Abstract
The left atrium is a dynamic chamber with peculiar characteristics. Stressors and disease mechanisms may deeply modify its structure and function, leading to left atrial remodelling and disease. Left atrial disease is a predictor of poor outcomes. It may be a consequence of left ventricular systolic and diastolic dysfunction and neurohormonal and inflammatory activation and/or actively contribute to the progression and clinical course of heart failure through multiple mechanisms such as left ventricular filling and development of atrial fibrillation and subsequent embolic events. There is growing evidence that therapy may improve left atrial function and reverse left atrial remodelling. Whether this translates into changes in patient's prognosis is still unknown.
In this review we report current data about changes in left atrial size and function across different stages of development and progression of heart failure. At each stage, drug therapies, lifestyle interventions and procedures have been associated with improvement in left atrial structure and function, namely a reduction in left atrial volume and/or an improvement in left atrial strain function, a process that can be defined as left atrial reverse remodelling and, in some cases, this has been associated with improvement in clinical outcomes. Further evidence is still needed mainly with respect of the possible role of left atrial reverse remodelling as an independent mechanism affecting the patient's clinical course and as regards better standardization of clinically meaningful changes in left atrial measurements. Summarizing current evidence, this review may be the basis for further studies.
Keywords: Heart failure, Left atrium, Reverse remodelling
Pathogenetic mechanism of atrial failure. Left atrial (LA) remodelling is a multifactorial clinical entity driven by different electrical, mechanical and metabolic stressors. These ones lead to volume/pressure overload, activate the renin–angiotensin–aldosterone (RAA) system and determine the release of different cytokines and hormones. The time‐dependent adaptation of atrial myocytes finally results in a chronic LA dilatation and a loss of elasticity, with a consequent stiffer chamber, less affected by changes in pressure. Without removing the causative stressor, this adaptive process inexorably progresses and the alterations become persistent. However, by treating the underlying diseases or risk factors and with heart failure therapies, atrial failure progression may be halted and, in some cases, reversed.
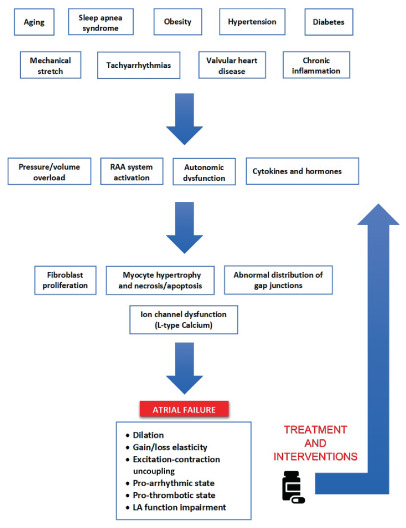
Introduction
The left atrium (LA) is a thin‐walled dynamic chamber that plays an important role in global cardiac performance, contributing to left ventricle (LV) filling and cardiac output and maintaining a dynamic interaction with the LV. 1 In response to varying stressors (i.e. electrical, mechanical and metabolic), the LA can undergo a remodelling process resulting in its persistent dilatation with functional impairment. 2 , 3 LA enlargement and dysfunction are well‐established markers of diastolic 4 and systolic dysfunction, 5 as well as predictors of cardiovascular (CV) outcomes, including atrial fibrillation (AF), 6 , 7 stroke, 8 , 9 heart failure (HF), and subsequent mortality. 10 This relationship is highly related to LV function and has been shown across all the ejection fraction, 11 , 12 , 13 , 14 even in advanced HF. 15 However, recent data suggest a possible independent contribution of LA dysfunction in HF. 16
In the past decades, the concept of LA remodelling has evolved, and the contribution of the LA to the pathophysiology of HF has grown in appreciation. 2 , 17 , 18 , 19 , 20 Indeed, changes in LA structure and function may be either a marker of HF severity or a mechanism actively contributing to the progression of cardiac dysfunction. One manifestation of LA disease, AF, contributes substantially to morbidity in patients with HF. Recent data suggest that timely therapeutic intervention may attenuate the process of deleterious LA remodelling and potentially promote reverse LA remodelling with a consequent improvement in clinical symptoms and outcomes. 2 , 21 In contrast, age and endurance exercise are related to LA enlargement even in absence of LA disease. 22 , 23
Prevention of LA dysfunction and treatment of LA disease may therefore be of critical importance. The relationship between changes in LV structure and function and subsequent CV outcome is well established. 24 We propose here a similar conceptual approach to the LA reflecting dynamic changes in relation to HF progression. The aim of this review is to summarize the concepts and the pathophysiologic mechanisms behind the potential beneficial effects of directed medical therapy on LA reverse remodelling and its clinical implications across all HF stages.
Definition of left atrial reverse remodelling
Atrial disease, as stated in the recent European Society of Cardiology (ESC) HF guidelines, can be defined as a complex of subclinical structural, electrophysiological, and functional changes that affect the atria with the potential to produce clinical consequences 25 (Graphical Abstract). Signs and symptoms may be those of HF and/or of atrial arrhythmias and be due to both a failing LV and a dysfunctional LA, proving the active role of atrial disease in HF pathophysiology 18 , 26 and in other pathophysiological processes. 27 Impaired atrial contractile reserve and LA enlargement independently predict mortality and CV morbidity, as well as AF onset or recurrence and severity of functional impairment in HF patients. 14 , 28 , 29 , 30
The temporal process occurring after the removal of external stressors, 21 leading to a reduction in LA volume and/or a restoration of specific functional parameters 2 is called reverse remodelling.
Only recently the attention shifted to quantifying and evaluating LA reverse remodelling as a potential biomarker that responds to specific therapeutic interventions, potentially providing prognostic information. One critical aspect is that the response to therapeutic interventions is potentially different in the acute or chronic settings or in the early or late stage of atrial failure. In the early stage, the LA is less abnormal and potentially more capable of responding to the changes in myocardial structure and function. When stressors act for a longer time, LA remodelling progresses and reversal might be less likely (Figure 1 ). Reversibility and, ultimately, the clinical significance of LA reverse remodelling in the late stages of atrial failure, have to be proven, yet. Some studies have indicated that LA dysfunction may precede changes in LA volume in different clinical settings 31 , 32 , 33 , 34 and in normal subjects. 35 , 36 Thus, changes in LA function may be more sensitive than changes in LA volume to detect abnormal myocardial function and the effects of therapeutic interventions. However, this has not been proven, yet.
Figure 1.
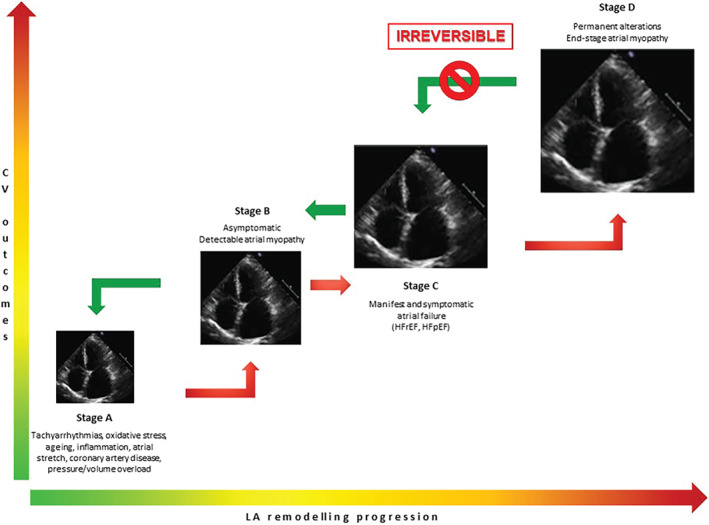
Progression of left atrial remodelling. The progression of left atrial remodelling goes through several stages, along with progressive worse cardiovascular outcomes. When causative stressors are not removed (by preventive measures or treatments), worsening atrial failure inexorably reaches an irreversible stage. On contrast, acting in the earlier phases may arrest and also reverse the pathophysiologic process. CV, cardiovascular; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Assessment of left atrial reverse remodelling
Assessment of LA remodelling is based on various non‐invasive parameters. To date, there are no definite cutoffs identifying a clinically relevant LA reverse remodelling. Hence, monitoring LA remodelling without such standardization makes routine examination challenging. Another major limitation of current evaluations of changes in LA function is that they are almost completely based on LA size. Measurements of LA strain and the different passive and active components might, on the other hand, better define atrial function in different clinical settings (Table 1 ). 37 , 38 , 39 , 40 Table 2 shows some of the definitions of LA reverse remodelling reported from the literature. 16 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 Most reports have used arbitrarily a 15% change of LA volume measured with either echocardiography and/or cardiac magnetic resonance (CMR). Functional aspects of LA reverse remodelling have been extensively described using strain analysis. A recent systematic review and meta‐analysis including more than 2500 patients showed reference values for reservoir, conduit and contractile strains. The variability was mostly explained by heart rate, body surface area, and sample size. 49 Nevertheless, little is known about the percentage change of LA strain which may be clinically significant. The difficulties related to the assessment of LA reverse remodelling are due to time‐consuming issues, the reliability of the measurement and the lack of standardization across inter‐vendor packages. However, the reproducibility of both LA volume and LA strain seems acceptable. 50 , 51
Table 1.
Imaging evaluation of left atrial reverse remodelling. Evaluation of left atrial size and function
| Parameter | Meaning | Advantages | Disadvantages |
|---|---|---|---|
| LA volume 2D‐TTE | ↓ LA volume = LA reverse remodelling |
Large body of evidence Good reproducibility |
Underestimation compared to 3D‐TTE, CT and CMR No LA functional assessment |
| LA volume 3D‐TTE | ↓ LA volume = LA reverse remodelling | Less geometric assumptions → correlation with CT and CMR |
Time‐consuming Low frame rate for 3D acquisition and consequent poor spatial resolution Few evidences of LA reverse remodelling |
| Peak LA longitudinal strain |
Quantification of functional reverse remodelling ↑ LA strain = ↓ LA fibrosis = LA reverse remodelling |
Semi‐automated Angle‐independent Less load‐dependent 37 Evaluation of phasic LA function 38 Acceptable reproducibility |
Time‐consuming Inter‐vendor variability Lack of standardization |
| LA volume CT |
↓ LA volume = LA reverse remodelling ↑ LA ejection fraction |
Short time for scanning and analysis More accurate than TTE Good correlation with CMR Anatomical characterization for AF ablation |
Need for contrast agent Need for breath‐hold |
| LA volume CMR |
↓ LA volume is the surrogate measure of LA reverse remodelling ↓ LA fibrosis = ↓ DE 39 |
Gold standard for LA volume assessment High spatial and temporal resolution Tissue characterization (DE) |
Time‐consuming Challenging with current technology Expensive Renal function Lack of evidence Availability/access |
2D, two‐dimensional; 3D, three‐dimensional; AF, atrial fibrillation; CMR, cardiac magnetic resonance; CT, computed tomography; DE, delayed enhancement; LA, left atrium; TTE, transthoracic echocardiography.
Table 2.
Definitions of left atrial reverse remodelling
| Author | Imaging modality | Definition | Type of choice | Reproducibility and agreement |
|---|---|---|---|---|
| Westenberg et al. 41 | CMR | ≥15% LAV reduction | Arbitrary, pre‐specified | Assessed: good |
| Antonini‐Canterin et al. 42 | TTE | ≥15% LAV reduction |
Arbitrary, pre‐specified Based on previously reported data |
Assessed on 10 pts: good |
| Tops et al. 43 | TTE | ≥15% LAV reduction | Arbitrary, pre‐specified | Assessed on 15 pts: good |
| Brenyo et al. 44 | TTE | High responders: ≥20% LAV reduction | Arbitrary | Not mentioned |
| Low responders: <20% LAV reduction | ≥lower‐quartile response in the CRT‐D group vs. <lower‐quartile response in the CRT‐D group | |||
| Marsan et al. 45 | 3D‐TTE | ≥15% LAV reduction |
Arbitrary Based on previously reported data |
Assessed on 20 pts: acceptable |
| Candan et al. 46 | TTE | >15% LAV index reduction | Arbitrary, pre‐specified | Assessed on 20 pts: good |
| Gelsomino et al. 47 | TTE | ≥15% LAV reduction | Arbitrary, pre‐specified | Assessed: acceptable |
| Kloosterman et al. 16 | TTE | ≥10% reduction in LAV | Arbitrary, pre‐specified | Not mentioned |
| Mathias et al. 48 | TTE | >30% LAV reduction | Arbitrary | Not mentioned |
| Median value of LAV percent decrease |
3D, three‐dimensional; CMR, cardiac magnetic resonance; CRT‐D, cardiac resynchronization therapy‐defibrillator; LAV, left atrial volume; pts, patients; TTE, transthoracic echocardiography.
Stages of heart failure
In line with the 2021 HF Society of America, HF Association of the ESC and Japanese HF Society universal definition of HF, 52 a four‐stage classification has been proposed for atrial disease. We discuss below the potential benefits of prevention and treatment on LA reverse remodelling, across the four HF stages.
Patients at risk for heart failure and with pre‐heart failure (stages A and B)
The importance of adopting effective measures to control risk factors and thus to prevent future HF development is an emerging public health need. 53 Even in the absence of overt HF, LA remodelling has a crucial role in this process. LA structural and functional reverse remodelling has been used to assess the therapeutic utility of different treatments, both in animal models and in humans (Table 3 ). 43 , 47 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 Risk of HF and pre‐HF, i.e. structural heart disease and/or abnormal cardiac function in the absence of symptoms (stages A and B) represent two crucial steps of the progression of LA remodelling in which preventive measures and targeted treatments have shown favourable results.
Table 3.
Left atrial reverse remodelling in patients at risk of heart failure and pre‐heart failure (stages A and B)
| Author | Type of study – sample | Population | Intervention/drug | Follow‐up | LA outcomes | Clinical outcomes |
|---|---|---|---|---|---|---|
| Arterial hypertension | ||||||
| Dernellis et al. 54 | Observational prospective – 48 | Essential hypertension | Enalapril ± chlorthalidone vs. untreated | 16 weeks | LARV: 35.4 to 29.3 ml (−17%), p < 0.05 LACV: 43.8 to 51.3 ml (+17%), p < 0.05 LA ejection force: 20.9 to 18.1 kdynes (−13%), p < 0.05 | |
| Mattioli et al. 55 | Observational prospective – 120 | Hypertensive patients with mild to moderate LV hypertrophy | Telmisartan | 12 months | LAV max: 35 to 32 ml (−9%), p < 0.05 | |
| Matsuyama et al. 56 | Observational | Animal: hypertension | Olmesartan | 8 weeks |
↓ LA fibrosis No difference in LA action potentials |
|
| Matsuyama et al. 56 | Observational prospective – 174 | Hypertension | Irbesartan or nebivolol | 12 months |
LAV max: Irbesartan 45.1 to 39.9 ml (−12%), p < 0.001 Nebivolol 47 to 40 ml (−15%), p < 0.001 |
|
|
LA‐GLS: Irbesartan 37.71% to 40.45% (+ 7%), p < 0.001 Nebivolol 36.4 to 41.52 (+14%), p < 0.001 |
||||||
| Atrial fibrillation | ||||||
| Shi et al. 57 | Observational prospective – 20 | Animal: CHF due to rapid atrial pacing | Enalapril vs. control | 5 weeks | ↓ LA fractional area shortening (−42%, p = 0.0001) | |
| ↓ LA fibrosis | ||||||
| Kumagai et al. 58 | Observational prospective – 20 | Animal: sustained AF by rapid right atrial pacing | Candesartan vs. control | 5 weeks | ↓ LA fibrosis | ↑ AF duration in control group |
| Cha et al. 59 | Observational prospective – 20 | Animal: CHF due to rapid ventricular pacing | Omapatrilat vs. control | 5 weeks | ↑ LA area index | |
| Lee et al. 60 | Observational prospective – 15 | Animal: CHF due to rapid ventricular pacing | Pirfenidone vs. control | 3 weeks | ↓ LA fibrosis | ↓ Arrhythmogenic atrial remodelling ↓ AF vulnerability |
| Li et al. 61 | Observational prospective – 27 | Animal: persistent AF by rapid right atrial pacing | Cilazapril or valsartan vs. control | 6 weeks | ↓ LAV ↑ LA ejection fraction | ↓ AF inducibility and duration |
| Kunamalla et al. 62 | Observational prospective – 21 | Animal: CHF due to rapid ventricular pacing | Gene‐based expression of dominant‐negative type II TGF‐β receptor | 3/4 weeks | ↓ Increase in conduction inhomogeneity ↓ LA fibrosis | ↓ AF episodes |
| Boldt et al. 63 | Observational prospective – 261 | Permanent AF versus sinus rhythm | ACEi (various) vs. control | 6 months | ↓ Collagen I expression | |
| Perea et al. 64 | Observational prospective – 90 | Paroxysmal AF | Catheter ablation | 4–6 months |
Recurrence of AF: LAV (CMR) 126.2 to 103.5 ml (−17%), p < 0.001 No recurrence of AF: LAV (CMR) 98 to 84.9 ml (−13%), p < 0.001 |
|
| Tops et al. 43 | Observational prospective – 148 | Paroxysmal or persistent AF | Catheter ablation | 13.2 ± 6.7 months |
Responders (63%): LAVI max 31 to 22 ml/m2 (−29%), p < 0.05 Non‐responders (37%): LAVI max 29 to 31 ml/m2 (+ 7%), p < 0.05 p‐value between groups: < 0.001 |
Baseline LA strain and LAVI max predictor of LARR at multivariate analysis |
|
Responders: LA strain 19% to 22% (+16%), p < 0.05 Non‐responders: LA strain 14% to 15% (+ 7%), NS p‐value between groups: < 0.001 |
||||||
| Gelsomino et al. 47 | Observational prospective – 33 | Paroxysmal AF | Minimally invasive atrial fibrillation surgery | 12 months | ↓ LAVI (LARR in 72%) ↑ LA strain | |
| Coronary artery disease | ||||||
| Milliez et al. 65 | Randomized – 88 | Animal: acute MI | Spironolactone ± lisinopril ± atenolol vs. untreated | 3 months | ↓ LA hyperexcitability ↓ LA fibrosis (spironolactone > atenolol and lisinopril) | |
| Yoon et al. 66 | Randomized – 38 | Animal: acute MI | Losartan vs. control | 4 weeks | ↓ Increase in LA diameter ↓ LA fibrosis (↓ connexin‐43 expression) | AF inducibility and duration not different between groups |
| Ahn et al. 67 | Observational prospective – 105 | Acute MI | PCI | 6 months | Occurrence of LA reverse remodelling after 6 months according to myocardial perfusion grade | |
| Other risk factors | ||||||
| Karason et al. 68 | Observational prospective – 63 | Obese patients | Bariatric surgery vs. control | 12 months | Surgery: LAV 71 to 63 ml (−11%), p < 0.05 Control: LAV 73 to 69 ml (−5%), NSp‐value between groups: NS | |
| Willens et al. 69 | Observational prospective – 17 | Obese patients | Bariatric surgery | 7.6 ± 3.6 months | LA diameter: 35 to 37 mm (+ 6%), NS | |
| Di Bello et al. 70 | Observational prospective – 39 | Obese patients | Bariatric surgery vs. control | 6–24 months | LA diameter: 37.9 to 33.5 (−12%), p < 0.05 | |
| Owan et al. 71 | Observational prospective – 882 | Obese patients | Bariatric surgery vs. untreated | 24 months | LAV: 55.3 to 54.4 ml (−2%), NS | |
| Luaces et al. 72 | Observational prospective – 41 | Obese patients | Bariatric surgery | 12 months | LAVI: 31.31 to 32.83 ml/m2 (+ 5%), NS | |
| Pathak et al. 73 | Observational prospective – 149 | Human: AF patients with BMI ≥27 kg/m2 and ≥1 CV risk factor | Risk factors management (more intensive vs. treating physician) after AF ablation | 42 months | Intensive: LAVI 42.5 to 30.4 ml/m2 (−28%), p < 0.001 Control: LAVI 42.4 to 39.5 ml/m2 (−7%), NSp‐value between groups: < 0.001 | Arrhythmia‐free survival greater and less symptoms in intensive management group |
| Pathak et al. 74 | Observational prospective – 308 | Paroxysmal/persistent AF and BMI ≥27 kg/m2 | Exercise programme and individual risk factors management; AF: medical therapy or ablation | 49 ± 19 months | No significant difference in LAVI between patients with cardiorespiratory fitness gain and without | Cardiorespiratory fitness predicts arrhythmia recurrence in obese individuals with symptomatic AF |
| Pathak et al. 75 | Observational prospective – 355 | AF and BMI ≥27 kg/m2 | Weight loss | 48 months | WL ≥10%: 37.6 to 30.9 ml/m2 (−18%), p < 0.001 WL 3%–9%: 39.5 to 34.7 ml/m2 (−12%), p < 0.001 WL <3%: 39 to 40.4 ml/m2 (+4%), p = 0.02p‐value between groups: < 0.001 | Long‐term sustained WL is associated with significant reduction in AF burden and maintenance of sinus rhythm |
ACEi, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; BMI, body mass index; CHF, chronic heart failure; CMR, cardiac magnetic resonance; CV, cardiovascular; LA, left atrium; LACV, left atrial conduit volume; LA‐GLS, left atrial global longitudinal strain; LARR, left atrial reverse remodelling; LARV, left atrial reservoir volume; LAV, left atrial volume; LAVI, left atrial volume index; LV, left ventricle; MI, myocardial infarction; NS, not significant; PCI, percutaneous coronary intervention; TGF‐β, transforming growth factor‐beta; WL, weight loss.
Hypertension
In hypertensive patients, evidence of LA reverse remodelling has been provided by the efficacy of specific therapies and a better blood pressure (BP) control. 76 In fact, some studies have demonstrated a reversal of LA dimension with associated functional improvement 54 , 55 , 56 , 77 , 78 (Table 3 ). Telmisartan showed a reduction in LA maximum and minimum volumes along with an increase in atrial ejection force and a decrease in BP, after 12 months of treatment. 55 Along with structural reverse remodelling, the use of antihypertensive therapy has also been shown to be associated with functional improvement in measures of LA strain. In a group of 160 patients treated with irbesartan or nebivolol, speckle tracking analysis showed a significant improvement in LA global peak reservoir strain after 12 months (from 37% to 40% and from 36% to 41% for irbesartan and nebivolol, respectively). 78 Whether these results may be translated to a clinically relevant LA reverse remodelling has yet to be demonstrated. Also, given the observational nature of these studies, results should be considered as exploratory.
The use of angiotensin‐converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs) has been hypothesized to be associated with LA reverse remodelling by a dual mechanism: an improved haemodynamic status, mediated by BP reduction, with a secondary improvement in LV diastolic function and a direct anti‐fibrotic effect. 56
Accordingly, careful monitoring of LA structure and function over time may serve as a surrogate marker to assess the efficacy of antihypertensive therapies. However, no study to date has assessed the prognostic significance of LA reverse remodelling among hypertensive patients.
Atrial fibrillation
The relationship between LA remodelling and AF is reciprocal. 79 The progression of LA disease may predispose to the occurrence of AF and, on the other hand, AF perpetuates and might worsen adverse LA remodelling in a vicious circle. The basis of this process seems to be related, at least in part, to an inflammatory state and the development of fibrosis. 80 In addition, the structural alterations associated with an enlarged LA lead to electrical remodelling. 81
Reverse remodelling identified by a shrinking LA volume has been associated with fewer AF recurrences in HF patients. 82 , 83 , 84 Several studies have demonstrated the benefits of both medical therapy and catheter ablation on LA reverse remodelling (Table 3 ). Even in animal models of HF due to rapid atrial pacing, the use of ACEi or ARB resulted in a significant LA size reduction and decreased amount of fibrosis. 85 , 86 The reduction of LA fibrosis has also been reported in one human study. The amount of collagen I was higher in AF versus sinus rhythm patients undergoing cardiac surgery. Interestingly, when divided into groups with or without ACEi treatment, there was a significant lower expression of collagen in AF with ACEi versus AF without ACEi. 63
The extent of pre‐existing fibrosis is a crucial determinant of interventional success in patients undergoing AF treatment. 87 , 88 Pre‐procedural LA strain is associated with rhythm outcome after LA catheter ablation. 89 Despite ablation itself may result in increased LA fibrosis, 90 , 91 performing the procedure has shown to favour LA reverse remodelling. Two studies reported LA response to catheter ablation or surgical treatment of AF. 43 , 47 In one study, LA reverse remodelling (Table 2 ) occurred in 63% of patients undergoing radiofrequency catheter ablation. 43 While LA maximum volume and LA minimum volume significantly decreased in the overall population, only the responder group reported a significant increase in LA strain. In this study, baseline LA strain and LA maximum volume were independent predictors of LA reverse remodelling. Similarly, in a cohort of patients who underwent surgical treatment of AF, 73% had LA reverse remodelling after a 12‐month follow‐up. 47
Coronary artery disease
Left atrial volume increases in patients with recent myocardial infarction (MI), especially in the early phase. LA remodelling after 1 month and baseline LA size are both independent predictors of morbidity and mortality. 14 LA enlargement often occurs in parallel with LV remodelling, even in the absence of LV ejection fraction (LVEF) deterioration. 92 , 93
Very few studies have assessed the direct effect of coronary interventions on LA reverse remodelling (Table 3 ). Ahn et al. 67 investigated the impact of myocardial perfusion on LA remodelling in 105 patients with acute MI treated with successful primary percutaneous coronary intervention. Despite no overall change of LA volume after 6 months, evidence of significant LA reverse remodelling according to myocardial perfusion grade was reported. In addition, perfusion grade and anterior MI location were independent determinants of LA remodelling at multivariate analysis (both p < 0.001).
In post‐MI animal models, renin–angiotensin–aldosterone system (RAAS) inhibition produced some effects on LA remodelling, preventing LA enlargement (Table 3 ). The anti‐fibrotic effect of ARBs was highlighted by a reduction in the expression of connexin 43. 66 Similarly, in ischaemic HF rats treated with ACEi, beta‐blocker or mineralocorticoid receptor antagonists (MRAs), atrial hyperexcitability was reduced by all drugs, but only spironolactone reduced atrial fibrosis. 65 The hypothesis generating nature of these findings should be replicated in humans to validate consistency in clinical practice.
Other risk factors
Recent evidence showed that dietary modification, physical activity and weight loss may reverse atrial abnormalities and may impact on arrhythmias incidence and recurrence in obese patients 2 (Table 3 ).
A recent meta‐analysis reported conflicting data about cardiac structural and functional changes after bariatric surgery. 94 Karason et al. 68 demonstrated LA reverse remodelling in obese patients following bariatric surgery when compared to the group with only dietary restrictions. Nevertheless, the difference in LA volume between the interventional and the dietary group was not significant either at baseline and after 1‐year follow‐up. In general, LA reverse remodelling occurred in parallel with LV mass reduction and LV filling pressure improvement.
Even in the absence of surgery, weight loss influenced LA reverse remodelling. Dietary modifications among obese patients have been shown to decrease LA volume along with a decrease in body mass index. 75
Other potentially modifiable risk factors such as sleep apnoea syndrome and arterial stiffness are associated with AF recurrence and LA remodelling. 95 , 96 Future studies will clarify whether targeted treatment of these conditions can reverse atrial remodelling and whether such changes are associated with a reduction in symptoms.
As opposed to LA reverse remodelling, exercise training may also be associated with LA enlargement. Young athletes tend to have larger LA volume when compared to active but not trained patients. 23 LA volume of athletes without AF showed considerable overlap with non‐athletes with AF. However, LA reservoir strain in AF patients was significantly impaired compared with patients in sinus rhythm, regardless of training status, 97 reflecting that the dysfunction acts as the basis of the arrhythmia.
Patients with heart failure (stage C)
Heart failure with reduced ejection fraction
Myocardial injury, haemodynamic changes, and neuro‐hormonal activation cause heart remodelling and this is a key factor for progression of HF with reduced ejection fraction (HFrEF). LA remodelling is a well‐studied entity, as it is associated with adverse CV events. LV reverse remodelling is a critical finding as a surrogate of the beneficial effects of most HF treatments and is associated with an improved prognosis. 98 Although some evidence on LA reverse remodelling has been reported from several HFrEF trials, the real impact on clinical outcomes remains less explored (Table 4 ) 16 , 42 , 44 , 45 , 46 , 48 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 .
Table 4.
Left atrial reverse remodelling in heart failure (stage C)
| Author | Type of study – sample | Stage of HF – NYHA class | Intervention | Follow‐up | LA outcomes | Clinical outcomes |
|---|---|---|---|---|---|---|
| Reduced ejection fraction | ||||||
| Desai et al. 99 | RCT – 413 | Stage C – NYHA I–III | ARNI vs. enalapril | 12 weeks |
ARNI: LAVI 30.4 to 28.2 ml/m2 (−7%) Enalapril: LAVI 29.8 to 30.5 ml/m2 (+2%)p‐value between groups: ≤ 0.001 |
|
| Januzzi et al. 100 | Observational prospective – 794 | Stage C – NYHA II–IV | ARNI | 6 months 12 months | ARNI: LAVI 37.76 to 32.80 (−13%) to 29.32 (−22%) ml/m2 (both p < 0.001) | ↓ NT‐proBNP (p < 0.001) |
| Brenyo et al. 44 | RCT (sub‐study) – 1378 | Stage C – NYHA II–III | CRT vs. ICD‐only | 12 months | Median LAV reduction: 29% (CRT; IQR 20%–36%) vs. 10% (ICD‐only; IQR 5%–14%) | ↓ Risk of supraventricular tachyarrhythmias, HF and deaths |
| Kloosterman et al. 16 | Observational retrospective – 365 | Stage C – NYHA II–IV | CRT | 6 months | LA + LVRR: LAVI 44 to 34 ml/m2 (−3%) LARR: LAVI 45 to 36 ml/m2 (−20%) LVRR: LAVI 40 to 42 ml/m2 (+5%) noRR: LAVI 43 to 47 ml/m2 (+9%)p‐value between groups: <0.001 | HFH and deaths: LARR not different from LARR + LVRR |
| Mathias et al. 48 | RCT (sub‐study) – 533 | Stage C – NYHA I–II | CRT vs. ICD‐only | 12 months | >30% LAV reduction in 60% of patients | Lower rate of HFH and deaths with LA + LVRR vs. LARR or noRR Lower rate of HFH and deaths with LARR vs. noRR |
| Valzania et al. 101 | Observational prospective – 30 | Stage C – NYHA III | CRT | 12 months | LA area: 26.6 to 23.1 cm2 (−13%) p < 0.05 | ↓ AF incidence (p = 0.05) ↓ NYHA class (p < 0.001) No difference for HFH |
| LA strain: 11.4% to 16.5% (+45%) p < 0.05 | ||||||
| St John Sutton et al. 102 | RCT – 419 | Stage C – NYHA I–II | CRT‐ON vs. CRT‐OFF (excluded from analysis) | 5 years (assessment every 6 months) | Overall LA area change not significant (p = 0.95) | No effect of LA area on mortality/morbidity |
| Singh et al. 103 | RCT – 56 | Stage C | Dapagliflozin vs. placebo | 12 months | LAVI: −2.6 ml/m2 (p = 0.464) | |
| Lee et al. 104 | RCT – 92 | Stage C – NYHA II–IV | Empagliflozin vs. placebo | 36 weeks | Empagliflozin: LAV (on CMR) 79 to 75.5 ml (−4%) Placebo: LAV (on CMR) 87.9 to 86.3 ml (−2%)p‐value between groups: 0.22 | |
| Preserved ejection fraction | ||||||
| Tsang et al. 105 | RCT – 21 | Diastolic dysfunction + LA enlargement | Quinapril vs. placebo | 6 and 12 months | Quinapril: LAVI 43 to 40 to 39 ml/m2 (−9%) Placebo: LAVI 38 to 40 to 44 ml/m2 (+16%)p‐value between groups: <0.05 | |
| Mak et al. 106 | RCT – 44 | Stage C – NYHA II–IV | Eplerenone vs. placebo | 6 and 12 months | Eplerenone: LAVI 50 to 49 to 52 ml/m2 (+4%) Placebo: LAVI 45 to 44 to 53 ml/m2 (+18%) Non‐significant difference between groups | |
| Kayrak et al. 107 | RCT – 110 | Post‐MI | Spironolactone vs. standard therapy | 6 months | Spironolactone: LAVI 52.3 to 52.2 ml/m2 (−0.2%) Control: LAVI 50.2 to 49.2 ml/m2 (+2%) Non‐significant difference between groups | |
| Spironolactone: LAEF 53% to 57% (+8%) Control: LAEF 50% to 47% (−6%)p‐value between groups: 0.013 | ||||||
| Edelmann et al. 108 | RCT – 64 | Stage C – NYHA II–III | Supervised exercise training vs. usual care | 3 months | Training: LAVI 27.9 to 24.3 ml/m2 (−13%) Control: LAVI 28.2 to 28.6 ml/m2 (+1%)p‐value between groups: 0.001 | Improved maximal exercise capacity |
| Solomon et al. 109 | RCT – 149 | Stage C – NYHA I–III | ARNI vs. valsartan | 36 weeks | ARNI: LAVI 35 to 32.4 ml/m2 (−7%) Valsartan: LAVI 36.8 to 37.1 ml/m2 (+1%)p‐value between groups: 0.007 | ↓ NYHA class (p = 0.05) |
| Deswal et al. 110 | RCT – 44 | Stage C – NYHA II–III | Eplerenone vs. placebo | 26 weeks | Eplerenone: LAV 73 to 64 ml (−12%) p = 0.02 Placebo: LAV 80 to 73 ml (−9%) p = 0.09 Non‐significant difference between groups | |
| Edelmann et al. 111 | RCT – 422 | Stage C – NYHA II–III | Spironolactone vs. placebo | 12 months | Spironolactone: LAVI 28.2 to 27.5 ml/m2 (−2%) Placebo: LAVI 27.8 to 27.6 ml/m2 (−1%) Non‐significant difference between groups | |
| Kurrelmeyer et al. 112 | RCT – 48 (only women) | Stage C – NYHA II–III | Spironolactone vs. placebo | 6 months | Spironolactone: LAVI 32.5 to 33.3 ml/m2 (+3%) Placebo: LAVI 35.7 to 36.7 ml/m2 (+3%) Non‐significant difference between groups | |
| ↓ Type III collagen levels | ||||||
| Shah et al. 113 | RCT – 239 | Stage C – NYHA I–IV | Spironolactone vs. placebo | 12–18 months | Spironolactone: LAV 59.3 to 60.3 ml (+2%) Placebo: LAVI 58.1 to 60.3 ml (+4%) Non‐significant difference between groups | Reduced composite endpoint of cardiovascular death, HFH, or aborted cardiac arrest due to overall LAV reduction |
| Soga et al. 114 | Observational prospective – 58 | Stage C (69% HFpEF, 13% HFrEF) | Dapagliflozin | 6 months | LAVI 31 to 26 ml/m2 (−16%) p = 0.001 | |
| Valvular heart disease | ||||||
| Antonini‐Canterin et al. 42 | Observational retrospective – 79 | Stage C | Mitral valve surgery (repair or replacement) for severe degenerative MR | 6 months | LAVI 68 to 47 ml/m2 (−29%) p < 0.001 | |
| 80% LARR Lower LAVI reduction in >45 years old and hypertensive patients | ||||||
| Kim et al. 115 | Observational prospective – 303 | Stage C – NYHA II–III | Percutaneous mitral valvuloplasty | After procedure, 1 year and 8 years | Total: LAVI 60.2 to 44.8 (−25%) to 69.1 (+15%) ml/m2, p < 0.001 SR: LAVI 48.8 to 35.7 (−27%) to 55.1 (+13%) ml/m2, p < 0.001 AF: LAVI 85.5 to 65 (−24%) to 100.9 (+18%) ml/m2, p < 0.001 | Pre‐procedural LAVI and percentage change of LAV immediately after procedure independent predictor of event‐free survival |
| Marsan et al. 45 | Observational prospective – 65 | Stage C | Early repair for severe MR (prolapse) | 6 and 12 months | LAVI 43 to 25 to 23 (−47%) ml/m2, both p < 0.05 | |
| 85% LARR (1 year) | ||||||
| D'Ascenzi et al. 116 | Observational prospective – 32 | Stage C – NYHA II–III–IV | TAVR | 40 days and 3 months | LAVI 47.3 to 42.8 to 43.5 (−8%) ml/m2, both p < 0.05 | |
| PALS 14.4% to 19% to 19.1% (+33%), p < 0.05 and p < 0.001 | ||||||
| Candan et al. 46 | Observational prospective – 53 | Stage C – NYHA I–II–III | Mitral valve surgery (repair or replacement) for severe MR | 6 months | LAVI 58.2 to 43.9 (−25%) ml/m2, p = 0.001 No difference between type of surgery | |
| Hatani et al. 117 | Observational retrospective – 83 | Stage C | Surgical AVR | 1 month, 1 year and 3 years | LAVI 51.3 to 43.7 (−15%) to 44.7 to 46.1 ml/m2, p < 0.001 | Residual LA dilatation at 1 year after AVR was associated with reduced event‐free survival |
| Toprak et al. 118 | Observational prospective – 25 | Stage C – NYHA II–IV (60%) | Transcatheter mitral valve repair (MitraClip implantation) | 12 months | 2D‐LAVI max: 51.5 to 50 ml/m2 (−3%) p = 0.512 3D‐LAVI max: 55.2 to 51.5 ml/m2 (−7%) p = 0.018 | Pre‐procedural 3D‐LAV and LA reservoir strain associated with MACE (univariate analysis) |
| LA reservoir strain: 7.66% to 11.15% (+46%), p < 0.001 | LA reservoir strain and 3D‐LAV min correlated with ↓ MR, ↓ NYHA class, ↑ 6MWD | |||||
| Avenatti et al. 119 | Observational retrospective – 35 | Stage C – NYHA III | Transcatheter mitral valve repair (MitraClip implantation) | 30 days | LAVI max: 74 to 53 ml/m2 (−28%), p = 0.008 LAVI max functional MR: 70 to 55 ml/m2 (−21%), p < 0.05 LAVI max primary MR: 65 to 49 ml/m2 (−25%), p < 0.05 | Significant inverse correlation between LA stiffness change and 6MWD in 14 patients |
| ↓ LA stiffness | ||||||
3D, three‐dimensional; 6MWD, 6‐min walking distance; 6MWT, 6‐min walking test; AF, atrial fibrillation; ARNI, angiotensin receptor–neprilysin inhibitor; AVR, aortic valve replacement; CMR, cardiac magnetic resonance; CRT, cardiac resynchronization therapy; HF, heart failure; HFH, hospitalization for heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter‐defibrillator; IQR, interquartile range; LA, left atrium; LAEF, left atrial emptying fraction; LARR, left atrial reverse remodelling; LAV, left atrial volume; LAVI, left atrial volume index; LV, left ventricle; LVRR, left ventricular reverse remodelling; MACE, major adverse cardiovascular event; MI, myocardial infarction; MR, mitral regurgitation; NYHA, New York Heart Association; PALS, peak atrial longitudinal strain; RCT, randomized clinical trial; RR, reverse remodelling; SR, sinus rhythm; TAVR, transcatheter aortic valve replacement.
The Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodelling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE‐HF) study 100 showed a reduction in LA volume index (from 37 to 29 ml/m2, p < 0.001) after 12 months in patients treated with angiotensin receptor–neprilysin inhibitor (ARNI), along with an increase in LVEF and decrease in LV volumes and E/e′ ratio. Similarly, in the multicentre randomized EVALUATE‐HF trial, 99 the ARNI group showed significant greater reductions in LA volume index, LV volumes and E/e′ ratio as compared to enalapril, while no significant change in LVEF was observed.
The rapid drop in N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) levels during treatment with ARNI 100 , 120 testifies the direct effect of sacubitril/valsartan on ventricular wall stress and a possible acute effect on filling pressures, perhaps related to increased venous capacitance or natriuresis. It has been demonstrated that the significant reduction in circulating NT‐proBNP (and high‐sensitivity cardiac troponin T) is correlated to the LA volume index reduction among HFrEF patients treated with ARNI. 121
Left atrial reverse remodelling can be considered an active player of the so‐called ‘complete left‐sided reverse remodelling’. 16 , 48 This concept has been elucidated by trials testing the structural response to cardiac resynchronization therapy (CRT) (Table 4 ). Results from the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT‐CRT) 44 study showed that reductions in LA volume with CRT‐defibrillator (CRT‐D) at 1 year were highly associated with reduction in LV volume, suggesting a cause–effect relationship. In this trial, most CRT‐D patients experienced ≥20% reduction in LA volume, while the implantable cardioverter‐defibrillator (ICD)‐only group had a significantly lower LA response. Patients who showed a favourable LA response to CRT‐D experienced a subsequent reduction in the risk of atrial tachycardia, HF events and death. Importantly, atrial tachycardia risk reduction appeared to be related to LA response independently of LV structural changes.
Two studies revealed that 20%–45% of patients with CRT showed LA reverse remodelling in absence of LV reverse remodelling 16 , 48 and contrasting results in terms of outcome have been reported (Table 4 ). Kloosterman et al. 16 retrospectively assessed LA reverse remodelling in 365 patients who were eligible for CRT. They reported that discordant left side reverse remodelling (LA without LV reverse remodelling) showed comparable mortality and HF hospitalization risk to patients with both LA and LV reverse remodelling. Thus, successful CRT can reduce LA size and improve LA pump function and LA reverse remodelling might improve outcome in CRT patients, even in the absence of LV remodelling.
The LA plays an active role in HFrEF pathophysiology and appears as a promising target to evaluate the efficacy of HF treatments. Although trials showed variable results, additional efforts should be addressed to clarify whether the assessment of LA reverse remodelling, as an independent entity, can provide prognostic information.
Heart failure with preserved ejection fraction
The assessment of LA response to different interventions in HF with preserved ejection fraction (HFpEF) patients has not been fully evaluated and available data have shown contrasting results.
Mineralocorticoid receptor antagonists decrease extracellular matrix turnover and myocardial collagen, one of the mechanisms of atrial disease. Studies investigating the impact of MRAs on cardiac function did not show significant LA reverse remodelling, despite many of them reported improvement in certain parameters of LV diastolic function (Table 4 ). In the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) 113 echocardiographic sub‐study, while spironolactone was not associated with significant change in either LA and LV structure over time, the overall reduction in LA volume was associated with an independent lower risk of subsequent occurrence of CV death, HF hospitalization, or aborted cardiac arrest. The reasons why the effects of antagonizing aldosterone activity did not show a direct effect on LA structure are uncertain. It is possible that the anti‐fibrotic effect of MRAs may require a longer time to result in functional or structural reverse remodelling.
In the phase II Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) trial, 109 , 122 while the use of ARNI did not show a significant effect on changes of LV structure or function, diastolic function, or LV mass after 12 and 36 weeks of treatment, LA size and volume significantly decreased after 36 weeks (Table 4 ). The concomitant inhibition of RAAS activity and the augmentation of the actions of natriuretic peptides have incremental effects on cardiac haemodynamics and structural remodelling. In the aforementioned study, the use of ARNI rapidly reversed LA remodelling without an apparent effect on LV size or diastolic function. This result may be explained by a rapid decrease in LV filling pressure, testified by the reduction of NT‐proBNP levels. LA remodelling reflects increased LV filling pressure in a more robust way than Doppler‐derived measures of diastolic function which are subject to greater variability. Also, the improvement of New York Heart Association (NYHA) functional class after 36 weeks may possibly explain the independent contribution of LA reverse remodelling to improvement of symptoms in HFpEF patients.
The Exercise training in Diastolic Heart Failure (Ex‐DHF) 108 study assessed the effect of structured exercise training compared to usual care in 64 patients with HFpEF. It demonstrated that exercise training positively affected exercise capacity and quality of life in HFpEF, through improvement in LV diastolic function and reduction in LA volume. BP values and body mass index did not change in either group, hence the effect on LA size reduction is not likely to be due to the haemodynamic effect. In fact, a significant reduction of procollagen type I plasma levels induced by exercise training was reported. It seems reasonable that LA reverse remodelling may be related to reduced collagen turnover.
The link between LA reverse remodelling and outcomes in patients with HFpEF needs to be evaluated in larger trials, possibly with longer follow‐up. However, targeting therapies based on LA response in HFpEF seems to be a rational approach.
Valvular heart disease
Mitral valve disease is a well‐studied model that represents the consequences of haemodynamic impairment on the LA. In mitral regurgitation (MR), volume overload has a direct effect on the LA causing chamber enlargement and myocyte hypertrophy to compensate the haemodynamic stress and to prevent pulmonary congestion. 123 At a cellular level, atrophy and interstitial fibrosis progressively impair LA function and decrease atrial elasticity. 124
Candan et al. 46 assessed the relationship between LA peak longitudinal reservoir strain and LA reverse remodelling in 53 patients with severe MR undergoing surgical valve repair or replacement (Table 4 ). LA volume index significantly decreased after surgery, regardless of the type of intervention. Also, pre‐operative higher LA volume, younger age and higher peak LA longitudinal strain values were independent predictor of LA reverse remodelling.
Left atrial function assessed by speckle tracking echocardiography has been associated with CV outcomes in patients with primary degenerative moderate asymptomatic MR. 125 Data from 87 subjects with degenerative MR enrolled in the Endovascular Valve Edge‐to‐Edge Repair Study II (EVEREST II) trial 126 displayed LA strain changes after both surgical or transcatheter mitral valve repair. LA strain improvement was dependent on baseline LA function as only in patients with normal or high baseline strain values, MR reduction resulted in normalization of LA strain. Moreover, in a cohort of 25 patients undergoing MitraClip, pre‐operative LA volume measured by three‐dimensional echocardiography (but not by two‐dimensional) and LA reservoir strain were associated with 1‐year major adverse CV events. 118
Recently, transcatheter indirect mitral annuloplasty showed benefits in reducing functional MR in HFrEF patients with a high procedural successful rate; an improvement in NYHA class was reported too. 127 One individual patient data meta‐analysis found that Carillon device implantation resulted in significant left‐sided reverse remodelling, as demonstrated by reduction in LV end‐diastolic and end‐systolic volume and reduction in LA volume. 128
The mechanisms behind LA remodelling in aortic stenosis (AS) are thought to be related to LV hypertrophy and increased LV filling pressure due to high afterload. The impact of aortic valve replacement (AVR) has yet to be explained. LV and LA reverse remodelling have been assessed in one cohort of patients with severe AS undergoing AVR 117 (Table 4 ). LA volume index rapidly decreased after 1 month along with a reduction in LV mass index. Patients who did not experience LA and LV reverse remodelling appeared to exhibit worse long‐term outcomes.
Even transcatheter AVR (TAVR) has been demonstrated to be associated with significant LA reverse remodelling. D'Ascenzi et al. 116 reported a significant reduction of LA size at a 40‐day follow‐up after TAVR. Alongside the recovery of LA structure, TAVR was accompanied by a significant increase in global peak atrial longitudinal reservoir strain. Pre‐procedural LA volume and trans‐aortic mean gradient change were reported as predictors of LA volume reduction 3 months following TAVR.
Advanced heart failure (stage D)
When reversal of LA remodelling fails to occur, the disease progresses to stage D of atrial disease. 1 The lack of LA volume reduction despite treatment identifies the irreversible advanced end‐stage, mainly related to a larger amount of fibrosis. Albeit not largely proven, in this stage, no therapeutic intervention may have beneficial consequences on LA function or structure. Only acting at earlier stages may prevent LA remodelling progression (Figure 1 ).
New treatments and future perspectives
While the effects of sodium–glucose cotransporter 2 inhibitors on atrial function have not been assessed, to date, 104 , 129 , 130 , 131 new drugs acting directly on cardiac function may have an impact also on the LA. Compared with placebo, the myosin activator omecamtiv mecarbil improved LA function, as shown by a reduced LA minimal volume and a larger emptying fraction, in the Chronic Oral Study of Myosin Activation to Increase Contractility in HF (COSMIC‐HF) trial and reduced the risk of new‐onset AF and stroke, likely through an improvement in LA function, in the larger Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility in HF (GALACTIC‐HF) trial. 132 , 133 , 134 , 135
Rhythm control for AF may improve LA function and this may improve patients' outcomes. 130 These effects, shown also after pulmonary vein isolation, may be particularly effective in patients with HFpEF such as 79% of the patients analysed in the Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial. 136 Improvement in LA function, as well as in outcomes, has been shown also in the Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation (CASTLE‐AF) trial, enrolling patients with reduced LVEF. 137
Our review shows that LA reverse remodelling may occur in clinical practice and be related with better outcomes. Changes in LA size and function may be related with progression of cardiac function and thus be a target of treatment or rather be a surrogate measurement of the severity of cardiac dysfunction. Also in this last case, changes in LA function may allow a more complete assessment of cardiac function and of the risk of cardiac events. LA function depends on both systolic and diastolic LV function and may predict not only HF events, as related with intracardiac pressure and congestion, but also other events including stroke and AF. However, even if measurements of LA function seem more stable than others, more data regarding their reproducibility and their clinically meaningful threshold are warranted. Future studies will also show whether LA function can be a target of treatment. Some interventions, such as pulmonary vein isolation for rhythm control in patients with AF may favourably affect LA function and this may mediate their effects on outcomes. 135 , 136 , 137 , 138 Also percutaneous treatment of MR may have an effect on LA function that may be larger than on LV function. 123
Conclusions
Left atrial remodelling and LA disease are markers of CV disease and may also have an impact on clinical outcomes. Counteracting the underlying mechanisms and removing the causative factors may restore LA structure and function. Increasing evidence indicates significant LA reverse remodelling after initiation of medical therapy across all the stages of development and progression of HF. Although a clear definition of LA reverse remodelling is needed, assessing LA response to targeted therapies appears to be a valid parameter to re‐define patients' risk stratification. Prospective clinical trials are required to establish the role of LA reverse remodelling as a marker of therapeutic response in clinical practice and to assess its potential independent role as a mechanism of development and progression of HF.
Acknowledgement
Open Access Funding provided by Università degli Studi di Brescia within the CRUI‐CARE Agreement.
Conflict of interest: T.B.S.: Steering Committee of the Amgen financed GALACTIC‐HF trial and the Boston Scientific financed LUX‐Dx TRENDS, Chief investigator of the Sanofi Pasteur financed NUDGEFLU trial, the DANFLU‐1 & DANFLU‐2 trials; research grants: Sanofi Pasteur & GE Healthcare; advisory boards: Sanofi Pasteur, Amgen & GSK; and speaker honoraria: Novartis, Sanofi Pasteur, GSK & Bayer. S.D.S. received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, US2.AI and has consulted for Abbott, Action, Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, GSK, Lilly, Merck, Myokardia, Novartis, Roche, Theracos, Quantum Genomics, Cardurion, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent, Sarepta, Lexicon, Anacardio, Akros, Puretech Health. M.M received personal fees of minimal amounts in the last 3 years from Actelion, Amgen, Livanova, Servier and Vifor pharma as member of Executive or Data Monitoring Committees of sponsored clinical trials and from AstraZeneca, Abbott Vascular, Bayer, Boehringer Ingelheim and Edwards Therapeutics for participation in advisory boards and/or speeches at sponsored meetings. All other authors have nothing to disclose.
References
- 1. Shen MJ, Arora R, Jalife J. Atrial myopathy. JACC Basic Transl Sci. 2019;4:640–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thomas L, Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging. 2017;10:65–77. [DOI] [PubMed] [Google Scholar]
- 3. Wakili R, Voigt N, Kääb S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:1961–77. [DOI] [PubMed] [Google Scholar]
- 5. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–60. [DOI] [PubMed] [Google Scholar]
- 6. Hsu PC, Lee WH, Chu CY, Lee HH, Lee CS, Yen HW, et al. Prognostic role of left atrial strain and its combination index with transmitral E‐wave velocity in patients with atrial fibrillation. Sci Rep. 2016;6:17318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarasunas J, Aidietis A, Aidietiene S. Left atrial strain – an early marker of left ventricular diastolic dysfunction in patients with hypertension and paroxysmal atrial fibrillation. Cardiovasc Ultrasound. 2018;16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sajeev JK, Kalman JM, Dewey H, Cooke JC, Teh AW. The atrium and embolic stroke: myopathy not atrial fibrillation as the requisite determinant? JACC Clin Electrophysiol. 2020;6:251–61. [DOI] [PubMed] [Google Scholar]
- 9. Yaghi S, Kamel H, Elkind MSV. Atrial cardiopathy: a mechanism of cryptogenic stroke. Expert Rev Cardiovasc Ther. 2017;15:591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huynh QL, Kalam K, Iannaccone A, Negishi K, Thomas L, Marwick TH. Functional and anatomic responses of the left atrium to change in estimated left ventricular filling pressure. J Am Soc Echocardiogr. 2015;28:1428–1433.e1. [DOI] [PubMed] [Google Scholar]
- 11. Blume GG, Mcleod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PM, et al. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr. 2011;12:421–30. [DOI] [PubMed] [Google Scholar]
- 12. Patel DA, Lavie CJ, Milani RV, Shah S, Gilliland Y. Clinical implications of left atrial enlargement: a review. Ochsner J. 2009;9:191–6. [PMC free article] [PubMed] [Google Scholar]
- 13. Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul study). Am J Cardiol. 2008;102:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meris A, Amigoni M, Uno H, Thune JJ, Verma A, Køber L, et al. Left atrial remodelling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the VALIANT Echo study. Eur Heart J. 2009;30:56–65. [DOI] [PubMed] [Google Scholar]
- 15. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015;8:295–303. [DOI] [PubMed] [Google Scholar]
- 16. Kloosterman M, Rienstra M, Mulder BA, Van Gelder IC, Maass AH. Atrial reverse remodelling is associated with outcome of cardiac resynchronization therapy. Europace. 2016;18:1211–9. [DOI] [PubMed] [Google Scholar]
- 17. Khan MS, Memon MM, Murad MH, Vaduganathan M, Greene SJ, Hall M, et al. Left atrial function in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Eur J Heart Fail. 2020;22:472–85. [DOI] [PubMed] [Google Scholar]
- 18. Reddy YNV, Borlaug BA. Left atrial myopathy in heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:486–8. [DOI] [PubMed] [Google Scholar]
- 19. Tamargo M, Obokata M, Reddy YNV, Pislaru SV, Lin G, Egbe AC, et al. Functional mitral regurgitation and left atrial myopathy in heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22:489–98. [DOI] [PubMed] [Google Scholar]
- 20. Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population‐based study. J Am Coll Cardiol. 2005;45:87–92. [DOI] [PubMed] [Google Scholar]
- 21. Rossi A, Gheorghiade M, Triposkiadis F, Solomon SD, Pieske B, Butler J. Left atrium in heart failure with preserved ejection fraction: structure, function, and significance. Circ Heart Fail. 2014;7:1042–9. [DOI] [PubMed] [Google Scholar]
- 22. Rønningen PS, Berge T, Solberg MG, Enger S, Nygård S, Pervez MO, et al. Sex differences and higher upper normal limits for left atrial end‐systolic volume in individuals in their mid‐60s: data from the ACE 1950 study. Eur Heart J Cardiovasc Imaging. 2020;21:501–7. [DOI] [PubMed] [Google Scholar]
- 23. Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Left atrial function in elite athletes: a meta‐analysis of two‐dimensional speckle tracking echocardiographic studies. Clin Cardiol. 2019;42:579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim GH, Uriel N, Burkhoff D. Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol. 2018;15:83–96. [DOI] [PubMed] [Google Scholar]
- 25. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 26. Mateescu AD, Călin A, Beladan CC, Roşca M, Enache R, Băicuş C, et al. Left atrial dysfunction as an independent correlate of heart failure symptoms in patients with severe aortic stenosis and preserved left ventricular ejection fraction. J Am Soc Echocardiogr. 2019;32:257–66. [DOI] [PubMed] [Google Scholar]
- 27. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–63. [DOI] [PubMed] [Google Scholar]
- 28. Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, et al.; I‐PRESERVE Investigators . Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–501. [DOI] [PubMed] [Google Scholar]
- 29. Cameli M, Sciaccaluga C, Loiacono F, Simova I, Miglioranza MH, Nistor D, et al. The analysis of left atrial function predicts the severity of functional impairment in chronic heart failure: the FLASH multicenter study. Int J Cardiol. 2019;286:87–91. [DOI] [PubMed] [Google Scholar]
- 30. Cameli M, Pastore MC, Mandoli GE, Nistor D, Lisi E, Tok ÖÖ, et al. Prognosis and risk stratification of patients with advanced heart failure (from PROBE). Am J Cardiol. 2019;124:55–62. [DOI] [PubMed] [Google Scholar]
- 31. Minamisawa M, Inciardi RM, Claggett B, Cuddy SAM, Quarta CC, Shah AM, et al. Left atrial structure and function of the amyloidogenic V122I transthyretin variant in elderly African Americans. Eur J Heart Fail. 2021;23:1290–5. [DOI] [PubMed] [Google Scholar]
- 32. Kadappu KK, Abhayaratna K, Boyd A, French JK, Xuan W, Abhayaratna W, et al. Independent echocardiographic markers of cardiovascular involvement in chronic kidney disease: the value of left atrial function and volume. J Am Soc Echocardiogr. 2016;29:359–67. [DOI] [PubMed] [Google Scholar]
- 33. Inciardi RM, Claggett B, Minamisawa M, Shin SH, Selvaraj S, Gonçalves A, et al. Association of left atrial structure and function with heart failure in older adults. J Am Coll Cardiol. 2022;79:1549–61. [DOI] [PubMed] [Google Scholar]
- 34. Inciardi RM, Claggett B, Gupta DK, Cheng S, Liu J, Echouffo Tcheugui JB, et al. Cardiac structure and function and diabetes‐related risk of death or heart failure in older adults. J Am Heart Assoc. 2022;11:e022308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morris DA, Takeuchi M, Krisper M, Köhncke C, Bekfani T, Carstensen T, et al. Normal values and clinical relevance of left atrial myocardial function analysed by speckle‐tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging. 2015;16:364–72. [DOI] [PubMed] [Google Scholar]
- 36. Boyd AC, Richards DAB, Marwick T, Thomas L. Atrial strain rate is a sensitive measure of alterations in atrial phasic function in healthy ageing. Heart. 2011;97:1513–9. [DOI] [PubMed] [Google Scholar]
- 37. Gan GCH, Ferkh A, Boyd A, Thomas L. Left atrial function: evaluation by strain analysis. Cardiovasc Diagn Ther. 2018;8:29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J Am Soc Echocardiogr. 2015;28:183–93. [DOI] [PubMed] [Google Scholar]
- 39. Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed‐enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–9. [DOI] [PubMed] [Google Scholar]
- 40. Inciardi RM, Galderisi M, Nistri S, Santoro C, Cicoira M, Rossi A. Echocardiographic advances in hypertrophic cardiomyopathy: three‐dimensional and strain imaging echocardiography. Echocardiography. 2018;35:716–26. [DOI] [PubMed] [Google Scholar]
- 41. Westenberg JJM, van der Geest RJ, Lamb HJ, Versteegh MIM, Braun J, Doornbos J, et al. MRI to evaluate left atrial and ventricular reverse remodeling after restrictive mitral annuloplasty in dilated cardiomyopathy. Circulation. 2005;112(9 Suppl):I437–42. [DOI] [PubMed] [Google Scholar]
- 42. Antonini‐Canterin F, Beladan CC, Popescu BA, Ginghina C, Popescu AC, Piazza R, et al. Left atrial remodelling early after mitral valve repair for degenerative mitral regurgitation. Heart. 2008;94:759–64. [DOI] [PubMed] [Google Scholar]
- 43. Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SAIP, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2011;57:324–31. [DOI] [PubMed] [Google Scholar]
- 44. Brenyo A, Link MS, Barsheshet A, Moss AJ, Zareba W, Wang PJ, et al. Cardiac resynchronization therapy reduces left atrial volume and the risk of atrial tachyarrhythmias in MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy). J Am Coll Cardiol. 2011;58:1682–9. [DOI] [PubMed] [Google Scholar]
- 45. Marsan NA, Maffessanti F, Tamborini G, Gripari P, Caiani E, Fusini L, et al. Left atrial reverse remodeling and functional improvement after mitral valve repair in degenerative mitral regurgitation: a real‐time 3‐dimensional echocardiography study. Am Heart J. 2011;161:314–21. [DOI] [PubMed] [Google Scholar]
- 46. Candan O, Ozdemir N, Aung SM, Hatipoglu S, Karabay CY, Guler A, et al. Atrial longitudinal strain parameters predict left atrial reverse remodeling after mitral valve surgery: a speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2014;30:1049–56. [DOI] [PubMed] [Google Scholar]
- 47. Gelsomino S, Lucà F, Rao CM, Parise O, Pison L, Wellens F, et al. Improvement of left atrial function and left atrial reverse remodeling after surgical treatment of atrial fibrillation. Ann Cardiothorac Surg. 2014;3:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mathias A, Moss AJ, McNitt S, Zareba W, Goldenberg I, Solomon SD, et al. Clinical implications of complete left‐sided reverse remodeling with cardiac resynchronization therapy: a MADIT‐CRT substudy. J Am Coll Cardiol. 2016;68:1268–76. [DOI] [PubMed] [Google Scholar]
- 49. Pathan F, D'Elia N, Nolan MT, Marwick TH, Negishi K. Normal ranges of left atrial strain by speckle‐tracking echocardiography: a systematic review and meta‐analysis. J Am Soc Echocardiogr. 2017;30:59–70.e8. [DOI] [PubMed] [Google Scholar]
- 50. Cho GY, Hwang IC. Left atrial strain measurement: a new normal for diastolic assessment? JACC Cardiovasc Imaging. 2020;13:2327–9. [DOI] [PubMed] [Google Scholar]
- 51. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two‐dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600. [DOI] [PubMed] [Google Scholar]
- 52. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, Anker SD, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail. 2021;23:352–80. [DOI] [PubMed] [Google Scholar]
- 53. Faggiano P, Bernardi N, Calvi E, Bonelli A, Faggiano A, Bursi F, et al. Stage a heart failure: modern strategies for an effective prevention. Heart Fail Clin. 2021;17:167–77. [DOI] [PubMed] [Google Scholar]
- 54. Dernellis JM, Vyssoulis GP, Zacharoulis AA, Toutouzas PK. Effects of antihypertensive therapy on left atrial function. J Hum Hypertens. 1996;10:789–94. [PubMed] [Google Scholar]
- 55. Mattioli AV, Zennaro M, Bonatti S, Bonetti L, Mattioli G. Regression of left ventricular hypertrophy and improvement of diastolic function in hypertensive patients treated with telmisartan. Int J Cardiol. 2004;97:383–8. [DOI] [PubMed] [Google Scholar]
- 56. Matsuyama N, Tsutsumi T, Kubota N, Nakajima T, Suzuki H, Takeyama Y. Direct action of an angiotensin II receptor blocker on angiotensin II‐induced left atrial conduction delay in spontaneously hypertensive rats. Hypertens Res. 2009;32:721–6. [DOI] [PubMed] [Google Scholar]
- 57. Shi Y, Li D, Tardif JC, Nattel S. Enalapril effects on atrial remodeling and atrial fibrillation in experimental congestive heart failure. Cardiovasc Res. 2002;54:456–61. [DOI] [PubMed] [Google Scholar]
- 58. Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003;41:2197–204. [DOI] [PubMed] [Google Scholar]
- 59. Cha YM, Dzeja PP, Redfield MM, Shen WK, Terzic A. Bioenergetic protection of failing atrial and ventricular myocardium by vasopeptidase inhibitor omapatrilat. Am J Physiol Heart Circ Physiol. 2006;290:H1686–92. [DOI] [PubMed] [Google Scholar]
- 60. Lee KW, Everett TH, Rahmutula D, Guerra JM, Wilson E, Ding C, et al. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114:1703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Y, Li W, Gong Y, Li B, Liu W, Han W, et al. The effects of cilazapril and valsartan on the mRNA and protein expressions of atrial calpains and atrial structural remodeling in atrial fibrillation dogs. Basic Res Cardiol. 2007;102:245–56. [DOI] [PubMed] [Google Scholar]
- 62. Kunamalla A, Ng J, Parini V, Yoo S, McGee KA, Tomson TT, et al. Constitutive expression of a dominant‐negative TGF‐β type II receptor in the posterior left atrium leads to beneficial remodeling of atrial fibrillation substrate. Circ Res. 2016;119:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boldt A, Scholl A, Garbade J, Resetar ME, Mohr FW, Gummert JF, et al. ACE‐inhibitor treatment attenuates atrial structural remodeling in patients with lone chronic atrial fibrillation. Basic Res Cardiol. 2006;101:261–7. [DOI] [PubMed] [Google Scholar]
- 64. Perea RJ, Tamborero D, Mont L, De Caralt TM, Ortiz JT, Berruezo A, et al. Left atrial contractility is preserved after successful circumferential pulmonary vein ablation in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:374–9. [DOI] [PubMed] [Google Scholar]
- 65. Milliez P, Deangelis N, Rucker‐Martin C, Leenhardt A, Vicaut E, Robidel E, et al. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J. 2005;26:2193–9. [DOI] [PubMed] [Google Scholar]
- 66. Yoon N, Cho JG, Kim KH, Park KH, Sim DS, Yoon HJ, et al. Beneficial effects of an angiotensin‐II receptor blocker on structural atrial reverse‐remodeling in a rat model of ischemic heart failure. Exp Ther Med. 2013;5:1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ahn SG, Shin JH, Koh BR, Choi JH, Kang SJ, Choi BJ, et al. Impact of myocardial perfusion on left atrial remodeling following primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2006;17:597–603. [DOI] [PubMed] [Google Scholar]
- 68. Karason K, Wallentin I, Larsson B, Sjöström L. Effects of obesity and weight loss on cardiac function and valvular performance. Obes Res. 1998;6:422–9. [DOI] [PubMed] [Google Scholar]
- 69. Willens HJ, Chakko SC, Byers P, Chirinos JA, Labrador E, Castrillon JC, et al. Effects of weight loss after gastric bypass on right and left ventricular function assessed by tissue Doppler imaging. Am J Cardiol. 2005;95:1521–4. [DOI] [PubMed] [Google Scholar]
- 70. Di Bello V, Santini F, Di Cori A, Pucci A, Talini E, Palagi C, et al. Effects of bariatric surgery on early myocardial alterations in adult severely obese subjects. Cardiology. 2008;109:241–8. [DOI] [PubMed] [Google Scholar]
- 71. Owan T, Avelar E, Morley K, Jiji R, Hall N, Krezowski J, et al. Favorable changes in cardiac geometry and function following gastric bypass surgery: 2‐year follow‐up in the Utah obesity study. J Am Coll Cardiol. 2011;57:732–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Luaces M, Cachofeiro V, García‐Muñoz‐Najar A, Medina M, González N, Cancer E, et al. Anatomical and functional alterations of the heart in morbid obesity. Changes after bariatric surgery. Rev Esp Cardiol (Engl Ed). 2012;65:14–21. [DOI] [PubMed] [Google Scholar]
- 73. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST‐AF cohort study. J Am Coll Cardiol. 2014;64:2222–31. [DOI] [PubMed] [Google Scholar]
- 74. Pathak RK, Elliott A, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO‐FIT study. J Am Coll Cardiol. 2015;66:985–96. [DOI] [PubMed] [Google Scholar]
- 75. Pathak RK, Middeldorp ME, Meredith M, Mehta AB, Mahajan R, Wong CX, et al. Long‐term effect of goal‐directed weight management in an atrial fibrillation cohort: a long‐term follow‐up study (LEGACY). J Am Coll Cardiol. 2015;65:2159–69. [DOI] [PubMed] [Google Scholar]
- 76. Eshoo S, Ross DL, Thomas L. Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging. 2009;2:93–9. [DOI] [PubMed] [Google Scholar]
- 77. Kokubu N, Yuda S, Tsuchihashi K, Hashimoto A, Nakata T, Miura T, et al. Noninvasive assessment of left atrial function by strain rate imaging in patients with hypertension: a possible beneficial effect of renin‐angiotensin system inhibition on left atrial function. Hypertens Res. 2007;30:13–21. [DOI] [PubMed] [Google Scholar]
- 78. Degirmenci H, Duman H, Demirelli S, Bakirci EM, Hamur H, Inci S, et al. Assessment of effect of irbesartan and nebivolol on the left atrium volume and deformation in the patients with mild‐moderate hypertension. Eur Rev Med Pharmacol Sci. 2014;18:781–9. [PubMed] [Google Scholar]
- 79. Inciardi RM, Giugliano RP, Claggett B, Gupta DK, Chandra A, Ruff CT, et al.; ENGAGE AF‐TIMI 48 Investigators . Left atrial structure and function and the risk of death or heart failure in atrial fibrillation. Eur J Heart Fail. 2019;21:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pessoa‐Amorim G, Mancio J, Vouga L, Ribeiro J, Gama V, Bettencourt N, et al. Impaired left atrial strain as a predictor of new‐onset atrial fibrillation after aortic valve replacement independently of left atrial size. Rev Esp Cardiol (Engl Ed). 2018;71:466–76. [DOI] [PubMed] [Google Scholar]
- 81. Bukowska A, Lendeckel U, Bode‐Böger SM, Goette A. Physiologic and pathophysiologic role of calpain: implications for the occurrence of atrial fibrillation. Cardiovasc Ther. 2012;30:e115–27. [DOI] [PubMed] [Google Scholar]
- 82. Vermes E, Tardif JC, Bourassa MG, Racine N, Levesque S, White M, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies of Left Ventricular Dysfunction (SOLVD) trials. Circulation. 2003;107:2926–31. [DOI] [PubMed] [Google Scholar]
- 83. Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin‐angiotensin‐aldosterone system (RAAS) for primary prevention of non‐valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2013;165:17–24. [DOI] [PubMed] [Google Scholar]
- 84. Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlöf B, et al. Angiotensin II receptor blockade reduces new‐onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention for End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–9. [DOI] [PubMed] [Google Scholar]
- 85. Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. 2000;101:2612–7. [DOI] [PubMed] [Google Scholar]
- 86. Cha TJ, Ehrlich JR, Chartier D, Qi XY, Xiao L, Nattel S. Kir3‐based inward rectifier potassium current: potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation. 2006;113:1730–7. [DOI] [PubMed] [Google Scholar]
- 87. Bax JJ, Marsan NA, Delgado V. Non‐invasive imaging in atrial fibrillation: focus on prognosis and catheter ablation. Heart. 2015;101:94–100. [DOI] [PubMed] [Google Scholar]
- 88. Leong DP, Delgado V, Bax JJ. Imaging for atrial fibrillation. Curr Probl Cardiol. 2012;37:7–33. [DOI] [PubMed] [Google Scholar]
- 89. Motoki H, Negishi K, Kusunose K, Popović ZB, Bhargava M, Wazni OM, et al. Global left atrial strain in the prediction of sinus rhythm maintenance after catheter ablation for atrial fibrillation. J Am Soc Echocardiogr. 2014;27:1184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tsao HM, Hu WC, Wu MH, Tai CT, Chang SL, Lin YJ, et al. The impact of catheter ablation on the dynamic function of the left atrium in patients with atrial fibrillation: insights from four‐dimensional computed tomographic images. J Cardiovasc Electrophysiol. 2010;21:270–7. [DOI] [PubMed] [Google Scholar]
- 91. Wylie JV, Peters DC, Essebag V, Manning WJ, Josephson ME, Hauser TH. Left atrial function and scar after catheter ablation of atrial fibrillation. Heart Rhythm. 2008;5:656–62. [DOI] [PubMed] [Google Scholar]
- 92. Yoon HJ, Jeong MH, Jeong Y, Kim KH, Song JE, Cho JY, et al. Progressive dilation of the left atrium and ventricle after acute myocardial infarction is associated with high mortality. Korean Circ J. 2013;43:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Popescu BA, Macor F, Antonini‐Canterin F, Giannuzzi P, Temporelli PL, Bosimini E, et al.; GISSI‐3 Echo Substudy Investigators . Left atrium remodeling after acute myocardial infarction (results of the GISSI‐3 Echo substudy). Am J Cardiol. 2004;93:1156–9. [DOI] [PubMed] [Google Scholar]
- 94. Cuspidi C, Rescaldani M, Tadic M, Sala C, Grassi G. Effects of bariatric surgery on cardiac structure and function: a systematic review and meta‐analysis. Am J Hypertens. 2014;27:146–56. [DOI] [PubMed] [Google Scholar]
- 95. Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. 2012;9:321–7. [DOI] [PubMed] [Google Scholar]
- 96. Lau DH, Middeldorp ME, Brooks AG, Ganesan AN, Roberts‐Thomson KC, Stiles MK, et al. Aortic stiffness in lone atrial fibrillation: a novel risk factor for arrhythmia recurrence. PLoS One. 2013;8:e76776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sørensen E, Myrstad M, Solberg MG, Øie E, Tveit A, Aarønæs M. Left atrial function in male veteran endurance athletes with paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2021;23:137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta‐analytic approach. J Am Coll Cardiol. 2010;56:392–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, et al.; EVALUATE‐HF Investigators . Effect of sacubitril‐valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019; 322:1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, et al.; PROVE‐HF Investigators . Association of change in N‐terminal pro‐B‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Valzania C, Gadler F, Boriani G, Rapezzi C, Eriksson MJ. Effect of cardiac resynchronization therapy on left atrial size and function as expressed by speckle tracking 2‐dimensional strain. Am J Cardiol. 2016;118:237–43. [DOI] [PubMed] [Google Scholar]
- 102. St John Sutton M, Linde C, Gold MR, Abraham WT, Ghio S, Cerkvenik J, et al. Left ventricular architecture, long‐term reverse remodeling, and clinical outcome in mild heart failure with cardiac resynchronization: results from the REVERSE trial. JACC Heart Fail. 2017;5:169–78. [DOI] [PubMed] [Google Scholar]
- 103. Singh JSS, Mordi IR, Vickneson K, Fathi A, Donnan PT, Mohan M, et al. Dapagliflozin versus placebo on left ventricular remodeling in patients with diabetes and heart failure: the REFORM trial. Diabetes Care. 2020;43:1356–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, et al. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR‐DM‐HF). Circulation. 2021;143:516–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tsang TSM, Barnes ME, Abhayaratna WP, Cha SS, Gersh BJ, Langins AP, et al. Effects of quinapril on left atrial structural remodeling and arterial stiffness. Am J Cardiol. 2006;97:916–20. [DOI] [PubMed] [Google Scholar]
- 106. Mak GJ, Ledwidge MT, Watson CJ, Phelan DM, Dawkins IR, Murphy NF, et al. Natural history of markers of collagen turnover in patients with early diastolic dysfunction and impact of eplerenone. J Am Coll Cardiol. 2009;54:1674–82. [DOI] [PubMed] [Google Scholar]
- 107. Kayrak M, Bacaksiz A, Vatankulu MA, Ayhan SS, Ari H, Kaya Z, et al. The effects of spironolactone on atrial remodeling in patients with preserved left ventricular function after an acute myocardial infarction: a randomized follow‐up study. Coron Artery Dis. 2010;21:477–85. [DOI] [PubMed] [Google Scholar]
- 108. Edelmann F, Gelbrich G, Düngen HD, Fröhling S, Wachter R, Stahrenberg R, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex‐DHF (Exercise Training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–91. [DOI] [PubMed] [Google Scholar]
- 109. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher‐Krainer E, et al.; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators . The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double‐blind randomised controlled trial. Lancet. 2012;380:1387–95. [DOI] [PubMed] [Google Scholar]
- 110. Deswal A, Richardson P, Bozkurt B, Mann DL. Results of the Randomized Aldosterone Antagonism in Heart Failure with Preserved Ejection Fraction trial (RAAM‐PEF). J Card Fail. 2011;17:634–42. [DOI] [PubMed] [Google Scholar]
- 111. Edelmann F, Wachter R, Schmidt AG, Kraigher‐Krainer E, Colantonio C, Kamke W, et al.; Aldo‐DHF Investigators . Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo‐DHF randomized controlled trial. JAMA. 2013;309:781–91. [DOI] [PubMed] [Google Scholar]
- 112. Kurrelmeyer KM, Ashton Y, Xu J, Nagueh SF, Torre‐Amione G, Deswal A. Effects of spironolactone treatment in elderly women with heart failure and preserved left ventricular ejection fraction. J Card Fail. 2014;20:560–8. [DOI] [PubMed] [Google Scholar]
- 113. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Deswal A, Anand IS, et al. Prognostic importance of changes in cardiac structure and function in heart failure with preserved ejection fraction and the impact of spironolactone. Circ Heart Fail. 2015;8:1052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, et al. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol. 2018;17:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kim KH, Kim YJ, Shin DH, Chang SA, Kim HK, Sohn DW, et al. Left atrial remodelling in patients with successful percutaneous mitral valvuloplasty: determinants and impact on long‐term clinical outcome. Heart. 2010;96:1050–5. [DOI] [PubMed] [Google Scholar]
- 116. D'Ascenzi F, Cameli M, Henein M, Iadanza A, Reccia R, Lisi M, et al. Left atrial remodelling in patients undergoing transcatheter aortic valve implantation: a speckle‐tracking prospective, longitudinal study. Int J Cardiovasc Imaging. 2013;29:1717–24. [DOI] [PubMed] [Google Scholar]
- 117. Hatani T, Kitai T, Murai R, Kim K, Ehara N, Kobori A, et al. Associations of residual left ventricular and left atrial remodeling with clinical outcomes in patients after aortic valve replacement for severe aortic stenosis. J Cardiol. 2016;68:241–7. [DOI] [PubMed] [Google Scholar]
- 118. Toprak C, Kahveci G, Kilicgedik A, Pala S, Kirma C, Tabakci MM, et al. Left atrial remodeling in patients undergoing percutaneous mitral valve repair with the MitraClip system: an advanced echocardiography study. Echocardiography. 2016;33:1504–11. [DOI] [PubMed] [Google Scholar]
- 119. Avenatti E, Little SH, Barker CM, Nagueh SF. Changes in left atrial function after transcutaneous mitral valve repair. Am J Cardiol. 2018;122:1204–9. [DOI] [PubMed] [Google Scholar]
- 120. Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al.; PIONEER‐HF Investigators . Angiotensin‐neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–48. [DOI] [PubMed] [Google Scholar]
- 121. Murphy SP, Prescott MF, Maisel AS, Butler J, Piña IL, Felker GM, et al. Association between angiotensin receptor‐neprilysin inhibition, cardiovascular biomarkers, and cardiac remodeling in heart failure with reduced ejection fraction. Circ Heart Fail. 2021;14:e008410. [DOI] [PubMed] [Google Scholar]
- 122. Zile MR, Jhund PS, Baicu CF, Claggett BL, Pieske B, Voors AA, et al.; Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction (PARAMOUNT) Investigators . Plasma biomarkers reflecting profibrotic processes in heart failure with a preserved ejection fraction: data from the Prospective Comparison of ARNI with ARB on Management of Heart Failure with Preserved Ejection Fraction study. Circ Heart Fail. 2016;9:e002551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Inciardi RM, Rossi A, Bergamini C, Benfari G, Maffeis C, Greco C, et al. Mitral regurgitation, left atrial structural and functional remodelling and the effect on pulmonary haemodynamics. Eur J Heart Fail. 2020;22:499–506. [DOI] [PubMed] [Google Scholar]
- 124. Braunwald E, Awe WC. The syndrome of severe mitral regurgitation with normal left atrial pressure. Circulation. 1963;27:29–35. [DOI] [PubMed] [Google Scholar]
- 125. Cameli M, Pastore MC, Righini FM, Mandoli GE, D'Ascenzi F, Lisi M, et al. Prognostic value of left atrial strain in patients with moderate asymptomatic mitral regurgitation. Int J Cardiovasc Imaging. 2019;35:1597–604. [DOI] [PubMed] [Google Scholar]
- 126. Gucuk Ipek E, Singh S, Viloria E, Feldman T, Grayburn P, Foster E, et al. Impact of the MitraClip procedure on left atrial strain and strain rate. Circ Cardiovasc Imaging. 2018;11:e006553. [DOI] [PubMed] [Google Scholar]
- 127. Witte KK, Lipiecki J, Siminiak T, Meredith IT, Malkin CJ, Goldberg SL, et al. The REDUCE FMR trial: a randomized sham‐controlled study of percutaneous mitral annuloplasty in functional mitral regurgitation. JACC Heart Fail. 2019;7:945–55. [DOI] [PubMed] [Google Scholar]
- 128. Giallauria F, Di Lorenzo A, Parlato A, Testa C, Bobbio E, Vigorito C, et al. Individual patient data meta‐analysis of the effects of the CARILLON® mitral contour system. ESC Heart Fail. 2020;7:3383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, et al.; DAPA‐HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 130. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, et al.; EMPEROR‐Reduced Trial Investigators . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–24. [DOI] [PubMed] [Google Scholar]
- 131. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al.; EMPEROR‐Preserved Trial Investigators . Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61. [DOI] [PubMed] [Google Scholar]
- 132. Teerlink JR, Felker GM, McMurray JJV, Solomon SD, Adams KF, Cleland JGF, et al.; COSMIC‐HF Investigators . Chronic Oral Study of Myosin Activation to Increase Contractility in Heart Failure (COSMIC‐HF): a phase 2, pharmacokinetic, randomised, placebo‐controlled trial. Lancet. 2016;388:2895–903. [DOI] [PubMed] [Google Scholar]
- 133. Teerlink JR, Diaz R, Felker GM, McMurray JJV, Metra M, Solomon SD, et al.;, GALACTIC‐HF Investigators . Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med. 2021;384:105–16. [DOI] [PubMed] [Google Scholar]
- 134. Biering‐Sørensen T, Teerlink JR, Felker GM, McMurray JJ, Malik FI, Honarpour N, et al. Cardiac myosin activator omecamtiv mecarbil improves left atrial structure and function in chronic heart failure (COSMIC‐HF). Circulation. 2016;134:A19108 (abstr). [Google Scholar]
- 135. Teerlink JR, Diaz R, Felker M, McMurray JJ, Solomon S, Metra M, et al.; GALACTIC‐HF Investigators and Patients . The effect of omecamtiv mecarbil on stroke in patients with heart failure and reduced ejection fraction in GALACTIC‐HF. Abstract 16605. Late‐Breaking Science Abstracts and Featured Science Abstracts from the American Heart Association's Scientific Sessions 2021 and Late‐Breaking Abstracts in Resuscitation Science from the Resuscitation Science Symposium 2021. Circulation. 2021;144:e564–93.34928705 [Google Scholar]
- 136. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, et al.; CABANA Investigators . Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al.; CASTLE‐AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 138. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS Focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–51. [DOI] [PubMed] [Google Scholar]


