Abstract
Neural oscillations in the alpha band (8–12 Hz) have been proposed as a key mechanism for the temporal resolution of visual perception. Higher alpha frequencies have been related to improved segregation of visual events over time, whereas lower alpha frequencies have been related to improved temporal integration. Similarly, also the phase of ongoing alpha has been shown to correlate with temporal integration/segregation. To test a causal relationship between alpha oscillations and perception, we here employed multi‐channel transcranial alternating current stimulation (mc‐tACS) over the right parietal cortex, whereas participants performed a visual temporal integration/segregation task that used identical stimuli with different instructions. Before and after mc‐tACS we recorded the resting‐state electroencephalogram (EEG) to extract the individual alpha frequency (IAF) and delivered electrical stimulation at slightly slower and faster frequencies (IAF±2 Hz). We hypothesized that this would not only drive endogenous alpha rhythms, but also affect temporal integration and segregation in an opposite way. However, the mc‐tACS protocol used here did not consistently increase or decrease the IAF after the stimulation and did not affect temporal integration/segregation accuracy as expected. Although we found some preliminary evidence for an influence of tACS phase on temporal integration accuracy, the ongoing phase of mc‐tACS oscillations did not reliably modulate temporal integration/segregation accuracy in a sinusoidal way as would have been predicted by an effective entrainment of brain oscillations. These findings may guide future studies using different stimulation montages for investigating the role of cortical alpha oscillations for human vision.
Keywords: EEG, neural oscillations, tES, timing, vision
Alpha oscillations (8–12 Hz) have been related to temporal sampling in vision. After estimating the individual alpha frequency (IAF) from the electroencephalogram, we delivered transcranial alternating current stimulation (tACS) at slightly slower and faster frequencies (±2 Hz), whereas the participants performed a temporal integration/segregation task. Contrarily to our hypothesis, right‐parietal tACS did not increase/decrease the IAF and did not lead to a modulation of the integration/segregation performance. Nonetheless, we observed preliminary evidence for an influence of tACS phase on temporal integration.
Abbreviations
- EEG
electroencephalography
- IAF
individual alpha frequency
- mc‐tACS
multi‐channels trancranial alternating current stimulation
1. INTRODUCTION
The continuous flow of information impacting our senses is not elaborated by the brain in a strictly analog fashion, even though we seem to experience perception as smooth and continuous. There is growing evidence that our brain discretizes sensory information based on specific sampling rhythms, both within and across the different sensory modalities (Buzsaki & Draguhn, 2004; Keil & Senkowski, 2018; Lakatos et al., 2007). In particular, perceptual processing of sensory inputs requires a balance between temporal integration and segregation (Pöppel, 2009; VanRullen, 2016). Temporal integration represents the capacity to combine information over time in a way that is advantageous for accurate and precise representation of objects that tend to remain stable over time (Wutz & Melcher, 2014). In contrast, temporal segregation describes the sensitivity to rapid changes in incoming sensory input due to a dynamic environment or our own actions in order to ensure a high temporal resolution and guidance of action (Ronconi & Melcher, 2017).
The brain may reach this balance between temporal segregation and integration through a rhythmic process. Early neurophysiological investigations proposed that perception depends on the rhythmic sampling of sensory information (Bishop, 1932; Harter, 1967; Lansing, 1957). The existence of a rhythmic process is supported by studies in nonhuman primates showing that spikes in sensory areas are more likely to occur at a specific phase of the local field potential oscillations (Haegens et al., 2011). The relationship between these sampling rhythms and perception has been corroborated using magneto‐ and electro‐encephalography (M/EEG) in humans, in particular by observing a consistent relationship between oscillations of the alpha rhythms (8–12 Hz) and sensitivity to new visual input (Busch et al., 2009; Dugué et al., 2011; Fiebelkorn et al., 2013; Mathewson et al., 2009) and, moreover, a relationship between theta rhythms and attentional sampling (Busch & VanRullen, 2010; Dugué et al., 2015; Landau et al., 2015; Senoussi et al., 2019).
Recent EEG/MEG studies have provided evidence for a role of the ongoing oscillatory phase, especially in the alpha (8–12 Hz) and theta (4–7 Hz) band (Varela et al., 1981; Mathewson et al., 2012; Wutz et al., 2016; Wutz et al., 2018; Wutz et al., 2014; Samaha & Postle, 2015; Milton & Pleydell‐Pearce, 2016; Ronconi & Melcher, 2017; Ronconi et al., 2017; Ronconi et al., 2018; for reviews see VanRullen, 2016; White, 2018), in the temporal resolution of visual perception, as defined by the capacity to perceive sequential stimuli as separate events rather than fusing them into a single percept. Although the precise role of theta and alpha in temporal processing needs to be further investigated, one possibility is that the segregation/integration of stimuli that alternate in close temporal proximity and in the same spatial position would mainly depend on alpha band activity, whereas stimuli separated by larger temporal intervals that also requires segregation/integration across space would mainly depend on slower frequencies within the theta band (Ronconi et al., 2017). The importance of ongoing neural oscillations for temporal segregation/integration has also been reported in the somatosensory domain, suggesting that the presence of discrete or quasi‐discrete perceptual cycles is not restricted to the visual modality. Baumgarten and colleagues, for example, showed that the phase of ongoing alpha and low‐beta oscillations predicts whether two successive tactile stimuli are perceived as distinct events or merged into a single event (Baumgarten et al., 2015, 2017).
According to the theoretical framework of rhythmic perception, the frequency of ongoing neural oscillations determines the temporal resolution of visual perception because higher frequencies imply shorter integration windows. As a result, higher frequencies should be associated with better segregation performance, but reduced integration performance (Figure 1). In agreement with this idea, it has been shown that individuals with higher prestimulus or resting‐state alpha frequencies exhibit better temporal segregation within (Samaha & Postle, 2015) and across sensory modalities (Cecere et al., 2015; Keil & Senkowski, 2017). Similarly, participants showed a higher peak alpha frequency on trials requiring rapid temporal segregation compared to other trials requiring temporal integration (Wutz et al., 2018).
FIGURE 1.
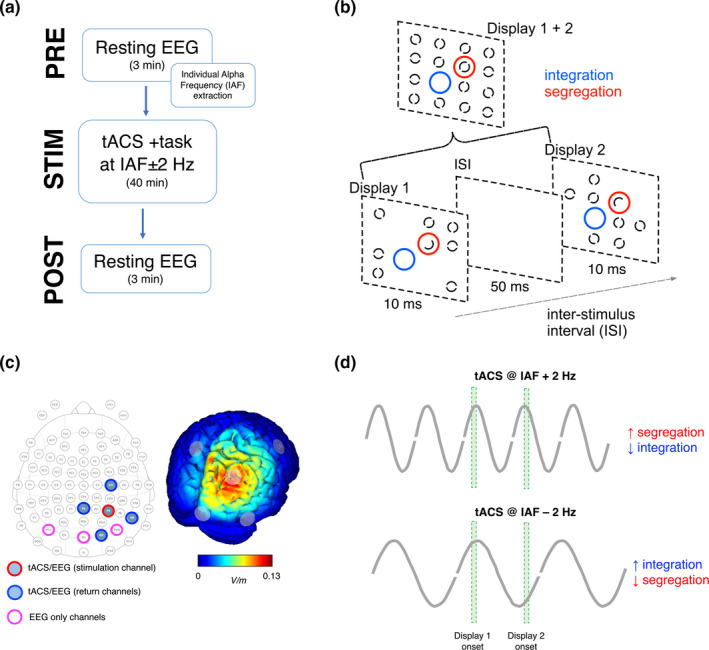
(a) Experimental design; (b) Task procedure: two target displays were presented on each trial and, in different blocks, participants were instructed to find the position of the single‐odd element (segregation trials) or to find the position of the single empty location (integration trials); no time constraints were imposed to subjects; (c) tACS/EEG montage employed and the estimated electric field distribution on the cortical surface; (d) Visual representation of the main hypothesis of the current study. According to the theoretical framework of rhythmic perception, higher frequencies imply shorter integration windows and should be associated with better segregation performance, but reduced integration performance. In contrast, lower frequency should facilitate integration, because stimuli are more likely to fall within the same oscillatory alpha cycle/integration window
While correlational evidence linking rhythmic brain activity to perception is accumulating, an increasing number of studies are trying to test a causal relationship between oscillations and perception. One strategy has been to apply sensory (i.e., visual and/or auditory) stimulation at a specific frequency in order to entrain ongoing neural oscillations, considering that this entrainment leads to resonance phenomena in neural and perceptual activity (Thut, et al., 2011; Mathewson et al., 2012; de Graaf et al., 2013; Spaak et al., 2014; for a review see Herrmann et al., 2016). In a few more recent studies, it has been observed that the alignment of the ongoing oscillations to a particular entrained rhythm can impact temporal perception (Chota & VanRullen, 2019; Ronconi et al., 2018; Ronconi & Melcher, 2017). In particular, it was found that temporal segregation of stimuli in a two‐flash fusion task can be influenced by sensory entrainment at frequencies that includes the alpha band (8–12 Hz) (Ronconi & Melcher, 2017). More recently, Ronconi et al. (2018) showed that sensory entrainment at the individual alpha frequency (IAF) + or −2 Hz was able to modulate both integration and segregation of two stimuli in close temporal proximity using a variant of the missing‐dot task (Eriksen & Collins, 1967; Hogben & Lollo, 1974) called the “SegInt” task (Ronconi et al., 2018, 2020; Sharp et al., 2018, 2019; Wutz et al., 2016, 2018). In this task, also employed in this study (Figure 1b), an identical sequence of two displays was used to measure temporal integration or segregation according to the specific task instruction. Specifically, participants were instructed either to find the position of the single “odd element,” which required segregating the displays over time, or to find the position of the single empty location, which requires integrating the displays over time.
Another approach to study a potential causal relationship between rhythmic brain activity and perception, instead of sensory entrainment, is the use of transcranial alternating current stimulation (tACS). In principle, tACS seems a particularly suitable method to directly modulate oscillatory signals, with some studies showing that tACS is able to interact with the brain's natural cortical oscillations, leading to oscillatory entrainment (Baltus et al., 2018; Fröhlich & McCormick, 2010; Helfrich et al., 2014) and driving the activity of cortical regions at (or towards) the frequency imposed by tACS. However, there is still an open question whether tACS effectively causes entrainment of endogenous neural oscillations or whether instead tACS influences oscillatory power through changes in neural plasticity (Vossen et al., 2015; Vosskuhl et al., 2018). Even though the precise neurophysiological mechanisms behind tACS remain to be clarified, tACS dependent behavioral effects on sensory and cognitive processes have been reported (for a review see Herrmann et al., 2016). Recently, tACS in the alpha band has also been shown to increase the probability of experiencing inattentional blindness to unexpected stimuli (Hutchinson et al., 2020).
Regarding the theoretical framework of rhythmic perception, at present only limited evidence exists for a causal relationship between the alpha band rhythm and the temporal resolution of perception. Cecere et al. (2015) used tACS to stimulate subjects over the occipital cortex at a frequency corresponding to their IAF + or −2 Hz. They probed the temporal resolution of multisensory perception using a sound‐induced two‐flash illusion, showing that lower frequency tACS enlarged the temporal window of illusion, whereas higher frequency tACS (higher than IAF) had the opposite effect.
With this study, we aimed to test the possibility of modifying the temporal resolution of visual perception using alpha tACS over right parietal cortex. Here, tACS was used to induce changes in the frequency of endogenous oscillations; EEG was used to verify such changes by comparing peak frequencies between the pre‐ and post‐stimulation resting‐state EEG signal. We used the SegInt task, which tests temporal integration and segregation using identical stimuli (see above; Eriksen & Collins, 1967; Hogben & Lollo, 1974; Ronconi et al., 2018, 2020; Sharp et al., 2018, 2019; Wutz et al., 2016), depending on a specific task instruction. tACS was delivered at the upper and lower boundary frequencies of the individually defined alpha band, by choosing for each participant the two stimulation frequencies based on the IAF peak obtained from the pre‐stimulation resting‐state EEG. We did not only want to confirm a causal role of alpha in the temporal aspects of perception, as this has been already suggested by the recent studies using sensory entrainment described above. We also wanted to provide a more precise identification of cortical areas that would be relevant for alpha‐related timing processes. Even if an increasing number of M/EEG studies found associations between alpha and timing in visual perception, as extensively reviewed above, the definition of a cortical “perceptual alpha network” remains to be clarified. Previous studies using a “standard” tACS montage (i.e., Oz‐Cz; Cecere et al., 2015; Minami & Amano, 2017) are an important starting point, but considering that in those cases the stimulated regions spanned a large portion of the occipital and parietal cortices, they are not optimal to define such a network. On the contrary, the multi‐channel tACS approach we adopted in this study had the advantage of allowing a more focal stimulation. We chose to stimulate the right parietal cortex for several reasons: Theoretical models based on TMS studies and investigations of patients with brain lesions proposed the existence of a “when” pathway in the human visual system involving primarily the right parietal lobe (Battelli et al., 2007). One of the main sources of pre‐stimulus alpha activity has been identified with MEG source imaging in the parietal cortex (van Dijk et al., 2008; Thut, et al., 2011). Moreover, right parietal transcranial magnetic stimulation (TMS) at alpha frequency (10 Hz) affects visual target visibility (Romei et al., 2010) and accuracy in a backward masking task (Jaegle & Ro, 2014). In particular, Jaegle and Ro (2014) demonstrated that repetitive TMS (rTMS) over the posterior parietal cortex at an alpha frequency (10 Hz), but not occipital or sham rTMS, entrained subsequent alpha oscillatory activity and produced phase‐dependent changes in backward masking accuracy, suggesting a role for right parietal alpha in shaping temporal processing in vision. This evidence is particularly relevant in the current context because backward masking is a well‐known proxy for temporal processing in the visual domain; in this family of tasks, the visibility of a visual target object is markedly impaired by the subsequent presentation of a non‐target object in the same (or nearby) spatial location (Enns & Di Lollo, 2000). Counterintuitively, the strongest masking effect does not occur when the mask is presented immediately after the target, but rather when a significant temporal gap is placed between the two, indicating a phenomenon that reflects recurrent processing in the visual system as also targeted by the SegInt task used here (Lamme & Roelfsema, 2000).
Provided that neurostimulation is effective in inducing a shift of the endogenous alpha rhythm, our main prediction was that a faster tACS rhythm (IAF + 2Hz) would improve temporal segregation, whereas a slower tACS rhythm (IAF–2Hz) would improve temporal integration, in agreement to what we previously found with sensory entrainment (Ronconi et al., 2018; Ronconi & Melcher, 2017).
Finally, given the evidence reviewed above of a significant relationship between visual temporal integration/segregation performance and the phase of endogenous EEG alpha oscillations, and given tACS ability to align such endogenous oscillations to the external electrical force, we expected to find a relationship between behavioral performance and tACS phase. Specifically, given that tACS current was alternating with a sinusoidal waveform, we expected a rhythmic, sinusoidal fluctuation of behavioral performance, phase‐locked to the tACS rhythm.
2. METHOD
2.1. Participants
Twenty‐one participants (9 male, mean age = 24.5, age range = 20–32) took part in this study. They were all students from the University of Münster. They provided informed consent, had normal or corrected to normal vision and normal hearing. All of them met the criteria for the application of Transcranial Alternating Current Stimulation (tACS) (Antal et al., 2017). This experiment was approved by the ethics committee of the Faculty for Psychology and Sport Sciences at the University of Münster (protocol n. 2017–20‐NB).
3. Apparatus and stimuli
All visual stimuli were displayed on a 22.5″ VIEWPixx monitor with a vertical refresh rate of 100 Hz on an intermediate gray background. The stimulus for the main task was made up of two displays which were shown sequentially, separated by a blank interval (see Figure 1b). The two target displays contained annuli placed within an invisible 4 × 4 quadratic element grid (each square was 1 × 1°). These stimuli were shown in two different and separated frames. Seven random locations (14 over both frames out of 16 total) were filled with a full black annulus on a uniform gray background (0.5° size, 0.06° line width; 0.5° space between grid locations). Each annulus was split by a central gap with a randomly chosen orientation that could be 0°, 45°, 90°, or 135°. In addition, there was one “odd element” with a half annulus in each of the two displays, such that the two half annuli complemented each other across displays. Finally, one location was left empty in both displays. The stimuli used were similar to those in previous studies measuring temporal integration and segregation (Ronconi et al., 2018; Sharp et al., 2018, 2019; Wutz et al., 2016, 2018). The experiment was programmed in Matlab, using the Psychtoolbox (Brainard, 1997).
4. Procedure
In different blocks, participants were instructed to localize either the odd element or the empty location across the two target displays. Finding the odd element requires segregating the displays over time, as temporal integration results in the fused percept of a full annulus, identical to the 14 other annuli. In contrast, finding the unique missing element (i.e., the only empty location) in both displays requires integrating the displays over time, as temporal segregation results in the perception of two separate displays with many empty locations. Given that both the odd and missing elements were shown in all trials, integration and segregation were tested in separate blocks using identical stimuli, with only the task instructions differing between block types. At the end of each trial, participants were asked to report the location of the odd element (half circle) in the segregation blocks, and the location of the missing element in the integration blocks, by clicking on the corresponding stimulus position in the grid. The response was given with no time constraints.
Each target display (containing the 7 circles and one half‐circle) was shown for 1 refresh cycle (10 ms) separated by a blank interval equal to five refresh cycles (50 ms). This value was chosen based on our previous studies (Ronconi et al., 2018; Sharp et al., 2018, 2019; Wutz et al., 2016, 2018) showing that this time interval was optimal to reach an intermediate accuracy level (across integration/segregation conditions) around 60%–70% (chance level = 6.25%), which gave us reasonable margins to test the eventual performance fluctuations over time caused by tACS. Importantly, a 50 ms time interval fits within the alpha duty cycle (if we consider 10 Hz as the central alpha frequency) (Jensen et al., 2012), and thus is particularly suitable to test variation in performance in relation to variation in the ongoing alpha phase and frequency.
Subjects participated in two sessions, taking place in two different days. In one session we delivered tACS stimulation at a frequency corresponding to IAF + 2 Hz and in the other session, we delivered tACS at IAF‐2 Hz. The order of the two sessions was counterbalanced across subjects, who were unaware of the specific tACS protocol administered on each session. Moreover, on each session, eyes‐closed resting‐state EEG was collected for 3 min before and 3 min after tACS (see below for further details). The tACS itself in both sessions lasted 40 min and during this period participants concurrently performed the temporal integration/segregation task. The total amount of trials administered on average for each participant over the two tACS sessions were 1,203 (SD = 74), consisting of 601 (SD = 46) segregation trials, and 602 (SD = 51) integration trials.
5. Stimulation setting and EEG recording
In this study, tACS was applied through a StarStim8 device, a hybrid wireless neurostimulation system for concurrent EEG/tACS controlled by the software Neuroelectrics Instrument Controller (NIC 2.0; http://www.neuroelectrics.com/products/software/nic2/). We used 5 PISTIM Ag/AgCl electrodes with 1 cm radius both for stimulation and EEG recording and 3 GELTRODE Ag/AgCl electrodes just for EEG recording, all arranged according to the 10–10 system. The stimulation electrode was placed at P4, whereas the four return electrodes were placed at C4, Pz, O2, and P8 (i.e., surrounding P4; see Figure 1c). Stimulation intensity was set at 1 mA (milliAmpere), with 0 mA offset (i.e., no additional direct current stimulation) and a sinusoidal ramp‐up phase of 20 s. This montage was chosen after carefully evaluating the electric field distribution with the software NIC 2.0. In particular, this 4 × 1 montage centered on P4 was optimal to stimulate the right parietal cortex as focal as possible and in radial direction (Figure 1b,c) (Dmochowski et al., 2011; Wagner et al., 2016; Khan et al., 2019). We created two protocols with different stimulation frequencies based on the IAF peak measured for each participant during an eyes‐closed resting‐state period of 3 min (Figure 1a). Resting‐state EEG at channel Pz was measured for 3 min before and after each tACS session (four times in total) with eyes closed, which was necessary for obtaining a sufficiently robust alpha rhythm for reliable estimation of the IAF peak.
Participants were asked to report whether they saw phosphenes at any time during the stimulation. None of them reported the presence of retinal phosphenes with either stimulation frequency (IAF + 2 Hz or IAF–2 Hz tACS). None of the participants reported disturbing skin sensations during the experiment; some participants reported mild skin sensation after the initial ramp‐up phase, but this sensation disappeared soon afterwards. The EEG signal was recorded at a sampling frequency of 500 Hz with 24‐bit digitization using eight electrodes (C4, Pz, P4, P8, PO8, PO7, Oz, O2) online referenced to Cz. Impedances were kept below 10 kΩ.
6. Data analysis: Resting‐state EEG data
Offline, eyes‐closed resting EEG data were filtered with a second‐order Butterworth IIR filter with half‐amplitude (−6 dB) frequency cut‐offs at 0.05 Hz and 40 Hz. Data were visually inspected to remove data segments contaminated by muscular or ocular artifacts. An independent component analysis (ICA) was used to correct for electrode artifacts when needed. The cleaned continuous data were segmented into 1‐s epochs before extracting the FFT spectrum. Zero padding was applied to increase the frequency resolution. The IAF peak frequency was identified as the frequency with the highest FFT amplitude within the frequency band of interest (8–12 Hz). Differences in IAF peak values before and after IAF + 2 Hz and IAF–2 Hz tACS were tested for each channel with paired samples t‐tests and Bonferroni correction for multiple comparisons (i.e., number of channels). Data analysis was performed using Matlab (MathWorks, Inc.) and EEGLAB (Delorme & Makeig, 2004).
7. Data analysis: temporal integration/segregation accuracy as a function of tACS frequency and phase
Two main analyses were performed for our study to test the relationship between brain stimulation and perception. The first analysis tested the relationship between tACS frequency (IAf + 2 Hz vs. IAF–2 Hz) and performance accuracy for segregation and integration trials. This analysis was performed initially by considering all trials irrespective of the position of target stimuli. However, tACS could in principle affect the perceptual performance specifically in the visual hemifield contralateral to the stimulus site. For this reason, we also conducted the main analysis on performance accuracy separately for left and right hemifield targets.
The second analysis was performed in order to study how the phase of the external stimulation influences temporal segregation and integration. As done in other previous studies (Neuling et al., 2012; Stonkus et al., 2016), we computed a Hilbert transform of the tACS stimulation sinewave to obtain for each trial the instantaneous phase at the onset of the first target display (the second target display was presented always at a constant delay after the first target). We then created eight bins along the tACS alpha cycle, evenly sized and non‐overlapping, ranged as follows: [ (Figure 3a). Each bin contained on average 37 trials (SD = 2) for each participant. We then analyzed performance across these phase bins. Considering that tACS stimulation might affect performance for different participants with a different phase‐lag, we analyzed both the original “raw” performance data and phase‐aligned data. To this end, each participant's performance data were phase‐shifted such that the bin with the best performance was arbitrarily assigned to bin 4. As this phase‐alignment yields consistently the highest performance at bin 4 by definition, this bin was then omitted from the analysis of phase‐aligned data (Asamoah et al., 2019). Differences in performance as a function of phase bin were tested with a repeated‐measures ANOVA across all 8 (for raw data) or 7 (for phase‐aligned data) phase bins, respectively. Additionally, we compared the accuracy of the bin with the best performance (omitting bin 4 for phase‐aligned data) to that of the bin with the worst performance with paired samples t‐tests.
FIGURE 3.
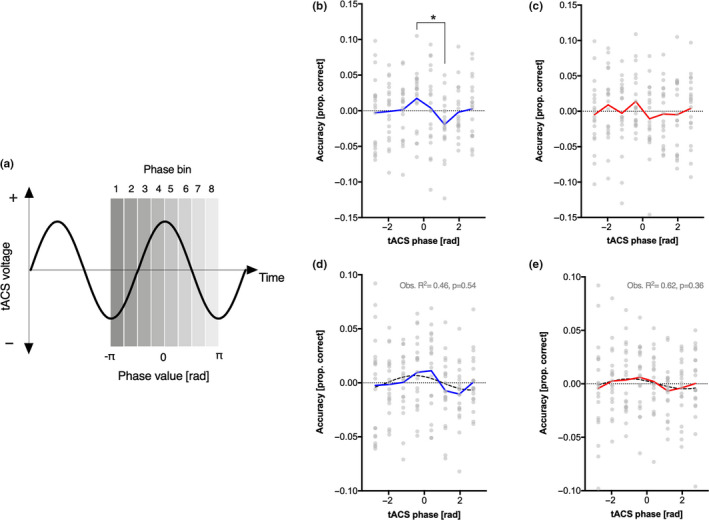
(a) Relationship between tACS voltage and phase bins/values extracted with a Hilbert transform; (b) Temporal integration and (c) segregation accuracy (raw values, centered on the individual mean) as a function of tACS phase bin (*=p<.05, Bonferroni‐corrected). (d) Temporal integration and (e) segregation accuracy (centered on the individual mean and smoothed with a moving average) as a function of tACS phase bin with superimposition (dotted line) of the best one cycle sinusoidal function; p‐values were obtained with permutation tests. See also FiguresS1‐S4for plots of individual data
In the third and last analysis, we aimed at evaluating whether the modulation of tACS on performance followed a single cycle sinusoidal function as predicted. In a first step, individual datapoints were smoothed by a moving average of two successive datapoints (Stonkus et al., 2016). Then, data were averaged across participants and the best fitting sinusoidal function was calculated separately for the two experimental conditions (segregation and integration). The sinusoidal function had a fixed frequency (i.e., one cycle across the datapoints) but free amplitude and phase. The goodness of fit (R 2) of the resulting best fitting function was compared with a null distribution obtained with 1,000 permutations of the real data. Specifically, for each individual dataset, we calculated 1,000 permutations obtained from the real data by randomizing the phase bin. Permuted data were smoothed with the same procedure described for real data before calculating the average across participants. The 1,000 measures of goodness of fit obtained from these permuted data constituted the null distribution against which we could compare the goodness of fit obtained from the real data and extract the p‐value.
8. RESULTS
8.1. The effect of IAF±2Hz tACS on IAF peak as measured with EEG
We measured the peak IAF for the two different tACS frequencies before and after the application of tACS. We did not find the expected IAF peak increment following IAF + 2 Hz tACS (one‐tailed t‐tests: all psuncor r > .24), suggesting that focal right parietal tACS was not effective in inducing an increment in the speed of the endogenous alpha rhythm as assessed in the post‐tACS resting‐state EEG activity. In contrast, we observed the expected decrement of the IAF peak following IAF‐2 Hz tACS. This difference was significant only for the more posterior (occipital and parieto/occipital) channels (Oz: t (19) = 4.26, p corr = 0.002; PO7: t (19) = 4.68, p corr < 0.001; PO8: t (19) = 2.94, p corr = 0.032; see Figure 2), but was not significant for the other channels, in particular not for the channel P4 where the stimulation was strongest and most focal and where thereby the effect was supposed to be strongest.
FIGURE 2.
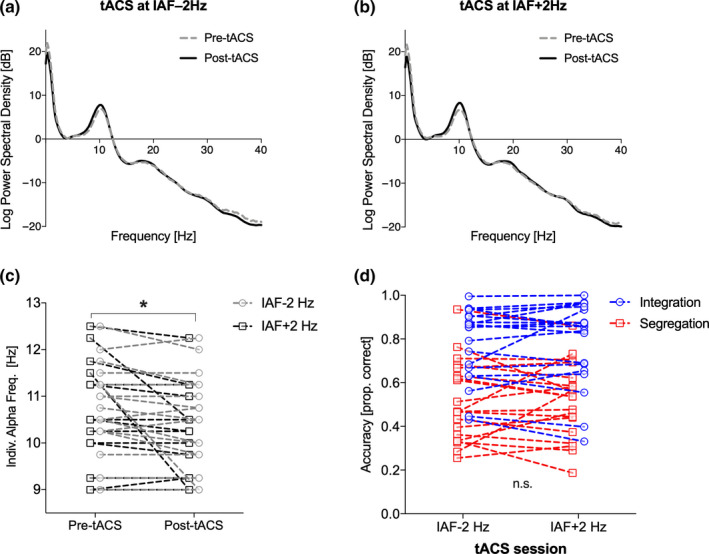
(a, b) Power spectrum of EEG data before and after tACS (channel Pz; similar but not statistically significant results were found for the channel P4; see Results section); frequency of stimulation was chosen based on the individual alpha frequency (IAF); (c) IAF changes before and after tACS as a function of stimulation session (IAF + 2 vs. IAF‐2 Hz) for the channel Pz; (d) Task accuracy as a function of task condition (integration and segregation) and stimulation session (IAF + 2 vs. IAF‐2 Hz). In (c) and (d) dots represent individual data
To check whether the decrement in IAF interacted with tACS frequency, we ran a 2 × 2 repeated measures ANOVA with tACS session (pre vs. post) and frequency (IAF + 2 vs. IAF–2 Hz) as within subject factors, and found a significant main effect of tACS session (Pz: F (1,18) = 5.57, p = .030; Oz: F (1,18) = 7.406, p = .014), but no significant main effect of tACS frequency or interaction (all Fs < 1). Thus, irrespective of the tACS frequency delivered, the IAF peak tended to decrease over time when compared before and after the experimental session, consistent with a recent report of decreasing alpha peak frequency with time‐on‐task, combined with an increase in alpha power (Benwell et al., 2019).
9. The effect of tACS frequency on temporal integration/segregation accuracy
In both segregation and integration trials, there was no evidence that tACS frequency‐impacted performance. Specifically, accuracy in integration trials was not significantly improved for the IAF–2 Hz stimulation (t (19) = −0.585, p = .717) and accuracy in segregation trials was not significantly improved for IAF + 2Hz stimulation (t (19) = −0.056, p = .478) (Figure 2b).
Stimuli were presented in foveal and parafoveal regions and thus the possibility of finding an effect specific for visual hemifield was not very strong. Nonetheless, we checked for a possible effect of stimulus hemifield with the hypothesis that any effect of a right parietal stimulation would be evident more (or exclusively) in the contralateral visual hemifield.
In integration trials, the results of a 2 × 2 repeated‐measures ANOVA with tACS frequency (IAF + 2 vs. IAF–2 Hz) and stimulus hemifield (left vs. right) as within‐subjects factors revealed only a main effect of hemifield (F (1,19) = 6.17, p = .022), with accuracy that was higher for the left hemifield as compared to right hemifield stimuli (left hemifield mean: 0.81, SD = 0.15; right hemifield mean: 0.75, SD = 0.21), but no significant main effect of tACS frequency and no significant interaction. In segregation trials, whereas no significant interaction emerged, there was a significant effect of stimulus hemifield, with accuracy that was higher for right hemifield target as compared to left hemifield target (right hemifield mean: 0.54, SD = 0.18; left hemifield accuracy: 0.49, SD = 0.18). There was also a main effect of frequency, with accuracy that was higher for IAF–2 Hz tACS (mean = 0.53, SD = 0.18) as compared to IAF + 2 Hz tACS (mean = 0.50, SD = 0.17). In the Supplementary Material, we have reported also the results of an additional three‐way ANOVA where accuracy was evaluated as a function of the type of trials (Integration vs. Segregation), Stimulus hemifield (Left vs. Right), and tACS frequency (IAF + 2 vs. IAF–2 Hz). Also these additional results did not support our original hypothesis.
10. The effect of tACS phase on temporal integration/segregation accuracy
The repeated‐measures ANOVA performed on raw accuracy data (non‐phase aligned) of integration trials did not reveal a main effect of phase bin (F (7,133) < 1, p = .48). Despite the absence of a significant main effect in the ANOVA, when comparing the peak and the trough of the accuracy as a function of tACS phase bin (phase bin 4 vs. phase bin 6), we found a significant Bonferroni‐corrected difference (t (19) = 3.024, p corr = 0.021) (Figure 3b). However, by comparing the grand average maximum versus minimum effect in our data we might have biased the results toward our hypothesis, that is, there is an effect of tACS phase on task accuracy (for a discussion of this potential issue, see Ruhnau et al., 2020). Thus, we performed a permutation test which represents an unbiased way to see whether the min versus max difference was reliable. This test was performed with 1,000 permutation of the real data to check if the t‐value of the observed max‐min difference was significantly larger than the max‐min differences obtained from the permuted data. This test, however, did not yield a significant result (p = .12; see Figure S7). As such, the claim that the effect was reliable cannot be made with confidence.
The repeated‐measures ANOVA on raw accuracy data (non‐phase aligned) of segregation trials, again, did not reveal a main effect of phase bin (F (7,133) < 1, p = .82). Moreover, in contrast with segregation trials, the comparison between the peak and the trough of the accuracy (phase bin 4 vs. phase bin 5) was not significant (t (19) = 1.589, p corr = 0.45) (Figure 3c).
A similar ANOVA computed on the phase‐aligned data did not yield any significant effects of phase bin in integration (F (6,114) < 1, p = .57) or segregation trials (F (6,114) < 1, p = .69).
An additional analysis that may add more information about a possible modulatory effect of tACS on accuracy was conducted by fitting a one‐cycle sinusoidal function to our data, and by comparing the results of this fitting with those obtained from a distribution of 1,000 permutations (see Method section for details). Results revealed for integration trials that the best one‐cycle sinusoidal fit led to an observed R 2 = 0.456, which was not significant (permutation tests p = .54) (Figure 3d). A similar result was found for segregation trials, where the best one‐cycle sinusoidal fit led to an observed R 2 = 0.68, which was again not significant (permutation tests p = .36) (Figure 3e).
Due to the high variability of tACS effects in the different participants, and considering that averaging across subjects does not take into account that tACS phase can affect performance with opposite trends (being the highest or lowest at the same tACS phase), we performed the one‐cycle sinusoidal fitting procedure also at the level of the single‐subject data, calculating the p‐value with the same smoothing and permutation tests procedure used for the average data. As can be seen in Figures S3 and S4, in integration trials, only two participants showed a significant sinusoidal modulation of task accuracy as a function of tACS phase, whereas for segregation trials none of the participants showed a significant sinusoidal modulation.
11. DISCUSSION
This study aimed to test the possibility of shifting the endogenous alpha rhythm (IAF) with parietal tACS in order to impact temporal aspects of perception. In particular, we were interested in testing the causal nature of the relationship between ongoing (prestimulus) oscillations in the alpha band and temporal integration and segregation in visual perception that has recently been the focus of several studies. We hypothesized that by delivering tACS to the parietal cortex at individually tailored oscillations, which were faster and slower relative to the endogenous alpha rhythm (i.e., IAF peak ±2 Hz) as measured with EEG, we would influence temporal integration and segregation of visual perception in an opposite way. Our hypothesis was motivated by increasing evidence showing a role for tACS as a method to shape perceptually relevant brain oscillations (Battaglini et al., 2020; Cecere et al., 2015; Helfrich et al., 2014; Neuling et al., 2012; Stonkus et al., 2016; Wolinski et al., 2018). To measure temporal segregation and integration we used an identical sequence of two displays but with different task instructions. We expected that accuracy in this task would vary as a function of tACS frequency, with higher tACS frequency promoting segregation and lower tACS frequency promoting integration. In addition, if alpha phase is relevant for temporal aspects of perception, we would expect also a significant relationship between tACS phase and performance in our task.
Contrary to our predictions, the present results showed no evidence of a modulation of the endogenous alpha rhythm after mc‐tACS over the right parietal cortex. Indeed, we observed only evidence of a slowing down of IAF after the end of the stimulation and task session that was independent of the frequency (IAF±2 Hz) used for stimulation. This observation seems to be in agreement with recent findings showing an alpha peak reduction with time on task that appears to be independent from the effect of the tACS itself (Benwell et al., 2019). Given that we did not measure EEG during tACS stimulation, our study could not show the instantaneous effects of tACS on ongoing brain activity. However, we can conclude that no long‐term effects were evident after tACS stimulation has ended.
Similarly, we did not find any evidence that temporal integration and segregation accuracy varied as the function of tACS frequency, neither when we considered performance across the entire visual field nor when we considered the accuracy split between targets presented in the two hemifields. The fact that we observed a significant main effect of hemifield in both integration and segregation trials, with accuracy being higher for the left hemifield, is possibly connected to the right hemisphere specialization for temporal perception and attention (Battelli et al., 2007; Corbetta & Shulman, 2011).
While a direct manipulation of temporal aspects of perception has been recently demonstrated with sensory entrainment (Chota & VanRullen, 2019; Ronconi et al., 2018; Ronconi & Melcher, 2017), to the best of our knowledge there are only few studies to date showing evidence that tACS can have a similar effect (Battaglini, et al., 2020; Cecere et al., 2015; Minami & Amano, 2017). Cecere et al. (2015), for example, directly addressed the possibility of using tACS to alter the temporal resolution of perception in the multisensory domain; they stimulated the occipital cortex at IAF±2 Hz and showed congruent modulation (expansion/contraction) of the temporal window for experiencing the sound‐induced two‐flash illusion. A comparable effect was not found in this study, which addressed a similar question in the unisensory visual domain. Cecere and colleagues, however, employed a standard montage with larger stimulation electrodes in occipito‐parietal areas (Oz‐Cz) that could lead to changes in activity in different cortical areas at the same time, possibly encompassing also parietal and frontal areas in addition to visual areas, whereas in this study the stimulation was focal on the right parietal cortex. Other findings that provide evidence regarding a possible influence of tACS on temporal aspects of perception have been reported by Minami and Amano (2017). The authors observed that illusory visual vibrations in a motion‐induced spatial conflict task were perceived at the same frequency as the individual alpha rhythm. Importantly, when tACS at IAF±1 Hz was used to stimulate with an Oz‐Pz montage and large electrodes (similar to that employed by Cecere et al., 2015), there was a corresponding change in the perceived jitter frequency.
Furthermore, we investigated whether a relationship between alpha tACS phase and temporal integration/segregation emerged. In integration trials, that is, where participants had to report the position of the missing element that could be individuated only when merging all filled positions across the two displays, we found that the phase of the ongoing alpha tACS had an impact on accuracy. This result was obtained by testing the difference between the maximum and the minimum of the distribution, which was defined as not a priori but post hoc based on the observed average phase‐sorted distribution. A more stringent permutation test, which represents an unbiased way to see whether such a difference is reliable, did not confirm such result. Despite therefore being of questionable reliability, we think the association between tACS phase and temporal integration accuracy is worth of a cautious consideration in light of the growing number of studies highlighting a relationship between performance and tACS phase at stimulus onset. For example, Polanía and colleagues (Polanía et al., 2012) showed a decrease in reaction times during a working memory task when a specific phase of 6Hz theta tACS was delivered over frontal and parietal areas. Helfrich and colleagues (Helfrich et al., 2014) using a visual oddball paradigm showed that the phase of the tACS modulates target detection performance. Similarly, Neuling et al. (2012) showed that the perception of auditory stimuli embedded in noise was modulated by the phase of tACS delivered within the alpha frequency. Here, we looked for a potential sinusoidal relationship between tACS phase and performance. Finding a specific sinusoidal relationship between tACS phase and any measure of task performance is essential to conclude that tACS is effective in leading to a significant entrainment of brain oscillations. In our data, however, we did not find evidence that the modulation of temporal integration (or segregation) accuracy as a function of tACS phase followed such sinusoidal function. Collectively, the present findings provide only preliminary evidence that the ascending phase of parietal tACS, close to the positive peak of the voltage variation, might be associated with a performance increment, but not necessarily as a result of neural entrainment. Such facilitation could be possibly attributed to effects occurring simultaneously or immediately after the modulations of target neurons by the external electrical field. Although they are only partially understood at the neurophysiological level, and largely neglected so far in the field of cognitive neuroscience, Liu and colleagues (Liu et al., 2018) recently identified several possible mechanisms of these short‐term tACS effects. For stimulation intensity in the range used in this study (<1 V/m) neural activity could be affected by: i) stochastic resonance and ii) rhythmic resonance. The first mechanism would take place when a small proportion of the applied field is affecting neurons that are near the threshold of spike generation, biasing their spike timing or probability thanks to the coincidence of intrinsic and extrinsic polarization. The second would occur when an AC field is applied at the same frequency of a regular endogenous rhythm, affecting the native oscillation at a similar phase during each cycle (Liu et al., 2018). It remains unclear why in our experimental design these immediate tACS effects would have influenced only temporal integration. One possibility is that the mechanism is related to the role of alpha in providing pulsed inhibition that reduces the processing capabilities of a given area (Jensen & Mazaheri, 2010). Our alpha tACS protocol, irrespective of the frequency employed, might have strengthened the alpha gating causing a phase‐dependent temporal smear of the two target displays that benefited only temporal integration, but not segregation.
An important limitation of this study is the specific montage used for stimulating the parietal cortex. The 4 × 1 montage employed here creates a radial field current in the parietal cortex, and the limited effect of tACS on perception that we found could be due to a sub‐optimal orientation and lower intensity of our electrical current relative to the target area. So, a future possibility might be to consider not only the location to stimulate but also to use an intensity‐optimizing (Khan et al., 2019) instead of the used focality‐optimizing approach (Wagner et al., 2016) and a range of different orientations that can increase tACS efficacy. Lastly, while multi‐channel tACS has the advantage of allowing focal action, in contrast to TMS it entails a multipolar stimulation with opposite excitatory and inhibitory effects at the anodes(s) and cathode(s). Thus, we might have stimulated the area under the anode (P4) in one direction and the directly neighboring areas in opposite directions, as also shown in the simulations by Wagner et al. (2016), which could have led to an unpredicted outcome given the nature of the alpha rhythm as reflecting wide cortical network activity (Donner & Siegel, 2011). In terms of possible alternatives, we cannot exclude the possibility that a stimulation on the same area with an extracephalic reference or with repetitive TMS could lead to a significant effect on temporal integration windows. Finally, given that we applied tACS at a frequency significantly below and above the participants’ IAF, it might be that entrainment effects are simply weaker and that a higher current intensity would have been needed to create entrainment at off‐peak frequencies.
12. CONCLUSION
Frequency‐specific brain stimulation is increasingly becoming a popular technique to establish a causal relationship between brain oscillations and cognition. In contrast to the growing literature on such effects, we demonstrate that stimulation near the individual endogenous alpha rhythm with parietal tACS did not lead to entrainment of performance in temporal integration/segregation task, even though the parietal cortex has been repeatedly identified as one of the cortical sources of the alpha rhythm. Nonetheless, we found preliminary evidence for an influence of tACS phase on temporal integration accuracy, although this post hoc and non‐predicted effect is of questionable reliability and therefore has to be replicated; this last result provides preliminary evidence linking parietal tACS, neural oscillations in the alpha band and temporal windows in perception, which, however, seem to reflect a neural mechanism that is potentially different from neural entrainment. These mixed findings are likely to stimulate future investigations with different focal stimulation montages. Testing their efficacy, also in direct comparisons with standard montages that have been applied in previous studies, could help to clarify the mechanism behind tACS influence on alpha oscillations and characterize the cortical alpha network subserving temporal aspects of human vision.
CONFLICT OF INTEREST
The author declares no competing financial interests.
AUTHORS’ CONTRIBUTION
L.R.: Conceptualization, Investigation, Methodology, Formal Analysis, Data curation, Visualization, Writing‐ Original draft, Writing ‐ Reviewing and Editing; D.M.: Conceptualization, Methodology, Supervision, Writing‐ Original draft, Writing ‐ Reviewing and Editing; M.J.: Resources, Methodology, Writing ‐ Reviewing and Editing; C.W.: Resources, Methodology, Writing‐ Original draft, Writing ‐ Reviewing and Editing; N.B.: Conceptualization, Project administration, Methodology, Resources, Supervision, Writing‐ Original draft, Writing ‐ Reviewing and Editing.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/ejn.15017.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a grant from the German Research Council (DFG) awarded to N.B. (BU 2400/8‐1), by a European Research Council grant “Construction of Perceptual Space‐Time” (StG Agreement 313658) awarded to D.M. and by the DFG priority program SPP1665, project WO1425/5‐2, awarded to C.H.W. We would like to thank Davina Hahn and Johanna Rehder for the precious help with participants' recruitment and data collection.
Ronconi L, Melcher D, Junghöfer M, Wolters CH, Busch NA. Testing the effect of tACS over parietal cortex in modulating endogenous alpha rhythm and temporal integration windows in visual perception. Eur J Neurosci. 2022;55(11–12): 3438–3450. 10.1111/ejn.15017
[Correction added on 17 December 2020, after first online publication: "Edited by" statement included in the first page.]
Edited By: Dr. Christian Keitel
DATA AVAILABILITY STATEMENT
The data supporting the results presented in this article are available upon request to the authors.
REFERENCES
- Antal, A. , Alekseichuk, I. , Bikson, M. , Brockmöller, J. , Brunoni, A. R. , Chen, R. , Cohen, L. G. , Dowthwaite, G. , Ellrich, J. , Flöel, A. , Fregni, F. , George, M. S. , Hamilton, R. , Haueisen, J. , Herrmann, C. S. , Hummel, F. C. , Lefaucheur, J. P. , Liebetanz, D. , Loo, C. K. , … Paulus, W. (2017). Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clinical Neurophysiology, 128, 1774–1809. 10.1016/j.clinph.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asamoah, B. , Khatoun, A. , & Mc Laughlin, M. (2019). Analytical bias accounts for some of the reported effects of tACS on auditory perception. Brain Stimulation, 12, 1001–1009. [DOI] [PubMed] [Google Scholar]
- Baltus, A. , Wagner, S. , Wolters, C. H. , & Herrmann, C. S. (2018). Optimized auditory transcranial alternating current stimulation improves individual auditory temporal resolution. Brain Stimulation, 11, 118–124. [DOI] [PubMed] [Google Scholar]
- Battaglini, L. , Ghiani, A. , Casco, C. , & Ronconi, L. (2020). Parietal tACS at beta frequency improves vision in a crowding regime. NeuroImage, 208, 116451. 10.1016/j.neuroimage.2019.116451 [DOI] [PubMed] [Google Scholar]
- Battaglini, L. , Mena, F. , Ghiani, A. , Casco, C. , Melcher, D. , & Ronconi, L. (2020). The effect of alpha tACS on the temporal resolution of visual perception. Frontiers in Psychology, 11, 1765. 10.3389/fpsyg.2020.01765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli, L. , Pascual‐Leone, A. , & Cavanagh, P. (2007). The “when” pathway of the right parietal lobe. Trends in Cognitive Sciences, 11, 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten, T. J. , Königs, S. , Schnitzler, A. , & Lange, J. (2017). Subliminal stimuli modulate somatosensory perception rhythmically and provide evidence for discrete perception. Scientific Reports, 7, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten, T. J. , Schnitzler, A. , & Lange, J. (2015). Beta oscillations define discrete perceptual cycles in the somatosensory domain. Proceedings of the National Academy of Sciences, 112(39), 12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell, C. S. Y. , London, R. E. , Tagliabue, C. F. , Veniero, D. , Gross, J. , Keitel, C. , & Thut, G. (2019). Frequency and power of human alpha oscillations drift systematically with time‐on‐task. NeuroImage, 192, 101–114. 10.1016/j.neuroimage.2019.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, G. H. (1932). Cyclic changes in excitability of the optic pathway of the rabbit. American Journal of Physiology Content, 103, 213–224. [Google Scholar]
- Brainard, D. H. (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Busch, N. A. , Dubois, J. , & VanRullen, R. (2009). The phase of ongoing EEG oscillations predicts visual perception. Journal of Neuroscience, 29, 7869–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch, N. A. , & VanRullen, R. (2010). Spontaneous EEG oscillations reveal periodic sampling of visual attention. Proceedings of the National Academy of Sciences of the United States of America, 107, 16048–16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki, G. , & Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science, 304, 1926–1929. 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- Cecere, R. , Rees, G. , & Romei, V. (2015). Individual differences in alpha frequency drive crossmodal illusory perception. Current Biology, 25, 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chota, S. , & VanRullen, R. (2019). Visual entrainment at 10 Hz causes periodic modulation of the flash lag illusion. Frontiers in Neuroscience, 13, 232. 10.3389/fnins.2019.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta, M. , & Shulman, G. L. (2011). Spatial neglect and attention networks. Annual Review of Neuroscience, 34, 569–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf, T. A. , Gross, J. , Paterson, G. , Rusch, T. , Sack, A. T. , & Thut, G. (2013). Alpha‐band rhythms in visual task performance: Phase‐locking by rhythmic sensory stimulation. PLoS One, 8(3), e60035. 10.1371/journal.pone.0060035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme, A. , & Makeig, S. (2004). EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134, 9–21. [DOI] [PubMed] [Google Scholar]
- Dmochowski, J. P. , Datta, A. , Bikson, M. , Su, Y. , & Parra, L. C. (2011). Optimized multi‐electrode stimulation increases focality and intensity at target. Journal of neural engineering, 8(4), 046011. [DOI] [PubMed] [Google Scholar]
- Donner, T. H. , & Siegel, M. (2011). A framework for local cortical oscillation patterns. Trends in Cognitive Sciences, 15, 191–199. [DOI] [PubMed] [Google Scholar]
- Dugué, L. , Marque, P. , & VanRullen, R. (2011). The phase of ongoing oscillations mediates the causal relation between brain excitation and visual perception. Journal of Neuroscience, 31, 11889–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugué, L. , Marque, P. , VanRullen, R. , Dugué, L. , Marque, P. , & VanRullen, R. (2015). Theta oscillations modulate attentional search performance periodically. Journal of Cognitive Neuroscience, 27, 945–958. [DOI] [PubMed] [Google Scholar]
- Enns, J. T. , & Di Lollo, V. (2000). What’s new in visual masking? Trends in Cognitive Sciences, 4, 345–352. [DOI] [PubMed] [Google Scholar]
- Eriksen, C. W. , & Collins, J. F. (1967). Some temporal characteristics of visual pattern perception. The Journal of Experimental Psychology, 74, 476–484. [DOI] [PubMed] [Google Scholar]
- Fiebelkorn, I. C. , Saalmann, Y. B. , & Kastner, S. (2013). Rhythmic sampling within and between objects despite sustained attention at a cued location. Current Biology, 23, 2553–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich, F. , & McCormick, D. A. (2010). Endogenous electric fields may guide neocortical network activity. Neuron, 67, 129–143. 10.1016/j.neuron.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens, S. , Nacher, V. , Luna, R. , Romo, R. , & Jensen, O. (2011). alpha‐Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proceedings of the National Academy of Sciences of the United States of America, 108, 19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter, M. R. (1967). Excitability cycles and cortical scanning: A review of two hypotheses of central intermittency in perception. Psychological Bulletin, 68, 47–58. 10.1037/h0024725 [DOI] [PubMed] [Google Scholar]
- Helfrich, R. F. , Schneider, T. R. , Rach, S. , Trautmann‐Lengsfeld, S. A. , Engel, A. K. , & Herrmann, C. S. (2014). Entrainment of brain oscillations by transcranial alternating current stimulation. Current Biology, 24, 333–339. [DOI] [PubMed] [Google Scholar]
- Herrmann, C. S. , Struber, D. , Helfrich, R. F. , & Engel, A. K. (2016). EEG oscillations: From correlation to causality. International Journal of Psychophysiology, 103, 12–21. [DOI] [PubMed] [Google Scholar]
- Hogben, J. H. , & di Lollo, V. (1974). Perceptual integration and perceptual segregation of brief visual stimuli. Vision Research, 14, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Hutchinson, B. T. , Pammer, K. , & Bandara, K. (2020). tACS stimulation at alpha frequency selectively induces inattentional blindness. Brain Topography, 33, 317–326. [DOI] [PubMed] [Google Scholar]
- Jaegle, A. , & Ro, T. (2014). Direct control of visual perception with phase‐specific modulation of posterior parietal cortex. Journal of Cognitive Neuroscience, 26, 422–432. [DOI] [PubMed] [Google Scholar]
- Jensen, O. , Bonnefond, M. , & VanRullen, R. (2012). An oscillatory mechanism for prioritizing salient unattended stimuli. Trends in Cognitive Sciences, 16, 200–206. [DOI] [PubMed] [Google Scholar]
- Jensen, O. , & Mazaheri, A. (2010). Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Frontiers in Human Neuroscience, 4, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil, J. , & Senkowski, D. (2017). Individual Alpha frequency relates to the sound‐induced flash illusion. Multisensory Research, 30, 565–578. [DOI] [PubMed] [Google Scholar]
- Keil, J. , & Senkowski, D. (2018). Neural oscillations orchestrate multisensory processing. Neuroscience, 107385841875535. [DOI] [PubMed] [Google Scholar]
- Khan, A. , Antonakakis, M. , Vogenauer, N. , Wollbrink, A. , Suntrup‐Krüger, S. , Schneider, T. R. , Hermann, C. S. , Nitsche, M. , Paulus, W. , Haueisen, J. , Wolters, C. H. (2019). Constrained maximum intensity optimized multi‐electrodetDCS targeting of human somatosensory network, 41st AnnualInternationalConference of the IEEE Engineering in Medicine and Biology Society (EMBC),Berlin, Germany. pp. 5894– 5897 [DOI] [PubMed]
- Lakatos, P. , Chen, C.‐M. , O’Connell, M. N. , Mills, A. , & Schroeder, C. E. (2007). Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron, 53, 279–292. 10.1016/j.neuron.2006.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme, V. A. F. , & Roelfsema, P. R. (2000). The distinct modes of vision offered by feedforward and recurrent processing. Trends in Neurosciences, 23, 571–579. [DOI] [PubMed] [Google Scholar]
- Landau, A. N. , Schreyer, H. M. , van Pelt, S. , & Fries, P. (2015). Distributed attention is implemented through theta‐rhythmic gamma modulation. Current Biology, 25, 2332–2337. [DOI] [PubMed] [Google Scholar]
- Lansing, R. W. (1957). Relation of brain and tremor rhythms to visual reaction time. Electroencephalography and Clinical Neurophysiology, 9, 497–504. [DOI] [PubMed] [Google Scholar]
- Liu, A. , Vöröslakos, M. , Kronberg, G. , Henin, S. , Krause, M. R. , Huang, Y. , Opitz, A. , Mehta, A. , Pack, C. C. , Krekelberg, B. , Berényi, A. , Parra, L. C. , Melloni, L. , Devinsky, O. , & Buzsáki, G. (2018). Immediate neurophysiological effects of transcranial electrical stimulation. Nature Communications, 9(1), 10.1038/s41467-018-07233-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson, K. E. , Gratton, G. , Fabiani, M. , Beck, D. M. , & Ro, T. (2009). To see or not to see: Prestimulus alpha phase predicts visual awareness. Journal of Neuroscience, 29, 2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson, K. E. , Prudhomme, C. , Fabiani, M. , Beck, D. M. , Lleras, A. , & Gratton, G. (2012). Making waves in the stream of consciousness: Entraining oscillations in EEG alpha and fluctuations in visual awareness with rhythmic visual stimulation. Journal of Cognitive Neuroscience, 24, 2321–2333. 10.1162/jocn_a_00288 [DOI] [PubMed] [Google Scholar]
- Milton, A. , & Pleydell‐Pearce, C. W. (2016). The phase of pre‐stimulus alpha oscillations influences the visual perception of stimulus timing. NeuroImage, 133, 53–61. 10.1016/j.neuroimage.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami, S. , & Amano, K. (2017). Illusory jitter perceived at the frequency of alpha oscillations. Current Biology, 27, 2344–2351.e4. 10.1016/j.cub.2017.06.033 [DOI] [PubMed] [Google Scholar]
- Neuling, T. , Rach, S. , Wagner, S. , Wolters, C. H. , & Herrmann, C. S. (2012). Good vibrations: Oscillatory phase shapes perception. NeuroImage, 63, 771–778. 10.1016/j.neuroimage.2012.07.024 [DOI] [PubMed] [Google Scholar]
- Polanía, R. , Nitsche, M. A. , Korman, C. , Batsikadze, G. , & Paulus, W. (2012). The importance of timing in segregated theta phase‐coupling for cognitive performance. Current Biology, 22, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Pöppel, E. (2009). Pre‐semantically defined temporal windows for cognitive processing. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei, V. , Gross, J. , & Thut, G. (2010). On the role of prestimulus alpha rhythms over occipito‐parietal areas in visual input regulation: Correlation or causation? Journal of Neuroscience, 30, 8692–8697. 10.1523/JNEUROSCI.0160-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi, L. , Busch, N. A. , & Melcher, D. (2018). Alpha‐band sensory entrainment alters the duration of temporal windows in visual perception. Scientific Reports, 8, 11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi, L. , & Melcher, D. (2017). The role of oscillatory phase in determining the temporal organization of perception: evidence from sensory entrainment. Journal of Neuroscience, 37, 10636–10644. 10.1523/JNEUROSCI.1704-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronconi, L. , Melcher, D. , & Franchin, L. (2020). Investigating the role of temporal processing in developmental dyslexia: Evidence for a specific deficit in rapid visual segmentation. Psychonomic Bulletin & Review, 27(4), 724–734. 10.3758/s13423-020-01752-5 [DOI] [PubMed] [Google Scholar]
- Ronconi, L. , Oosterhof, N. N. , Bonmassar, C. , & Melcher, D. (2017). Multiple oscillatory rhythms determine the temporal organization of perception. Proceedings of the National Academy of Sciences of the United States of America, 114, 13435–13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhnau, P. , Rufener, K. S. , Heinze, H. J. , & Zaehle, T. (2020). Pulsed transcranial electric brain stimulation enhances speech comprehension. Brain Stimulation, 13, 1402–1411. 10.1016/j.brs.2020.07.011 [DOI] [PubMed] [Google Scholar]
- Samaha, J. , & Postle, B. R. (2015). The speed of alpha‐band oscillations predicts the temporal resolution of visual perception. Current Biology, 25, 2985–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoussi, M. , Moreland, J. C. , Busch, N. A. , & Dugué, L. (2019). Attention explores space periodically at the theta frequency. Journal of Visual Communication, 19, 1–17. [DOI] [PubMed] [Google Scholar]
- Sharp, P. , Melcher, D. , & Hickey, C. (2018). Endogenous attention modulates the temporal window of integration. Attention, Perception, Psychophys, 80(5), 1214–1228. 10.3758/s13414-018-1506-y [DOI] [PubMed] [Google Scholar]
- Sharp, P. , Melcher, D. , & Hickey, C. (2019). Different effects of spatial and temporal attention on the integration and segregation of stimuli in time. Attention, Perception, Psychophysics, 81, 433–441. [DOI] [PubMed] [Google Scholar]
- Spaak, E. , de Lange, F. P. , & Jensen, O. (2014). Local entrainment of alpha oscillations by visual stimuli causes cyclic modulation of perception. Journal of Neuroscience, 34, 3536–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stonkus, R. , Braun, V. , Kerlin, J. R. , Volberg, G. , & Hanslmayr, S. (2016). Probing the causal role of prestimulus interregional synchrony for perceptual integration via tACS. Scientific Reports, 6, 32065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut, G. , Schyns, P. G. , & Gross, J. (2011). Entrainment of perceptually relevant brain oscillations by non‐invasive rhythmic stimulation of the human brain. Frontiers in Psychology, 2, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut, G. , Veniero, D. , Romei, V. , Miniussi, C. , Schyns, P. , & Gross, J. (2011). Rhythmic TMS causes local entrainment of natural oscillatory signatures. Current Biology, 21, 1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk, H. , Schoffelen, J.‐M. , Oostenveld, R. , & Jensen, O. (2008). Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. Journal of Neuroscience, 28, 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen, R. (2016). Perceptual cycles. Trends in Cognitive Sciences, 20, 723–735. [DOI] [PubMed] [Google Scholar]
- Varela, F. J. , Toro, A. , John, E. R. , & Schwartz, E. L. (1981). Perceptual framing and cortical alpha rhythm. Neuropsychologia, 19, 675–686. 10.1016/0028-3932(81)90005-1 [DOI] [PubMed] [Google Scholar]
- Vossen, A. , Gross, J. , & Thut, G. (2015). Alpha power increase after transcranial alternating current stimulation at alpha frequency (α‐tACS) reflects plastic changes rather than entrainment. Brain Stimulation, 8, 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosskuhl, J. , Strüber, D. , & Herrmann, C. S. (2018). Non‐invasive brain stimulation: A paradigm shift in understanding brain oscillations. Frontiers in Human Neuroscience, 12, 10.3389/fnhum.2018.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, S. , Burger, M. , & Wolters, C.H. (2016). An optimization approach for well‐targeted transcranial direct current stimulation. SIAM Journal on Applied Mathematics, 76(6), 2154–2174. 10.1137/15M1026481 [DOI] [Google Scholar]
- White, P. A. (2018). Is conscious perception a series of discrete temporal frames? Consciousness and Cognition, 60, 98–126. [DOI] [PubMed] [Google Scholar]
- Wolinski, N. , Cooper, N. R. , Sauseng, P. , & Romei, V. (2018). The speed of parietal theta frequency drives visuospatial working memory capacity. PLoS Biology, 16, e2005348. 10.1371/journal.pbio.2005348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz, A. , & Melcher, D. (2014). The temporal window of individuation limits visual capacity. Frontiers in Psychology, 5, 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz, A. , Melcher, D. , & Samaha, J. (2018). Frequency modulation of neural oscillations according to visual task demands. Proceedings of the National Academy of Sciences, 115, 1346–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz, A. , Muschter, E. , van Koningsbruggen, M. G. , Weisz, N. , & Melcher, D. (2016). Temporal integration windows in neural processing and perception aligned to saccadic eye movements. Current Biology, 26, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz, A. , Weisz, N. , Braun, C. , & Melcher, D. (2014). Temporal windows in visual processing: “prestimulus brain state” and “poststimulus phase reset” segregate visual transients on different temporal scales. Journal of Neuroscience, 34, 1554–1565. 10.1523/JNEUROSCI.3187-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data supporting the results presented in this article are available upon request to the authors.


