Summary
Background and objective
Pityriasis rosea (PR), a common skin disease in young adults, may adversely affects the course of pregnancy and the unborn child.
Patients and methods
Data from forty‐six pregnant women with PR seen in the dermatological university clinic between 2003 and 2018 were analyzed and compared with patient data (n = 53) from previously published studies to determine the incidence and risk factors for an unfavorable pregnancy outcome after PR infection.
Results
Unfavorable pregnancy outcomes (defined as miscarriage, preterm delivery before week 37 of gestation, or birth weight < 2,500 g) were significantly less frequent in our study population than in a pooled cohort obtained from previously published studies (10.9 % vs. 39.6 %; P = 0.0012). Analysis of pooled data from our study and from previous studies revealed that the week of pregnancy at onset of PR was inversely associated with an unfavorable outcome (odds ratio [OR] = 0.937; 95 % CI 0.883 to 0.993). In addition, duration of PR (OR = 1.432; 95 % CI 1.129 to 1.827), additional extracutaneous symptoms (OR = 4.112; 95 % CI 1.580 to 10.23), and widespread rash distribution (OR 5.203, 95 % CI 1.702 to 14.89) were directly associated with unfavorable outcome.
Conclusion
In most cases, PR does not influence pregnancy or birth outcomes.
Introduction
Pityriasis rosea (PR) is an acute, self‐limiting rash that primarily affects young adults aged 15 to 35 [1, 2]. It typically begins with a single plaque, followed by exanthematous spread after a latency period of 10–14 days. The rash may last from two weeks to a few months and may be accompanied by additional symptoms such as fever, headache, malaise, or lymph node swelling [3, 4, 5, 6, 7]. The differential diagnosis of PR includes nummular eczema, pityriasis lichenoides chronica, guttata psoriasis, drug eruption, syphilis, and, in the case of pregnancy, polymorphic and atopic eruptions of pregnancy [3, 4]. Pityriasis rosea typically occurs once in a lifetime but may recur, is communicable and is associated with other factors such as socioeconomic background [3, 8, 9, 10, 11, 12, 13, 14, 15]. There is strong evidence of an infectious origin, in particular relating to human herpesvirus (HHV) 6 and 7 [16, 17, 18, 19, 20, 21]. The reported incidence of PR in pregnancy is 18 % versus 6 % in the general population [22]. A few studies have linked PR in pregnancy to an unfavorable outcome [3]. In a study of 61 pregnant women with PR, Drago and colleagues observed miscarriage, preterm delivery, low birth weight, weak motility, hypotonia, and a low Apgar score at birth [23, 24]. They also described high spontaneous abortion rates (57–62 %) in women who developed PR in the first 15 weeks of gestation [23, 24, 25]. However, there are only a limited number of studies investigating pregnancy outcomes in women with PR.
Patients and methods
Study design
This retrospective study was carried out at the Department of Dermatology of the Medical University of Graz. It included data from 59 pregnant women who presented with PR between January 2003 and July 2018. These 59 women had a total of 63 births and miscarriages. Data were obtained from the electronic hospital health system and patient record database. The study was approved by the ethics committee of the Medical University of Graz (Application number: 30‐405‐ex‐17/18).
Patient identification and data extraction
We initially identified potential candidates for our study by electronically searching our hospital database for the following keywords: “pityriasis”, “rosea”, “exanthema”, and “pregnancy”. Of the patients identified, only those patients were selected for the study in whom the diagnosis of PR could be clearly established clinically. We then manually scrutinized the electronic and/or paper hospital records of the patients, so identified and extracted the following data: age when PR appeared, previous pregnancies, pregnancy week when PR first occurred, rash duration, additional/extracutaneous symptoms, rash location, gestation week at time of birth, and body weight and length at time of birth. Laboratory tests for anti‐HHV‐6 titer were retrieved when available. To complete and expand the resulting database, we sent a written questionnaire to all women enroled in the study. The questionnaire contained questions about the course of the PR‐associated pregnancy and previous pregnancies, the course of the PR disease, and the outcome of medical examinations recommended in the Austrian pregnancy passport. This passport includes information on the newborn’s weight, length, gestation week, other complications at time of birth and examinations during the infant’s first year of life. For our analysis, the gestation week was rounded up or down in 0.5 increments.
Case series analysis and literature review
For case series analysis, we systematically searched the PubMed databases for the period from 1968 to 2020. The literature search was based on the following keywords: “pregnancy” and “pityriasis rosea”. References from selected articles were screened for further relevant studies that were not directly identified by the PubMed search. In total, we identified seven papers from which we extracted detailed descriptions and datasets for 53 women; these data were included in our case series analysis and risk calculations (Table 1).
Table 1.
Study characteristics of women with pityriasis rosea in pregnancy
| Source | Location | Total number of cases | Pregnancy outcome | Mean age of cases (years) | |
|---|---|---|---|---|---|
| Unfavorable (%)* | Favorable (%) | ||||
| Drago et al. [23] 2008 | Italy | 38 | 14 (36.8) | 24 (63.2) | 28.8 |
| Drago et al. [24] 2014 | Italy | 9 | 6 (66.7) | 3 (33.3) | 30.6 |
| Alame et al. [7] 2018 | Lebanon | 1 | 0 (0) | 1 (100) | 30 |
| Chuh et al. [26] 2005 | China | 2 | 0 (0) | 2 (100) | 30.5 |
| Cruz et al. [27] 2012 | Portugal | 1 | 0 (0) | 1 (100) | 28 |
| Overton et al. [28] 1968 | USA | 1 | 1 (100) | 0 (0) | 24 |
| Loh et al. [29] 2016 | USA | 1 | 0 (0) | 1 (100) | 28 |
| Summary of previous studies | 53 | 21 (39.6)*** | 32 (60.4) | 29.1 | |
| This study, 2020 | Austria | 46** | 5 (10.9)*** | 41 (89.1) | 28.4 |
| Summary of all study data | 99 | 26 (26.3) | 73 (73.7) | 28.8 | |
Criteria for an unfavorable pregnancy outcome were: preterm delivery before 37 weeks of gestation, birth weight lower than 2,500 grams and miscarriage.
6 cases of twin pregnancies were excluded, as outlined in materials and methods and the results section.
In total, unfavorable pregnancy outcome was significantly lower in our cohort compared to previously published data (P = 0.0012).
Pregnancy outcome definition
The women included in this study were categorized by pregnancy outcome (favorable or unfavorable) according to published criteria [7, 23, 24, 26–29]. A favorable outcome was defined as a pregnancy of normal duration and birth of a healthy newborn of normal weight. An unfavorable outcome was defined as preterm delivery before week 37 of gestation, birth weight lower than 2,500 g, or miscarriage. These criteria are stricter than the ones used in the studies conducted by Drago et al. [23, 24, 25]. In particular, we excluded non‐severe events at birth such as transient weak motility, hypotonia, and hydramnios from our definition of unfavorable outcome. Twin pregnancies were also excluded from the final statistical analysis.
Statistics and case series analysis
All data obtained from the patients of this study and from previous published papers were anonymized and analyzed. Odds ratios (ORs) and the associated 95 % confidence intervals (Cis) and P values were calculated with SPSS version 25. P values < 0.05 were considered statistically significant. Graphics were created with Prism V8.4.1.
Results
Data from the current study
Fifty‐nine pregnant women were identified and selected for analysis in this study. The median age at PR onset was 28 years (online supplementary Table S1). More than half of the women were primiparous (54.5 %); the others already had one (31 %) or more children (14.5 %). Of 55 women who provided information on abortion or miscarriage, 46 (84 %) had no such history. The median duration of pregnancy was 39.5 weeks. For newborns, the median birth weight was 3,225 g, and the median birth length was 50.5 cm. In total, the 59 women had 63 babies; however, birth datasets for eleven of those babies were not available. In 5 out of 46 complete data sets the pregnancy outcome was unfavorable. Six newborns from twin pregnancies and four newborns from singleton pregnancies were delivered prematurely before week 37 of gestation. However, all newborns of the women in our study population had an Apgar‐Score of ≥ 8 after five minutes. Eleven newborns had a birth weight less than 2,500 g, including the six newborns from twin pregnancies. In our study population, PR developed most often in the first trimester (53 %, n = 32) and decreasingly less often in the second (35 %, n = 21) and third (12 %, n = 7) trimester. The median time of PR onset in pregnancy was earlier in women with an unfavorable outcome than in those with a favorable outcome (6 vs. 14 weeks of pregnancy; P = 0.27). The median rash duration was shorter in women with an unfavorable outcome (3 vs. 4 weeks; P = 0.31). Widespread rash distribution (involving trunk and extremities) occurred more often in women with an unfavorable outcome than in those with a favorable outcome (80 % [4/5] vs. 58 % [23/40 with complete data]; P = 0.63). Likewise, additional extracutaneous symptoms occurred more often in those with an unfavorable outcome (75 % [3/4] vs. 30 % [12/40]; P = 0.11) (online supplementary Table S1). Of all patients, 34 were given short‐term topical steroids, mostly methylprednisolone aceponate, four were given oral antihistamines, and all other patients received moisturizing care ointments only or no treatment. Birth weight was lower in those with an unfavorable outcome compared to favorable outcome (2,350 g vs. 3,320 g). Demographic data are presented in Table 2. In one case, the mother had been diagnosed with PR twice in two consecutive pregnancies (online supplementary Table S1, patient number 15 and 31). The children from her two pregnancies were born on the expected due dates and had normal birth weights.
Table 2.
Characteristics and clinical outcome of patients from this study and previously published reports
| Pregnancy outcome (our data) | Pregnancy outcome (previously published papers) | Pregnancy outcome (summarized) | ||||
|---|---|---|---|---|---|---|
| Unfavorable | Favorable | Unfavorable | Favorable | Unfavorable | Favorable | |
| Median age (range) | 28 (18 to 32) | 28 (20 to 37) | 29 (24 to 34) | 29.5 (24 to 34) | 29 (18 to 34) | 29 (20 to 37) |
| Rate of primiparae (percentage) | 4 of 5 (80) | 18 of 41 (44) | 9 of 21 (43) | 16 of 31 (52) | 13 of 26 (50) | 34 of 72 (47) |
| Median week at PR onset (range) | 6 (6 to 29) | 14 (3 to 37) | 15 (6 to 29) | 24 (8 to 32) | 14.5 (6 to 29) | 19 (3 to 37) |
| Median duration of the rash (range) | 3 (3 to 3) | 4 (2 to 8) | 8 (3 to 13) | 5 (4 to 10) | 7 (3 to 13) | 5 (2 to 10) |
| Additional symptoms (percentage) | 3 of 4 (75) | 12 of 40 (30) | 11 of 20 (55) | 6 of 31 (19) | 14 of 24 (58) | 18 of 71 (25) |
| Extended distribution of the rash (percentage) | 4 of 5 (80) | 23 of 40 (57) | 18 of 21 (86) | 14 of 32 (44) | 22 of 26 (85) | 37 of 72 (51) |
| Median time of birth in weeks (range) | 26 (6 to 37.5) | 40 (37 to 42) | 33 (11 to 36) | 38 (37 to 41) | 32.5 (6 to 37.5) | 39 (37 to 42) |
| Median weight at time of birth in grams (range) | 2,350 (848 to 2,454) | 3,320 (2,580 to 4,282) | 2,800 (1,900 to 3,200) | 3,300 (2,640 to 3,900) | 2,650 (848 to 3,200) | 3,305 (2,580 to 4,282) |
| Median length at time of birth in centimeters (range) | 47 (46 to 48) | 50 (48 to 55) | – | – | – | – |
The examination of the patient medical records revealed that in 16 out of 58 patients blood had been drawn for anti‐HHV‐6 titer. Of these 16 patients, 15 had slightly to moderately elevated IgG antibodies to HHV‐6 and one patient also had borderline elevated IgM antibodies. Five women in our study had an unfavorable pregnancy outcome (patients 1, 2, 7, 10, and 11), two of them (patient 1, 10) had a miscarriage. HHV‐6 laboratory findings were available in three of these five patients (1, 2, and 10, all with anti‐HHV‐6 titers of IgG 1 : < 64, and IgM negative). Patient 1 had had a cold for four weeks before being diagnosed with PR at her first consultation at a special outpatient clinic for pregnancy dermatoses. Laboratory tests initially revealed a slightly elevated CRP level, but normal CRP levels on repeat testing. This patient had a missed abortion three weeks later. She delivered by caesarean section two and four years later. Apart from gestational hypertension in the first pregnancy, these two pregnancies were unremarkable. Patient 2 had had a normal pregnancy two years before the onset of PR. However, the second pregnancy (that followed PR onset) ended in a spontaneous abortion due to cervical insufficiency. No abnormal laboratory findings were detected in patient 7. The pregnancy of patient 10 ended in a missed abortion in week 6. She had underlying Hashimoto thyroiditis and had four more pregnancies after being diagnosed with PR, one of which was also a missed abortion. Patient 11 had received blood circulation‐enhancing therapy after medical ENT consultation for blast trauma, but had otherwise no risk factors for an unfavorable pregnancy outcome.
Pooled data from previous studies
Pooled data from the 53 pregnant women identified by our literature search of previous studies were analyzed. The median age was 29 years in those with an unfavorable outcome (n = 21) and 29.5 years in those with a favorable outcome (n = 32). The median time to PR onset was significantly earlier (15 vs. 24 weeks of pregnancy; P = 0.002) and the rash lasted longer (median of 8 vs. 5 weeks; P = 0.014) in those with an unfavorable outcome. Additional extracutaneous symptoms occurred significantly more often in those with an unfavorable outcome than in those with a favorable outcome (55 % [11/20] vs. 19 % [6/31]; P = 0.008). Extended rash distribution occurred significantly more often in women with an unfavorable outcome than in those with a favorable outcome (86 % [18/21] vs. 44 % [14/32]; P = 0.003). The median time of birth was earlier (33 vs. 38 weeks) and the median birth weight was lower (2,800 g vs. 3,300 g) in women with an unfavorable outcome.
Case series analysis
To compare unfavorable and favorable outcomes in the combined cohorts from this study and from previously published studies, all cases with complete birth data from this and previously published papers [7, 23, 24, 26–29] were subjected to a case series analysis according to the statistical criteria defined in Methods. Of note, unfavorable pregnancy outcomes were significantly less frequent in our cohort than in the pooled cohort obtained from previously published studies (10.9 % vs. 39.6 %; P = 0.0012). In our case series analysis, 99 women were enrolled (46 from this study and 53 from previous studies). The characteristics of the two cohorts are summarized in Table 2.
In our case series analysis of the combined cohorts, the newborns in the unfavorable‐outcome group had a shorter median time of birth (32.5 vs. 39 weeks), lower median weight at birth (2,650 g vs. 3,305 g), and shorter median length at birth (47 cm vs. 50 cm) than did the favorable‐outcome group. Risk analysis revealed that the week of pregnancy at PR onset was inversely associated with an unfavorable outcome (OR = 0.937; 95 % CI 0.883 to 0.993; P = 0.03) (Figure 1). The median onset of PR in the case series analysis occurred earlier in the unfavorable‐outcome group (14.5 vs. 19 weeks) (Table 2). Duration of PR (OR = 1.432; 95 % CI 1.129 to 1.827; P = 0.003), additional extracutaneous symptoms (OR = 4.112; 95 % CI 1.580 to 10.23; P = 0.003), and extended rash distribution (OR = 5.203; 95 % CI 1.702 to 14.89; P = 0.004) were significantly associated with an unfavorable outcome (Figure 1). The PR rash lasted longer in women with an unfavorable outcome (median, 7 vs. 5 weeks). Additional extracutaneous symptoms were seen more often in women with unfavorable outcomes (58 % [14/24] vs. 25 % [18/71]). In addition, the rash was more often spread over a larger area of the body (than limited to the trunk) in women with unfavorable outcomes (85 % [22/26] vs. 51 % [37/72]). The median mother’s age at PR onset was 29 years in both groups. Primiparous women were equally distributed between the unfavorable and favorable outcome groups (50 % [13/26] vs. 47 % [34/72]). All data from the case series analysis are reported in online supplementary Tables S1 and S2 and online supplementary Figures S1 and S2.
Figure 1.
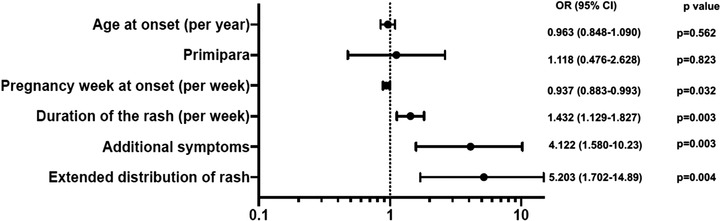
Odds ratio for unfavorable outcome (case‐series and literature).
Discussion
This study and the pertinent case series analysis revealed that early onset, extensive spread, long rash duration, and presence of additional extracutaneous symptoms were directly associated with an unfavorable pregnancy outcome in women with PR. Of note, the ORs for women from our study were similar to those observed in previous studies, but did not reach statistical significance. This may be due to the relatively low number of unfavorable pregnancy outcomes in the women seen at our clinic. Moreover, in comparing our study’s data with data from previously published studies, we noted that women seen at our clinic more often had PR in the first trimester of pregnancy (54 % [25/46] vs. 29 % [15/52]). Despite this clear difference, the patients of our study still had a lower rate of complications than those in previous studies [23, 24].
However, a limiting factor in those previous studies was that they did not primarily investigate the effect of PR on pregnancy [30], but rather the intrauterine transmission of HHV‐6/7 in women in whom PR developed during pregnancy [24]. Furthermore, investigators in those previous studies did not document who had made the primary clinical diagnosis of PR or whether the clinical diagnosis excluded differential diagnoses. Skin rashes, which can present the clinical picture of PR, are common in pregnancy. Frequently, these are atopic eruption of pregnancy (AEP) or polymorphic eruption of pregnancy (PEP) that are relatively harmless pregnancy dermatoses and pose no risk to the fetus [31, 32]. If additional symptoms such as fever, headache or oropharyngeal enanthema occur, these can also indicate infectious diseases, and any infectious illness that elicits eruptions in pregnant patients may be a risk factor for fetal loss [21]. Drago et al. reported that all eight miscarrying women in their study had atypical PR (skin lesions unusually widespread and long duration with constitutional symptoms associated) but none carried HHV‐6/7 IMG antibodies [24]. In an effort to exclude differential diagnoses as reliably as possible, we diagnosed and documented PR in most of the patients during consultation in our special outpatient clinic for pregnancy dermatoses. However, from a statistical point of view, the overall good pregnancy outcome in our study population was a limiting factor for risk estimation itself. Therefore, to identify and define potential risk factors, we pooled our data with the data from previous studies and conducted our case series analysis.
This case series analysis revealed that extensive spread of the PR rash was seen more often in women with unfavorable outcomes than in those with favorable outcomes (85 % [22/26] vs. 51 % [37/72]). In previous studies, PR lesions involving > 50 % of the body area had been described in 37 % of women with pregnancy or neonatal complications and in only 3 % of women with lower body area involvement [25]. Women with unfavorable outcomes also had longer‐lasting rashes than did those with favorable outcomes (median, 7 vs. 5 weeks). This finding compares well with a duration of six to seven weeks for patients with PR in general [3]. Fifteen of 44 women in our study population (34 %) and 17 of 51 (33 %) in previously published studies [7, 23, 24, 26–29] showed additional extracutaneous symptoms shortly before and/or simultaneously with the PR rash. In studies of nonpregnant women with PR, extracutaneous symptoms have been described in up to 69 % of 613 cases examined [3, 33]. It may be that PR rashes that are widespread, longer lasting, and accompanied by extracutaneous symptoms are the result of higher viral loads, stronger immune responses, or both. These three clinical characteristics of PR may thus represent important prognostic factors for pregnancy outcomes. Many studies suggest that human HHV‐6 is likely to play a role in PR. It is interesting that HHV‐6 can also be chromosomally integrated (iciHHV‐6) and be genetically transmitted from mother to child. One study in pregnant women with iciHHV‐6 showed an increased risk of spontaneous abortion [34]. Intriguingly, 15 of 16 (93 %) tested patients of our study had a slightly to moderately elevated anti‐HHV‐6 IgG antibody titer.
As outlined in previous reports, the abortion rates in pregnant women with PR is not substantially different from those in pregnant women in general [35, 36]. Indeed, with the two abortions in our study population (4 %, 2/46), the overall abortion rate was within the range of that for women under 35 years of age in whom fetal cardiac activity was detected between gestational weeks 10 and 12 [37].
Limitation
Because the literature concerning PR during pregnancy is very limited, the comparability of our findings with those of other studies is restricted. Apart from case studies [7, 26–29], the only other studies with adequate numbers of patients are those by Drago and colleagues [23, 24] published in 2008 and 2014. The main restriction of this study, however, is its retrospective character and the limited number of PR cases.
Conclusions
In most of the cases (74 %, 73/99) included in this case series analysis, PR did not influence pregnancy outcome. Indeed, the total abortion rate of 13 % in pregnant women with PR in previous studies [35, 38] is within the range of that of the general population or even lower as evidenced by analysis of our study data (Table 1). However, if PR starts early in pregnancy, is long‐lasting, spreads widely, and is accompanied by extracutaneous symptoms, the risk of an unfavorable outcome may increase. Consequently, close gynecological monitoring of pregnant women with PR is recommended.
Previous publications or related manuscripts
The content of the manuscript is partially presented in the diploma thesis of Lena Wenger‐Oehn, which was written as part of her studies in human medicine at the Medical University of Graz. Otherwise, the paper has not been published previously or submitted for publication elsewhere and will not be submitted for publication elsewhere before a decision is reached concerning publication in the JDDG.
IRB approval status: 30–405 ex 17/18
Conflict of interest
None.
Acknowledgment
The authors thank the women for participating in this study. They are also very grateful to Honnavara N. Ananthaswamy, Houston, Texas, for critical reading of the manuscript and Jude Richard, Austin, Texas, for the editing of the manuscript. This work has been conducted as part of a diploma thesis (LW‐O).
References
- 1. Chuang TY, Ilstrup DM, Perry HO, Kurland LT. Pityriasis rosea in Rochester, Minnesota, 1969 to 1978. J Am Acad Dermatol 1982; 7(1): 80–9. [DOI] [PubMed] [Google Scholar]
- 2. Sharma L, Srivastava K. Clinicoepidemiological study of pityriasis rosea. Indian J Dermatol Venereol Leprol 2008; 74(6): 647–9. [DOI] [PubMed] [Google Scholar]
- 3. Drago F, Ciccarese G, Rebora A et al. Pityriasis rosea: a comprehensive classification. Dermatology 2016; 232(4): 431–7. [DOI] [PubMed] [Google Scholar]
- 4. Stulberg DL, Wolfrey J. Pityriasis rosea. Am Fam Physician 2004; 69(1): 87–91. [PubMed] [Google Scholar]
- 5. Bianca S, Ingegnosi C, Ciancio B et al. Pityriasis rosea in pregnancy. Reprod Toxicol 2007; 24(3–4): 277–8. [DOI] [PubMed] [Google Scholar]
- 6. Chuh AA. Rash orientation in pityriasis rosea: a qualitative study. Eur J Dermatol 2002; 12(3): 253–6. [PubMed] [Google Scholar]
- 7. Alame MM, Chamsy DJ, Zaraket H. Pityriasis rosea‐like eruption associated with ondansetron use in pregnancy. Br J Clin Pharmacol 2018; 84(5): 1077–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheong WK, Wong KS. An epidemiological study of pityriasis rosea in Middle Road Hospital. Singapore Med J 1989; 30(1): 60–2. [PubMed] [Google Scholar]
- 9. Miller TH. Pityriasis rosea: report of three cases in one family, with clinical variations in two of them. Arch Derm Syphilol 1941; 44(1): 66–8. [Google Scholar]
- 10. Messenger AG, Knox EG, Summerly R et al. Case clustering in pityriasis rosea: support for role of an infective agent. Br Med J (Clin Res Ed) 1982; 284(6313): 371–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chuh AA, Lee A, Molinari N. Case clustering in pityriasis rosea: a multicenter epidemiologic study in primary care settings in Hong Kong. Arch Dermatol 2003; 139(4): 489–93. [DOI] [PubMed] [Google Scholar]
- 12. Chuh AAT, Molinari N, Sciallis G et al. Temporal case clustering in pityriasis rosea: a regression analysis on 1379 patients in Minnesota, Kuwait, and Diyarbakir, Turkey. Arch Dermatol 2005; 141(6): 767–71. [DOI] [PubMed] [Google Scholar]
- 13. Cameron D, Jones IG. Case clustering in pityriasis rosea: support for role of an infective agent. Br Med J (Clin Res Ed) 1982; 284(6323): 1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chuang TY, Perry HO, Ilstrup DM, Kurland LT. Recent upper respiratory tract infection and pityriasis rosea: a case‐control study of 249 matched pairs. Br J Dermatol 1983; 108(5): 587–91. [DOI] [PubMed] [Google Scholar]
- 15. Traore A, Korsaga‐Some N, Niamba P et al. [Pityriasis rosea in secondary schools in Ouagadougou, Burkina Faso]. Ann Dermatol Venereol 2001; 128(5): 605–9. [PubMed] [Google Scholar]
- 16. Parsons JM. Pityriasis rosea update: 1986. J Am Acad Dermatol 1986; 15(2 Pt 1): 159–67. [DOI] [PubMed] [Google Scholar]
- 17. Chuh A, Chan H, Zawar V. Pityriasis rosea: evidence for and against an infectious aetiology. Epidemiol Infect 2004; 132(3): 381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El‐Ela MA, Shaarawy E, El‐Komy M et al. Is there a link between human herpesvirus infection and toll‐like receptors in the pathogenesis of pityriasis rosea? a case‐control study. Acta Dermatovenerol Croat 2016; 24(4): 282–7. [PubMed] [Google Scholar]
- 19. Rebora A, Drago F, Broccolo F. Pityriasis rosea and herpesviruses: facts and controversies. Clin Dermatol 2010; 28(5): 497–501. [DOI] [PubMed] [Google Scholar]
- 20. Broccolo F, Drago F, Careddu AM et al. Additional evidence that pityriasis rosea is associated with reactivation of human herpesvirus‐6 and ‐7. J Invest Dermatol 2005; 124(6): 1234–40. [DOI] [PubMed] [Google Scholar]
- 21. Rebora A, Ciccarese G, Herzum A et al. Pityriasis rosea and other infectious eruptions during pregnancy: possible life‐threatening health conditions for the fetus. Clin Dermatol 2020; 38(1): 105–12. [DOI] [PubMed] [Google Scholar]
- 22. Corson EF, Luscombe HA. Coincidence of pityriasis rosea with pregnancy. AMA Arch Derm Syphilol 1950; 62(4): 562–4. [DOI] [PubMed] [Google Scholar]
- 23. Drago F, Broccolo F, Zaccaria E et al. Pregnancy outcome in patients with pityriasis rosea. J Am Acad Dermatol 2008; 58(5 Suppl 1): 78–83. [DOI] [PubMed] [Google Scholar]
- 24. Drago F, Broccolo F, Javor S et al. Evidence of human herpesvirus‐6 and ‐7 reactivation in miscarrying women with pityriasis rosea. J Am Acad Dermatol 2014; 71(1): 198–9. [DOI] [PubMed] [Google Scholar]
- 25. Drago F, Ciccarese G, Herzum A et al. Pityriasis rosea during pregnancy: major and minor alarming signs. Dermatology 2018; 234(1–2): 31–6. [DOI] [PubMed] [Google Scholar]
- 26. Chuh AA, Lee A, Chan PK. Pityriasis rosea in pregnancy: specific diagnostic implications and management considerations. Aust N Z J Obstet Gynaecol 2005; 45(3): 252–3. [DOI] [PubMed] [Google Scholar]
- 27. Cruz MJ, Baudrier T, Azevedo F. Atypical pityriasis rosea in a pregnant woman: first report associating local herpes simplex virus 2 reactivation. J Dermatol 2012; 39(5): 490–2. [DOI] [PubMed] [Google Scholar]
- 28. Overton RW. Pityriasis rosea in pregnancy: a case report. J Iowa Med Soc 1968; 58(12): 1239–40. [PubMed] [Google Scholar]
- 29. Loh TY, Cohen PR. Pityriasis rosea in pregnancy: report of a spousal occurrence and craniosynostosis in the healthy newborn. Dermatol Pract Concept 2016; 6(3): 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rebora A, Ciccarese G, Herzum A et al. Pityriasis rosea and other infectious eruptions during pregnancy: Possible life‐threatening health conditions for the fetus. Clin Dermatol 2020; 38(1): 105–12. [DOI] [PubMed] [Google Scholar]
- 31. Ambros‐Rudolph CM, Müllegger RR, Vaughan‐Jones SA et al. The specific dermatoses of pregnancy revisited and reclassified: Results of a retrospective two‐center study on 505 pregnant patients. J Am Acad Dermatol 2006; 54(3): 395–404. [DOI] [PubMed] [Google Scholar]
- 32. Sachdeva S. The dermatoses of pregnancy. Indian J Dermatol 2008; 53(3): 103–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sharma PK, Yadav TP, Gautam RK et al. Erythromycin in pityriasis rosea: a double‐blind, placebo‐controlled clinical trial. J Am Acad Dermatol 2000; 42(2 Pt 1): 241–4. [DOI] [PubMed] [Google Scholar]
- 34. Miura H, Kawamura Y, Ohye T et al. Inherited chromosomally integrated human herpesvirus 6 is a risk factor for spontaneous abortion. J Infect Dis 2021; 223: 1717–23. [DOI] [PubMed] [Google Scholar]
- 35. Monastirli A, Pasmatzi E, Badavanis G, Tsambaos D. Gestational pityriasis rosea: Suggestions for approaching affected pregnant women. Acta Dermatovenerol Croat 2016; 24(4): 312–3. [PubMed] [Google Scholar]
- 36. Stashower J, Bruch K, Mosby A et al. Pregnancy complications associated with pityriasis rosea: A multicenter retrospective study. J Am Acad Dermatol 2021; v85: 1648–9. [DOI] [PubMed] [Google Scholar]
- 37. Berle P, Weiss E. Rate of spontaneous abortion in relation to the time of fetal viability assessment. Geburtshilfe Frauenheilkd 1990; 50(12): 959–63. [DOI] [PubMed] [Google Scholar]
- 38. Fölster‐Holst R. Infectious exanthemas in childhood. J Dtsch Dermatol Ges 2020; 18(10): 1128–55. [DOI] [PubMed] [Google Scholar]


