Thus, in the most advanced stages of HFpEF exercise limitation, a thorough analysis of VE/VCO2 slope determinants, i.e. VD/VT ratio and PaCO2 may offer valuable insights for planning therapeutic interventions. 55
Figure 6 reports a 9‐plot analysis of a 72‐year‐old overweight patient complaining initial exertional dyspnoea, arterial hypertension, pre‐diabetes, persistent atrial fibrillation, sleep apnoea syndrome and COPD, Gold class 2. His HFA‐PEFF score is 4 points and H2FPEF score is 0.5 pointing to a 85% probability of HFpEF. His CPET performance excludes a respiratory mechanical limitation (breathing reserve of 20%) and, despite a quite relatively preserved peak VO2 (17.3 ml/kg/min; 78% of predicted) and O2 pulse (11.1 ml; 92% of predicted), exhibits a severely impaired ventilatory efficiency (VE/VCO2 slope of 43.3 and end‐tidal of CO2 of 24 mmHg) with a Y‐intercept in the upper normal range, a picture suggesting to investigate a potential underlying pulmonary vascular limitation due to impaired vasomotility and increased pulmonary vascular resistances. 81 CPET supported clinical management with (i) exclusion of respiratory mechanical limitation as a cause of dyspnoea, (ii) providing evidence of severe VE inefficiency and prospecting PH under exertion as a major cause of dyspnoea; (iii) showing the need for better rate control of atrial fibrillation; (iv) suggesting a relatively preserved aerobic capacity of the peripheral muscles; and (v) documenting a 10‐fold increased risk for incident HF hospitalizations, compared to HFpEF patients with a VE/VCO2 slope <30.
Figure 6.
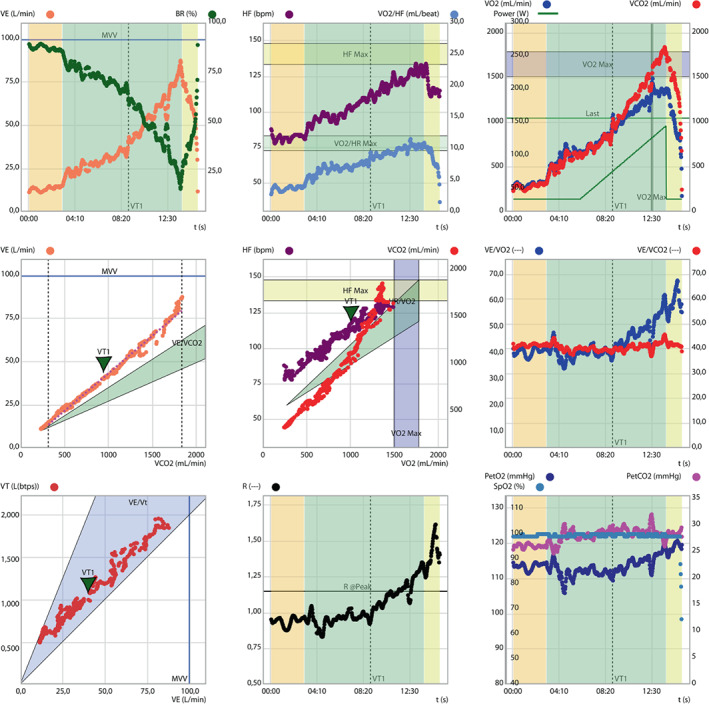
Nine‐plot analysis of a middle aged man with initial exertional dyspnoea presenting with a different cardiopulmonary exercise test phenotype. See text for explanation. HF, heart frequency; HR, heart rate; PetCO2, end‐tidal carbon dioxide tension; PetO2, end‐tidal oxygen tension; VCO2, carbon dioxide ouput; VE, minute ventilation; VO2, oxygen uptake; VT, ventilatory threshold.
Exercise stress echocardiography
The study of LV filling adaptations/maladaptations during dynamic exercise is a priority that can be pursued by performing exercise stress echocardiography. 6 Most of the interest has been focused on diastolic adaptation and on the study of LV filling by E/e' changes 88 primarily for diagnostic purposes integrating also with the parallel changes in TR peak velocity and estimated pulmonary pressures during effort. 89 , 90 Among the technical requirements, the semi‐recumbent position is suggested for a better Doppler evaluation.
Incremental ramps at low workload (8–15 W/min) are preferable for a comprehensive image acquisition. Loop storage of adequate duration (5 beats) is required in order to perform the averaging of measures, especially for Doppler parameters, accounting for physiological respiratory variations. Pulsed‐wave Doppler echocardiography also enables measurement of CO at rest and during exercise, which can be of great value in distinguishing patients with predominant central versus peripheral abnormalities. 45
The HFA‐PEFF recommendations suggest to perform exercise stress echo at step 3 (F1), primarily looking at mitral E/e' and the TR peak velocity. 1 Compared to invasively determined PCWP, an E/e' >13 has been identified as a pathological cut‐off. 91 The isolated increase in TR is not specific for HFpEF because it may depend on intrinsic RV dysfunction and/or PVD. Remarkably, in approximately 30% of cases, TR velocity cannot be reliably assessed. 92 Also, its correlation with the invasive RV to right atrium gradient at peak exercise is moderate (r = 0.72) with a small bias (−1 mmHg). 93 Echo Doppler is less consistent to measure pulmonary pressures during exercise because right atrial pressure cannot be reliably estimated and in case of RV to Pc uncoupling during effort, the contribution of right atrial pressure can be even higher than the RV to right atrium gradient estimate. Technically, an improved definition of TR velocity signal may be obtained with agitated gelofusine administration yielding to a 87% feasibility and the correlation with invasive measures is significantly improved (+2.9 mmHg). 94
Cardiopulmonary exercise test imaging
Cardiopulmonary exercise test imaging is a comprehensive and expanding approach which combines the advantage to address the exercise physiological implications with non‐invasive haemodynamic data by echocardiography. Cardiac functional reserve is extended to the integrated analysis of measures of chamber volumes, geometry, valvular status, systolic and diastolic function, including the assessment of LA dynamics and response of the right heart. 45 , 95 The full non‐invasive nature and the considerable amount of clinical information are complementary and synergic to those obtained with invasive CPET. However, caution should be applied when comparing gas exchange information obtained in the sitting position due to the different impact of preload changes during exercise.
Application of CPET imaging in HFpEF is expanding and covers the wide spectrum of clinical presentation from the early diagnostic process to the advanced right heart involvement taking advantage of the combined pathophysiological and prognostic insights of gas exchange‐derived variables. 96
An initial study combining CPET and Doppler analysis led to the ultimate diagnosis of HFpEF in the subset of patients presenting with an elevated VE/VCO2 slope combined with an average E/e' >15 at peak exercise. 97 Subsequent studies have implemented the interest for E/e' to the role of LV deformation primarily assessed by speckle‐tracking analysis 98 and lung congestion by the analysis of exercise‐induced B‐lines 99 which have been shown to predict HFpEF better than standard echocardiographic estimation of filling pressure. 100 However, recent findings by invasive simultaneous study have shown that around 50% of HFpEF patients with exercise PCWP elevations do not present with B‐lines. 3
Most recent findings have also focused in depth on LA dynamics 17 highlighting the putative role of an impaired LA deformation (LA strain) during exercise as a key step in the backward and forward haemodynamic impairment and symptom cascade. 45 Indeed, among all echocardiographic data obtained at rest, abnormalities in LA strain seem to be most strongly correlated with haemodynamic abnormalities that develop during exercise. 45 Evidence has been brought also on the role of mitral regurgitation 19 and its dynamic component during maximal exercise 101 to physical limitation along with its remarkable prognostic value. 19 , 45
Figure 7 reports an example of advanced CPET imaging application in HFpEF (gas exchange data in Figure 3 ) by studying LV systolic dynamics (3D strain analysis) and filling (E/A and E/e'), LA dynamics (LA strain analysis) and RV function analysis (RV to Pc coupling by tricuspid annular plane systolic excursion/systolic pulmonary artery pressure [TAPSE/PASP] ratio and RV ejection fraction by three‐dimensional acquisition) at rest and at peak exercise. Data show a preserved LV deformation analysis with exercise increase in LV filling pressure (E/e': from 12.6 to 16.9); a severely limited LA strain (10.5% at rest and 9.6% at peak exercise) and a loss in RV ejection fraction (57% at rest and 45% at peak exercise) with exercise‐induced PH (PASP of 41 mmHg at rest and 58 mmHg at peak exercise). The CPET‐derived 9‐plot analysis fits with the documentation of cardiogenic limitation and a ventilatory pattern common in left‐sided PH.
Figure 7.
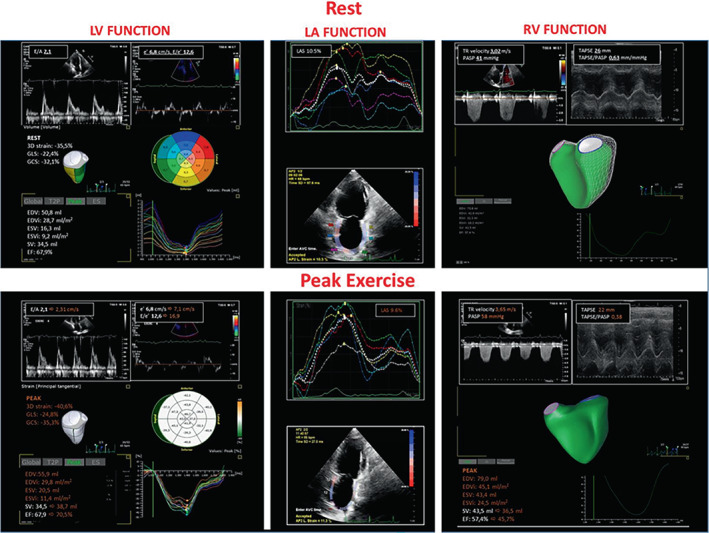
Cardiopulmonary exercise test imaging rest to peak exercise analysis of the same case as Figure 5 . Measures obtained by stress echocardiography (rest to peak exercise). The analysis was performed analysing the diastolic (E/e') and systolic (three‐dimensional longitudinal and circumferential strain) left ventricular (LV) function; the adaptive left atrial (LA) dynamics by LA strain (LAS); right ventricular (RV) function (RV ejection fraction 3D analysis) and its coupling with the pulmonary circulation by the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure (TAPSE/PASP) ratio. Data are reported at rest (white) and at peak exercise (orange) with the changes occurring in the main variables from rest to peak. TR, tricuspid regurgitation.
Invasive cardiopulmonary exercise test
In the last 10 years, there has been a progressive reappraisal of invasive CPET as the gold standard approach for the thorough characterization of the haemodynamic reasons for exercise limitations, precisely dissecting central and peripheral mechanisms throughout direct measures of LV filling pressures, pulmonary haemodynamics, CO, and a–v O2 differences. In HFpEF, pulmonary haemodynamic measurements during exercise, especially PCWP and mPAP, may yield to incremental prognostic value compared with evaluation at rest only. 102 Although technically challenging, invasive assessment of pulmonary haemodynamics is more sensitive and specific compared to fluid loading for detection of an abnormal rise. 103 , 140
Borlaug et al. 38 first reported the potential to suspect HFpEF in subjects with unexplained dyspnoea, whilst euvolaemic with normal levels of B‐type natriuretic peptide and without clear signs and symptoms of HF at rest. In half of the subjects, the observed increase in PAWP during exercise was concordant with LV end‐diastolic pressure, though it was preliminary to a diagnosis. Studies have then well established that an increased mean PCWP ≥25 mmHg at peak exercise, even in the absence of elevations in pulmonary vascular resistance, indicates HFpEF. 105 Some groups have advocated for assessment of the PCWP increase during exercise to CO relationship, with a cutoff ≥2 mmHg/L/min shown to be associated with adverse outcomes. Furthermore, the analysis of biventricular interaction and changes in right atrial pressure versus PCWP implement the diagnostic information with the pressure‐induced unfavourable RV to LV interaction mechanisms, intended as a decrease in the pressure gradient between the left and right ventricle, and a change in septumbecoming less convex toward the right ventricle is documented even at earlier stages of HFpEF. 22 , 53
In the most advanced cases, accurate assessment of the pressure–flow relationship during exercise by plotting mPAP versus CO provides a robust indication of abnormalities in RV to Pc coupling. 53 A mPAP/CO relationship >3 mmHg/L/min is reflective of a pulmonary hypertensive response, indicating abnormalities in pulmonary vascular reserve, often associated with a high VE/VCO2 slope. 106 Even more, the occurrence of RV to Pc uncoupling is responsible for a delayed VO2 on kinetics during early exercise. 29
Incorporating exercise testing within the HFA‐PEFF algorithm
The HFA‐PEFF algorithm includes ergometry and 6MWT, however CPET is not recommended as a typical element of the initial HFpEF workup, mainly because of the low specificity to diagnose HFpEF. 75 Nonetheless, the role of cardiac versus pulmonary predominance in generating symptoms is crucial.
The use of CPET along the steps of the HFA‐PEFF appears also quite relevant for implementing the diagnostic and clinical oriented approach. Peak VO2 should be paralleled by the VE/VCO2 slope analysis in the search for a right heart involvement and PH coexistence, adding both specificity and specificity to HFpEF diagnosis. 81
The CPET may then play a role in an in‐depth phenotyping of the functional response assisting in the identification and relative importance of the individual defective mechanisms in the O2 cascade, allowing assignment of patients to a specific exercise‐based HFpEF phenotype. 5
Therefore, translating these concepts to the HFA‐PEFF algorithm would enable important implementations in the diagnosis and clinical workup of HFpEF. Accordingly, we propose that CPET assessment should be referred in more details in Step 1 (P, pre‐assessment) to ascertain the degree of functional limitation and to address toward the primary origin of symptoms, and in Step 4 (F, final aetiology) for a comprehensive analysis of O2 cascade organ‐related defective mechanisms. This conceivable pathophysiological‐oriented supported approach would certainly require dedicated studies aimed at exactly defining the prioritization order to get an ideal operationalization of gas exchange analysis even better when combined with imaging and invasive haemodynamic evaluation 76 , 107 (Graphical Abstract). Information in terms of prognosis and clinical work‐up should be derived at all steps proposed.
Step 1 (P): pre‐assessment
Exercise gas exchange can delineate cardiac and extracardiac reserve capacity impairments contributing to exertional intolerance. 76 , 77 Importantly, abnormal findings under resting (e.g. spirometry, echocardiography) may anticipate but not definitively prove their relevance to exertional dyspnoea. If the diagnosis of HFpEF is ruled out by the HFA‐PEFF score (Step 2 [E]), or by a diastolic stress test (Step 3 [F1]), the collected data at CPET evaluation could provide alternative explanations of cardiac and non‐cardiac reasons of exertional dyspnoea, or at least provide evidence to pursue further examinations to determine the true source of symptoms.
Step 4 (F): final aetiology
If the diagnosis of HFpEF is classified according to the HFA‐PEFF criteria, CPET may additionally rank‐order multiorgan system limitations, illustrate O2 pathway defects, and support aetiological work‐up, risk stratification, and therapeutic guidance. 40 , 108 Specifically, the combination of CPET data with findings of chronotropic incompetence, elevated PCWP, 75 PH, exercise‐induced mitral regurgitation and RV dysfunction may definitively secure HFpEF diagnosis and potentially enhance care through improved phenotyping. 76 Compared to HFrEF, the heterogeneous manifestation of HFpEF phenotype 87 , 108 , 109 may well explain how a robust use of CPET‐derived variables in clinical risk stratification is lacking. 110
However, emerging evidence suggests that also in HFpEF, CPET variables, namely peak VO2 and the VE/VCO2 slope, provide incremental prognostic value beyond clinical variables based on the C‐statistic, net reclassification improvement, and integrated diagnostic improvement. 108 Notably, in a study by Nadruz et al., 108 the magnitude of association of peak VO2 and VE/VCO2 slope with adverse outcomes was greater in HFpEF versus HFrEF. Additional risk definition can actually be derived from invasive CPET and CPET imaging (Table 1 ). 14 , 40 , 73 , 76 , 77 , 81 , 85 , 87 , 93 , 96 , 102 , 105 , 108 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121
Table 1.
Cardiopulmonary exercise testing variables delineating oxygen pathway defects, and risk stratification in heart failure with preserved ejection fraction
| Variable | Cut‐off | Interpretation |
|---|---|---|
| Quantification of exercise intolerance | ||
| RER | <1.0 ≥ 1.0, preferably ≥1.1 | Definition of submaximal or maximal exercise testing 73 , 112 |
| Peak VO2 (ml/kg/min) | Weber class A >20.0, B 16.0–20.0, C 10.0–15.9 D <10.0 or age‐ and sex‐specific cut‐offs | Categorization of cardiorespiratory fitness can be used in maximal exercise tests either classified based on Weber or on healthy adult cohorts 73 , 113 |
| OUES (L/min/log[L/min]) | Age‐ and sex‐specific cut‐offs | Submaximal parameter that correlates with peak VO2 113 , 114 |
| VO2@VT1 (ml/kg/min) | Age and sex‐specific cut‐offs | Submaximal parameter that correlates with peak VO2 113 |
| Ventilatory mechanical limitation | ||
| BR (%) | <15–20 | Ventilatory limitation 77 |
| VFL/VT (%) | >50 | Expiratory airflow limitation 77 |
| IC (ml) | Decrease >140 | Dynamic hyperinflation 77 |
| EELV (ml) | Increase instead of decrease | Dynamic hyperinflation 77 |
| Pulmonary vascular limitations defined by gas exchange abnormalities and/or haemodynamics | ||
| VE/VCO2 slope (L/min/ml/kg/min) | >30 | Reduced ventilatory efficiency due to increased ventilation and/or increased death space ventilation. 112 Elevations associated with higher PVR and more severe diseases in HFpEF patients with PH 115 |
| VE intercept | <2.64 L/min | May discriminate HFpEF from COPD HFpEF 116 |
| SaO2 (%) | Decrease ≥5 | Gas exchange abnormalities, most commonly related to V/Q mismatch 73 , 77 |
| VD/VT (%) a | No decrease from baseline or blunted response | Increased dead space ventilation related to V/Q mismatch and/or rapid shallow breathing, 77 associated with increased PVR and PH in HFpEF 115 |
| PA–aO2 a (mmHg) | Increase above age‐ and sex‐specific normal values | Gas exchange abnormalities, most commonly related to V/Q mismatch 77 , 117 |
| PaO2 (mmHg) a | Decrease ≥10 | Gas exchange abnormalities, most commonly related to V/Q mismatch 77 |
| Exercise PCWP (mmHg) b | ≥25 | Cut‐off for exercise‐induced PH with limited validity 76 , 93 |
| ΔPAP/ΔCO (mmHg/L/min) b | >3 | Alternative marker of exercise‐induced PH 76 , 93 |
| ΔTPG/ΔCO (mmHg/L/min) b | >1 | Pre‐capillary PH 76 , 93 |
| Cardiovascular limitations defined by gas exchange abnormalities and/or haemodynamics | ||
| VO2/work rate trajectory (ml/kg/min/W) | Flattening or decline | LV dysfunction due to myocardial ischaemia, 118 or right‐sided cardiac dysfunction and PH in HF 85 |
| O2 pulse trajectory (ml/kg/min/bpm) | Flattening or decline | LV dysfunction due to myocardial ischaemia 118 |
| HR/VO2 slope (bpm/ml/kg/min) | >50 | Relative tachycardia to VO2 77 |
| MCR | ≤0.80 or <0.62 on beta‐blocker | Chronotropic incompetence 40 |
| ΔPCWP/ΔCO slope (mmHg/L/min) b | >2 | Impaired LV reserve capacity 76 , 111 |
| Exercise RAP (mmHg) b | >PCWP | RV dysfunction 76 |
| ΔCO/ΔVO2 slope (ml blood/ml O2) b | <4.8 | Impaired CO reserve due to cardiac limitations or preload reserve failure 14 |
| Peripheral muscle limitations | ||
| VO2@VT1 (ml/kg/min) | <40% of predicted | Early first ventilatory threshold suggests peripheral muscle limitation 77 |
| Peak C(a–v)O2 (ml/dl) b | <0.8*haemoglobin | Impaired peripheral O2 utilization 76 |
| VO2 kinetics | MRT <60 s | Impaired peripheral O2 utilization in HFpEF, 119 may also indicate impaired RV pulmonary vascular function in HFrEF 120 |
| Risk stratification | ||
| VO2peak (ml/kg/min) | <14 | Predicts higher risk of HF hospitalization and the composite outcome of all‐cause death, LVAD implantation, or heart transplantation, in particular when combined with VE/VCO2 slope >30 108 |
| VE/VCO2 slope | >30 |
Predicts higher risk of HF hospitalization and the composite outcome of all‐cause death, LVAD implantation, or heart transplantation, in particular when combined with VO2peak <14 ml/kg/min 108 Predicts mortality in HFpEF patients with PH 81 |
| EOV | Present | Predicts higher risk of CV death 87 |
| HRR at 1 min (bpm) | <12 decrease | Predicts higher risk of CV death 121 |
| PCWP/CO slope (mmHg/L/min) b | >2 | Predicts higher risk of the composite outcome of CV death, HF hospitalization, or abnormal resting PCWP on future right heart catheterization 111 |
| PCWP/workload/kg (mmHg/W/kg) b | >25.5 | Predicts higher risk of all‐cause mortality, independently of baseline PCWP 105 |
| PAP/CO slope (mmHg/L/min) b | >3 | Predicts higher risk of first HF hospitalization or all‐cause mortality, both in patients with or without resting PH 96 , 102 |
BR, breathing reserve; C(a–v)O2, difference in oxygen content in arterial and mixed venous blood; CO, cardiac output; COPD, chronic obstructive pulmonary disease; CV, cardiovascular; EELV, end‐expiratory lung volume; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, heart rate; HRR, heart rate reserve; IC, inspiratory capacity; LV, left ventricular; LVAD, left ventricular assist device; MCR, metabolic–chronotropic relationship; OUES, oxygen uptake efficiency slope; PA‐aO2, alveolar–arterial oxygen gradient; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RER, respiratory exchange ratio; SaO2, arterial oxygen saturation; TPG, transpulmonary gradient; VCO2, carbon dioxide output; VD/VT, ratio of dead‐space ventilation to tidal ventilation; VFL/VT, percent of the tidal breath that expiratory airflow exceeds the maximal flow/volume envelope; VE, minute ventilation; VE/VCO2, minute ventilation to carbon dioxide output; VO2, oxygen uptake; VT, ventilatory threshold (VT1/VT2 corresponding to anaerobic threshold/respiratory compensation point).
Derived from additional arterial blood gas analysis.
Derived from additional invasive measurement (right heart catheterization).
Testing effectiveness of interventions through functional evaluation
Functional capacity has been addressed as an endpoint in several interventional trials of HFpEF focusing on peak VO2 as the main reference variable. Pharmacological trials in HFpEF have historically been unsuccessful in improving functional capacity and symptoms on effort, 70 , 122 , 123 though more recent human data on levosimendan (Reference 124) and animal model of exercise ‐induced PH treated with sodium–glucose cotransporter 2 inhibitor have revealed promising effects on functional capacity. 103 , 124
In addition to pharmacologic treatments, ET interventions have particularly become accepted since the earlier evidence on their effectiveness to modulate dyspnoea on exertion and to increase peak VO2. 125 , 126 , 127 In these studies, CPET has acquired a primary role in both planning ET interventions and measuring the extent of benefits.
In parallel, lifestyle interventions may be effective to prevent HFpEF, 128 to favourably affect several abnormalities of the HFpEF syndrome 129 and to effectively improve peak V̇O2, but prospective data on prevention are lacking. In patients with prevalent HFpEF, a landmark lifestyle intervention trial targeting weight loss, examined the effects of ET and caloric restriction in HFpEF versus controls on changes in peak V̇O2 over 20 weeks of treatment. 130 ET and caloric restriction resulted in similar changes in peak V̇O2 (average effect of ET: 1.2 ml/kg/min vs. diet 1.3 ml/kg/min) and weight loss. This is impactful as most patients with HFpEF are overweight or obese, and body mass index is a main determinant of peak V̇O2 131 and New York Heart Association functional class. 132
Effectiveness of ET programmes in HF have been further scrutinized by performing high‐intensity interval training (HIIT) in addition to traditionally prescribed moderate continuous training (MCT). The largest randomized controlled trial performed in HFpEF so far is the OptimEx trial (Optimizing Exercise in HFpEF), 133 which compared MCT versus HIIT, revealing that both ET intensities of moderate as well as high intensity may improve peak V̇O2 after 3 months of supervised endurance ET in stable HFpEF patients. Specifically, ET resulted in a mean increase of peak V̇O2 by +1.1 ml/kg/min for HIIT and +1.6 ml/kg/min for MCT. These changes were less compared to findings of a previous meta‐analysis of six smaller studies (n = 276 patients) over 12–24 weeks of ET (+2.7 ml/kg/min; 95% confidence interval 1.79–3.65). 134 Overall, data have shown that ET carries beneficial effects that are primarily mediated by peripheral rather than central determinants, e.g. myocardial diastolic function did not change significantly. 133 , 135 Rather, the effects seem to be related to training adherence, as the most adherent patients exhibited the most effects on peak V̇O2 and diastolic function. 133 Prevention of HFpEF may be different, as recent data indicate that sustained ET can improve LV diastolic stiffness in adults without HF. 136 This may relate to greater plasticity of myocardial dysfunction prior to HF onset, or a greater dose and duration of ET applied.
Data obtained by CPET can be extremely helpful for prescribing exercise intensities in HFpEF. 28 Beyond intensity modalities, e.g. Borg rating of perceived exertion (RPE), percentage of maximal HR (%HRmax), or percentage of HRR (%HRR), ventilatory thresholds, e.g. VT1 and VT2, may clearly differentiate individual metabolic and respiratory exercise intensity levels. Although a rough estimate can be given for e.g. 60–70% HRR, which equals VT1 and 80% of HRR, which equals VT2, precise HR corridors for exercise prescription are needed. This is especially relevant in HFpEF, as these patients have a high prevalence of CI, which affects the estimation of exercise HR by using the fixed HRmax or HR reserve formula. In these cases, the prescribed HR range, e.g. for MCT, is narrow and target training intensities may be falsely calculated when using traditional parameters, e.g. %HR max. 137
Moreover, individual responses to exercise vary widely despite similar exercise interventions as well as levels of adherence. This response heterogeneity is typical of HFpEF, as it is known to be a multifactorial and highly heterogeneous disease 138 and patients are almost exclusively suffering from multiple defects affecting the convective and diffusive O2 delivery. 5 In the OptimEx trial, the adaptive range to improve exercise capacity varied significantly among HFpEF patients, thus suggesting the potential value of personalized prescription of exercise intensity, which can be materially aided by the evaluation of baseline CPET parameters.
Conclusions and perspectives
In HFpEF, exercise intolerance is a hallmark manifestation, characterized by impairment in the physiological reserve capacity of multiple organ systems that is the cardiac dynamics itself and/or related comorbid conditions and extracardiac factors. The relative cardiac and extracardiac deficits vary among individuals. Therefore, detailed measurements made during exercise are necessary to identify and rank‐order the multiorgan system limitations in exercise reserve capacity. In this context, the value of CPET is well established in clinical practice in its ability to assess a multitude of derived variables, to address the specific phenotypes of exercise impairment, providing insightful information in the multistep limitation of the O2 cascade and directing attention towards the cardiac or non‐cardiac reasons for exercise limitation. While CPET is most useful to differentiate HFpEF from non‐cardiac dyspnoea at the extremes of peak VO2, it also provides valuable insight into potential pulmonary causes of dyspnoea, supporting its use earlier in the diagnostic evaluation. Advantages of the use of CPET also extend to planning of ET programmes as well as to the documentation of the effectiveness of therapeutic interventions. For these reasons an implementation of CPET use in the early and advanced diagnostic steps of the HFA‐PEFF score is adopted. A similar rationale applies to patients evaluated using the H2FPEF score, where CPET can be helpful in the initial diagnostic workup, as well as to guide medical decision making in patients where the diagnosis is secured. The use of gas exchange analysis with stress echocardiography by CPET imaging and/or invasive assessment remarkably increases the amount of diagnostic, pathophysiological and therapeutic insights. Under the European perspective, there is a need to expand CPET‐derived knowledge to HFpEF by implementing in clinical cardiology with infrastructure and expertise that may be lacking. Accordingly, the new ESC/EAPC curricula for core cardiology and subspecialty training aim at these goals. 139 , 140
Acknowledgement
Open Access Funding provided by Universita degli Studi di Milano within the CRUI‐CARE Agreement.
Conflict of interest: none declared.
References
- 1. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40:3297–317. [DOI] [PubMed] [Google Scholar]
- 2. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verbrugge FH, Omote K, Reddy YNV, Sorimachi H, Obokata M, Borlaug BA. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur Heart J. 2022;43:1941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON‐HF trial. Circ Heart Fail. 2018;11:e004962. [DOI] [PubMed] [Google Scholar]
- 5. Houstis NE, Eisman AS, Pappagianopoulos PP, Wooster L, Bailey CS, Wagner PD, et al. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O2 pathway analysis. Circulation. 2018;137:148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol. 2017;70:1618–36. [DOI] [PubMed] [Google Scholar]
- 7. Eckhardt CM, Balte PP, Barr RG, Bertoni AG, Bhatt SP, Cuttica M, et al. Lung function impairment and risk of incident heart failure: the NHLBI pooled cohorts study. Eur Heart J. 2022;43:2196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, et al. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 2020;8:172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mooney L, Hawkins NM, Jhund PS, Redfield MM, Vaduganathan M, Desai AS, et al. Impact of chronic obstructive pulmonary disease in patients with heart failure with preserved ejection fraction: insights from PARAGON‐HF. J Am Heart Assoc. 2021;10:e021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. AbouEzzeddine OF, Davies DR, Scott CG, Fayyaz AU, Askew JW, McKie PM, et al. Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yunis A, Doros G, Luptak I, Connors LH, Sam F. Use of ventilatory efficiency slope as a marker for increased mortality in wild‐type transthyretin cardiac amyloidosis. Am J Cardiol. 2019;124:122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank‐Starling mechanism. J Am Coll Cardiol. 1991;17:1065–72. [DOI] [PubMed] [Google Scholar]
- 14. Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarma S, Howden E, Lawley J, Samels M, Levine BD. Central command and the regulation of exercise heart rate response in heart failure with preserved ejection fraction. Circulation. 2021;143:783–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sugimoto T, Bandera F, Generati G, Alfonzetti E, Bussadori C, Guazzi M. Left atrial function dynamics during exercise in heart failure: pathophysiological implications on the right heart and exercise ventilation inefficiency. JACC Cardiovasc Imaging. 2017;10:1253–64. [DOI] [PubMed] [Google Scholar]
- 18. Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. 2020;76:1051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tamargo M, Obokata M, Reddy YNV, Pislaru SV, Lin G, Egbe AC, et al. Functional mitral regurgitation and left atrial myopathy in heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:489–98. [DOI] [PubMed] [Google Scholar]
- 20. Ahmad A, Corban MT, Toya T, Verbrugge FH, Sara JD, Lerman LO, et al. Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:765–72. [DOI] [PubMed] [Google Scholar]
- 21. Obokata M, Reddy YNV, Melenovsky V, Kane GC, Olson TP, Jarolim P, et al. Myocardial injury and cardiac reserve in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2018;72:29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parasuraman SK, Loudon BL, Lowery C, Cameron D, Singh S, Schwarz K, et al. Diastolic ventricular interaction in heart failure with preserved ejection fraction. J Am Heart Assoc. 2019;8:e010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fermoyle CC, Stewart GM, Borlaug BA, Johnson BD. Simultaneous measurement of lung diffusing capacity and pulmonary hemodynamics reveals exertional alveolar‐capillary dysfunction in heart failure with preserved ejection fraction. J Am Heart Assoc. 2021;10:e019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reddy YNV, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, et al. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2017;70:136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weavil JC, Thurston TS, Hureau TJ, Gifford JR, Kithas PA, Broxterman RM, et al. Heart failure with preserved ejection fraction diminishes peripheral hemodynamics and accelerates exercise‐induced neuromuscular fatigue. Am J Physiol Heart Circ Physiol. 2021;320:H338–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martens P, Claessen G, Van De Bruaene A, Verbrugge FH, Herbots L, Dendale P, et al. Iron deficiency is associated with impaired biventricular reserve and reduced exercise capacity in patients with unexplained dyspnea. J Card Fail. 2021;27:766–76. [DOI] [PubMed] [Google Scholar]
- 28. Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 Focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2018;39:1144–61. [DOI] [PubMed] [Google Scholar]
- 29. Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8:286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ho JE, Zern EK, Wooster L, Bailey CS, Cunningham T, Eisman AS, et al. Differential clinical profiles, exercise responses, and outcomes associated with existing HFpEF definitions. Circulation. 2019;140:353–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, et al. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:640–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mishra S, Kass DA. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2021;18:400–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered hemodynamics and end‐organ damage in heart failure: impact on the lung and kidney. Circulation. 2020;142:998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roberto S, Mulliri G, Milia R, Solinas R, Pinna V, Sainas G, et al. Hemodynamic response to muscle reflex is abnormal in patients with heart failure with preserved ejection fraction. J Appl Physiol (1985). 2017;122:376–85. [DOI] [PubMed] [Google Scholar]
- 35. Pandey A, Shah SJ, Butler J, Kellogg DL Jr, Lewis GD, Forman DE, et al. Exercise intolerance in older adults with heart failure with preserved ejection fraction: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;78:1166–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Soetkamp D, Gallet R, Parker SJ, Holewinski R, Venkatraman V, Peck K, et al. Myofilament phosphorylation in stem cell treated diastolic heart failure. Circ Res. 2021;129:1125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Burrage MK, Hundertmark M, Valkovic L, Watson WD, Rayner J, Sabharwal N, et al. Energetic basis for exercise‐induced pulmonary congestion in heart failure with preserved ejection fraction. Circulation. 2021;144:1664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilkoff BL, Miller RE. Exercise testing for chronotropic assessment. Cardiol Clin. 1992;10:705–17. [PubMed] [Google Scholar]
- 40. Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123:1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Palau P, Seller J, Dominguez E, Sastre C, Ramon JM, de La Espriella R, et al. Effect of beta‐blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021;78:2042–56. [DOI] [PubMed] [Google Scholar]
- 42. Mesquita T, Zhang R, Cho JH, Zhang R, Lin YN, Sanchez L, et al. Mechanisms of sinoatrial node dysfunction in heart failure with preserved ejection fraction. Circulation. 2022;145:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Singleton MJ, Nelson MB, Samuel TJ, Kitzman DW, Brubaker P, Haykowsky MJ, et al. Left atrial stiffness index independently predicts exercise intolerance and quality of life in older, obese patients with heart failure with preserved ejection fraction. J Card Fail. 2021;28:567–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Telles F, Nanayakkara S, Evans S, Patel HC, Mariani JA, Vizi D, et al. Impaired left atrial strain predicts abnormal exercise haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21:495–505. [DOI] [PubMed] [Google Scholar]
- 45. Sugimoto T, Barletta M, Bandera F, Generati G, Alfonzetti E, Rovida M, et al. Central role of left atrial dynamics in limiting exercise cardiac output increase and oxygen uptake in heart failure: insights by cardiopulmonary imaging. Eur J Heart Fail. 2020;22:1186–98. [DOI] [PubMed] [Google Scholar]
- 46. Pandey A, Vaduganathan M, Arora S, Qamar A, Mentz RJ, Shah SJ, et al. Temporal trends in prevalence and prognostic implications of comorbidities among patients with acute decompensated heart failure: the ARIC study community surveillance. Circulation. 2020;142:230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kitzman DW, Haykowsky MJ, Tomczak CR. Making the case for skeletal muscle myopathy and its contribution to exercise intolerance in heart failure with preserved ejection fraction. Circ Heart Fail. 2017;10:e004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chirinos JA, Segers P, Hughes T, Townsend R. Large‐artery stiffness in health and disease: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;74:1237–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Namasivayam M, Lau ES, Zern EK, Schoenike MW, Hardin KM, Sbarbaro JA, et al. Exercise blood pressure in heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2022;10:278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2012;302:H1050–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sorimachi H, Burkhoff D, Verbrugge FH, Omote K, Obokata M, Reddy YNV, et al. Obesity, venous capacitance, and venous compliance in heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:1648–58. [DOI] [PubMed] [Google Scholar]
- 52. Guazzi M, Ghio S, Adir Y. Pulmonary hypertension in HFpEF and HFrEF: JACC review topic of the week. J Am Coll Cardiol. 2020;76:1102–11. [DOI] [PubMed] [Google Scholar]
- 53. Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39:2825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Guazzi M, Naeije R. Right heart phenotype in heart failure with preserved ejection fraction. Circ Heart Fail. 2021;14:e007840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Van Iterson EH, Johnson BD, Borlaug BA, Olson TP. Physiological dead space and arterial carbon dioxide contributions to exercise ventilatory inefficiency in patients with reduced or preserved ejection fraction heart failure. Eur J Heart Fail. 2017;19:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J. 2019;40:3721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Omote K, Sorimachi H, Obokata M, Reddy YNV, Verbrugge FH, Omar M, Dubrock HM, Redfield MM, Borlaug B. Pulmonary vascular disease in pulmonary hypertension due to left heart disease: pathophysiologic implications. Eur Heart J. 2022, https://doi.10.1093/eurheartJ/ehac184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kitzman DW, Nicklas B, Kraus WE, Lyles MF, Eggebeen J, Morgan TM, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:H1364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Molina AJ, Bharadwaj MS, Van Horn C, Nicklas BJ, Lyles MF, Eggebeen J, et al. Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. JACC Heart Fail. 2016;4:636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parcha V, Patel N, Kalra R, Bhargava A, Prabhu SD, Arora G, et al. Clinical, demographic, and imaging correlates of anemia in heart failure with preserved ejection fraction (from the RELAX trial). Am J Cardiol. 2020;125:1870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barandiaran Aizpurua A, Sanders‐van Wijk S, Brunner‐La Rocca HP, Henkens M, Weerts J, Spanjers MHA, et al. Iron deficiency impacts prognosis but less exercise capacity in heart failure with preserved ejection fraction. ESC Heart Fail. 2021;8:1304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid‐range and preserved ejection fraction. Acta Cardiol. 2018;73:115–23. [DOI] [PubMed] [Google Scholar]
- 63. Koepp KE, Obokata M, Reddy YNV, Olson TP, Borlaug BA. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rayner JJ, Peterzan MA, Watson WD, Clarke WT, Neubauer S, Rodgers CT, et al. Myocardial energetics in obesity: enhanced ATP delivery through creatine kinase with blunted stress response. Circulation. 2020;141:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113:1211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maack C, Lehrke M, Backs J, Heinzel FR, Hulot JS, Marx N, et al. Heart failure and diabetes: metabolic alterations and therapeutic interventions: a state‐of‐the‐art review from the Translational Research Committee of the Heart Failure Association‐European Society of Cardiology. Eur Heart J. 2018;39:4243–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wolsk E, Kaye D, Borlaug BA, Burkhoff D, Kitzman DW, Komtebedde J, et al. Resting and exercise haemodynamics in relation to six‐minute walk test in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2018;20:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zotter‐Tufaro C, Mascherbauer J, Duca F, Koell B, Aschauer S, Kammerlander AA, et al. Prognostic significance and determinants of the 6‐min walk test in patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2015;3:459–66. [DOI] [PubMed] [Google Scholar]
- 69. Maldonado‐Martin S, Brubaker PH, Eggebeen J, Stewart KP, Kitzman DW. Association between 6‐minute walk test distance and objective variables of functional capacity after exercise training in elderly heart failure patients with preserved ejection fraction: a randomized exercise trial. Arch Phys Med Rehabil. 2017;98:600–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Armstrong PW, CSP Lam, Anstrom KJ, Ezekowitz J, Hernandez AF, O'Connor CM, et al.; VITALITY‐HFpEF Study Group . Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY‐HFpEF randomized clinical trial. JAMA. 2020;324:1512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shah SJ, Cowie MR, Wachter R, Szecsody P, Shi V, Ibram G, et al. Baseline characteristics of patients in the PARALLAX trial: insights into quality of life and exercise capacity in heart failure with preserved ejection fraction. Eur J Heart Fail. 2021;23:1541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guazzi M, Dickstein K, Vicenzi M, Arena R. Six‐minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights. Circ Heart Fail. 2009;2:549–55. [DOI] [PubMed] [Google Scholar]
- 73. Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, et al.; EACPR and AHA . EACPR/AHA Joint Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2012;33:2917–27. [DOI] [PubMed] [Google Scholar]
- 74. Agostoni P, Dumitrescu D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int J Cardiol. 2019;288:107–13. [DOI] [PubMed] [Google Scholar]
- 75. Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6:665–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Nayor M, Houstis NE, Namasivayam M, Rouvina J, Hardin C, Shah RV, et al. Impaired exercise tolerance in heart failure with preserved ejection fraction: quantification of multiorgan system reserve capacity. JACC Heart Fail. 2020;8:605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Laveneziana P, Di Paolo M, Palange P. The clinical value of cardiopulmonary exercise testing in the modern era. Eur Respir Rev. 2021;30:200187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Boucly A, Morelot‐Panzini C, Garcia G, Weatherald J, Jais X, Savale L, et al. Intensity and quality of exertional dyspnoea in patients with stable pulmonary hypertension. Eur Respir J. 2020;55:1802108. [DOI] [PubMed] [Google Scholar]
- 79. Neder JA, Arbex FF, Alencar MC, O'Donnell CD, Cory J, Webb KA, et al. Exercise ventilatory inefficiency in mild to end‐stage COPD. Eur Respir J. 2015;45:377–87. [DOI] [PubMed] [Google Scholar]
- 80. Teopompi E, Tzani P, Aiello M, Gioia MR, Marangio E, Chetta A. Excess ventilation and ventilatory constraints during exercise in patients with chronic obstructive pulmonary disease. Respir Physiol Neurobiol. 2014;197:9–14. [DOI] [PubMed] [Google Scholar]
- 81. Klaassen SHC, Liu LCY, Hummel YM, Damman K, van der Meer P, Voors AA, et al. Clinical and hemodynamic correlates and prognostic value of VE/VCO2 slope in patients with heart failure with preserved ejection fraction and pulmonary hypertension. J Card Fail. 2017;23:777–82. [DOI] [PubMed] [Google Scholar]
- 82. Alenezi F, Covington TA, Mukherjee M, Mathai SC, Yu PB, Rajagopal S. Novel approaches to imaging the pulmonary vasculature and right heart. Circ Res. 2022;130:1445–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Reddy YNV, Kaye DM, Handoko ML, Van de Bovenkamp AA, Tedford RJ, Keck C, Anderson MJ, Sharma K, Trivedi RK, Carter RE, Obokata M, Vergrubbe FH, Redfield MM, Borlaug BA. Diagnosis of heart failure with preserved ejection fraction with unexplained dyspena. JAMA Cardiol. 2022. https://doi.101001/jamacardio.2022.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Barron A, Francis DP, Mayet J, Ewert R, Obst A, Mason M, et al. Oxygen uptake efficiency slope and breathing reserve, not anaerobic threshold, discriminate between patients with cardiovascular disease over chronic obstructive pulmonary disease. JACC Heart Fail. 2016;4:252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Popovic D, Arena R, Guazzi M. A flattening oxygen consumption trajectory phenotypes disease severity and poor prognosis in patients with heart failure with reduced, mid‐range, and preserved ejection fraction. Eur J Heart Fail. 2018;20:1115–24. [DOI] [PubMed] [Google Scholar]
- 86. Guazzi M, Dixon D, Labate V, Beussink‐Nelson L, Bandera F, Cuttica MJ, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. 2017;10:1211–21. [DOI] [PubMed] [Google Scholar]
- 87. Guazzi M, Myers J, Peberdy MA, Bensimhon D, Chase P, Arena R. Exercise oscillatory breathing in diastolic heart failure: prevalence and prognostic insights. Eur Heart J. 2008;29:2751–9. [DOI] [PubMed] [Google Scholar]
- 88. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 89. Donal E, Lund LH, Oger E, Reynaud A, Schnell F, Persson H, et al. Value of exercise echocardiography in heart failure with preserved ejection fraction: a substudy from the KaRen study. Eur Heart J Cardiovasc Imaging. 2016;17:106–13. [DOI] [PubMed] [Google Scholar]
- 90. Shim CY, Kim SA, Choi D, Yang WI, Kim JM, Moon SH, et al. Clinical outcomes of exercise‐induced pulmonary hypertension in subjects with preserved left ventricular ejection fraction: implication of an increase in left ventricular filling pressure during exercise. Heart. 2011;97:1417–24. [DOI] [PubMed] [Google Scholar]
- 91. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation. 2017;135:825–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Obokata M, Kane GC, Sorimachi H, Reddy YNV, Olson TP, Egbe AC, et al. Noninvasive evaluation of pulmonary artery pressure during exercise: the importance of right atrial hypertension. Eur Respir J. 2020;55:1901617. [DOI] [PubMed] [Google Scholar]
- 93. Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J. 2017;50:1700578. [DOI] [PubMed] [Google Scholar]
- 94. Claessen G, La Gerche A, Voigt JU, Dymarkowski S, Schnell F, Petit T, et al. Accuracy of echocardiography to evaluate pulmonary vascular and RV function during exercise. JACC Cardiovasc Imaging. 2016;9:532–43. [DOI] [PubMed] [Google Scholar]
- 95. Martens P, Herbots L, Timmermans P, Verbrugge FH, Dendale P, Borlaug BA, et al. Cardiopulmonary exercise testing with echocardiography to identify mechanisms of unexplained dyspnea. J Cardiovasc Transl Res. 2021;15:116–30. [DOI] [PubMed] [Google Scholar]
- 96. Guazzi M, Borlaug B, Metra M, Losito M, Bandera F, Alfonzetti E, et al. Revisiting and implementing the weber and ventilatory functional classifications in heart failure by cardiopulmonary imaging phenotyping. J Am Heart Assoc. 2021;10:e018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nedeljkovic I, Banovic M, Stepanovic J, Giga V, Djordjevic‐Dikic A, Trifunovic D, et al. The combined exercise stress echocardiography and cardiopulmonary exercise test for identification of masked heart failure with preserved ejection fraction in patients with hypertension. Eur J Prev Cardiol. 2016;23:71–7. [DOI] [PubMed] [Google Scholar]
- 98. Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol. 2009;54:402–9. [DOI] [PubMed] [Google Scholar]
- 99. Simonovic D, Coiro S, Deljanin‐Ilic M, Kobayashi M, Carluccio E, Girerd N, et al. Exercise‐induced B‐lines in heart failure with preserved ejection fraction occur along with diastolic function worsening. ESC Heart Fail. 2021;8:5068–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hubert A, Girerd N, Le Breton H, Galli E, Latar I, Fournet M, et al. Diagnostic accuracy of lung ultrasound for identification of elevated left ventricular filling pressure. Int J Cardiol. 2019;281:62–8. [DOI] [PubMed] [Google Scholar]
- 101. Bandera F, Barletta M, Fontana M, Boveri S, Ghizzardi G, Alfonzetti E, et al. Exercise‐induced mitral regurgitation and right ventricle to pulmonary circulation uncoupling across the heart failure phenotypes. Am J Physiol Heart Circ Physiol. 2021;320:H642–53. [DOI] [PubMed] [Google Scholar]
- 102. Ho JE, Zern EK, Lau ES, Wooster L, Bailey CS, Cunningham T, et al. Exercise pulmonary hypertension predicts clinical outcomes in patients with dyspnea on effort. J Am Coll Cardiol. 2020;75:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Satho T, Wang L, Espinosa‐Diez C, Wang B, Hahn SA, Noda K, Rochon ER et al Metabolic syndrome mediates ROS‐miR‐193b‐NFYA‐dependent downregulation of soluble guanylate cyclase and contributes to exercise‐induced pulmonary hypertension in heart failure with preserved ejection fraction. Circulation. 2021;144:615–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Andersen MJ, Hwang SJ, Kane GC, Melenowski V, Olson TP, Fetterly K, Borlaug BA. Enhanced pulmonary vasodilator reserve and abnormal right ventricular pulmonary artery coupling in heart failure with preserved ejection fraction. Circ Heart Fail. 2015;8:542–50. [DOI] [PubMed] [Google Scholar]
- 105. Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, et al. Pulmonary capillary wedge pressure during exercise and long‐term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35:3103–12. [DOI] [PubMed] [Google Scholar]
- 106. Lewis GD, Bossone E, Naeije R, Grunig E, Saggar R, Lancellotti P, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128:1470–9. [DOI] [PubMed] [Google Scholar]
- 107. Jain CC, Borlaug BA. Performance and interpretation of invasive hemodynamic exercise testing. Chest. 2020;158:2119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nadruz W Jr, West E, Sengelov M, Santos M, Groarke JD, Forman DE, et al. Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J Am Heart Assoc. 2017;6:e006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Shafiq A, Brawner CA, Aldred HA, Lewis B, Williams CT, Tita C, et al. Prognostic value of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. The Henry ford HospITal CardioPulmonary EXercise testing (FIT‐CPX) project. Am Heart J. 2016;174:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Corra U, Agostoni PG, Anker SD, Coats AJS, Crespo Leiro MG, de Boer RA, et al. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:3–15. [DOI] [PubMed] [Google Scholar]
- 111. Eisman AS, Shah RV, Dhakal BP, Pappagianopoulos PP, Wooster L, Bailey C, et al. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail. 2018;11:e004750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Malhotra R, Bakken K, D'Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016;4:607–16. [DOI] [PubMed] [Google Scholar]
- 113. Takken T, Mylius CF, Paap D, Broeders W, Hulzebos HJ, Van Brussel M, et al. Reference values for cardiopulmonary exercise testing in healthy subjects – an updated systematic review. Expert Rev Cardiovasc Ther. 2019;17:413–26. [DOI] [PubMed] [Google Scholar]
- 114. Sun XG, Hansen JE, Stringer WW. Oxygen uptake efficiency plateau: physiology and reference values. Eur J Appl Physiol. 2012;112:919–28. [DOI] [PubMed] [Google Scholar]
- 115. Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39:2810–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Smith JR, Van Iterson EH, Johnson BD, Borlaug BA, Olson TP. Exercise ventilatory inefficiency in heart failure and chronic obstructive pulmonary disease. Int J Cardiol. 2019;274:232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Petersson J, Glenny RW. Gas exchange and ventilation‐perfusion relationships in the lung. Eur Respir J. 2014;44:1023–41. [DOI] [PubMed] [Google Scholar]
- 118. Belardinelli R, Lacalaprice F, Carle F, Minnucci A, Cianci G, Perna G, et al. Exercise‐induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur Heart J. 2003;24:1304–13. [DOI] [PubMed] [Google Scholar]
- 119. Hearon CM Jr, Sarma S, Dias KA, Hieda M, Levine BD. Impaired oxygen uptake kinetics in heart failure with preserved ejection fraction. Heart. 2019;105:1552–8. [DOI] [PubMed] [Google Scholar]
- 120. Chatterjee NA, Murphy RM, Malhotra R, Dhakal BP, Baggish AL, Pappagianopoulos PP, et al. Prolonged mean VO2 response time in systolic heart failure: an indicator of impaired right ventricular‐pulmonary vascular function. Circ Heart Fail. 2013;6:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart‐rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–7. [DOI] [PubMed] [Google Scholar]
- 122. Udelson JE, Lewis GD, Shah SJ, Zile MR, Redfield MM, Burnett J Jr, et al. Effect of praliciguat on peak rate of oxygen consumption in patients with heart failure with preserved ejection fraction: the CAPACITY HFpEF randomized clinical trial. JAMA. 2020;324:1522–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42:700–10. [DOI] [PubMed] [Google Scholar]
- 124. Burkhoff D, Borlaug BA, Shah SJ, Zolty R, Tedford RJ, Thenappan T, et al. Levosimendan improves hemodynamics and exercise tolerance in PH‐HFpEF: results of the randomized placebo‐controlled HELP trial. JACC Heart Fail. 2021;9:360–70. [DOI] [PubMed] [Google Scholar]
- 125. Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex‐DHF (Exercise Training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–91. [DOI] [PubMed] [Google Scholar]
- 127. Mueller S, Haller B, Halle M; OptimEx‐Clin Study Group . Effect of training on peak oxygen consumption in patients with heart failure with preserved ejection fraction‐reply. JAMA. 2021;326:772–3. [DOI] [PubMed] [Google Scholar]
- 128. Kraigher‐Krainer E, Lyass A, Massaro JM, Lee DS, Ho JE, Levy D, et al. Association of physical activity and heart failure with preserved vs. reduced ejection fraction in the elderly: the Framingham Heart Study. Eur J Heart Fail. 2013;15:742–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wolsk E, Kaye D, Komtebedde J, Shah SJ, Borlaug BA, Burkhoff D, et al. Central and peripheral determinants of exercise capacity in heart failure patients with preserved ejection fraction. JACC Heart Fail. 2019;7:321–32. [DOI] [PubMed] [Google Scholar]
- 132. Dalos D, Mascherbauer J, Zotter‐Tufaro C, Duca F, Kammerlander AA, Aschauer S, et al. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:189–99. [DOI] [PubMed] [Google Scholar]
- 133. Mueller S, Winzer EB, Duvinage A, Gevaert AB, Edelmann F, Haller B, et al.; OptimEx‐Clin Study Group . Effect of high‐intensity interval training, moderate continuous training, or guideline‐based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2021;325:542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta‐analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, et al. Dose‐response relationship between physical activity and risk of heart failure: a meta‐analysis. Circulation. 2015;132:1786–94. [DOI] [PubMed] [Google Scholar]
- 136. Hieda M, Sarma S, Hearon CM Jr, MacNamara JP, Dias KA, Samels M, et al. One‐year committed exercise training reverses abnormal left ventricular myocardial stiffness in patients with stage B heart failure with preserved ejection fraction. Circulation. 2021;144:934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hansen D, Abreu A, Ambrosetti M, Cornelissen V, Gevaert A, Kemps H, et al. Exercise intensity assessment and prescription in cardiovascular rehabilitation and beyond: why and how: a position statement from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2022;29:230–45. [DOI] [PubMed] [Google Scholar]
- 138. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, et al. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134:73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Tanner FC, Brooks N, Fox KF, Gonçalves L, Kearney P, Michalis L, et al. ESC core curriculum for the cardiologist. Eur Heart J. 2020;41:3605–92. [DOI] [PubMed] [Google Scholar]
- 140. Wilhelm M, Abreu A, Adami PE, Ambrosetti M, Antonopoulou M, Biffi A, et al. EACP core curriculum for preventive cardiology. Eur J Prev Cardiol. 2021;29:251–74. [DOI] [PubMed] [Google Scholar]


