Abstract
Lorlatinib is a third‐generation, brain‐penetrant anaplastic lymphoma kinase (ALK) and c‐ros oncogene 1 (ROS1) tyrosine kinase inhibitor (TKI) with robust intracranial activity in patients with ALK‐ or ROS1‐positive non‐small cell lung cancer (NSCLC). Data from the ongoing open‐label, single‐arm, multicenter, phase‐1/2 study of lorlatinib in patients with metastatic ALK‐ or ROS1‐positive NSCLC were used to further investigate the potential brain penetration of lorlatinib. Patients received escalating lorlatinib doses (10–200 mg once daily or 35–100 mg twice daily) or the approved dosing (100 mg daily). Plasma was collected from all patients, and cerebrospinal fluid (CSF) was collected at baseline and during the study from 5 patients with suspected or confirmed leptomeningeal carcinomatosis or carcinomatous meningitis. For those 5 patients, lorlatinib concentrations ranged from 2.64 to 125 ng/mL in the CSF and from 12.7 to 457 ng/mL in the plasma; free plasma concentrations ranged from 4.318 to 155.385 ng/mL. The CSF/free plasma ratio was 0.77 (R2 = 0.96 and P < .001). Using a post‐hoc population pharmacokinetic model, the average steady‐state unbound plasma concentration of lorlatinib was derived and the CSF concentration was estimated for all patients. Known minimum efficacy concentrations (Ceff) for wild‐type and mutated (L1196M and G1202R) ALK were used to derive central nervous system (CNS) Ceff. Estimated CNS concentrations exceeded the derived CNS Ceff values in all patients for wild‐type ALK and the ALK L1196M mutation, and in 35.8% of patients for the ALK G1202R mutation. Projected lorlatinib CNS concentrations were consistent with the high intracranial response rates reported in clinical trials and provide further evidence of the potent CNS penetration of lorlatinib.
Keywords: central nervous system, clinical pharmacology, clinical research, clinical trials, oncology
Anaplastic lymphoma kinase (ALK)‐positive and c‐ros oncogene 1 (ROS1)‐positive non‐small cell lung cancers (NSCLCs) have been shown to be sensitive to targeted tyrosine kinase inhibitors (TKIs) such as the first‐generation ALK/ROS1 TKI crizotinib and the additional second‐generation ALK TKIs ceritinib and alectinib. 1 , 2 , 3 , 4 , 5 , 6 These earlier generation ALK TKIs demonstrated strong clinical potency against the ALK‐rearranged oncogene in the lung. However, these ALK TKIs demonstrated a relatively low intracranial response rate as first‐line and later treatment, 7 probably because of the relatively low intracranial concentrations achieved by these drugs. The ratio of the concentration of alectinib in cerebral spinal fluid (CSF) and in plasma is reported to be between 0.002 and 0.005. 8 This ratio was also low for crizotinib (range: 0.0006–0.026). 9 , 10 , 11 The low levels of central nervous system (CNS) penetration from these early‐generation ALK TKIs likely made patients prone to disease progression, resulting from the eventual development of metastases of the CNS or through the acquisition of resistance to second‐generation TKIs. 12 Progression in this setting affects the longer‐term efficacy of treatments and survival, which prompted the development of lorlatinib, a third‐generation ALK and ROS1 TKI specifically designed to cross the blood–brain barrier (BBB) and exhibit potent CNS activity. 12 , 13 , 14
P‐glycoprotein 1 (P‐gp), also known as multidrug resistance protein 1 (MDR1), and breast cancer resistance protein (BCRP) are among the main efflux transporters involved in limiting drug penetration into the brain. 15 Results of nonclinical permeability studies in MDCKII‐MDR1 and MDCKII‐LE‐BCRP cells showed a lorlatinib efflux ratio of 1.8 and 1.32 with P‐gp and BCRP, respectively, after 90 minutes of incubation (unpublished data). Hence, lorlatinib was designed and optimized (from crizotinib) for increased potency against ALK mutants as well as for brain penetration to treat brain metastases (ie, specifically designed to not be a substrate of P‐gp).
Lorlatinib has shown potent overall and intracranial antitumor activity in a phase‐1/2 study of patients with advanced ALK‐ or ROS1‐positive NSCLC having progressed after previous TKIs or who were naïve to treatment (NCT01970865), 12 , 16 and in a phase‐3 study versus crizotinib in patients with ALK‐positive NSCLC who were naïve to treatment in the metastatic setting (NCT03052608). 17 In animal studies, lorlatinib has demonstrated the capability to cross the BBB, 18 , 19 and, in phase‐1/2 and phase‐3 studies, it has demonstrated robust intracranial efficacy. 12 , 17 , 20 , 21
Here, data from the phase‐1/2 study have been analyzed to further investigate the brain penetration potential of lorlatinib, through an evaluation of the relationship between free drug concentration in plasma and total drug concentration in CSF, in a subset of patients that was then modeled to predict CSF concentrations for the entire study population.
At diagnosis, brain metastases are present in around 25% of patients with ALK‐positive NSCLC and in nearly 60% of patients 3 years after diagnosis. 22 Higher frequencies of alterations in known cancer genes have been observed in brain metastases compared with primary lung adenocarcinoma. 23 Molecular characteristics of brain metastases that occur after treatment is initiated may differ from those of the primary tumor. Natural therapeutic selection may drive tumor heterogeneity. 24 With systemic therapy, drugs may achieve high potency in the systemic compartment, which can create selective pressure through the elimination of sensitive cellular clones with specific genomic and epigenetic alterations or microenvironmental features (in a specific stromal niche). However, these same drugs might achieve subpar exposure in the brain, 24 leading to a very different selective pressure on brain metastases. The differential selective pressure may contribute to the high level of heterogeneity between primary tumors and brain metastases. 25 This heterogeneity has been documented in both melanoma and breast cancer. 26 , 27
Lorlatinib has also demonstrated the broadest coverage of ALK resistance mutations among the ALK TKIs identified to date. 14 Here we utilized the estimated CSF/free plasma ratio determined from previously published CSF pharmacokinetic (PK) samples, as well as the predicted average concentrations from a previously published population PK model, to predict lorlatinib CSF concentrations for all patients receiving 100 mg lorlatinib once daily (QD) enrolled in this registrational study. The projected lorlatinib CSF levels were evaluated in relation to the unbound effective target concentration for the inhibition of wild‐type ALK and 2 key resistance mutations: L1196M, which is the most common resistance mutation to the first‐generation ALK TKI crizotinib 28 ; and G1202R, which has been associated with high‐level resistance to crizotinib and second‐generation ALK TKIs. 14 This analysis was undertaken with the goal of further exploring the use of lorlatinib in patients with NSCLC and brain metastases.
Methods
Study Design
The institutional review board or independent ethics committee at each participating center approved the protocol, which complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. All patients provided written, informed consent before participation.
The study design, objectives, and eligibility criteria for the lorlatinib phase‐1/2 study have been published previously. 12 , 16 , 29 In brief, this ongoing, open‐label, single‐arm, multicentered study enrolled patients with ALK‐positive or ROS1‐positive metastatic NSCLC. Patients (n = 54) enrolled in phase 1 received lorlatinib in escalating doses of 10–200 mg QD or 35–100 mg twice daily, whereas patients (n = 276) in phase 2 received the approved dose of lorlatinib 100 mg QD administered in continuous 21‐day cycles. 12 , 16
Evaluation of the CSF concentration of lorlatinib, where possible, was a prespecified exploratory protocol end point. In patients with suspected or confirmed leptomeningeal carcinomatosis, not visualized on magnetic resonance imaging, or with carcinomatous meningitis, CSF was to be collected at baseline and during the study. In those instances, CSF was also used to determine lorlatinib concentrations, and a blood sample for PK analysis was collected at approximately the same time as the postdose CSF sample. Plasma samples for lorlatinib PK were also collected from all other patients and analyzed according to methods previously published. 30
For ALK molecular profiling, the extraction and analysis of cell‐free DNA (cfDNA) was performed using a validated, commercially available 73‐gene cfDNA next‐generation sequencing (NGS) assay (Guardant360, panel version 2.10, bioinformatics pipeline version 3.0; Guardant Health, Inc., Redwood City, California), as previously described. 31 , 32
Statistical Analysis
For an evaluation of the relationship between unbound plasma concentrations and CSF concentrations of lorlatinib, a linear regression model via the generalized linear models function in R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) was applied. The slope for the plasma/CSF concentration curve that reflected the study population CSF to plasma ratio was then utilized to estimate the average steady‐state CSF concentration (CSFss,avg) for the entire study population of enrolled patients.
Post hoc population PK model estimates of average lorlatinib steady‐state plasma concentration (Css,avg) were used to derive the average steady‐state unbound plasma concentration (Css,avg,u) based on the known lorlatinib human protein binding (fraction unbound of 0.34). 30 , 33 , 34 The CSF concentration in each patient was then estimated as the product of plasma Css,avg,u and the study‐specific CSF to unbound plasma concentration ratio derived earlier by linear regression. The known minimum efficacy concentrations (Ceff) for wild‐type ALK and the L1196M and G1202R ALK resistance mutations were multiplied by the fraction of unbound value (0.34) to derive the effective Ceff in the CNS. 16 , 30 The projected CSF concentrations were compared with the CNS Ceff to evaluate the ratio of patients achieving coverage of these mutations. 16
Results
Patients
Among all enrolled patients in the lorlatinib phase‐1/2 study, 5 underwent lumbar puncture as part of their clinical treatment and lorlatinib concentrations were measured in the CSF. The demographics of the group of 5 patients with available CSF samples are summarized in Table S1. Four patients had ALK‐positive NSCLC and 1 had ROS1‐positive NSCLC. Four patients were assigned to the 100 mg QD dose and 1 patient was part of the 150 mg QD lorlatinib cohort.
Lorlatinib CSF and Plasma Concentrations
The concentrations of lorlatinib in the CSF and blood plasma from the 5 patients showed CSF concentrations ranging from 2.64 to 125 ng/mL and plasma concentrations ranging from 12.7 to 457 ng/mL. Free plasma concentrations ranged from 4.318 to 155.385 ng/mL. The CSF/free plasma ratios were relatively consistent, ranging from 0.6114 to 0.9574 (Table 1). Patient 1 had stopped taking lorlatinib 8 days before the lumbar puncture and patient 5 had stopped taking lorlatinib 2 days before the lumbar puncture. The ratio of CSF/free plasma concentration was considered reliable in these individuals and their data were included in the analysis.
Table 1.
Observed Clinical Lorlatinib CSF and Plasma Concentrations
| Patient | Assigned Dose Regimen (mg QD) | Dose on Date of CSF Collection (mg) | CSF Concentration (ng/mL) | Plasma Concentration (ng/mL) | Free (Unbound) Plasma Concentration (ng/mL) | CSF/Free Plasma Ratio |
|---|---|---|---|---|---|---|
| 1 | 150 | 0 | 2.64 a | 12.7 | 4.318 a | 0.6114 a |
| 2 | 100 | 100 | 125 a | 384 | 130.562 a | 0.9574 a |
| 3 | 100 | 100 | 101 a | 457 | 155.385 a | 0.65 a |
| 4 | 100 | 100 | 81.8 a | 311 | 105.739 a | 0.7736 a |
| 5 | 100 | 0 | 38.1 | 165 | 56.104 | 0.6791 |
CSF, cerebrospinal fluid; QD, once daily.
Note that patient 1 was on a drug holiday at the time of CSF collection, and patient 5 had discontinued lorlatinib 2 days before the collection of the CSF sample via lumbar puncture.
Data previously published. 16
CSF/Free Plasma Ratio Model
The regression analysis based on the sample of 5 patients with available CSF and estimated unbound plasma concentration data showed a CSF/free plasma concentration ratio of 0.77, with an adjusted R2 = 0.96 and P < .001 (Figure 1).
Figure 1.
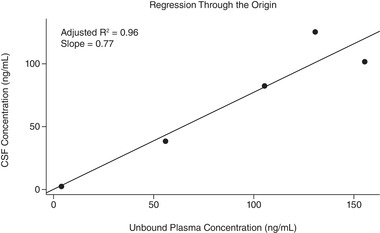
Linear regression analysis for the prediction of overall CSF/free (unbound) plasma ratio for patients with measured CSF concentrations of lorlatinib. CSF, cerebrospinal fluid.
Intracranial Response
Among the 5 patients with available CSF concentrations, the intracranial objective response reported for each was a complete response for patients 2, 3, and 4, stable disease for patient 1, and non‐evaluable response for patient 5.
Projected Wild‐Type ALK and Resistance Mutation Coverage
The analysis of coverage over wild‐type ALK and the 2 main ALK resistance mutations of interest, L1196M and G1202R, showed that the estimated CSFss,avg for lorlatinib would achieve CNS coverage for the inhibition of wild‐type ALK (unbound target concentration of 2.58 ng/mL) and the L1196M ALK resistance mutation (unbound target concentration of 21.08 ng/mL) in all patients enrolled in the study (Figures 2 and 3A). According to the projection from the model, the estimated CSFss,avg would exceed the target coverage for the G1202R ALK resistance mutation (unbound target concentration of 67.06 ng/mL) in 35.8% of all patients (Figure 2). Based on plasma cfDNA analysis, all patients with confirmed L1196M mutation and 26.3% of those with confirmed G1202R mutation had an estimated CSFss,avg for lorlatinib that would achieve CNS coverage for the inhibition of L1196M (Figure 3A) or G1202R (Figure 3B), respectively.
Figure 2.
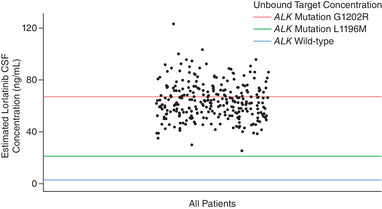
Coverage of estimated CSF Css,avg over ALK inhibition targets in patients receiving 100 mg QD lorlatinib dosing. The blue line indicates the unbound (free) target concentration for the inhibition of wild‐type ALK (2.58 ng/mL). The green line indicates the unbound (free) target concentration for the inhibition of L1196M (21.08 ng/mL). The red line indicates the unbound (free) target concentration for the inhibition of G1202R (67.06 ng/mL). 29 ALK, anaplastic lymphoma kinase; CSF, cerebrospinal fluid; Css,avg, average steady‐state plasma concentration; QD, once daily.
Figure 3.
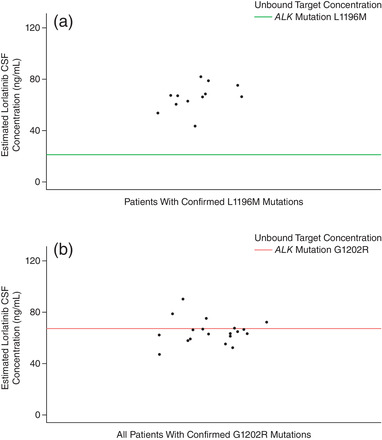
Coverage of estimated CSF Css,avg over ALK inhibition targets in patients receiving 100 mg QD lorlatinib dosing and a confirmed L1196M (a) or G1202R (b) mutation. The green line shows the unbound target concentrations for L1196M (21.08 ng/mL) inhibition. The red line shows the unbound target concentrations for G1202R (67.06 ng/mL) inhibition. 29 ALK, anaplastic lymphoma kinase; CSF, cerebrospinal fluid; Css,avg, average steady‐state plasma concentration; QD, once daily.
Discussion
In the current analysis, we observed a relationship between concentrations of lorlatinib in the CSF and plasma in patients with ALK‐ or ROS1‐positive NSCLC in this phase‐1/2 study. The projected ratio of CSF and free plasma lorlatinib concentration of 0.77 (R2 = 0.96 and P < .001) based on the regression model indicates potent brain penetration, with 77% of systemic unbound lorlatinib penetrating the brain (Figure 1). The robustness of the regression model warranted the extrapolation of the results to estimate the CSFss,avg for all remaining patients in the current analysis receiving lorlatinib doses of 100 mg QD (and with only plasma samples available). The CSF/free plasma ratios observed in this analysis (range: 0.61–0.96) were much higher than the CSF/plasma ratios observed with the first‐generation ALK/ROS1 TKI crizotinib (range: 0.0006–0.026) 9 , 10 , 11 and the second‐generation ALK TKI alectinib (0.002–0.005). 8 Lorlatinib PK at single dose and at steady state are largely dose proportional. 30 Lorlatinib has a plasma elimination half‐life of approximately 24 hours after a single dose. 34
One patient (patient 1) had low concentrations of lorlatinib in both the CSF and plasma because of a dosing holiday. The patient had been off their lorlatinib dose for 8 days before having an unplanned lumbar puncture. Despite this, because of a presumed concurrent drop of lorlatinib concentrations in both the CSF and the plasma, the patient had a CSF/free plasma ratio of around 0.6, which is consistent with the other patients in the analysis. Of note, another patient (patient 5) was off drug for 2 days before the unplanned lumbar puncture and had a CSF/free plasma ratio of around 0.7, also consistent with the other patients in the analysis.
Our projected lorlatinib concentration results are consistent with the high clinical intracranial response rates (intracranial objective response rate, IC‐ORR) observed in patients with CNS lesions at baseline receiving lorlatinib 100 mg QD in phase 2 of this phase‐1/2 trial who were either treated previously with ALK TKIs with/without chemotherapy (IC‐ORR 63.0%; 95% CI 51.5–73.4%) or naïve to treatment (IC‐ORR 66.7%; 95% CI 9.4–99.2%). 12
Further, our analysis showed that the estimated CNS concentrations exceed the minimum Ceff in all patients for both wild‐type ALK and the L1196M ALK resistance mutation, supporting broad coverage of both, and in 35.8% of patients for the G1202R ALK resistance mutation. This supports earlier findings highlighting the potent penetration of lorlatinib into the CNS as well as robust intracranial clinical responses. However, it should be noted that the mutation status of patients was determined in plasma and may not reflect the mutation status of brain metastases. In fact, brain metastases established before ALK treatment are likely to be harboring ALK fusion mutations only, whereas metastases that develop on treatment as part of a resistance mechanism are more likely to harbor additional mutations, including ALK kinase domain mutations. 35
There are several limitations to this analysis. First, this is an unplanned post hoc analysis of data from the clinical trial. Second, the analysis was limited by the low number of patients with available CSF samples, including 4 of 5 measurements that have been previously published; however, an analysis of alectinib has been reported with similar sample sizes (CSF samples from 5 patients), 8 , 36 and the high R2 value (0.96) suggests a robust CSF/free plasma ratio. Per protocol, patients with suspected or confirmed leptomeningeal carcinomatosis for whom a CSF draw was medically indicated were to provide a portion of the CSF for the measurement of lorlatinib; few patients in this large study actually progressed via the development of leptomeningeal carcinomatosis, and this is reflected by the measurement of lorlatinib from the CSF of 5 patients only. Third, the true shape of the (potentially nonlinear) relationship between CSF and plasma concentrations across a wide range of concentrations/doses is unknown. Despite the confirmation of a linear relationship for concentration/dose in the current analysis (100–150 mg QD), a less linear relationship could exist at higher doses. Fourth, the current analysis is based on plasma levels as the sole driver for lorlatinib CSF levels. Arguably, this assumption ignores the potential additional contributions from individual patient factors such as the expression of transporters at the BBB (P‐gp and BCRP), brain metastases tumor load, and other pharmacodynamic parameters. As previously mentioned, molecular profiling of the brain metastases was not performed, and the only available molecular information was derived from cfDNA analysis. Finally, this analysis assumes that the CNS Ceff, after correction for protein binding, is the same as the known systemic Ceff (ie, Ceff = CNS Ceff). This assumption also ignores the potential overexpression of numerous functional polymorphisms of multidrug resistance proteins, similar to the aforementioned point.
Conclusions
This analysis provides additional evidence for the ability of lorlatinib to markedly cross the BBB. This key property differentiates lorlatinib from the first‐generation ALK/ROS1 TKI crizotinib, which can cross the BBB but to a much lesser degree. 9 Alectinib also demonstrated intracranial activity both in first‐line and in pretreated settings, 3 , 36 , 37 , 38 but it has a lower reported CSF/plasma ratio than that demonstrated in the current analysis of lorlatinib. 8 The high intracranial response rate observed in clinical trials investigating lorlatinib is mirrored by the current results, demonstrating the highest CSF/plasma ratio among currently approved ALK TKIs. 12 , 17 , 20 , 21 This potent CNS penetration paired with the coverage of 2 key ALK resistance mutations (L1196M and G1202R) in a meaningful percentage of patients offers additional support for its current indication in patients with ALK‐positive NSCLC.
Conflicts of Interest
Y.K.P., J.‐F.M., and J.C. are full‐time employees of Pfizer Inc. and own stock or stock options in Pfizer. S.S. was a summer intern (contractor) at Pfizer Clinical Pharmacology at the time that this analysis was conducted and owns stock or stock options in Pfizer.
Data Sharing
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de‐identified participant data. For more information, see https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results.
Funding
This study was sponsored by Pfizer Inc.
Author Contributions
Y.K.P. and S.S. formed the design and conception of the study. J.‐F.M. collected and assembled the cfDNA data, Y.K.P. and J.C. collected and assembled the plasma and CSF concentration data, and S.S. conducted the R version 3.5.1 analysis. S.S., Y.K.P., J.‐F.M., and J.C. conducted the data analysis and interpretation, manuscript writing and reviewing, and final approval of the article, and are accountable for all aspects of the work.
Supporting information
Supplemental Information
Acknowledgments
We would like to thank Charity Henrich for providing helpful comments following a review of this analysis. Editorial and medical writing support was provided by Marius Dettmer, PhD, of CMC AFFINITY, McCann Health Medical Communications, and Ravi Subramanian, PhD, of ClinicalThinking, Inc., which were funded by Pfizer.
Prior presentation: These data have been presented previously in poster format at the American Society for Clinical Pharmacology and Therapeutics (ASCPT) 122nd Annual Meeting, 2021 (poster PII‐013).
Steven Sun was a summer intern (contractor) at Pfizer Clinical Pharmacology at the time that this analysis was conducted.
This study was sponsored by Pfizer Inc.
References
- 1. Soria J‐C, Tan DSW, Chiari R, et al. First‐line ceritinib versus platinum‐based chemotherapy in advanced ALK‐rearranged non‐small‐cell lung cancer (ASCEND‐4): a randomised, open‐label, phase 3 study. Lancet. 2017;389(10072):917‐929. [DOI] [PubMed] [Google Scholar]
- 2. Solomon BJ, Mok T, Kim D‐W, et al. First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med. 2014;371(23):2167‐2177. [DOI] [PubMed] [Google Scholar]
- 3. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK‐positive non‐small‐cell lung cancer. N Engl J Med. 2017;377(9):829‐838. [DOI] [PubMed] [Google Scholar]
- 4. Camidge DR, Kim HR, Ahn M‐J, et al. Brigatinib versus crizotinib in ALK‐positive non‐small‐cell lung cancer. N Engl J Med. 2018;379(21):2027‐2039. [DOI] [PubMed] [Google Scholar]
- 5. Shaw AT, Ou S‐H, Bang Y‐J, et al. Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med. 2014;371(21):1963‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1‐rearranged advanced non‐small‐cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019;30(7):1121‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petrelli F, Lazzari C, Ardito R, et al. Efficacy of ALK inhibitors on NSCLC brain metastases: a systematic review and pooled analysis of 21 studies. PLoS One. 2018;13(7):e0201425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Food and Drug Administration Center for Drug Evaluation and Research . Clinical Pharmacology and Biopharmaceutics Review: 208434Orig1s000. Food and Drug Administration Center for Drug Evaluation and Research; https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/208434Orig1s000ClinPharmR.pdf. Accessed September 14, 2021. [Google Scholar]
- 9. Okimoto T, Tsubata Y, Hotta T, et al. A low crizotinib concentration in the cerebrospinal fluid causes ineffective treatment of anaplastic lymphoma kinase‐positive non‐small cell lung cancer with carcinomatous meningitis. Intern Med. 2019;58(5):703‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443‐e445. [DOI] [PubMed] [Google Scholar]
- 11. Metro G, Lunardi G, Floridi P, et al. CSF concentration of crizotinib in two ALK‐positive non‐small‐cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol. 2015;10(5):e26‐e27. [DOI] [PubMed] [Google Scholar]
- 12. Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK‐positive non‐small‐cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654‐1667. [DOI] [PubMed] [Google Scholar]
- 13. Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK‐rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first‐ and second‐generation ALK inhibitors in ALK‐rearranged lung cancer. Cancer Discov. 2016;6(10):1118‐1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Urquhart BL, Kim RB. Blood‐brain barrier transporters and response to CNS‐active drugs. Eur J Clin Pharmacol. 2009;65(11):1063‐1070. [DOI] [PubMed] [Google Scholar]
- 16. Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non‐small‐cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open‐label, single‐arm first‐in‐man phase 1 trial. Lancet Oncol. 2017;18(12):1590‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shaw AT, Bauer TM, de Marinis F, et al. First‐line lorlatinib or crizotinib in advanced ALK‐positive lung cancer. N Engl J Med. 2020;383(21):2018‐2029. [DOI] [PubMed] [Google Scholar]
- 18. Collier TL, Maresca KP, Normandin MD, et al. Brain penetration of the ROS1/ALK inhibitor lorlatinib confirmed by PET. Mol Imaging. 2017;16:1536012117736669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collier TL, Normandin MD, Stephenson NA, et al. Synthesis and preliminary PET imaging of (11)C and (18)F isotopologues of the ROS1/ALK inhibitor lorlatinib. Nat Commun. 2017;8:15761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Felip E, Shaw AT, Bearz A, et al. Intracranial and extracranial efficacy of lorlatinib in patients with ALK‐positive non‐small‐cell lung cancer previously treated with second‐generation ALK TKIs. Ann Oncol. 2021;32(5):620‐630. [DOI] [PubMed] [Google Scholar]
- 21. Bauer TM, Shaw AT, Johnson ML, et al. Brain penetration of lorlatinib: cumulative incidences of CNS and non‐CNS progression with lorlatinib in patients with previously treated ALK‐positive non‐small‐cell lung cancer. Target Oncol. 2020;15(1):55‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR‐mutated or ALK‐rearranged non‐small‐cell lung cancers. Lung Cancer. 2015;88(1):108‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shih DJH, Nayyar N, Bihun I, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet. 2020;52(4):371‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ali S, Górska Z, Duchnowska R, Jassem J. Molecular profiles of brain metastases: a focus on heterogeneity. Cancers (Basel). 2021;13(11):2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. De Mattos‐Arruda L, Ng CKY, Piscuoglio S, et al. Genetic heterogeneity and actionable mutations in HER2‐positive primary breast cancers and their brain metastases. Oncotarget. 2018;9(29):20617‐20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fischer GM, Jalali A, Kircher DA, et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 2019;9(5):628‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kay M, Dehghanian F. Exploring the crizotinib resistance mechanism of NSCLC with the L1196M mutation using molecular dynamics simulation. J Mol Model. 2017;23(11):323. [DOI] [PubMed] [Google Scholar]
- 29. Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1‐positive non‐small‐cell lung cancer: a multicentre, open‐label, single‐arm, phase 1–2 trial. Lancet Oncol. 2019;20(12):1691‐1701. [DOI] [PubMed] [Google Scholar]
- 30. Chen J, O'Gorman MT, James LP, Klamerus KJ, Mugundu G, Pithavala YK. Pharmacokinetics of lorlatinib after single and multiple dosing in patients with anaplastic lymphoma kinase (ALK)‐positive non‐small cell lung cancer: results from a global phase I/II study. Clin Pharmacokinet. 2021;60(10):1313‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Odegaard JI, Vincent JJ, Mortimer S, et al. Validation of a plasma‐based comprehensive cancer genotyping assay utilizing orthogonal tissue‐ and plasma‐based methodologies. Clin Cancer Res. 2018;24(15):3539‐3549. [DOI] [PubMed] [Google Scholar]
- 32. Lanman RB, Mortimer SA, Zill OA, et al. Analytical and clinical validation of a digital sequencing panel for quantitative, highly accurate evaluation of cell‐free circulating tumor DNA. PLoS One. 2015;10(10):e0140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen J, Houk B, Pithavala YK, Ruiz‐Garcia A. Population pharmacokinetic model with time‐varying clearance for lorlatinib using pooled data from patients with non‐small cell lung cancer and healthy participants. CPT Pharmacometrics Syst Pharmacol. 2021;10(2):148‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pfizer Inc . LORBRENA® (lorlatinib): Prescribing Information. Pfizer Inc.; 2021. http://labeling.pfizer.com/ShowLabeling.aspx?id=11140. Accessed August 10, 2021. [Google Scholar]
- 35. Tabbò F, Reale ML, Bironzo P, Scagliotti GV. Resistance to anaplastic lymphoma kinase inhibitors: knowing the enemy is half the battle won. Transl Lung Cancer Res. 2020;9(6):2545‐2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib‐resistant ALK‐rearranged non‐small‐cell lung cancer (AF‐002JG): results from the dose‐finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119‐1128. [DOI] [PubMed] [Google Scholar]
- 37. Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK‐positive non‐small‐cell lung cancer (J‐ALEX): an open‐label, randomised phase 3 trial. Lancet. 2017;390(10089):29‐39. [DOI] [PubMed] [Google Scholar]
- 38. Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib‐refractory ALK‐rearranged non‐small‐cell lung cancer: a phase II global study. J Clin Oncol. 2016;34(7):661‐668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Information


