Abstract
Aims
We aimed to conduct a systematic review and meta‐analysis of randomised controlled clinical trials (RCTs) assessing separately and together the effect of the three distinct categories of continuous glucose monitoring (CGM) systems (adjunctive, non‐adjunctive and intermittently‐scanned CGM [isCGM]), compared with traditional capillary glucose monitoring, on HbA1c and CGM metrics.
Methods
PubMed, Web of Science, Scopus and Cochrane Central register of clinical trials were searched. Inclusion criteria were as follows: randomised controlled trials; participants with type 1 diabetes of any age and insulin regimen; investigating CGM and isCGM compared with traditional capillary glucose monitoring; and reporting glycaemic outcomes of HbA1c and/or time‐in‐range (TIR). Glycaemic outcomes were extracted post‐intervention and expressed as mean differences and 95%CIs between treatment and comparator groups. Results were pooled using a random‐effects meta‐analysis. Risk of bias was assessed using the Cochrane Rob2 tool.
Results
This systematic review was conducted between January and April 2021; it included 22 RCTs (15 adjunctive, 5 non‐adjunctive, and 2 isCGM)). The overall analysis of the pooled three categories showed a statistically significant absolute improvement in HbA1c percentage points (mean difference (95% CI): −0.22% [−0.31 to −0.14], I 2 = 79%) for intervention compared with comparator and was strongest for adjunctive CGM (−0.26% [−0.36, −0.16]). Overall TIR (absolute change) increased by 5.4% (3.5 to 7.2), I 2 = 71% for CGM intervention compared with comparator and was strongest with non‐adjunctive CGM (6.0% [2.3, 9.7]).
Conclusions
For individuals with T1D, use of CGM was beneficial for impacting glycaemic outcomes including HbA1c, TIR and time‐below‐range (TBR). Glycaemic improvement appeared greater for TIR for newer non‐adjunctive CGM technology.
Keywords: adjunctive CGM, CGM metrics, continuous glucose monitoring, HbA1c, isCGM, non‐adjunctive CGM, type 1 diabetes
Novelty statement:
The impact of real‐time continuous glucose monitoring (RT‐CGM) compared with Self‐Monitoring of Blood Glucose on CGM metrics as shown by previous meta‐analyses has been either modest or non‐significant. However, separate assessment of the distinct categories of CGM systems (adjunctive, nonadjunctive and intermittently‐scanned‐CGM) on HbA1c and CGM metrics has not been done previously.
This meta‐analysis showed significant improvement in HbA1c percentage points with the strongest noticed for adjunctive CGM. Time‐in‐range increased for CGM especially with non‐adjunctive CGM.
The use of CGM improved glycaemic outcomes; the greater improvement for TIR was noticed with the newer non‐adjunctive CGM technology.
1. INTRODUCTION
Glucose monitoring is an integral aspect of type 1 diabetes (T1D) management. 1 , 2 However, given the limitations of self‐monitored blood glucose (SMBG), 3 , 4 , 5 alternative methods have been sought. This has led to considerable advances in interstitial continuous glucose monitoring (CGM) over the past two decades. Since 1999 when the first system was marketed, 6 modern CGM systems have become smaller, more user friendly, and more accurate. 7
Modern interstitial CGM is broadly divided into: real‐time continuous glucose monitoring (RT‐CGM); and intermittently scanned continuous glucose monitoring (isCGM). 8 , 9 These systems measure interstitial glucose levels through a subcutaneous glucose sensor at frequent intervals. 8 , 9 RT‐CGM allows real‐time access to glucose data, predictive glucose alerts to mitigate or prevent hypoglycaemia and/or hyperglycaemia, and inter‐operability with insulin pump or closed‐loop systems. 9 , 10 On a divergent CGM pathway are isCGM, which provide glucose readings only on demand when the user scans (using near field communication) the sensor with a reader device, and lacks the ability for continuous real‐time remote monitoring and the provision of glucose threshold alerts (although the recently released Abbott FreeStyle Libre 2™ provides limited vibrational alerts). 9 , 11 , 12 Continuous glucose monitoring can also be defined as adjunctive or non‐adjunctive, with early generation CGM sensors designed to be used as an adjunct to SMBG to make treatment decisions. More recently, as accuracy and reliability has improved, CGM sensors have become non‐adjunctive, enabling treatment decisions without finger‐stick confirmation. 13 Devices with current non‐adjunctive approvals from the United States Food and Drug Administration (FDA) are: isCGM systems ‐ Abbott Freestyle Libre 1 and 2, and the following RT‐CGM systems ‐ Dexcom G5, Dexcom G6, and Eversense. 13 , 14 The Guardian™ 4 system has non‐adjunctive CE mark approval in Europe only. 15
Many studies spanning multiple design types, including observational real‐world data and trials, have now assessed CGM efficacy, often using HbA1c or a variety of CGM metrics as their primary outcomes. Although, only relatively recently has CGM metric reporting become standardised 16 , 17 , 18 to now include key factors such as time‐in‐range (TIR) [70–180 mg/dl (3.9–10 mmol/L)], time below range (TBR) [<70 mg/dl (<3.9 mmol/L)], time‐above‐range (TAR) [>180 mg/dl (>10 mmol/L)] and glucose variability. Previous meta‐analyses of randomised controlled trials (RCTs) have suggested an improvement in these parameters after the use of RT‐CGM compared with SMBG. 11 , 19 However, the overall effect size of these findings has been modest; and some meta‐analyses have shown no significant improvements in these parameters. 12 , 20 In addition, a weakness of prior meta‐analyses has been the combining of all past studies together, be they older adjunctive, newer non‐adjunctive technologies, or isCGM together with RTCGM. Some have only included older technologies in the analysis. 11 , 19
The aim of the present study was to conduct a systematic review and meta‐analysis of RCT investigating separately and together the effect of the three broad categories of divergent CGM systems for T1D (adjunctive, non‐adjunctive and isCGM) and their effect on HbA1c and TIR (as well as a range of broader secondary glycaemic variables).
2. METHODS
This meta‐analysis follows the 2009 Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines (registered at PROSPERO, https://www.crd.york.ac.uk/prospero, at CRD42021234019). The PRISMA checklist is provided in the Supplementary Data.
2.1. Data sources and searches
A search of the databases PubMed, Web of Science, Scopus and Cochrane Central register of clinical trials was conducted up to April 2021. Studies not in English language were excluded. No other filters were applied. The complete search terms list used were as follows: (‘type 1 diabetes’ OR t1d OR ‘insulin dependent diabetes’ OR iddm) AND (‘continuous glucose monitor*’ OR ‘flash glucose monitor*’ OR ‘continuous subcutaneous glucose’ OR ‘glucose sensor’ OR ‘glucose‐sensor’ OR cgm OR rtcgm OR fgm OR icgm OR iscgm OR ‘diabetes technology’ OR ‘sensor‐guided’ OR ‘sensor guided’ OR ‘sensor augmented’ OR ‘sensor‐augmented’ OR sap). A full list of search terms for each database is found in Table S3.
2.2. Study selection
RCTs were only included if they compared RT‐CGM or isCGM with traditional SMBG and if glycaemic measurements of TIR and/or change in HbA1c were reported (co‐primary outcomes). Results were also collected for TBR, TAR, and glucose variability (%CV or SD) if these were reported in these trials. Trials were restricted to participants with T1D of any age, receiving either multiple daily injections of insulin (MDI) or continuous subcutaneous infusion (CSII). In addition, we selected RCTs if they had a minimum intervention duration for 6 weeks for TIR and, 12 weeks for HbA1c measurements, and used CGM for at least 50% of the intervention. Exclusion criteria included type 2 diabetes, and non‐randomised extensions of previous studies or studies with incomplete data. Trials were also excluded if the insulin administration method differed between the control and intervention group, or if the trial involved any level of insulin automation, that is, low glucose suspend, threshold suspend, predictive low glucose suspend or closed loop systems. Trials directly comparing isCGM with RT‐CGM were also excluded (as not compared with SMBG). Two independent investigators (ME and HS) assessed each RCT’s eligibility based on the titles and abstracts, and did the data extraction for the systematic review independently, with input from a third investigator (JH) to extract data required for meta‐analysis as needed.
2.3. Outcomes of interest
The two primary outcomes of this meta‐analysis were as follows: TIR and HbA1c between individuals using traditional SMBG compared with rt‐CGM or isCGM. Secondary outcomes were TBR and TAR, and glycaemic variability (%coefficient of variation [%CV]).
2.4. Data extraction
All search results from the databases were downloaded into EndNote X9 (Clarivate Analytics, London, United Kingdom), and duplicates were removed. Using Rayyan (a web application for systematic reviews), 21 titles and abstracts were screened to determine relevance. The full text of remaining studies was then closely examined to determine eligibility. Where eligibility could not be decided (based mainly on missing or unclear information), authors were contacted to clarify issues. Data were extracted into a predesigned spreadsheet to help with data extraction for both meta‐analysis and systematic review. This included information about author, country, publication year, trial design and duration, number of participants and participants’ demographic characteristics, rate of attrition, device type and duration of usage and differences in primary and secondary outcomes with the inclusion of p‐values and/or confidence intervals (CI).
We assessed risk of bias for studies’ estimates of the effect of assignment to the intervention (intention‐to‐treat) for all outcomes, using the Cochrane RoB 2 tool. 22 The RoB 2 looks at potential for bias across 5 domains: the randomisation process, deviations from the intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. Risk of bias for each domain and overall were designated as ‘low’, ‘some concerns’ or ‘high’ by two independent reviewers (SK and BG). Discrepancies between reviewers were resolved by discussion and by a third reviewer (BW) if consensus could not be reached.
2.5. Data synthesis and meta‐analysis
For each RCT, the mean changes from the baseline within the control and the intervention groups and its CI were extracted. The effect size was the estimated difference between the mean changes. If the effect size was not available, then it was calculated as the difference between the post‐intervention means. If the post‐intervention means were not reported in the RCT, it was calculated as the difference between the post‐intervention medians. 23 The 95% CI of the effect sizes were reported if available. If these were not reported and the p‐value was reported, the p‐value was used to calculate the 95% CI. 24 If the SD of the differences was reported, then the CI was calculated using the method outlined in Cochrane Handbook. 25 If there was only an interquartile range reported, this was used to estimate the SD by multiplying by 1.35 (again, as outlined in the Cochrane Handbook). If the units of time (for TIR, TAR and TBR) were reported in minutes or hours of the day, these were converted to percentage of the day on the assumption that data were collected for 24 h. Those studies that had the time converted to percentage were included in a meta‐regression to assess if this time conversion influenced the summary effects. Where estimates were reported by age group or other demographic group (such as pregnant or planning to get pregnant), these were included separately.
Restricted maximum likelihood random effects meta‐analyses were undertaken for each outcome by whether the active intervention was adjunctive, non‐adjunctive, or intermittent scanning. These were undertaken using ‘meta’ in Stata 17.0 (StataCorp, Texas). Effect sizes, 95% CI, and p‐values were calculated for each sub‐group and for all studies together. Forest plots were generated to display results. Heterogeneity statistics were also calculated: τ2, I 2 and H2.
Some studies reported medians instead of means for TBR because of skewed data. The difference in the medians was used as an approximation for the mean difference, however, when data are skewed this may not be an accurate approximation. Because of this, a sensitivity analysis was undertaken that only included studies that reported mean difference.
To further explore sources of heterogeneity, meta‐regression models were calculated by whether the study was carried out in adults or children; whether baseline HbA1c was >58 mmol/mol > 7.5% (indicative of inadequate control); whether the study was a cross‐over or parallel trial; and whether the trial reported effect sizes that were adjusted for baseline. The meta‐regressions were undertaken for the adjunctive and non‐adjunctive studies together and were not undertaken if one subgroup had less than three studies. Mean differences and 95% CI were calculated from the meta‐regression, which represent the mean difference in effect size by the subgroup. If a mean difference was large enough to indicate potential moderation, forest plots were also generated by these subgroups to illustrate trends.
3. RESULTS
3.1. Search results
The process of study selection is illustrated in Figure 1. In total, 3835 records were identified with the initial search process. After excluding duplicates, we included 2259 records. A total of 2172 records were further excluded on the basis of reviewing titles and abstracts. Further excluded trials (n = 64) were mainly reviews, comments, editorials, and observational studies. Finally, 22 RCTs were included in the quantitative synthesis and meta‐analysis.
FIGURE 1.
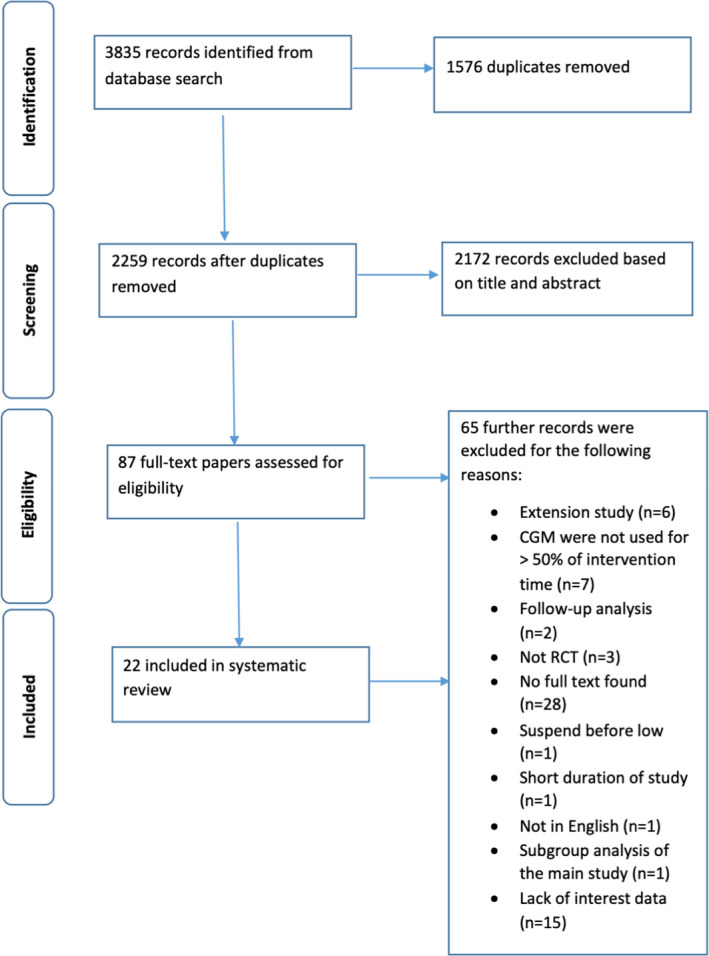
Flowchart of the recruitment process. CGM – continuous glucose monitoring; RCT – randomised controlled trail
3.2. Study and participant characteristics
Of 22 RCTs, there were 15 studies with adjunctive CGM, five studies with non‐adjunctive CGM, and two isCGM studies. Details of the 22 trials are presented in Table S1.
Clinical trials of adjunctive CGM included Medtronic Paradigm 722 (n = 1) 26 ; FreeStyle Navigator (n = 4) 27 , 28 , 29 , 30 ; Guardian REAL‐Time (n = 3) 31 , 32 , 33 ; Dexcom G4 Platinum (n = 2) 34 , 35 ; Dexcom SEVEN (n = 2) 27 , 28 ; MiniMed Paradigm (n = 2) 27 , 28 ; MiniMed Paradigm REAL‐Time (n = 5) 27 , 28 , 36 , 37 , 38 ; MiniMed MiniLink (n = 2) 29 , 33 ; Enlite (n = 1) 39 ; Paradigm Veo system with a MiniLink transmitter/Enlite sensor (n = 2). 39 , 40 Clinical trials of non‐adjunctive CGM included Dexcom G5 (n = 4), 41 , 42 , 43 , 44 and Dexcom G6 (n = 1). 45 Some of these RCTs investigated more than one sensor. 27 , 28 , 29 , 39 Funding sources were from the industry in the majority (n = 18) with only four studies supported via independent grants. 27 , 28 , 40 , 46 Insulin delivery varied by study, with MDI alone in three studies, 34 , 35 , 43 CSII alone in five studies 26 , 31 , 36 , 37 , 38 and by both MDI and CSII in fourteen studies. 27 , 28 , 29 , 30 , 32 , 33 , 39 , 40 , 41 , 42 , 44 , 45 , 46 , 47
Different primary outcomes were assessed in the included RCTs. Change in HbA1c was investigated in 13 studies, 26 , 27 , 29 , 31 , 32 , 33 , 34 , 35 , 37 , 38 , 41 , 46 , 48 TIR in four studies 36 , 39 , 44 , 45 and TBR in four studies 28 , 30 , 42 , 47 ; one study investigated the difference in hypoglycaemia awareness 40 and another reported the number of hypoglycaemic events. 43 The definition of TIR as a primary outcome varied across the included studies; three studies reported it as the time spent between 70 and 180 mg/dl, 36 , 44 , 45 and one study reported it as the time spent between 72 and 180 mg/dl. 39 In addition, the definition of the time spent in hypoglycaemia showed some variability as four studies defined it as <70 mg/dl, 28 , 42 , 47 , 49 and one study measured it <63 mg/dl. 30 The variation in the definition of targets for TIR and the different levels cut‐off levels for the time spent in hypoglycaemia was also obvious when both glycaemic outcomes were measured as secondary outcomes. Difference in hypoglycaemia awareness, 40 and the number of hypoglycaemic events 43 were reported as primary outcomes in two studies.
3.3. Adjunctive
Fifteen RCTs were included; these studies were published between 2006 and 2017. In total, 2218 participants were included, of these 1226 were in the intervention group. Three studies used the cross‐over design, 31 , 34 , 39 with intervention period ranging from 16 to 28 weeks.
Only two studies recruited children, 29 , 38 whereas five studies recruited only adults, 26 , 33 , 34 , 39 , 40 and eight recruited both children and adults. 27 , 28 , 30 , 31 , 32 , 35 , 36 , 37 Mean participant age ranged from 7.5 to 51 years. All included adjunctive RCTs recruited both males and females with the exception of one study that recruited only pregnant females and females who were planning to get pregnant. 33 The level of baseline HbA1c was reasonably in target in 4 RCTs, 28 , 30 , 36 , 39 whereas the other 12 adjunctive RCTs started with out of target level of HbA1c. Mean baseline HbA1c ranged from 6.4% to 11.5% (46 to 102). Of these 15 studies, 6 26 , 31 , 34 , 37 , 38 , 39 did not report that they used statistical analyses that adjusted for baseline levels, and a further 3 studies 29 , 32 , 33 did not report adjusted effect sizes. Three studies 27 , 28 , 30 reported effects adjusted for baseline HbA1c but not for any other outcome.
3.4. Non‐adjunctive continuous glucose monitoring
Five RCTs were included with one study published in 2018 43 and the other four studies published in 2020. 41 , 42 , 44 , 45 One 45 used a cross‐over design with a duration of intervention of 8 weeks, whereas the other four studies 41 , 42 , 43 , 44 used a parallel design with a duration of intervention ranging from 24 to 26 weeks. Mean baseline HbA1c ranged from 7.3% to 9.3%. Overall, 658 participants with T1D were included, of these, 326 were in the intervention group. Most non‐adjunctive RCTs recruited adults and/or teenagers (16 years and above) with the exception of one study 44 which recruited children. Study mean participant age ranged from 5.2 to 68 years. One study did not report effect sizes adjusted for baseline. 45
3.5. Intermittent continuous glucose monitoring
Two studies with parallel design were included. One study started with considerably out of target HbA1c at baseline and its primary outcome was the change in HbA1c after the use of isCGM for six months. 46 The primary outcome of the other study was the change in time spent in hypoglycaemia after a period of intervention of 6 months. 47 The total number of participants was 303, of these, 152 were in the intervention group. One study recruited youth aged 13–20 years, 46 whereas one recruited only adults. 47 Mean participant age ranged from 16.5 to 45 years, and mean baseline HbA1c ranged from 6.8% to 11.2%.
3.6. Primary outcomes from meta‐analysis
The overall analysis of the pooled three categories (adjunctive, non‐adjunctive and isCGM) showed a statistically significant absolute improvement in HbA1c percentage points (mean difference (95% CI): −0.22% (−0.31 to −0.14)) for intervention compared with control, with heterogeneity of 79% (Figure 2). The effects were strongest with adjunctive technology (−0.26% [−0.36, −0.16]), and no evidence of a difference in HbA1c was seen for isCGM. Overall absolute TIR increased by 5.4% (3.5 to 7.2) for CGM intervention compared with control, with heterogeneity (I2) of 71% (Figure 3). The effects were strongest with non‐adjunctive technology (6.0% [2.3, 9.7]). A meta‐regression comparing effects between studies where results were reported in % of time compared with studies reporting effects in minutes or hours did not suggest that the unit conversion influenced results (Table S2).
FIGURE 2.
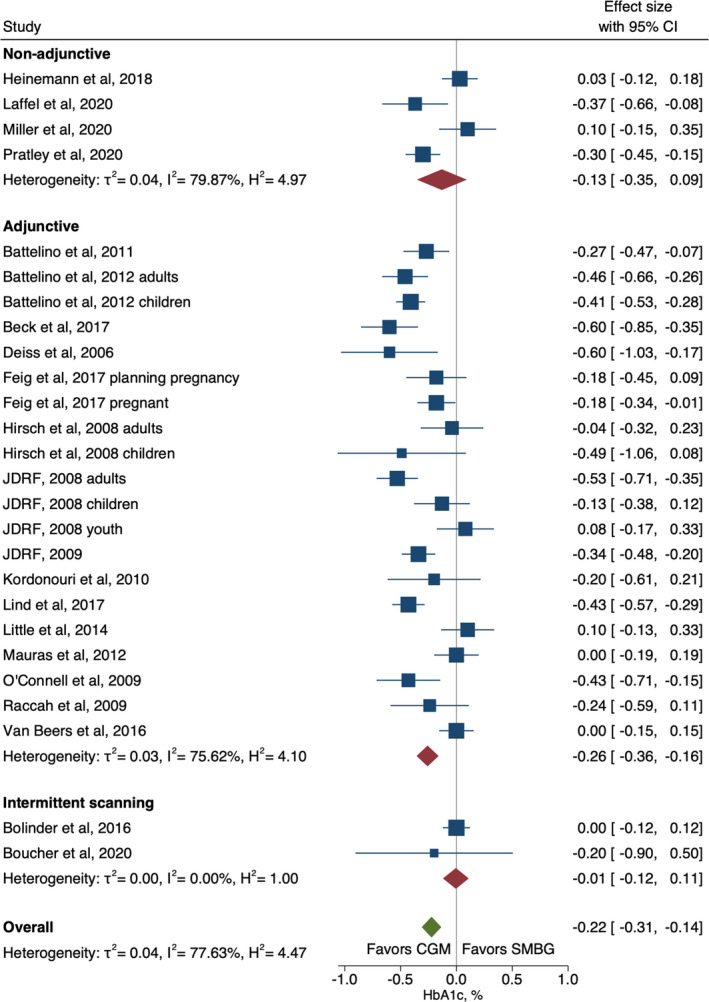
Forest plot of meta‐analysis of continuous glucose monitoring randomised controlled trials for HbA1c by non‐adjunctive, adjunctive and intermittent scanning technologies
FIGURE 3.
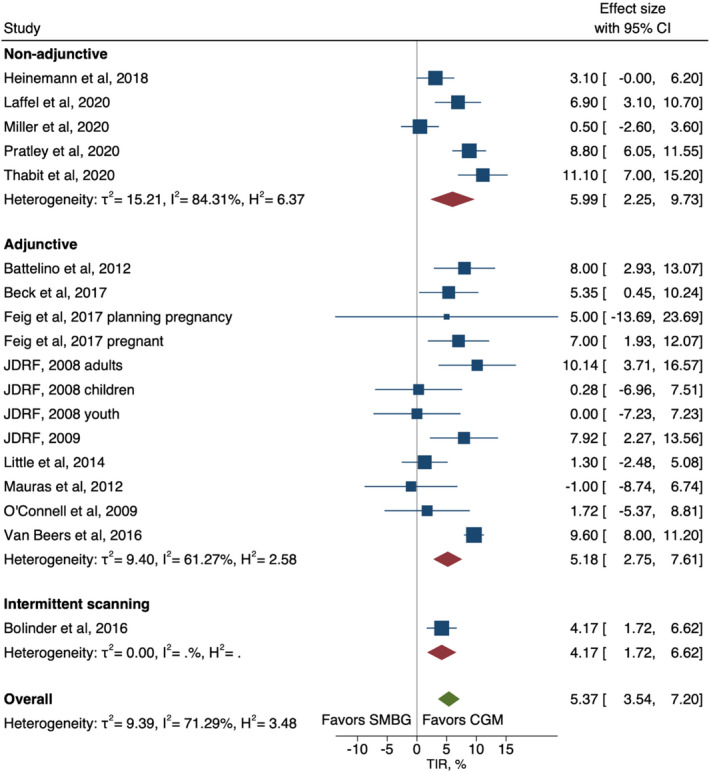
Forest plot of meta‐analysis of randomised controlled trails for time‐in‐range by non‐adjunctive, adjunctive and intermittent scanning technologies
Meta‐regression explored potential sources of heterogeneity (Table S2). Study design was indicated as a potential moderator for trials investigating HbA1c, where cross‐over trials showed stronger effects (−0.32 [−0.54, −0.11]) than parallel trials (−0.21 [−0.31, −0.11]) (Figure S3). Similarly, a higher increase in TIR was seen in studies with cross‐over design; 9.7% (8.2 to 11.1) compared with parallel design; 4.3% (95% CI 2.3 to 6.3) (Figure S4). Age group and baseline HbA1c were also effect modifiers for TIR, where the effect in adults was much stronger than in children (6.4% [3.4, 9.4] and 0.3% [−2.4, 3.0], respectively) (Figure S5) and studies of participants with HbA1c within target levels (≤58 mmol/mol/≤7.5%) were more effective than studies of participants who had HbA1c above target levels (6.9% [4.3, 9.5] and 4.5% [1.8, 7.3], respectively) (Figure S6).
3.7. Secondary outcomes
Overall, the use of non‐adjunctive and adjunctive RT‐CGM combined showed a significant decrease in TAR; −3.6% (95% CI −5.9 to −1.3), with a heterogeneity (I2) of 75% (Figure S1). The 3.6% decrease in TAR can be interpreted to an average of 0.87 h decrease in time spent in hyperglycaemia (>180 mg/dl [3.9 mmol/l]) per day. Non‐adjunctive and adjunctive RT‐CGM showed similar effectiveness in reducing TAR. When exploring potential sources of heterogeneity through meta‐regression, both age group and study design were indicated to be potential effect modifiers. Figure S7 shows that RT‐CGM was only effective at lowering TAR in adults (−4.6% [−7.8, −1.3]) but not in children (2.1 (−0.4, 4.5) %). RT‐CGM was also more effective in cross‐over trials than in parallel trials (−7.3% [−11.2, −3.3] and −2.4% [−4.8, 0.1], respectively) (Figure S8). The meta‐regression by studies with converted time units (to %) also indicated that these conversions did not result in meaningfully different effect estimates (Table S2).
Overall, with all divergent CGM technology combined there was a significant absolute decrease in TBR by 1.8% (95% CI −2.7 to −0.8) for intervention compared with control, with a heterogeneity (I2) of 84% (Figure 4). Non‐adjunctive and adjunctive RT‐CGM performed similarly and there was only one study investigating the effect of isCGM. Eight studies reported medians instead of means for TBR because of skewed data; therefore, we undertook a sensitivity analysis that excluded these studies. This showed that the overall effect size was strengthened, estimating a significant reduction in TBR of −2.2% [95% CI −3.6 to −0.7] (Figure S9), suggesting that including studies that reported medians does not inflate effect estimates. When exploring sources of heterogeneity through meta‐regression, studies with baseline HbA1c above or below the target level (58 mmol/mol / 7.5%) was indicated as a potential moderator (Table S2). Studies with participants who started within the target level of HbA1c at baseline showed a greater effect of RT‐CGM (−3.0% (−4.5 to −1.5) compared with studies with participants who had baseline HbA1c above the target level (−0.9% (−1.70 to −0.04)) (Figure S10)
FIGURE 4.
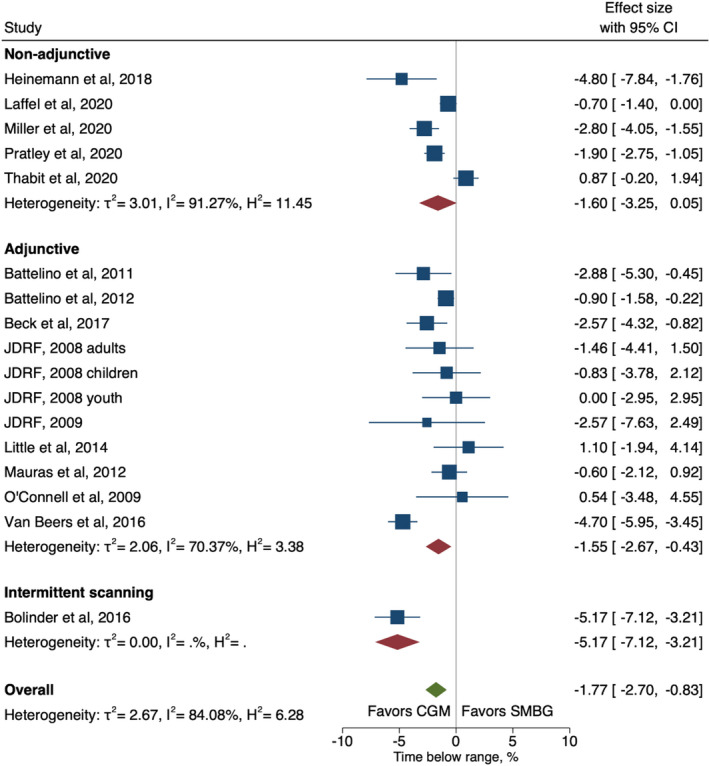
Forest plot of meta‐analysis of randomised controlled trails for time‐below‐range (<3.9 mmol/L) by non‐adjunctive, adjunctive real‐time continuous glucose monitoring and intermittent scanning
There were only five studies that reported glucose variability (%CV), and these were all in non‐adjunctive RT‐CGM. Overall there was no evidence of an effect on %CV (−0.6% [−5.1, 3.8]) and heterogeneity (I2) was high at 97% (Figure S2).
4. RISK OF BIAS
The majority of studies (17/22) had low overall risk of bias with 13/22 having one or more domain‐specific risks of bias (Figure S12). Uncertain risk was noted for each domain as follows: most commonly for missing outcome data, with 11 studies having a total amount of missingness or imbalance between groups which may allow bias 26 , 30 , 31 , 32 , 33 , 34 , 37 , 39 , 40 , 43 , 47 ; four studies for the randomisation process because of insufficient detail about sequence generation or allocation concealment 26 , 32 , 37 , 38 ; three studies for measurement of the outcome, due to differences in the CGM devices used to compare outcomes between groups (intervention CGM calibrated in real time, masked CGM calibrated retrospectively) 33 , 39 or not describing the masked device 29 ; two studies had enough participants deviating from study protocols to potentially affect outcomes, 32 , 40 and two studies for selection of the reported result, due to lack of prospectively published outcome information. 26 , 32
5. DISCUSSION
To our knowledge, this is the first systematic review and meta‐analysis investigating the glycaemic efficacy of CGM systems according to their diverging type (non‐adjunctive, adjunctive [many now not commercially available] and isCGM). In addition, more recent standardised metrics of glycaemic control have been presented where available. Overall use of RT‐CGM resulted in significant absolute improvement in HbA1c (by −0.22%) regardless of adjunctive or non‐adjunctive indications, whereas isCGM did not show improvement in HbA1c. Improvement in absolute TIR and reduction in absolute TAR and hypoglycaemia were seen with all CGM technologies irrespective of type. Finally, all divergent technologies showed improvements in hypoglycaemia reduction both when analysed separately and combined.
Our finding of a 6% absolute increase in TIR with the non‐adjunctive CGM use contributes to understanding the effectiveness of using this evolving technology to achieve glycaemic targets and minimise hypoglycaemia. Importantly, this improvement was achieved at the same time overall burden of use was reduced by allowing non‐adjunctive decision making, and now more recently factory calibrated systems. Interestingly, despite this improvement in TIR, the use of the non‐adjunctive CGM showed only a modest improvement in HbA1c. This could reflect study design, as these five non‐adjunctive studies were arguably specifically targeting less studied, more challenging and under‐served populations i.e. 3/5 in youth/young adults, and one in older adults with risk of hypoglycaemia. However, the older generation adjunctive studies did clearly show significant improvements in both HbA1c and TIR, confirming the overall potential of RT‐CGM to lower HbA1c. These findings are consistent with past CGM meta‐analyses. 11 , 19 , 20 This variable result for HBA1c highlights the limitations of a traditional focus on Hba1c to measure CGM effectiveness. 50 The most important limitation is that Hba1c fails to adequately identify the complexities of glycaemic variability for those living with diabetes, especially for those experiencing problematic hypoglycaemia. 42 , 43 , 50 For this reason, CGM metrics, which more clearly identify the nuances of glucose fluctuations, are likely to play an ever‐increasing role in measuring efficacy of advanced diabetes technologies. 18 The more recent standardisation of CGM metric reporting has also considerably improved the ability to compare findings of the more recent trials, 18 which was an important limitation of older generation studies.
One of the more complex aspects of a systematic review and meta‐analysis of these divergent CGM technologies is the substantial heterogeneity seen between studies. Heterogeneity >50% is considered to be substantial, 51 thus highlighting how high the >70% heterogeneity we have seen for both co‐primary outcomes are. Therefore, all results should be interpreted with caution. Particular attention in interpretation needs to focus on differences in trial design, study population (children vs. youth vs. older adults) and differences in primary outcomes (such as HbA1c/TIR vs hypoglycaemia reduction). All of these impact study findings, for example one should not realistically expect improvements in HbA1c to be seen in a study designed to improve hypoglycaemia in well‐controlled adults.
This may well represent the last or one of the last systematic reviews and meta‐analyses of stand‐alone CGM for type 1 diabetes. This is likely for a number reasons. Firstly, uptake of CGM is rapid in the developed world. 52 Secondly, CGM is now frequently integrated into increasingly sophisticated levels of decision support and insulin automation/closed‐loop. This last point, for type 1 diabetes in particular, may begin to make standalone data less relevant (as opposed to Type 2 diabetes where there is an increasing focus on the role of CGM). Highlighting this, recent systematic review and meta‐analyses of first generation closed loop technology reveal TIR improvements in the order of 10–12%. 53 , 54
The strengths of this meta‐analysis include the thorough and in‐depth systematic literature review with the inclusion of studies with various technical aspects and different demographic characteristics. Highlighting each diverging technology is also a considerable strength rather than solely combining all together. Our findings are limited by the moderate and high heterogeneity (as previous noted). Although most studies were rated as being at low risk of overall bias, generalisability to the real world may be an issue, with some studies having run‐in periods and screening out participants with reduced adherence. Another limitation was seen in the eight studies only reporting the median of the outcomes of interest. We addressed this by using calculated means in our main analysis, and by running a sensitivity analysis which showed a strengthened effect size. Another important factor is that most studies were funded/sponsored by industry which may impact on the duration and design of the included studies. Our criteria also did not allow for inclusion of studies of the effect of transitioning from one CGM system to another, like ALERTT1 (i.e. isCGM to RT‐CGM). 55 Finally, although a focus on randomised trials is vital, there is an important and growing place in understanding efficacy of diabetes technology using less robust forms of evidence such as real‐world data. For isCGM and RT‐CGM in particular, while excluded in this review, the impressive findings seen in the recent real‐world data have an important role in understanding CGM efficacy, 56 , 57 as well as highlighting that key benefits depend on sustained use, which appears to be improving with more modern iterations of CGM. 58
In conclusion, this systematic review and meta‐analysis highlights the favourable evolution in CGM with regards to TIR. TIR appears to be more sensitive than HbA1c at detecting benefits of CGM technology in that TIR appropriately incorporates health gains made from reducing hypoglycaemia, traditionally one of the most feared acute complications of T1D. Given the rapid evolution of diabetes technology and in particular decision support tools and automated insulin delivery, future trials and studies are likely to reduce their focus on CGM alone, but instead pivot to the role of CGM as but one vital component of more advanced integrated diabetes technology.
CONFLICT OF INTEREST
BJW has previously received research funding from Dexcom, Medtronic and iSENS. NO has received research funding from Dexcom, Roche Diabetes and Medtronic Diabetes and has participated in advisory boards for Dexcom, Roche Diabetes and Medtronic Diabetes. MIdB received Research funding from: Novo Nordisk, Medtronic, Dexcom, Pfizer and Research support from Medtronic, Dexcom, SOOIL, Honoraria from Medtronic. VNS reported receiving research supports through University of Colorado from Sanofi, Novo Nordisk, Eli‐Lilly, Insulet, Dexcom, Abbott, vTv Therapeutics, JDRF and NIH. VNS’ employer also received honoraria from Dexcom, insulet, Medscape and Sanofi for speaking, consulting or being on advisory board. ME, HS, JH, BG and SK declare no conflict of interest.
AUTHOR CONTRIBUTIONS
BJW is the guarantor of this work. BJW, MIdB, ME, NO, VS, JH and BG were involved with the design of the study and protocol. ME and HS conducted the review. SK and BG independently conducted the risk of bias assessment. ME and JH conducted the meta‐analysis. ME produced the first draft of the manuscript, and all authors worked collaboratively to review and prepare the final manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Open access publishing facilitated by University of Otago, as part of the Wiley ‐University of Otago agreement via the Council of Australian University Librarians. [Correction added on 14 May 2022, after first online publication:CAUL funding statement has been added.]
Elbalshy M, Haszard J, Smith H, et al. Effect of divergent continuous glucose monitoring technologies on glycaemic control in type 1 diabetes mellitus: A systematic review and meta‐analysis of randomised controlled trials. Diabet Med. 2022;39:e14854. doi: 10.1111/dme.14854
Prospero registration ID: CRD42021234019
Funding information
Funding for this work was provided in an unrestricted way by Dexcom.
REFERENCES
- 1. DiMeglio LA, Evans‐Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449‐2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bloomgarden ZT. Type 1 diabetes and glucose monitoring. Diabet Care. 2007;30(11):2965‐2971. [DOI] [PubMed] [Google Scholar]
- 3. Patton SR, Clements MA. Continuous glucose monitoring versus self‐monitoring of blood glucose in children with type 1 diabetes‐are there pros and cons for both? US Endocrinol Summer. 2012;8(1):27‐29. [PMC free article] [PubMed] [Google Scholar]
- 4. Heinemann L. Finger pricking and pain: a never ending story. J Diabet Sci Technol. 2008;2(5):919‐921. 10.1177/193229680800200526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blackwell M, Wheeler BJ. Clinical review: the misreporting of logbook, download, and verbal self‐measured blood glucose in adults and children with type I diabetes. Acta Diabetol. 2017;54(1):1‐8. 10.1007/s00592-016-0907-4 [DOI] [PubMed] [Google Scholar]
- 6. Pfeiffer E‐F. The, “Ulm Zucker Uhr System” and its consequences. Horm Metab Res. 1994;26(11):510‐514. [DOI] [PubMed] [Google Scholar]
- 7. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabet Technol Ther. 2016;18(S2):S2‐3‐S2‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Funtanilla VD, Caliendo T, Hilas O. Continuous glucose monitoring: a review of available systems. P and T. 2019;44(9):550. [PMC free article] [PubMed] [Google Scholar]
- 9. Cappon G, Vettoretti M, Sparacino G, Facchinetti A. Continuous glucose monitoring sensors for diabetes management: a review of technologies and applications. Diabet Metab J. 2019;43(4):383‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blauw H, Onvlee AJ, Klaassen M, van Bon AC, DeVries JH. Fully closed loop glucose control with a bihormonal artificial pancreas in adults with type 1 diabetes: an outpatient, randomized, crossover trial. Diabet Care. 2021;44(3):836‐838. [DOI] [PubMed] [Google Scholar]
- 11. Maiorino MI, Signoriello S, Maio A, et al. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta‐analysis of randomized controlled trials. Diabet Care. 2020;43(5):1146‐1156. [DOI] [PubMed] [Google Scholar]
- 12. Castellana M, Parisi C, Di Molfetta S, et al. Efficacy and safety of flash glucose monitoring in patients with type 1 and type 2 diabetes: a systematic review and meta‐analysis. BMJ Open Diabet Res Care. 2020;8(1):e001092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beck RW, Brown SA, Lum JW, Kovatchev BP. Nonadjunctive use of continuous glucose monitoring: the end of fingersticks? Diabet Technol Ther. 2020;22(2):67‐68. [DOI] [PubMed] [Google Scholar]
- 14. FDA Advisory Panel . FDA advisory panel votes to recommend non‐adjunctive use of Dexcom G5 mobile CGM. Diabetes Technol Ther. 2016;18:512‐516. [DOI] [PubMed] [Google Scholar]
- 15.Accessed 31/12/2021, 2021. https://news.medtronic.com/2021‐05‐26‐Medtronic‐Secures‐Two‐CE‐Mark‐Approvals‐for‐Guardian‐4‐Sensor‐for‐InPen‐MDI‐Smart‐Insulin‐Pen
- 16. Rodbard D. Glucose time in range, time above range, and time below range depend on mean or median glucose or HbA1c, glucose coefficient of variation, and shape of the glucose distribution. Diabet Technol Ther. 2020;22(7):492‐500. [DOI] [PubMed] [Google Scholar]
- 17. Gabbay MAL, Rodacki M, Calliari LE, et al. Time in range: a new parameter to evaluate blood glucose control in patients with diabetes. Diabetol Metab Syndr. 2020;12(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabet Care. 2019;42(8):1593‐1603. 10.2337/dci19-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dicembrini I, Cosentino C, Monami M, Mannucci E, Pala L. Effects of real‐time continuous glucose monitoring in type 1 diabetes: a meta‐analysis of randomized controlled trials. Acta Diabetol. 2021;58(4):401‐410. [DOI] [PubMed] [Google Scholar]
- 20. Benkhadra K, Alahdab F, Tamhane S, et al. Real‐time continuous glucose monitoring in type 1 diabetes: a systematic review and individual patient data meta‐analysis. Clin Endocrinol. 2017;86(3):354‐360. [DOI] [PubMed] [Google Scholar]
- 21. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898‐ 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 23. McGrath S, Sohn H, Steele R, Benedetti A. Meta‐analysis of the difference of medians. Biom J. 2020;62(1):69‐98. [DOI] [PubMed] [Google Scholar]
- 24. Altman DG, Bland JM. How to obtain the confidence interval from a P value. BMJ. 2011;343:d2090. [DOI] [PubMed] [Google Scholar]
- 25. Collaboration C . Obtaining standard deviations from standard errors and confidence intervals for group means. In: Higgins JPT, Green S, eds. Cochrane Handbook for systematic reviews of interventions. 2011;5. Version 5.1.0. [Google Scholar]
- 26. Hirsch IB, Abelseth J, Bode BW, et al. Sensor‐augmented insulin pump therapy: results of the first randomized treat‐to‐target study. Diabet Technol Ther. 2008;10(5):377‐383. [DOI] [PubMed] [Google Scholar]
- 27. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464‐1476. [DOI] [PubMed] [Google Scholar]
- 28. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . The effect of continuous glucose monitoring in well‐controlled type 1 diabetes. Diabet Care. 2009;32(8):1378‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mauras N, Beck R, Xing D, et al. A randomized clinical trial to assess the efficacy and safety of real‐time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to<10 years. Diabet Care. 2012;35(2):204‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabet Care. 2011;34(4):795‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55(12):3155‐3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deiss D, Bolinder J, Riveline J‐P, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real‐time continuous glucose monitoring. Diabet Care. 2006;29(12):2730‐2732. [DOI] [PubMed] [Google Scholar]
- 33. Feig DS, Donovan LE, Corcoy R, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390(10110):2347‐2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379‐387. [DOI] [PubMed] [Google Scholar]
- 35. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371‐378. [DOI] [PubMed] [Google Scholar]
- 36. O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient‐led use of sensor‐guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009;52(7):1250‐1257. [DOI] [PubMed] [Google Scholar]
- 37. Raccah D, Sulmont V, Reznik Y, et al. Incremental value of continuous glucose monitoring when starting pump therapy in patients with poorly controlled type 1 diabetes: the RealTrend study. Diabet Care. 2009;32(12):2245‐2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kordonouri O, Pankowska E, Rami B, et al. Sensor‐augmented pump therapy from the diagnosis of childhood type 1 diabetes: results of the Paediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia. 2010;53(12):2487‐2495. [DOI] [PubMed] [Google Scholar]
- 39. van Beers CAJ, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open‐label, crossover trial. Lancet Diabet Endocrinol. 2016;4(11):893‐902. [DOI] [PubMed] [Google Scholar]
- 40. Little SA, Leelarathna L, Walkinshaw E, et al. Recovery of hypoglycemia awareness in long‐standing type 1 diabetes: a multicenter 2× 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self‐monitoring (HypoCOMPaSS). Diabet Care. 2014;37(8):2114‐2122. [DOI] [PubMed] [Google Scholar]
- 41. Laffel LM, Kanapka LG, Beck RW, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388‐2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pratley RE, Kanapka LG, Rickels MR, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2397‐2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heinemann L, Freckmann G, Ehrmann D, et al. Real‐time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367‐1377. [DOI] [PubMed] [Google Scholar]
- 44. Group StENCUiECS, Group: StENCUiECS . A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabet Care. 2021;44(2):464‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thabit H, Prabhu JN, Mubita W, et al. Use of factory‐calibrated real‐time continuous glucose monitoring improves time in target and HbA1c in a multiethnic cohort of adolescents and young adults with type 1 diabetes: the MILLENNIALS study. Diabet Care. 2020;43(10):2537‐2543. [DOI] [PubMed] [Google Scholar]
- 46. Boucher SE, Gray AR, Wiltshire EJ, et al. Effect of 6 months of flash glucose monitoring in youth with type 1 diabetes and high‐risk glycemic control: a randomized controlled trial. Diabet Care. 2020;43(10):2388‐2395. [DOI] [PubMed] [Google Scholar]
- 47. Bolinder J, Antuna R, Geelhoed‐Duijvestijn P, Kröger J, Weitgasser R. Novel glucose‐sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non‐masked, randomised controlled trial. Lancet. 2016;388(10057):2254‐2263. [DOI] [PubMed] [Google Scholar]
- 48. Sequeira PA, Montoya L, Ruelas V, et al. Continuous glucose monitoring pilot in low‐income type 1 diabetes patients. Diabet Technol Ther. 2013;15(10):855‐858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Oskarsson P, Antuna R, Geelhoed‐Duijvestijn P, Krӧger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre‐specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018;61(3):539‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wright LA‐C, Hirsch IB. Metrics beyond hemoglobin A1C in diabetes management: time in range, hypoglycemia, and other parameters. Diabet Technol Ther. 2017;19(S2):S‐16‐S‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sandercock P. The authors say: ‘The data are not so robust because of heterogeneity’ ‐ so, how should I deal with this systematic review? Meta‐analysis and the Clinician. Cerebrovasc Dis. 2011;31(6):615‐620. 10.1159/000326068 [DOI] [PubMed] [Google Scholar]
- 52. Prahalad P, Ebekozien O, Alonso GT, et al. Multi‐clinic quality improvement initiative increases continuous glucose monitoring use among adolescents and young adults with type 1 diabetes. Clin Diabet. 2021;39(3):264‐271. 10.2337/cd21-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta‐analysis of outpatient randomised controlled trials. Lancet Diabet Endocrinol. 2017;5(7):501‐512. 10.1016/S2213-8587(17)30167-5 [DOI] [PubMed] [Google Scholar]
- 54. Bekiari E, Kitsios K, Thabit H, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta‐analysis. BMJ. 2018;361:k1310. 10.1136/bmj.k1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Visser MM, Charleer S, Fieuws S, et al. Comparing real‐time and intermittently scanned continuous glucose monitoring in adults with type 1 diabetes (ALERTT1): a 6‐month, prospective, multicentre, randomised controlled trial. Lancet. 2021;397(10291):2275‐2283. 10.1016/S0140-6736(21)00789-3 [DOI] [PubMed] [Google Scholar]
- 56. Dunn TC, Xu Y, Hayter G, Ajjan RA. Real‐world flash glucose monitoring patterns and associations between self‐monitoring frequency and glycaemic measures: a European analysis of over 60 million glucose tests. Diabet Res Clin Pract. 2018;137:37‐46. 10.1016/j.diabres.2017.12.015 [DOI] [PubMed] [Google Scholar]
- 57. Karter AJ, Parker MM, Moffet HH, Gilliam LK, Dlott R. Association of real‐time continuous glucose monitoring with glycemic control and acute metabolic events among patients with insulin‐treated diabetes. JAMA. 2021;325(22):2273‐2284. 10.1001/jama.2021.6530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van der Linden J, Welsh JB, Walker TC. Sustainable use of a real‐time continuous glucose monitoring system from 2018 to 2020. Diabet Technol Ther. 2021;23(7):508‐511. 10.1089/dia.2021.0014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


