Abstract
In the context of ACTH‐dependent Cushing's syndrome, ectopic ACTH secretion from a neuroendocrine tumour is not uncommon, and needs to be carefully differentiated from pituitary‐dependent Cushing's syndrome, Cushing's disease, in order to optimise therapy. Some cases may be quite obvious, while in others the diagnosis may be difficult to confirm and the source of ACTH problematic, as many clinical and biochemical tests may overlap with Cushing's disease. Imaging is essential, but needs to be interpreted in the light of both anatomical as well as functional imaging modalities. In this review we summarise some of the main diagnostic problems, and emphasise the multimodal and interdisciplinary nature of the diagnostic pathways.
Keywords: ACTH, carcinoid, Cushing's, diagnosis, ectopic, neuroendocrine tumour
1. INTRODUCTION
The ectopic ACTH syndrome (EAS) remains one of the most challenging differential diagnoses in endocrinology. In the past, patients with the EAS typically presented with the abrupt onset of the clinical features associated with severe hypercortisolism with aggressive malignancies, such as small cell lung cancer (SCLC); however, probably due to improved imaging techniques, the spectrum of tumours associated with the EAS has been extended, and well‐differentiated lung neuroendocrine tumours (NETs), or carcinoid tumours, are now considered the most frequent cause. 1 Discriminating the EAS from Cushing's disease is now especially challenging, as ACTH‐secreting lung NETs can often be small and difficult to detect, with biochemical features similar or even identical to that of pituitary corticotroph tumours. Moreover, patients with ACTH‐secreting NETs often present with the gradual onset of classical symptoms and signs of Cushing's syndrome, indistinguishable from Cushing's disease. It is therefore essential to undertake a meticulous assessment to differentiate the EAS from Cushing's disease, incorporating clinical factors, dynamic biochemical tests, (frequently) inferior petrosal sinus sampling and multimodal imaging, with an astute appreciation of the caveats and pitfalls of each. Of course, it is vital to confirm the presence of Cushing's syndrome initially, firstly to distinguish it from so‐called pseudo‐Cushing's syndrome, and then to confirm its ACTH‐dependence (see Balomenaki et al: Diagnostic workup for Cushing's syndrome, in this supplement). This article is part of a special issue on the “Update of Cushing's Syndrome: 100 years after Minnie G”.
1.1. Prevalence and nonpituitary source of ACTH
The EAS is rare. It is reported in approximately 1%–5% of patients with SCLC, 2 , 3 3% of patients with lung or pancreatic NETs (excluding MEN1) 4 and 0.7% of patients with medullary thyroid cancer (MTC), 5 but may occur in up to 25% of thymic NETs 6 , 7 (including MEN1). Contemporary studies suggest that more cases of EAS are being identified, potentially due to increased awareness. 8 However, when looking at ACTH‐dependent Cushing's syndrome overall, some 10%–20% of such patients may harbour an ectopic source. 9 , 10 The prevalence of EAS in patients with ACTH‐dependent Cushing's syndrome has likely increased over the years due to vastly improved imaging techniques, and it may also be found to be more common in patients with NETs if more detailed endocrine studies were performed.
The most frequent site of ectopic ACTH is the lung. The largest published collection of series, with 383 EAS patients, suggests that lung NETs are the most common tumour (>25%) with a roughly equal prevalence of typical and atypical histopathology, followed by SCLC (~20%). 1 The next most common tumours are thymic (11%) and pancreatic NETs (8%), medullary thyroid cancer (6%) and phaeochromocytomas (5%). However, up to 20% of ACTH‐secreting tumours remain undiscovered, 9 , 10 , 11 being referred to as “occult” despite repeated evaluation, although progressively smaller numbers are reported in recent studies due to more sophisticated imaging techniques and, probably, longer follow‐up intervals. 11 The lung nevertheless remains the major occult source where tumours are eventually demonstrated, although this may take many years to become apparent. 9
1.2. Epidemiological and clinical features of the EAS
The epidemiological features that can help discriminate between the EAS and Cushing's disease include age and gender. The age of onset is normally higher in the EAS, with a mean age of onset varying between 38 and 50 years, compared to 30–40 years in Cushing's disease. 10 , 12 , 13 , 14 The gender ratio also differs, with EAS occurring only slightly more often in women (female‐to‐male ratio 1:1 to 2:1) compared to the marked female predominance in adult Cushing's disease (female‐to‐male ratio 3–5:1). 9 , 10 , 14 , 15 , 16 , 17
The clinical features of the EAS are particularly heterogeneous, and are influenced by the malignant potential of the underlying tumour and the severity of the hypercortisolism. While patients with the EAS, especially associated with SCLC, tend to have higher ACTH and cortisol levels, there remains significant overlap between the EAS and Cushing's disease cohorts. 9 , 10 It should also be noted that while disease activity in Cushing's disease can be fluctuant, this may also be observed with the EAS. 18 , 19 Accordingly, the clinical presentation ranges from the abrupt onset of the signs and symptoms of severe hypercortisolism, including hyperpigmentation, weight loss and mineralocorticoid effects (i.e., hypertension, hypokalaemia and peripheral oedema) often associated with aggressive malignancies like SCLC, to the gradual and slow onset of the classical signs and symptoms of Cushing's syndrome. These may be indistinguishable from the presentation of Cushing's disease, which is typically seen with well‐differentiated NETs. 9 , 10
Both lung NETs and pituitary corticotroph tumours are often small and difficult to detect. 9 , 20 ACTH‐secreting lung NETs, or carcinoid tumourlets, have been reported as small as 2–3 mm. 9 , 14 Furthermore, up to 10% of the population will have incidental non‐secreting pituitary corticotroph tumours demonstrated on magnetic resonance imaging (MRI), and pituitary imaging should be interpreted with caution. 21 This problem is even more complex with the increasing power of new MRI machines of higher resolution. It is therefore essential to rely on biochemical testing to direct the imaging to the appropriate site.
1.3. Dynamic noninvasive tests
After confirming endogenous hypercortisolism (overnight dexamethasone suppression testing or low‐dose dexamethasone suppression testing, urinary free cortisol and midnight serum or especially salivary cortisol assessments) and a readily detectable plasma ACTH (usually above 20–30 ng/l), the next challenge is to determine the source (Figure 1).
FIGURE 1.
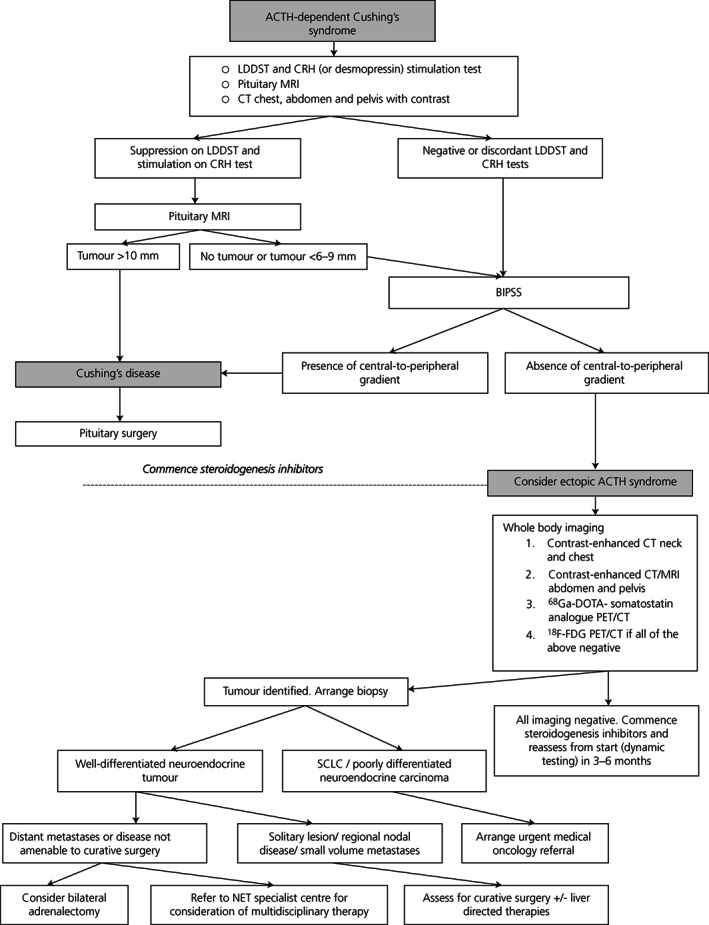
Establishing the cause of ACTH‐dependent Cushing's syndrome and management (adapted from 22 ). Abbreviations: ACTH, adrenocorticotropic hormone; LDDST, low dose dexamethasone suppression test; CRH, corticotropin releasing hormone; MRI, magnetic resonance imaging; BIPSS, bilateral inferior petrosal sinus sampling; CT, computed tomography; SCLC, small cell lung cancer
The rationale for most biochemical tests is that the majority of pituitary corticotroph tumours retain glucocorticoid receptors with the ability to inhibit ACTH secretion when exposed to high‐dose dexamethasone, as well as vasopressin V2 and V1b (V3) receptors and CRH receptors, whilst ectopic ACTH‐secreting tumours typically do not. 23 , 24 , 25 Hence, increased plasma ACTH and cortisol levels after CRH or desmopressin administration usually indicates Cushing's disease. 25 Nevertheless, this distinction is far from absolute, especially for small lung NETs, which can occasionally express some or all of these receptors and thus lead to false positive results. 4 , 9 , 10 , 26
The most commonly used dynamic tests to discriminate patients with EAS from Cushing's disease are the CRH stimulation test or the desmopressin stimulation test, either alone or in combination with CRH, although the desmopressin test has not been as useful as originally proposed. 27 The high‐dose (8 mg) dexamethasone suppression test (HDDST) is still used in some centres but is considered to have relatively low diagnostic accuracy, 25 and has the associated risks of the administration of corticosteroids in a patient already exposed to excessive levels.
In the CRH stimulation test, recombinant human or ovine‐sequence CRH is given as an intravenous bolus of either 1 μg/kg or, more typically, 100 μg. An ACTH rise of at least 35% (sensitivity 93%, specificity 100%) and a serum cortisol rise at least 20% (sensitivity 91%, specificity 88%) above baseline values is considered indicative of Cushing's disease when ovine CRH is used, 28 and at least a 105% ACTH increase (sensitivity 70%, specificity 100%) and a 14% cortisol increase (85% sensitivity; 100% specificity) when human CRH is used. 29 However, diagnostic errors (false positives and false negatives) occur in 7%–15% of patients even if the best discriminating criteria are applied, with most errors being false negative results in Cushing's disease patients who fail to respond. 30 , 31 , 32 , 33 Therefore, the CRH stimulation test on its own does not consistently discriminate between Cushing's disease and the EAS, although responses to either the CRH stimulation test or the desmopressin stimulation test clearly shift the probability in favour of Cushing's disease. 27 , 34 , 35
Desmopressin can stimulate ACTH secretion from a high proportion of corticotroph adenomas via activation of vasopressin receptors (V2, V1b). 27 , 34 However, because many ectopic ACTH‐secreting tumours also express these receptors, the desmopressin test has somewhat less utility than CRH in the differential diagnosis of ACTH‐dependent Cushing's syndrome, 27 , 36 even when combined with CRH. 37 Furthermore, up to 20% of those with Cushing's disease do not respond. 38
Whilst each test on its own may have limited diagnostic accuracy, the combination of the results of HDDST and CRH testing were shown to have a greater discriminatory capacity. 39 , 40 In one study of 245 patients with ACTH‐dependent Cushing's syndrome, the combination of cortisol suppression on HDDST and stimulation on CRH had a sensitivity of 95% and a specificity of 93% to detect Cushing's disease. 41 Furthermore, when using optimal criteria, the combination of the results of the low‐dose dexamethasone suppression test (LDDST) and CRH test had similar diagnostic accuracy (sensitivity 94%, specificity 97%) compared with the combination of the HDDST and CRH tests, so it can be argued that the HDDST provides little additional information. 41 Nevertheless, up to 25% of EAS patients may have discordant dynamic test results, 9 , 10 and tumour and epidemiological factors including age, sex and severity of hypercortisolism, can all impact on the results. 25
1.4. Invasive testing
Most clinicians will diagnose Cushing's disease if a patient with ACTH‐dependent Cushing's syndrome has concordant results on the dexamethasone and CRH and/or desmopressin test suggestive of Cushing's disease, and the demonstration of a focal lesion of 10 mm or more on pituitary MRI. 25 , 42 There is a recent consensus that pituitary tumours <6 mm should have bilateral inferior petrosal sinus sampling (BIPSS) while tumours ≥10 mm do not require BIPSS. 25 Expert opinions differ regarding tumours 6–9 mm, although the majority would recommend BIPSS in this setting. In addition, up to 40% of patients with proven Cushing's disease will have a negative MRI, 43 and BIPSS is also particularly suitable for this cohort. However, CT scanning of chest, abdomen and pelvis is a simple and readily‐available imaging technique which will rapidly diagnose any gross lesion, and may direct further investigation to an ectopic source even in the absence of BIPSS: it should probably be offered to all patients with confirmed ACTH‐dependent Cushing's syndrome, similar to MRI of the pituitary. 11 , 35
BIPSS, in experienced hands, is the gold standard test to identify a pituitary versus ectopic source of ACTH with a sensitivity and specificity of approximately 95%. 25 , 42 , 44 In a recent meta‐analysis of 20 retrospective and three prospective studies including 1,642 patients, BIPSS was shown to have a sensitivity of 94% and specificity of 89%, although 17% of studies did not use CRH or desmopressin stimulation. 45 This is discussed in more detail in another review in this issue. A pituitary source is identified by a central‐to‐peripheral ACTH gradient of more than two at baseline and more than three following CRH or desmopressin administration. 42 However, false negative, 46 , 47 and rare false positive results have been described. 48 , 49 False positive responses can occur in EAS patients with mild hypercortisolism, 48 , 50 and some centres recommend documentation of a two‐fold or greater increase in urinary free cortisol for 6–8 weeks prior to BIPSS to ensure suppression of normal corticotrophs. We would simply check the serum cortisol within 24 h of the test to ensure it remained elevated. Treatment with cortisol‐lowering agents may also theoretically cause desuppression of normal corticotrophs and subsequent responsivity to CRH or desmopressin. 48 Other rare causes of false positive results in EAS patients include those with cyclical hypercortisolism or in CRH‐producing tumours which may induce corticotroph hyperplasia. 42 , 51 False negative results can occur in the setting of poor catheter placement, petrosal sinus hypoplasia or anomalous venous drainage, or low peak inferior petrosal sinus ACTH levels. 49 , 52 , 53 Measurement of prolactin (to normalise ACTH values) in cases without a gradient can confirm adequacy of sampling 54 , 55 although prolactin‐corrected ACTH values may threaten the sensitivity and specificity of the test, and a recent study found the measurement of prolactin unhelpful and confusing 56 ; it is best used in select cases. In addition, in patients with confirmed Cushing's disease, a high rate of false negative responses has been reported in patients with low peak inferior petrosal sinus ACTH values (<400 ng/l before or after CRH), and so in the setting of an absent central‐to‐peripheral gradient, low inferior petrosal sinus ACTH values should be interpreted with caution. 53 There is little evidence that sampling from the cavernous sinuses is advantageous, and is technically challenging.
1.5. Biomarkers
Calcitonin and plasma or urinary metanephrines have been shown to be the only biomarkers of specific diagnostic value in the EAS 9 , 10 and they should be performed in all patients to exclude MTC or phaeochromocytoma, respectively. Interestingly, serum calcitonin is the most frequently elevated tumour marker in the EAS regardless of tumour type, and is elevated in 44%–69% of EAS cases including MTC, NETs including gastrinomas, phaeochromocytoma, and occult tumours. 9 , 10 , 57 Fasting plasma gut hormones, most commonly gastrin, may be elevated in functioning pancreatic NETs, although in a recent series only one of nine ACTH‐secreting islet cell tumours cosecreted gastrin. 4 A useful discriminative test in the future may include the measurement of ACTH precursors. Ectopic ACTH‐secreting tumours typically do not process POMC efficiently, leading to increased prevalence of ACTH precursors in the circulation. 58 A higher POMC or pro‐ACTH to ACTH ratio has been found in the EAS compared with Cushing's disease. 58
1.6. Immunohistochemistry
Tumour immunostaining for ACTH can be negative in up to 30% of ACTH‐secreting tumours 4 , 10 and cannot be used to retrospectively validate biochemical tests. Whilst this finding has been attributed to the high secretory capacity of some tumours, negative ACTH immunostaining has also been reported in occult tumours with modest hormone secretion. 59 Other explanations for negative immunostaining include the presence of ACTH precursors which do not react with the antibodies used, poor fixation during immunohistochemistry or, of course, that the identified tumour has been wrongly assumed to be the ectopic source. 4 , 59
1.7. Imaging and tumour localisation
Whilst aggressive malignancies are often identified rapidly, lung NETs can be more difficult to localise due to their small size and usual location in the middle third of the lung adjacent to pulmonary vasculature, from which they cannot readily be differentiated on computed tomography (CT) or MRI. 60 Lung NETs, particularly “typical” carcinoids, can also often be solitary, and identification is critical to permit surgery with curative intent and to avoid unnecessary adrenalectomy.
The introduction of molecular imaging has greatly increased our capacity to diagnose NETs, including those that cause the EAS (Figure 2). 61 In one of the largest systematic reviews of 231 EAS cases (with only small numbers of SCLC), approximately half of EAS sources were readily identified on cross‐sectional imaging, while extensive investigations were needed to discover “covert” cases in up to 30%. 11 In such covert cases, nuclear medicine imaging, including 111In‐pentetreotide scintigraphy and especially 68Ga‐DOTA‐somatostatin analogue (SSA) PET/CT, identified 80% of tumours not seen on conventional imaging. In this review, 19% of ACTH‐secreting tumours remained occult despite intensive investigation; however, in nine patients with “covert” disease who underwent 68Ga‐DOTA‐SSA PET/CT, all had tumours identified, suggesting its potential superiority over all other imaging techniques in which a significant number of tumours remained occult. Of the entire cohort, they reported a sensitivity of 82% (18/22) for 68Ga‐DOTA‐SSA PET/CT compared to 66% (137/202) for conventional CT; however, for covert cases this changed to a sensitivity of 100% (9/9) for 68Ga‐DOTA‐SSA PET/CT and 44% (24/55) for CT, although the sample size of covert cases who underwent 68Ga‐DOTA‐SSA PET was small. 11 In another systematic review of 69 EAS cases (49% with lung NETs, 10% with thymic NETs), including 10 with occult disease, a per‐lesion analysis was performed. 62 They documented 57 68Ga‐DOTA‐SSA PET positive lesions and 32 68Ga‐DOTA‐SSA PET negative lesions (including occult cases), resulting in an overall sensitivity of 64%; however, this is difficult to compare with the per‐patient analysis above. Of the 10 cases they considered to remain “occult”, 68Ga‐DOTA‐SSA PET identified some ‐ but not all ‐ lesions in two patients. Furthermore, one patient who initially had a negative 68Ga‐DOTA‐SSA PET had tumours which became avid after treatment with ketoconazole 62 : it is well known that some NETs may not express somatostatin receptor subtype‐2 in the presence of high circulating glucocorticoids, but these may be visualised when such levels are lowered medically. 63
FIGURE 2.
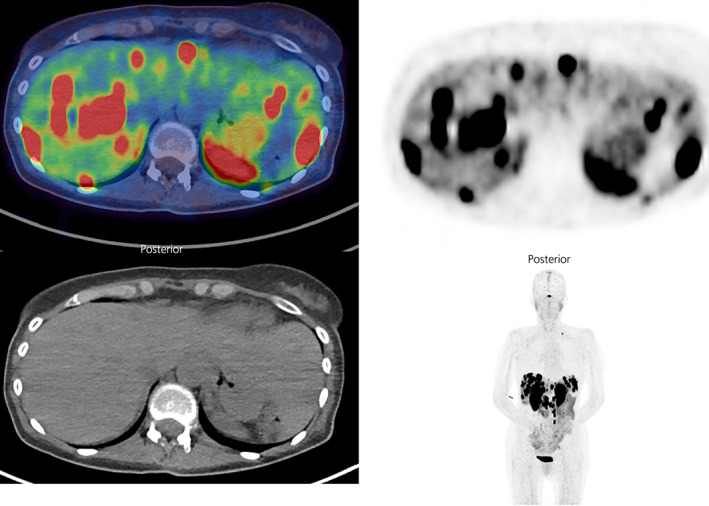
68Ga‐DOTATATE PET/CT showing intensely avid hepatic metastases and abdominal and left supraclavicular nodal metastases in a 55 year old woman with a well‐differentiated, grade 2 (Ki‐67 8%), pancreatic neuroendocrine tumour (primary tumour previously resected) and ectopic ACTH syndrome (images courtesy of Dr Shaunak Navalkissoor, Department of Nuclear Medicine, Royal Free Hospital)
There is a role for 111In‐pentetreotide scintigraphy which is considered a specific test (92%–100%) to detect NETs but is considerably less sensitive (60%–80%) compared with 68Ga‐DOTA‐SSA PET (88%–93%), 61 and its use may be to confirm abnormalities detected on CT or MRI.
There may also be a role for 18F‐FDG PET; some studies have shown that in EAS cases that remain occult after 68Ga‐DOTA‐SSA PET, the next best imaging to identify ACTH‐secreting tumours is 18F‐FDG PET. 11 18F‐FDG PET has been traditionally reserved for high grade (grade 3) NET or poorly‐differentiated neuroendocrine carcinomas (NEC), which tend to have higher glycolytic metabolism and less somatostatin expression. The sensitivity of 18F‐FDG PET to detect high grade NET or NEC is >90%; however, it is still quite substantial (40%–60%) in low grade (grade 1 or grade 2) NETs. 64 , 65 Furthermore, some EAS series have demonstrated a high proportion of patients with grade 2 and grade 3 NETs, and this may justify the use of 18F‐FDG PET/CT in EAS patients with occult disease and negative somatostatin receptor imaging. 4 , 14
Apart from patients with “typical” carcinoid tumours of the lung, more than half of patients with ACTH‐secreting NETs present with metastatic disease. 14 Complete staging incorporating a multimodal approach, is therefore critical to determine appropriate candidates for surgery (Figure 1). CT imaging for liver metastases and pancreatic NETs is inferior to that of MRI, and dynamic contrast‐enhanced MRI is therefore the preferred modality for imaging of the pancreas and liver. 61 68Ga‐DOTA‐SSA PET/CT provides high sensitivity (88%–93%) and specificity (88%–95%) for the diagnosis of NETs and is considered imperative for their complete staging and to direct management 61 (Figure 2). It is more specific than conventional imaging in well‐differentiated NETs, and will often diagnose lymph node, bone and peritoneal metastases not characterised on CT or MRI. 61 , 66 As a theranostic, it also indicates the potential efficacy of peptide receptor radionuclide therapy (PRRT) in the setting of considerable tumour uptake. 14 , 61
2. CONCLUSIONS
While the ectopic ACTH syndrome is relatively uncommon in NETs in general, it remains problematic in the context of a patient diagnosed with ACTH‐dependent Cushing's syndrome. Originally considered to be a very rare differential diagnosis, it is now known to occur in a sizeable minority of such patients, even in childhood, and may cause not insignificant diagnostic problems, less a “needle in a haystack” and more “hiding in plain sight.” It contributes considerable excess morbidity and mortality in a cancer patient population. When the clinical, metabolic and biochemical alterations are severe and the tumour readily detectable on cross‐sectional imaging, such “overt” tumours may be rapidly identified and treated. However, not infrequently the biochemical and clinical features markedly overlap with Cushing's disease, the tumour may not be immediately obvious, and detailed molecular imaging as well as bilateral inferior petrosal sinus sampling are required to locate such “covert” tumours. And if all else fails and the tumour remains “occult”, then one should consider “curing” the Cushing's syndrome with bilateral adrenalectomy. In conclusion, the diagnosis of the ectopic ACTH syndrome remains difficult in many cases, with the necessity of consideration of the probabilistic nature of diagnosis, while fine clinical judgement is as ever essential.
This review is an updated and revised version of our recently published review. 22
This article is part of an update series on the diagnosis and treatment of Cushing's syndrome. 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83
AUTHOR CONTRIBUTIONS
Aimee R Hayes: conceptualization; writing ‐ original draft; writing ‐ review and editing. Ashley B. Grossman: conceptualization, supervision, writing ‐ review and editing.
CONFLICT OF INTEREST
The authors maintain they have no conflicts of interest for this review. No external funding or grants were employed.
Hayes AR, Grossman AB. Distinguishing Cushing's disease from the ectopic ACTH syndrome: Needles in a haystack or hiding in plain sight? J Neuroendocrinol. 2022;34(8):e13137. doi: 10.1111/jne.13137
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Isidori AM, Lenzi A. Ectopic ACTH syndrome. Arq Bras Endocrinol Metabol. 2007;51(8):1217‐1225. [DOI] [PubMed] [Google Scholar]
- 2. Limper AH, Carpenter PC, Scheithauer B, Staats BA. The Cushing syndrome induced by bronchial carcinoid tumors. Ann Intern Med. 1992;117(3):209‐214. [DOI] [PubMed] [Google Scholar]
- 3. Delisle L, Boyer MJ, Warr D, et al. Ectopic corticotropin syndrome and small‐cell carcinoma of the lung: clinical features, outcome, and complications. Arch Intern Med. 1993;153(6):746‐752. [PubMed] [Google Scholar]
- 4. Kamp K, Alwani RA, Korpershoek E, Franssen GJH, De Herder WW, Feelders RA. Prevalence and clinical features of the ectopic ACTH syndrome in patients with gastroenteropancreatic and thoracic neuroendocrine tumors. Eur J Endocrinol. 2016;174(3):271‐280. [DOI] [PubMed] [Google Scholar]
- 5. Barbosa SL‐S, Rodien P, Leboulleux S, et al. Ectopic adrenocorticotropic hormone‐syndrome in medullary carcinoma of the thyroid: a retrospective analysis and review of the literature. Thyroid. 2005;15(6):618‐623. [DOI] [PubMed] [Google Scholar]
- 6. Filosso PL, Yao X, Ahmad U, et al. Outcome of primary neuroendocrine tumors of the thymus: a joint analysis of the international Thymic malignancy interest group and the European Society of Thoracic Surgeons databases. J Thorac Cardiovasc Surg. 2015;149(1):103‐109.e2. doi: 10.1016/j.jtcvs.2014.08.061 [DOI] [PubMed] [Google Scholar]
- 7. Soga J, Yakuwa Y, Osaka M. Evaluation of 342 cases of mediastinal/thymic carcinoids collected from literature: a comparative study between typical carcinoids and atypical varieties. Ann Thorac Cardiovasc Surg. 1999;5(5):285‐292. [PubMed] [Google Scholar]
- 8. Wengander S, Trimpou P, Papakokkinou E, Ragnarsson O. The incidence of endogenous Cushing's syndrome in the modern era. Clin Endocrinol (Oxf). 2019;91(2):263‐270. doi: 10.1111/cen.14014 [DOI] [PubMed] [Google Scholar]
- 9. Ilias I, Torpy DJ, Pacak K, Mullen N, Wesley RA, Nieman LK. Cushing's syndrome due to ectopic corticotropin secretion: twenty years' experience at the national institutes of health. J Clin Endocrinol Metab. 2005;90(8):4955‐4962. [DOI] [PubMed] [Google Scholar]
- 10. Isidori AM, Kaltsas GA, Pozza C, et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long‐term follow‐up. J Clin Endocrinol Metab. 2006;91(2):371‐377. [DOI] [PubMed] [Google Scholar]
- 11. Isidori AM, Sbardella E, Zatelli MC, et al. Conventional and nuclear medicine imaging in ectopic Cushing's syndrome: a systematic review. J Clin Endocrinol Metab. 2015;100(9):3231‐3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Beuschlein F, Hammer GD. Ectopic pro‐opiomelanocortin syndrome. Endocrinol Metab Clin North Am. 2002;31(1):191‐234. [DOI] [PubMed] [Google Scholar]
- 13. Salgado LR, Fragoso MCBV, Knoepfelmacher M, et al. Ectopic ACTH syndrome: our experience with 25 cases. Eur J Endocrinol. 2006;155(5):725‐733. [DOI] [PubMed] [Google Scholar]
- 14. Davi MV, Cosaro E, Piacentini S, et al. Prognostic factors in ectopic Cushing's syndrome due to neuroendocrine tumors: a multicenter study. Eur J Endocrinol. 2017;176(4):453‐461. doi: 10.1530/EJE-16-0809 [DOI] [PubMed] [Google Scholar]
- 15. Steffensen C, Bak AM, Rubeck KZ, Jørgensen JOL. Epidemiology of Cushing's syndrome. Neuroendocrinology. 2010;92(Suppl. 1):1‐5. [DOI] [PubMed] [Google Scholar]
- 16. Ejaz S, Vassilopoulou‐Sellin R, Busaidy NL, et al. Cushing syndrome secondary to ectopic adrenocorticotropic hormone secretion. Cancer. 2011;117(19):4381‐4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Valassi E, Santos A, Yaneva M, et al. The European registry on Cushing's syndrome: 2‐year experience. Baseline demographic and clinical characteristics. Eur J Endocrinol. 2011;165(3):383‐392. [DOI] [PubMed] [Google Scholar]
- 18. Trott MJ, Farah G, Stokes VJ, Wang LM, Grossman AB. A thymic neuroendocrine tumour in a young female: a rare cause of relapsing and remitting Cushing's syndrome. Endocrinol Diabetes Metab Case Reports. 2016;2016(1):EDM160018. doi: 10.1530/EDM-16-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnaldi G, Mancini T, Kola B, et al. Cyclical Cushing's syndrome in a patient with a bronchial neuroendocrine tumor (typical carcinoid) expressing ghrelin and growth hormone Secretagogue receptors. J Clin Endocrinol Metab. 2003;88(12):5834‐5840. doi: 10.1210/jc.2003-030514 [DOI] [PubMed] [Google Scholar]
- 20. Woo YS, Isidori AM, Wat WZ, et al. Clinical and biochemical characteristics of adrenocorticotropin‐secreting macroadenomas. J Clin Endocrinol Metab. 2005;90(8):4963‐4969. [DOI] [PubMed] [Google Scholar]
- 21. Aron DC, Howlett TA. Pituitary incidentalomas. Endocrinol Metab Clin North Am. 2000;29(1):205‐221. [DOI] [PubMed] [Google Scholar]
- 22. Hayes AR, Grossman AB. The ectopic ACTH syndrome: Rarely easy, always challenging. Endocrinol Clin North Am. 2018;47:409‐425. [DOI] [PubMed] [Google Scholar]
- 23. Liddle GW, Nicholson WE, Island DP, Orth DN, Abe K, Lowder SC. Clinical and laboratory studies of ectopic humoral syndromes. Recent Prog Horm Res. 1969;25:283‐314. [DOI] [PubMed] [Google Scholar]
- 24. Liddle GW. Tests of pituitary‐adrenal suppressibility in the diagnosis of Cushing's syndrome. J Clin Endocrinol Metab. 1960;20(12):1539‐1560. [DOI] [PubMed] [Google Scholar]
- 25. Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing's disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847‐875. doi: 10.1016/s2213-8587(21)00235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strott CA, Nugent CA, Tyler FH. Cushing's syndrome caused by bronchial adenomas. Am J Med. 1968;44(1):97‐104. [DOI] [PubMed] [Google Scholar]
- 27. Tsagarakis S, Tsigos C, Vasiliou V, et al. The desmopressin and combined CRH‐desmopressin tests in the differential diagnosis of ACTH‐dependent Cushing's syndrome: constraints imposed by the expression of V2 vasopressin receptors in tumors with ectopic ACTH secretion. J Clin Endocrinol Metab. 2002;87(4):1646‐1653. [DOI] [PubMed] [Google Scholar]
- 28. Nieman LK, Oldfield EH, Wesley R, Chrousos GP, Loriaux DL, Cutler GB Jr. A simplified morning ovine corticotropin‐releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin‐dependent Cushing's syndrome. J Clin Endocrinol Metab. 1993;77(5):1308‐1312. [DOI] [PubMed] [Google Scholar]
- 29. Newell‐Price J, Morris DG, Drake WM, et al. Optimal response criteria for the human CRH test in the differential diagnosis of ACTH‐dependent Cushing's syndrome. J Clin Endocrinol Metab. 2002;87(4):1640‐1645. [DOI] [PubMed] [Google Scholar]
- 30. Kaye TB, Crapo L. The Cushing syndrome: an update on diagnostic tests. Ann Intern Med. 1990;112:434‐444. [DOI] [PubMed] [Google Scholar]
- 31. Nieman LK, Cutler GB Jr, Oldfield EH, Loriaux DL, Chrousos GP. The ovine corticotropin‐releasing hormone (CRH) stimulation test is superior to the human CRH stimulation test for the diagnosis of Cushing's disease. J Clin Endocrinol Metab. 1989;69(1):165‐169. [DOI] [PubMed] [Google Scholar]
- 32. Nakahara M, Shibasaki T, Shizume K, et al. Corticotropin‐releasing factor test in normal subjects and patients with hypothalamic‐pituitary‐adrenal disorders. J Clin Endocrinol Metab. 1983;57(5):963‐968. [DOI] [PubMed] [Google Scholar]
- 33. Chrousos GP, Schulte HM, Oldfield EH, Gold PW, Cutler GB Jr, Loriaux DL. The corticotropin‐releasing factor stimulation test: an aid in the evaluation of patients with Cushing's syndrome. N Engl J Med. 1984;310(10):622‐626. [DOI] [PubMed] [Google Scholar]
- 34. Luque RM, Ibáñez‐Costa A, López‐Sánchez LM, et al. A cellular and molecular basis for the selective desmopressin‐induced ACTH release in Cushing disease patients: key role of AVPR1b receptor and potential therapeutic implications. J Clin Endocrinol Metab. 2013;98(10):4160‐4169. [DOI] [PubMed] [Google Scholar]
- 35. Frete C, Corcuff JB, Kuhn E, et al. Non‐invasive diagnostic strategy in ACTH‐dependent cushing's syndrome. J Clin Endocrinol Metab. 2020;105(10):3273‐3284. doi: 10.1210/clinem/dgaa409 [DOI] [PubMed] [Google Scholar]
- 36. Newell‐Price J. The desmopressin test and Cushing's syndrome: current state of play. Clin Endocrinol (Oxf). 1997;47(2):173‐174. [PubMed] [Google Scholar]
- 37. Arlt W, Dahia PLM, Callies F, et al. Ectopic ACTH production by a bronchial carcinoid tumour responsive to desmopressin in vivo and in vitro. Clin Endocrinol (Oxf). 1997;47(5):623‐627. [DOI] [PubMed] [Google Scholar]
- 38. Vassiliadi DA, Tsagarakis S. The role of the desmopressin test in the diagnosis and follow‐up of Cushing's syndrome. Eur J Endocrinol. 2018;178(5):R201‐R214. doi: 10.1530/EJE-18-0007 [DOI] [PubMed] [Google Scholar]
- 39. Nieman LK, Chrousos GP, Oldfield EH, Avgerinos PC, Cutler GB Jr, Loriaux DL. The ovine corticotropin‐releasing hormone stimulation test and the dexamethasone suppression test in the differential diagnosis of Cushing's syndrome. Ann Intern Med. 1986;105(6):862‐867. [DOI] [PubMed] [Google Scholar]
- 40. Hermus A, Pesman G, Benraad T, Pieters G, Smals A, Kloppenborg P. The corticotropin‐releasing‐hormone test versus the high‐dose dexamethasone test in the differential diagnosis of Cushing's syndrome. Lancet. 1986;328(8506):540‐544. [DOI] [PubMed] [Google Scholar]
- 41. Isidori AM, Kaltsas GA, Mohammed S, et al. Discriminatory value of the low‐dose dexamethasone suppression test in establishing the diagnosis and differential diagnosis of Cushing's syndrome. J Clin Endocrinol Metab. 2003;88(11):5299‐5306. [DOI] [PubMed] [Google Scholar]
- 42. Newell‐Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet. 2006;367(9522):1605‐1617. [DOI] [PubMed] [Google Scholar]
- 43. Invitti C, Giraldi FP, De Martin M, Cavagnini F. Diagnosis and management of Cushing's syndrome: results of an Italian multicentre study. J Clin Endocrinol Metab. 1999;84(2):440‐448. [DOI] [PubMed] [Google Scholar]
- 44. Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88(12):5593‐5602. [DOI] [PubMed] [Google Scholar]
- 45. Wang H, Ba Y, Xing Q, Cai RC. Differential diagnostic value of bilateral inferior petrosal sinus sampling (BIPSS) in ACTH‐dependent Cushing syndrome: a systematic review and meta‐analysis. BMC Endocr Disord. 2020;20(1):1‐11. doi: 10.1186/s12902-020-00623-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kaltsas GA, Giannulis MG, Newell‐Price JDC, et al. A critical analysis of the value of simultaneous inferior petrosal sinus sampling in Cushing's disease and the occult ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1999;84(2):487‐492. [DOI] [PubMed] [Google Scholar]
- 47. Lopez J, Barcelo B, Lucas T, et al. Petrosal sinus sampling for diagnosis of Cushing's disease: evidence of false negative results. Clin Endocrinol (Oxf). 1996;45(2):147‐156. [DOI] [PubMed] [Google Scholar]
- 48. Yamamoto Y, Davis DH, Nippoldt TB, Young WF Jr, Huston J III, Parisi JE. False‐positive inferior petrosal sinus sampling in the diagnosis of Cushing's disease: report of two cases. J Neurosurg. 1995;83(6):1087‐1091. [DOI] [PubMed] [Google Scholar]
- 49. Swearingen B, Katznelson L, Miller K, et al. Diagnostic errors after inferior petrosal sinus sampling. J Clin Endocrinol Metab. 2004;89(8):3752‐3763. [DOI] [PubMed] [Google Scholar]
- 50. Findling JW, Raff H. Diagnosis and differential diagnosis of Cushing's syndrome. Endocrinol Metab Clin North Am. 2001;30(3):729‐747. [DOI] [PubMed] [Google Scholar]
- 51. Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015;386(9996):913‐927. [DOI] [PubMed] [Google Scholar]
- 52. Doppman JL, Chang R, Oldfield EH, Chrousos G, Stratakis CA, Nieman LK. The hypoplastic inferior petrosal sinus: a potential source of false‐negative results in petrosal sampling for Cushing's disease. J Clin Endocrinol Metab. 1999;84(2):533‐540. [DOI] [PubMed] [Google Scholar]
- 53. Wind JJ, Lonser RR, Nieman LK, DeVroom HL, Chang R, Oldfield EH. The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing's disease. J Clin Endocrinol Metab. 2013;98(6):2285‐2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Findling JW, Kehoe ME, Raff H. Identification of patients with Cushing's disease with negative pituitary adrenocorticotropin gradients during inferior petrosal sinus sampling: prolactin as an index of pituitary venous effluent. J Clin Endocrinol Metab. 2004;89(12):6005‐6009. [DOI] [PubMed] [Google Scholar]
- 55. Sharma ST, Raff H, Nieman LK. Prolactin as a marker of successful catheterization during IPSS in patients with ACTH‐dependent Cushing's syndrome. J Clin Endocrinol Metab. 2011;96(12):3687‐3694. doi: 10.1210/jc.2011-2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. De Sousa S, McCormack AI, McGrath S, Torpy DJ. Prolactin correction for adequacy of petrosal sinus cannulation may diminish diagnostic accuracy in Cushing's disease. Clin Endocrinol (Oxf). 2017;87:515‐522. doi: 10.1111/cen.13401 [DOI] [PubMed] [Google Scholar]
- 57. Howlett TA, Drury PL, Perry L, Doniach I, Rees LH, Besser GM. Diagnosis and management of ACTH‐dependent Cushing's syndrome: comparison of the features in ectopic and pituitary ACTH production. Clin Endocrinol (Oxf). 1986;24(6):699‐713. [DOI] [PubMed] [Google Scholar]
- 58. Oliver RL, Davis JRE, White A. Characterisation of ACTH related peptides in ectopic Cushing's syndrome. Pituitary. 2003;6(3):119‐126. [DOI] [PubMed] [Google Scholar]
- 59. Grossman AB, Kelly P, Rockall A, Bhattacharya S, McNicol A, Balwick T. Cushing's syndrome caused by an occult source: difficulties in diagnosis and management. Nat Rev Endocrinol. 2006;2(11):642‐647. [DOI] [PubMed] [Google Scholar]
- 60. Doppman JL, Pass HI, Nieman LK, et al. Detection of ACTH‐producing bronchial carcinoid tumors: MR imaging vs CT. AJR Am J Roentgenol. 1991;156(1):39‐43. [DOI] [PubMed] [Google Scholar]
- 61. Sundin A, Arnold R, Baudin E, et al. ENETS consensus guidelines for the standards of Care in Neuroendocrine Tumors: radiological, nuclear medicine and hybrid imaging. Neuroendocrinology. 2017;105(3):212‐244. doi: 10.1159/000471879 [DOI] [PubMed] [Google Scholar]
- 62. Varlamov E, Hinojosa‐Amaya JM, Stack M, Fleseriu M. Diagnostic utility of Gallium‐68‐somatostatin receptor PET/CT in ectopic ACTH‐secreting tumors: a systematic literature review and single‐center clinical experience. Pituitary. 2019;22(5):445‐455. doi: 10.1007/s11102-019-00972-w [DOI] [PubMed] [Google Scholar]
- 63. de Bruin C, Hofland LJ, Nieman LK, et al. Mifepristone effects on tumor somatostatin receptor expression in two patients with Cushing's syndrome due to ectopic adrenocorticotropin secretion. J Clin Endocrinol Metab. 2012;97(2):455‐462. doi: 10.1210/jc.2011-1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Carideo L, Prosperi D, Panzuto F, et al. Role of combined [68Ga]Ga‐DOTA‐SST analogues and [18F]FDG PET/CT in the management of GEP‐NENs: a systematic review. J Clin Med. 2019;8(7):1032. doi: 10.3390/jcm8071032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hayes AR, Furtado O'Mahony L, Quigley A‐M, et al. The combined interpretation of 68Ga‐DOTATATE PET/CT and 18F‐FDG PET/CT in metastatic Gastroenteropancreatic neuroendocrine tumors. Clin Nucl Med. 2022;47(1):26‐35. doi: 10.1097/rlu.0000000000003937 [DOI] [PubMed] [Google Scholar]
- 66. Norlén O, Montan H, Hellman P, Stålberg P, Sundin A. Preoperative 68Ga‐DOTA‐somatostatin analog‐PET/CT hybrid imaging increases detection rate of intra‐abdominal small intestinal neuroendocrine tumor lesions. World J Surg. 2018;42(2):498‐505. doi: 10.1007/s00268-017-4364-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Millar RP, Karavitaki N, Kastelan D. Cushing's syndrome update: 100 years after Minnie G. J Neuroendocrinol. 2022;34(8):e13167. doi: 10.1111/jne.13167 [DOI] [PubMed] [Google Scholar]
- 68. Simon J, Theodoropoulou M. Genetics of Cushing's disease. J Neuroendocrinol. 2022;34(8):e13148. doi: 10.1111/jne.13148 [DOI] [PubMed] [Google Scholar]
- 69. Salehidoost R, Korbonits M. Glucose and lipid metabolism abnormalities in Cushing's syndrome. J Neuroendocrinol. 2022;34(8):e13143. doi: 10.1111/jne.13143 [DOI] [PubMed] [Google Scholar]
- 70. Dekkers AJ, Amaya JM, van der Meulen M, Biermasz NR, Meijer OC, Pereira AM. Long‐term effects of glucocorticoid excess on the brain. J Neuroendocrinol. 2022;34(8):e13142. doi: 10.1111/jne.13142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Clayton RN. Cardiovascular complications of Cushings Syndrome: impact on morbidity and mortality. J Neuroendocrinol. 2022;34(8):e13175. doi: 10.1111/jne.13175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Honegger J, Nasi‐Kordhishti I. Surgery and perioperative management of patients with Cushing's disease. J Neuroendocrinol. 2022;34(8):e13177. doi: 10.1111/jne.13177 [DOI] [PubMed] [Google Scholar]
- 73. Lasolle H, Vasiljevic A, Jouanneau E, Ilie MD, Raverot G. Aggressive corticotroph tumors and carcinomas. J Neuroendocrinol. 2022;34(8):e13169. doi: 10.1111/jne.13169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guignat L, Bertherat J. Long‐term follow‐up and predictors of recurrence of Cushing's disease. J Neuroendocrinol. 2022;34(8):e13186. doi: 10.1111/jne.13186 [DOI] [PubMed] [Google Scholar]
- 75. Balomenaki M, Margaritopoulos D, Vassiliadi DA, Tsagarakis S. Diagnostic workup of Cushing's syndrome. J Neuroendocrinol. 2022;34(8):e13111. doi: 10.1111/jne.13111 [DOI] [PubMed] [Google Scholar]
- 76. Braun LT, Vogel F, Reincke M. Long‐term morbidity and mortality in patients with Cushing's syndrome. J Neuroendocrinol. 2022;34(8):e13113. doi: 10.1111/jne.13113 [DOI] [PubMed] [Google Scholar]
- 77. Valassi E. Clinical presentation and etiology of Cushing's syndrome: Data from ERCUSYN. J Neuroendocrinol. 2022;34(8):e13114. doi: 10.1111/jne.13114 [DOI] [PubMed] [Google Scholar]
- 78. Hamblin R, Coulden A, Fountas A, Karavitaki N. The diagnosis and management of Cushing's syndrome in pregnancy. J Neuroendocrinol. 2022;34(8):e13118. doi: 10.1111/jne.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Castinetti F. Medical management of Cushing's disease: When and how? J Neuroendocrinol. 2022;34(8):e13120. doi: 10.1111/jne.13120 [DOI] [PubMed] [Google Scholar]
- 80. Bonneville J‐F, Potorac I, Petrossians P, Tshibanda L, Beckers A. Pituitary MRI in Cushing's disease ‐ an update. J Neuroendocrinol. 2022;34(8):e13123. doi: 10.1111/jne.13123 [DOI] [PubMed] [Google Scholar]
- 81. Losa M, Albano L, Bailo M, Barzaghi LR, Mortini P. Role of radiosurgery in the treatment of Cushing's disease. J Neuroendocrinol. 2022;34(8):e13134. doi: 10.1111/jne.13134 [DOI] [PubMed] [Google Scholar]
- 82. Drouin J. The corticotroph cells from early development to tumorigenesis. J Neuroendocrinol. 2022;34(8):e13147. doi: 10.1111/jne.13147 [DOI] [PubMed] [Google Scholar]
- 83. Balasko A, Zibar Tomsic K, Kastelan D, Dusek T. Hypothalamic–pituitary–adrenal axis recovery after treatment of Cushing's syndrome. J Neuroendocrinol. 2022;34(8):e13172. doi: 10.1111/jne.13172 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


