The success of cancer immunotherapy strongly depends on the (re)invigoration of cancer immunosurveillance. Therapeutically relevant anticancer immune responses can be stimulated by a restricted panel of chemotherapeutics, targeted agents, oncolytics as well as by radiotherapy, all of which are activators of defined immunogenic cell stress and cell death (ICD) circuitries.1 The spatially defined sequential emission of danger associated molecular patterns (DAMPs) in the course of ICD defines the level of adjuvanticity of succumbing malignant cells and the consequent initiation of T cell-mediated adaptive immunity. DAMPs emitted in the course of ICD trigger the attraction, activation and maturation and thus the functional engagement of antigen presenting dendritic cells (DCs). The attraction and homing of DCs is driven by the liberation of adenosine triphosphate (ATP) and annexin A1 (ANXA1) by malignant cells, respectively. Moreover, relocation and subsequent exposure of calreticulin (CALR) serves as a phagocytic signal for DCs. CALR exposure, together with the production of type I interferons and the exodus of high mobility group box 1 (HMGB1), triggers tumor antigen transfer and DC maturation. In sum, ICD elicits the tumor antigen-specific DC-mediated priming of cytotoxic T lymphocytes (CTL), resulting in anticancer immunity, tumor lysis and disease control that finally outlasts treatment discontinuation.2
The increased expression in certain types of cancer of indoleamine 2,3-dioxygenase 1 (IDO1) catalyzes the conversion of tryptophan to immunosuppressive kynurenine, in turn inducing CTL anergy while increasing the abundancy of regulatory T cells (Treg) in the tumor microenvironment (TME), altogether impairing antitumor immunity and resulting in disease progression.3 Therapeutic regimens combining immunometabolic regulators such as IDO1 inhibitors with immunogenic cell death inducers can synergistically enhance the therapeutic efficacy of immunotherapy and render tumors responsive to subsequent immune checkpoint blockade.4
Accumulating preclinical and clinical evidence supports the idea that locally applied anticancer regimens including cytotoxicants, radiotherapy, photodynamic therapy and thermal ablation facilitate the onset of anticancer immunity, in particular when combined with systemic immune checkpoint blockade.5–7 In a recent paper published in Advanced Science, Wang et al described the development of a near-infrared photo-immunometabolic cancer therapy (PICT) with programmed raspberry-structured nanoadjuvants (PRNMT).8 PRNMTs are self-assembling structures that consist of the IDO1 inhibitor 1-methyl-tryptophan (1-MT) and CuS5 nanoparticles, together with a TME-responsive polymeric matrix, that triggers PRN disintegration and cargo delivery to the site of the tumor. Inhibition of IDO1 together with photothermal therapy (PTT) of the tumor by deep tissue penetrating NIR-II irradiation reportedly triggered the CuS5-mediated induction of ICD, reduced the growth of subcutaneous murine mammary carcinoma 4T1 tumors and synergized with programmed cell death protein 1 (PD-1) blockade to inhibit the manifestation of pulmonary metastasis when 4T1 cells were injected intravenously (Figure 1).
Figure 1.
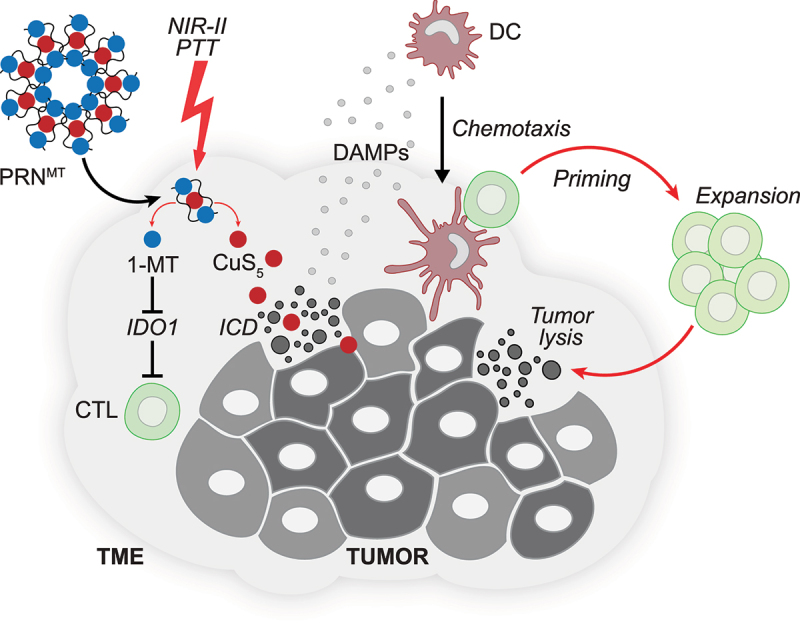
Nanoparticles releasing immunometabolism regulators together with immunogenic cell death inducers render tumors responsive to immune checkpoint blockade. Near-infrared photo-immunometabolic cancer therapy (PICT) employs programmed raspberry-structured nanoadjuvants (PRNMT) that facilitate the tumor-directed deployment of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor 1-methyl-tryptophan (1-MT) and CuS5 nanoparticles. Inhibition of IDO1 together with deep tissue penetrating photothermal therapy (PTT) of the tumor by NIR-II irradiation triggers the induction of immunogenic cell death (ICD) and can be advantageously combined with immune checkpoint blockade.
Abbreviations: CTL, cytotoxic T lymphocyte; DAMP, danger associated molecular pattern; PTT, photothermal therapy; TME, tumor microenvironment.
Preclinical and clinical studies support the notion that, ICD inducing therapies can be advantageously combined when administered sequentially with systemic immune checkpoint blockade and that such regimens are superior to concomitant combinations or monotherapies.9,10 It is thus tempting to speculate that local inhibition of immunosuppressive circuitries together with the PTT-mediated release of ICD-associated DAMPs by malignant cells will strongly sensitize cancers to immune checkpoint blockade. Although it remains to be determined whether the protocol developed by Wang et al.8 will be validated in clinical trials, it appears more and more plausible that progress in galenic formulations, as well as biophysical methods facilitating the local activation of immunotherapeutics, will yield successful combination therapies designed to ignite unrestrained anticancer immune responses.
Acknowledgments
OK receives funding by the DIM ELICIT initiative of the Ile de France and Institut National du Cancer (INCa); GK are supported by the Ligue contre le Cancer (équipes labellisées, Program “Equipe labelisée LIGUE”; no. EL2016.LNCC (VT/PLP)); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; INCa; Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM).
Data availability statement
All data that led to the conclusions in this manuscript have been referenced and all sources have been described.
Disclosure statement
OK is a cofounder of Samsara Therapeutics. GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sotio, Tollys, Vascage and Vasculox/Tioma. GK has been consulting for Reithera. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders.
References
- 1.Kroemer G, Galassi C, Zitvogel L, Galluzzi L.. Immunogenic cell stress and death. Nat Immunol. 2022;23(4):487–2. doi: 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- 2.Yatim N, Cullen S, Albert ML. Dying cells actively regulate adaptive immune responses. Nat Rev Immunol. 2017;17:262–275. [DOI] [PubMed] [Google Scholar]
- 3.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–452. [DOI] [PubMed] [Google Scholar]
- 4.Peng J, Xiao Y, Li W, Yang Q, Tan L, Jia Y, Qu Y, Qian Z. Photosensitizer Micelles Together with IDO Inhibitor Enhance Cancer Photothermal Therapy and Immunotherapy. Adv Sci. 2018;5:1700891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kepp O, Marabelle A, Zitvogel L, Kroemer G. Oncolysis without viruses - inducing systemic anticancer immune responses with local therapies. Nat Rev Clin Oncol. 2020;17:49–64. [DOI] [PubMed] [Google Scholar]
- 6.Kepp O, Kroemer G. Pseudovirus for immunotherapy. Nat Cancer. 2020;1:860–861. [DOI] [PubMed] [Google Scholar]
- 7.Kepp O, Kroemer G. A nanoparticle-based tour de force for enhancing immunogenic cell death elicited by photodynamic therapy. Oncoimmunology. 2022;11:2098658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Jiang W, Su Y, Zhan M, Peng S, Liu H, Lu L. Self-Splittable Transcytosis Nanoraspberry for NIR-II Photo-Immunometabolic Cancer Therapy in Deep Tumor Tissue. Adv Sci. 2022;3:e2204067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer G, Kepp O. Small cell lung cancer responds to immunogenic chemotherapy followed by PD-1 blockade. Oncoimmunology. 2021;10:1996686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki T, Buque A, Ames TD, Galluzzi L. PT-112 induces immunogenic cell death and synergizes with immune checkpoint blockers in mouse tumor models. Oncoimmunology. 2020;9:1721810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that led to the conclusions in this manuscript have been referenced and all sources have been described.


