Abstract
Although seaweeds exhibit many benefits as a food source, few studies have characterized their sensory attributes. An expert nine-member panel developed a vocabulary with 25 descriptors to describe the appearance, aroma, flavor, texture, and aftertaste of raw and cooked seaweeds consumed in Chile: Durvillaea antarctica, Pyropia spp., and Ulva lactuca. Subsequently, the vocabulary was used in a ranking descriptive analysis (RDA) to evaluate the sensory properties and relate them with physicochemical and physical data. Sensory attributes of the three seaweeds were very different from each other but similar between treatments (raw and cooked). Pyropia spp., both cooked and hydrated, had the highest glutamate content (310 and 324 mg (100 g) −1 d.w., respectively), and was perceived by the sensory panel as having the most umami taste. Cooked D. antarctica was perceived as sweeter, had more caramel notes than the hydrated seaweed and was sensed as cartilaginous and hard in accordance with its mechanical properties. Generalized Procrustes analysis revealed that D. antarctica exhibited most of the desirable descriptors, such as caramel, umami and marine aromas while U. lactuca was described as bitter and moldy. This primary vocabulary can assist food scientists and chefs in the development of seaweed products and dishes for the consumer market.
Keywords: Seaweeds, Sensory analysis, Texture profile analysis, Umami components, Generalized procrustes analysis
Introduction
Most seaweeds are novel foods in the Western world and have a great potential given their abundance, claimed nutritional and functional properties as healthy foods, and a tradition of uses in Oriental and Polynesian gastronomy. However, these positive attributes are not sufficient to attract consumers’ preferences (Prager 2020). In fact, the unique textures and flavors of seaweeds are unfamiliar to most people in the Western world, except for a few seaweed species and their local use in some traditional dishes.
Seaweeds are called “the vegetables of the sea” They are subject to environmental conditions different from plants, therefore, their chemical composition, morphology and structural properties are quite different from leafy terrestrial products. Botanists describe seaweeds as having a cartilaginous thallus and elastic fronds. Unfamiliar consumers perceive flavors of seaweeds as marine, iodized, slightly bitter and fishy (Figueroa et al. 2021). Research articles, magazine reviews and books as well as famous chefs, have promoted the consumption of seaweeds in Western countries (Mouritsen 2013; O’Connor 2017; Figueroa et al. 2021). One major hindrance to increase their gastronomic applications is the limited knowledge of the specific sensory properties deemed undesirable by consumers. In Chile, Durvillaea antarctica (“cochayuyo”) is by far the most consumed seaweed species. Other seaweeds used in traditional dishes are Pyropia spp. (ex Porphyra spp. "luche"), Ulva lactuca, Chondracanthus chamissoi and Callophyllis variegata (Aguilera 2021).
Key sensory descriptors (texture, aroma, flavor, aftertaste, etc.) of food products or dishes are expressed as words, terms or sentences associated with the human perception (Giboreau et al. 2007; Lawless and Civille 2013). They are widely used to identify, characterize, and compare sensory characteristics of foods, relate them to instrumental data, and inform and educate consumers on the gastronomic traits of novel foods (Suwonsichon 2019). A well-developed sensory vocabulary helps to conduct precise sensory analysis and the resulting descriptors become the universe of terms for untrained panelists and consumers alike (Hayakawa et al. 2010). Developing a sensory terminology is particularly relevant for novel and unfamiliar foods to better comprehend the sensory traits that may preclude or limit their consumption and how to overcome them (Yang and Lee 2019). Sensory quality is a major factor behind neophobia or the reluctance to eat new foods (Tan et al. 2017; Tuorila and Hartmann 2019; Yang et al. 2020).
The development of sensory lexicons requires trained panelists while sensory vocabularies pretend to describe in words the sensory characteristics of a food. For example, Talavera-Bianchi et al. (2010), Baker et al. (2014) and Chun et al. (2020) used six panelists to derive their lexicons for fresh leafy vegetables, caviar, and mushrooms, respectively. The degree of training of panelists also varies. Sharma et al. (2020) developed a lexicon for potato varieties with five expert panelists, while Sato et al. (2017) relied on untrained students to define sensory properties of the same tuber. Yang et al. (2020) used only four expert panelists to conceive a bilingual flavor lexicon for Sichuan pepper, but Galán-Soldevilla et al. (2005) trained eight subjects to develop a sensory vocabulary for the odor and flavor characteristics of floral honeys from Spain. Wu et al. (2017) in their panel for quinoa, used four habitual consumers and five others that had rarely consumed the food. Regarding seaweeds, Chapman et al. (2015), developed flavor sensory descriptors using fifteen assessors, none of which had a great experience with sensory profiling of seaweeds. Kato et al. (2015) utilized six skilled panelists to evaluate the appearance, taste, firmness, and stickiness of kombu softened by enzymatic treatments.
Well-defined and referenced descriptors provide valuable information on the sensory qualities of food products. Currently, scientific studies on edible macroalgae use several terms to describe their appearance, flavor and texture that are mostly adopted from other foods (Table 1).
Table 1.
Terms commonly used to describe sensory properties of seaweeds
| Seaweed | Appearance | Texture | Flavor | Reference |
|---|---|---|---|---|
| Ulva spp. |
Bright green Thin and transparent sheet |
Thin, cartilaginous, slightly plastic Roasted: crispy |
Fresh, slightly bitter, reminiscent of green and wild herbs | Pérez-Lloréns et al. 2017; Porto-Muiños 2021 |
| Durvillaea antarctica |
Fresh: green Dried: reddish brown |
Fleshy, elastic, and firm consistency. Crunchy, damp, and spongy | Intense taste of the sea; flavor of wild mushrooms | Mansilla et al. 2012; Pérez-Lloréns et al. 2017 |
| Macrocystis pyrifera | Olive green, with rough fronds along their length |
Fresh: slightly slimy Dried: crunchy |
Smooth taste of the sea | Mansilla et al. 2012 |
| Pyropia spp. |
Thin and transparent sheet Fresh: violet Toasted, cooked: green |
Fine and cartilaginous |
Dried: mushrooms Toasted, cooked: roasted sardines |
Pérez-Lloréns et al. 2017; Porto-Muiños 2021; Kreischer and Schuttelaar 2016 |
| Pyropia columbina | Greenish or pinkish brown | Fresh: elastic and slightly cartilaginous | Taste of the sea | Mansilla et al. 2012 |
| Callophyllis variegata | Intense red |
Fresh; cartilaginous Dried: crunchy |
Intense, with hints of crustacean | Mansilla et al. 2012 |
| Laminaria digitata | Dark olive green | Meaty and slightly cartilaginous | Iodised, lightly smoked, mild honey flavor, salty and seafood-like taste | Peinado et al. 2014; Pérez-Lloréns et al. 2017; Porto-Muiños 2021 |
| Chondrus crispus |
Small and ramified Fresh: red Cooked: green |
Cartilaginous | Crustacean flavor | Peinado et al. 2014; Pérez-Lloréns et al. 2017 |
| Undaria pinnatifida |
Elongated and wavy sheets Fresh: yellow to brown Cooked: green |
Fine, crispy, and somewhat meaty | Fishy, marine; reminds oysters, sweet | Kreischer and Schuttelaar 2016; Mouritsen et al. 2019; Porto-Muiños 2021 |
| Palmaria palmata |
Palm shape Deep red, brown or purple |
Cartilaginous, soft, and dissolves easily | Sweet and slightly iodised flavour; strong marine aroma | Kreischer and Schuttelaar 2016; Mouritsen et al. 2012; Pérez-Lloréns et al. 2017 |
| Saccharina japonica/ Saccharina lattisima |
Slimy Yellow green |
Fleshy and slightly cartilaginous | Mild taste of sea, sweet; intense umami, mushroom aroma | Mouritsen et al. 2019; Pérez-Lloréns et al. 2017 |
| Himanthalia elongata |
Narrow strips Fresh: brown Cooked: green |
Crunchy and fleshy | Soft, reminds a land vegetable | Porto-Muiños 2021 |
A traditionally used method to obtain detailed information on the sensory profile and quantitative data of attributes is the Quantitative Descriptive Analysis (QDA). To provide reliable and consistent results, QDA uses a sensory panel trained with benchmarks of the product and ingredient (Meilgaard et al. 2016). This method has some limitations, namely, it requires plenty of time to train the panelists and it may generate some inconsistencies typical of the intensity evaluation method (Richter et al. 2010). Alternatively, the Ranking Descriptive Analysis method (RDA) compares multiple samples with different intensities of a given attribute. It is a relatively simple method, where the evaluators can be consumers or panelists with different levels of training (Richter et al. 2010; Chizoti et al. 2018). In RDA the evaluators classify samples using an ordinal scale, for example from 1 to 10, that facilitates achieving a final consensus in the panel (Mamede and Benassi 2016). RDA generally produces good results with respect to sample discrimination and it is cheaper and requires fewer samples than QDA. However, a shortcoming of RDA is that it does not provide the magnitude of the differences between samples. To compare the results of panels based on individual ranking data and a measure of variance, the RDA uses Generalized Procrustes Analysis (GPA), a statistical method of analysis (Guerrero et al. 2001).
Texture is a major factor in the acceptability of fresh and processed seaweeds as foods (Birch et al. 2018). The texture of most seaweeds is often described as “leathery, fibrous, and sticky” by Western consumers, traits that are positively appreciated by the Japanese (Tanaka 1986). Flavor is another important factor in the acceptability of seaweeds. The taste of seaweeds is mainly due to sugars, polyols, free amino acids and nucleotides, and organic acids. Umami, due to the presence of L-glutamate, L-aspartate, and 5'-ribonucleotides such as inosinate and guanylate, is the taste most often associated with seaweeds (Mouritsen 2013; Figueroa et al. 2021). Volatile compounds are fundamental contributors to the aroma of seaweeds with halogenated compounds providing notes like marine, crustacean, and herbaceous (López-Pérez et al. 2017; Santos and Narendra 2018).
The aims of this research are: (i) to develop a vocabulary with adequate descriptors for Chilean seaweeds; (ii) to determine the sensory properties of these seaweeds; (iii) to characterize the seaweeds in terms of chemical and physical properties; and (iv) to relate the outcomes of the sensory panel evaluation with desirable characteristics of the three seaweeds.
Materials and methods
Materials
Dried samples of Durvillea antarctica, Pyropia spp., and Ulva lactuca were purchased from the commercial purveyor Kaiso Spa (Chile). Seaweeds were harvested in April 2021, sun-dried near Puerto Montt (approximately 41°N, 72°W) and are representative of products used for culinary uses in local dishes. After purchase, they were kept in sealed plastic bags at room temperature (approximately 20 °C) until used in rehydrated and cooked forms.
Proximate analysis
The proximate composition of seaweeds was determined in duplicate samples, according to methods described in AOAC (2012). Moisture was determined by the oven method at 105 °C (AOAC 934.01). Total protein was determined following the Kjeldahl procedure (N × 6.25) (AOAC 2000.11). Lipids were extracted with petroleum ether in a Soxhlet and determined gravimetrically (AOAC 991.36). The ash content was gravimetrically obtained after heating at 550 °C in a muffle furnace (AOAC 930.05). The total carbohydrate content in the samples was estimated by the anthrone method (Osborne and Voogt 1986). All results were expressed on a dry weight basis (d.w.). More details of the analytical methodologies are in Ortiz et al. (2006).
Lexicon development
Panel
Nine panelists were selected among individuals that actively participated in the development and tasting of seaweed products during the three previous years (Figueroa et al. 2021). All panel members (6 women and 3 men, all Chileans and aged between 25 and 74 y.o.) were food technologists with previous training in sensory evaluation and familiar with local dishes containing the three seaweeds.
Sample preparation
Due to the Covid-19 pandemic, development of the sensory vocabulary was performed with coded dry samples sent to the homes of seven panelists, along with detailed instructions of the hydration, cooking and tasting of samples. Two of the panelists living on the outskirts of Santiago de Chile and familiar with products and procedures bought similar dried seaweeds at local markets and followed the preparation instructions. All panelists were present in these online sessions. Portions of approximately 200 mL dry seaweed were hydrated in 1 L of water for 3 h and part of them were also cooked in water at 100 °C for 20 min (traditional method to cook seaweed).
Descriptor generation
Test sessions took place by videoconference. Panelists participated in four preliminary sessions led by a sensory evaluation expert (A.B.), each of approximately 90 min. In the first three sessions, the panelists tasted raw and cooked seaweed and described the samples in as many terms as possible regarding their appearance, aroma, taste, mouthfeel, texture, and residual sensation. In a fourth session, the descriptors were commented on, discussed, and redundant or irrelevant terms were eliminated, resulting in a consensus classification of 25 words or key terms for sensory descriptors.
Sensory evaluation
The sensory evaluation activity aimed at qualitatively assessing the seaweed samples according to the selected descriptors and took place in the premises of our laboratory in the Gastronomic Unit of the Pontificia Universidad Católica de Chile. This evaluation consisted of two sessions, one to verify that all panelists (present in both sessions) understood the descriptors and the evaluation method, and the other for sensory evaluation itself.
Sample preparation for sensory evaluation
The traditional way to prepare the seaweeds was adapted to avoid the loss of flavor compounds in the cooking water, thus to better evaluate the natural sensory characteristics. Samples were presented in two formats: raw-rehydrated and hydrated-cooked (six samples in total). The rehydrated samples were prepared and standardized as follows: D. antarctica, 1 g dry weight: 3 g water; Pyropia spp., 1 g dry weight: 2.5 g of water; and U. lactuca, 1 g dry weight: 2 g water. All the samples were rehydrated for 1 h. For the cooked seaweed, the same hydration treatment was followed, but then U. lactuca and Pyropia spp. were cooked inside sealed plastics bags for 15 min and D. antarctica for 20 min in water at 100 °C. The hydrated and cooked samples were prepared the day before, stored at 5 °C until used and served to the panel in closed white plastic cups at room temperature (20 °C), identified with a randomly selected 3-digit code.
Final training for the evaluation of samples
Each panelist received six samples (three seaweed species and two treatments of each) and an answer sheet with the 25 sensory descriptors obtained from the vocabulary development meetings. The objective of this session was to confirm that the panelists understood the sensory descriptors of appearance, aromatic and taste components, texture, and aftertaste of the newly standardized samples. The panelists first tasted the D. antarctica, both hydrated and cooked, and commented on the descriptors presented in the sensory descriptor guide. The attributes were condensed and discussed in the following order: appearance, aroma, flavor, mouthfeel, texture, and aftertaste. Later, the same attributes were discussed for Pyropia spp. and U. lactuca (hydrated and cooked).
Ranking descriptive analysis
In the sensory evaluation session, the six seaweed samples were analyzed by the RDA method in a sequential monadic order (Richter et al. 2010). The instruction was to evaluate and compare each sample with the other samples and record the impressions. Every panelist received deionized water to clean the palate between tastings. The comparative evaluation consisted in ranking the six samples for each descriptor, using a ranking order from 1 to 6. Results are expressed as the sum of rankings by the nine panelists for each descriptor: the smaller the sum, the smaller the intensity of the descriptor.
Physico-chemical analysis
Umami compounds
Free amino acids were determined by a modification of the method of Segura-Campos et al. (2011). For hydrated seaweed, 35 mL of water was added to 1 g of ground dried seaweed and allowed to hydrate for 1 h. For the cooked samples, hydrated U. lactuca and Pyropia spp. were cooked in boiling water for 15 min and D. antarctica for 20 min. In both cases, the aqueous extract was separated, filtered, and used for analysis.
A sample of 200 µL of the aqueous extract was dissolved in 2.8 mL of borate buffer (1 M, pH 9.0) and derivatized with 2.4 µL of diethyl ethoxymethylene malonate at 50 °C for 50 min under agitation. Quantification of free amino acids was performed in a UHPLC UltiMate 3000 system (Thermo Scientific, USA) following the procedures for the separation of derivatives by Segura-Campos et al. (2011). Results are expressed in mg (100 g) −1 of dry seaweed.
Nucleotides were extracted with water and hydrochloric acid, after centrifugation (Peinado et al. 2014). For hydrated samples, dry seaweed (0.6 g) was weighed into a falcon tube and distilled water (10 mL) was added and allowed to hydrate for 1 h. For cooked seaweed, the samples were hydrated following the previous steps and cooked at 100 °C for 15 min (U. lactuca and Pyropia spp.) and 20 min (D. antarctica). Then hydrochloric acid (10 mL, 0.01 N) was added followed by stirring at 90 °C for 90 min. The mixture was allowed to stand for another 20 min and then filtered through a gauze. The supernatant was centrifuged at 8500 × g for 15 min. Quantification was performed by UHPLC UltiMate 3000 system (Thermo Scientific) with a C18 column (4.6 × 100 mm, 5 µm particle size) at 30 °C. The mobile phases were A: 20 mmol L−1 KH2PO4: 20 mmol L−1 K2HPO4 (v:v 1:1), adjusted to pH 5.8 with phosphoric acid and B: Methanol at a flow rate of 0.7 mL min−1. The gradient program used was as follows: 0–9 min 8% B. A period of 6 min with initial conditions was sufficient time for a subsequent analysis run. UV detection was at a wavelength of 254 nm. Results are expressed in mg (100 g) −1 of dry seaweed.
Equivalent umami concentration (EUC)
The EUC value reflects the impact on umami flavor intensity given by a mixture of the free amino acids glutamic acid and aspartic acid (L-Glu and L-Asp) and free nucleotides IMP (disodium inosinate) and GMP (disodium guanylate), and is represented by the following equation (Yamaguchi 1991).
EUC is expressed as g of monosodium glutamate in 100 g of sample (dry weight). Values of ai and aj denote the concentrations (g (100 g)−1) of amino acids (L-Asp or L-Glu) and nucleotides (GMP or IMP), respectively. Parameter bi represents the relative concentration of umami (RUC) for each amino acid (L-Glu: 1; L-Asp: 0.077) and bj is the RUC for the 5'-nucleotides (IMP: 1; GMP: 2.3). The coefficient 1218 is a synergistic constant based on the concentration (g (100 g)−1) used (Chen and Zhang 2007).
Texture profile analysis
The mechanical behavior of seaweed samples was assessed by the texture profile analysis (TPA) protocol after adapting the assay to the morphological characteristics of each seaweed (cylindrical shape or extended sheets). Durvillea antarctica (around 1.5 cm diameter) was cut into pieces 2 cm long while the foliose seaweeds U. lactuca and Pyropia spp. were weighed (8 g) and placed directly in a cylindrical sample holder (4 cm diam.; 3 cm height). Compression testing was done with a stainless-steel flat probe 3 cm in diameter. TPA test parameters were pre-test and post-test speed, 3.0 mm s−1; test speed, 1.0 mm s−1. U. lactuca and Pyropia spp. were compressed twice for 8 mm and D. antarctica to 50% deformation (ten replicates each). Six parameters were generated from the force–deformation graph: hardness (N), the maximum load applied to the samples during the first compression cycle; adhesiveness (N s), the negative force area for the first bite; cohesiveness (dimensionless), ratio of the area under the second peak to that under the first peak; springiness (dimensionless), the reversed sample deformation in the second compression obtained as the ratio of the distance of the detected height of sample on the second compression to that of the original compression; and, chewiness (N mm), the product of gumminess and springiness (Ansari et al. 2014).
Color
Color of hydrated and cooked seaweeds was measured with a computer vision system (DVS-Lab, Digital Vision Solutions, Chile) and expressed according to the CIE coordinates of lightness (L*), redness (a*), and yellowness (b*) (Luna and Aguilera 2014).
Data analysis
The statistical analysis of instrumental data was performed using the SPSS version 19.0 (SPSS, IBM, USA). Seaweed samples were grouped for analysis according to the treatment (raw or cooked seaweed). The normality of results was analyzed by the Shapiro–Wilk test, and the Levene test was applied to determine the homogeneity of the variance. Significance of instrumental data (p = 0.05) was evaluated by the t-Student or Mann–Whitney U method. Results of the ranking descriptive analysis (RDA) were analyzed using the Generalized Procrustes Analysis (GPA) and the XLSTAT 2015.6 software (Mamede and Benassi 2016). Data were arranged as nine individual matrices (one per judge) of six lines (corresponding to sample treatments) and 25 columns (descriptors). Correlations of instrumental and sensory data were performed using SPSS version 19.0 (SPSS, IBM, USA), by the Spearman method with a significance of p = 0.05.
Results
Chemical characterization of seaweeds
Proximate analysis of samples provides a valuable chemical characterization of the materials in the study given the high variability exhibited by the same seaweed species depending on location, time of harvest, part of the frond, etc. Pyropia spp. and U. lactuca exhibited a high protein content, 19.6 and 23.3 g (100 g)−1 d.w., respectively (Table 2) while the protein content in D. antarctica was approximately 7 g (100 g)−1 d.w. Seaweeds contained only a small amount of lipids (Table 2). U. lactuca had the highest fat content, 0.41 g g (100 g)−1 d.w. while the lipid content of Pyropia spp. and D. antarctica were 0.1- 0.2 g (100 g)−1 d.w., respectively. Regarding carbohydrates, U. lactuca had the lowest carbohydrate content (41.6 g g (100 g)−1 d.w.) while D. antarctica exhibited the highest amount (69.6 g (100 g)−1 d.w.) (Table 2). Seaweeds are known for their ability to accumulate minerals and their high ash content compared to land plants, e.g., 8- 40% (Rupérez 2002; Munoz and Díaz 2022). Ulva lactuca had the highest ash content (20.2 g (100 g)−1 d.w.) and D. antarctica the lowest (15.2 g (100 g)−1 d.w.) (Table 2).
Table 2.
Chemical composition of raw seaweeds
| Seaweed |
D. antarctica g (100 g)−1 d.w |
Pyropia spp. g (100 g)−1 d.w |
U. lactuca g (100 g)−1 d.w |
|---|---|---|---|
| Moisture | 8.1 ± 0.3 | 9.3 ± 0.0 | 18.2 ± 0.2 |
| Ash | 15.2 ± 0.1 | 18.8 ± 0.2 | 20.2 ± 0.7 |
| Protein | 7.0 ± 0.1 | 23.3 ± 0.4 | 19.6 ± 0.1 |
| Carbohydrates | 69.6 ± 0.2 | 48.5 ± 0.5 | 41.6 ± 0.1 |
| Crude fiber | 53.0 ± 1.5 | 21.7 ± 1.1 | 33.7 ± 0.9 |
| Fat | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 |
Values are expressed as mean ± standard (n = 2)
Development of a sensory vocabulary and definition of descriptors
From the online evaluation session, 25 descriptors were generated for aroma, flavor, mouthfeel, texture, and residual sensation. Table 3 shows some examples of sensory descriptors and terms used in the food science literature that allowed the panelists guide and compare the descriptors selected for seaweeds (Table 4).
Table 3.
Definition of selected sensory descriptors for seaweeds
| Descriptor | Definition | Suggested references | References |
|---|---|---|---|
| Appearance | |||
| Shiny | Light is reflected from the surface |
Brilliant: tomato, candy Opaque: cookies, bread |
Meilgaard et al. 2016 |
|
Translucent/ opaque |
Light goes through sample, but clear images cannot be seen through it |
Translucent: apple juice, fried onion Opaque: cookies, cheese |
FAO 1999 |
| Rough | Contains irregularities, bumps, or grains on the surface |
Mild: apple peel Rough: peel of Hass avocado |
Meilgaard et al. 2016 |
| Turgid | The surface appears swollen or stretched (tense) due to hydrated cells underneath |
Flaccid: raisins, dehydrated fruits Turgent: fresh grape, celery |
Taniwaki and Sakurai 2010 |
| Aroma | |||
| Marine | Related to the smell of the sea, wet rocks, fresh fish, shellfish | Nori, fresh fish | Baker et al. 2014; Chapman et al. 2015; Stévant et al. 2020 |
| Herbal | Reminds of freshly cut grass and fresh green leafy vegetables | Fresh spinach, matcha tea, parsley, freshly cut grass | Smyth et al. 2012; Talavera-Bianchi et al. 2010 |
| Earthy /mouldy | Associated with humus, including moist soil, decaying of vegetation or basement scent | Fresh mushrooms | Talavera-Bianchi et al. 2010 |
| Mineral | Associated with an aromatic and mouthfeel of metallic aroma and sea salts | Blood, metal cans, Al foil, salt solution | Sharma et al. 2020; Talavera-Bianchi et al. 2010 |
| Caramel | Associated with the impression of sweet substances, aromas of caramel. Sweet, honey, toasted | Honey, caramel | Chun et al. 2020; Wu et al. 2017 |
| Taste | |||
| Sweet | Sensation stimulated by sucrose and low-calorie sweeteners | Honey, candies, sugar | Bueno de Godoy et al. 2020; Galán-Soldevilla et al. 2005 |
| Bitter | Taste stimulated by substances like quinine, caffeine, and hop bitters | Ristretto coffee, IPA beers, grapefruit, tonic water | Chapman et al. 2015; Talavera-Bianchi et al. 2010 |
| Salty | Taste stimulated by sodium salts, such as sodium chloride and sodium glutamate | Salty snacks, jerky, salt-packed anchovies | Chapman et al. 2015; Talavera-Bianchi et al. 2010 |
| Acid | Taste stimulated by acids, such as citric, acetic, malic, phosphoric, etc | Lemon juice, vinegar, sour apples | Chapman et al. 2015; Talavera-Bianchi et al. 2010 |
| Umami | The basic taste produced by monosodium glutamate or disodium inosinate | Soy sauce, aged cheeses, soup broths | Chapman et al. 2015; Talavera-Bianchi et al. 2010 |
| Mouthfeel | |||
| Astringent | Produces the shrinkage or puckering of the tongue's surface | Red wine, immature fruit | Bueno de Godoy et al. 2020; Wu et al. 2017 |
| Slimy | The textural property that produces the sensation of wet. Slipperiness at the surfaces of the oral cavity | Natto (fermented soybeans), okra | ISO 2008 |
| Texture | |||
| Sticky | During chewing the food adheres to surfaces in the palate, teeth and tongue | Okra | ISO2008 |
| Elastic | The degree to which the sample returns to its original shape after exerting a force |
Plastic: butter Elastic: squid, marshmallows, gummies |
Jowitt 1974; Meilgaard et al. 2016 |
| Crunchy | Food emits noise while it breaks or fractures, characterized by few significant breaks | Raw carrot, apple, celery, pig's ear | Aguirre et al. 2018; Wu et al. 2017 |
| Cohesive | Difficult to break/cut and bite resistant requires chewing |
Low: muffin Medium: cheeses High: chewing gum |
Aguirre et al. 2018; Wu et al. 2017 |
| Cartilaginous | Associated with cartilage- a combination of hardness and crispness | Pig’s ear, chicken cartilage | Texture Analysis Professionals Blog 2017 |
| Hard | Requires force to compress between the molars to bring the teeth together |
Soft: cream cheese Medium hard: peanuts Hard: hard candies |
Jowitt 1974; Stévant et al. 2020; Wu et al. 2017 |
| Residual sensation | |||
| Toothstick | Amount of product that sticks to the teeth and palate after swallowing |
Low level: mushrooms Intense: chewy candy |
Meilgaard et al. 2016 |
| Bitter | Lingering bitter sensation remaining in the mouth after the product is swallowed | Ristretto coffee, high-hops beers, grapefruit, tonic water | Talavera-Bianchi et al. 2010 |
| Mineral | Lingering salty or metallic sensation remaining in the mouth after swallowing | Blood, some mineral waters | Talavera-Bianchi et al. 2010 |
Table 4.
Characterization of samples by RDA
| Descriptor | Descriptor | DH | DC | PH | PC | UH | UC |
|---|---|---|---|---|---|---|---|
| Appearance | Brilliant | 51 a | 41 a,b | 38 a,b | 27 b,c | 18 c | 14 c |
| Translucent | 20 b,c | 15 c | 47 a | 47 a | 28 b,c | 32 a,b | |
| Rough | 47 a | 48 a | 20 b | 25 b | 24 b | 25b | |
| Turgid | 53 a | 42 a,b | 35 b | 28 b,c | 19 c,d | 12 d | |
| Aroma | Marine | 46 a | 27 b | 42 a | 23 b | 21 b | 25 b |
| Herbal | 23 b,c | 15 c | 34 a,b | 30 a,b | 41 a | 40 a | |
| Earthy/mouldy | 16 c | 12 c | 25 b,c | 35 a,b | 38 a,b | 42 a | |
| Mineral | 35 a | 20 a | 38 a | 38 a | 29 a | 29 a | |
| Caramel | 37 b | 54 a | 25b,c,d | 35 b,c | 17 d | 21 c,d | |
| Taste | Sweet | 35 a | 43 a | 39 a | 41 a | 17 b | 14 b |
| Salty | 25 b,c | 18 c | 42 a | 37 a,b | 33a,b,c | 34 a,b | |
| Acid | 21 c,d | 14 d | 31 b,c | 31 b,c | 45 a,b | 47 a | |
| Umami | 33 a | 36 a | 43 a | 48 a | 15 b | 14 b | |
| Bitter | 21 b,c | 13 c | 26 b,c | 30 b | 49 a | 50 a | |
| Mouthfeel | Astringent | 19 b | 16 b | 28 b | 27 b | 49 a | 50 a |
| Slimy | 50 a | 45 a,b | 33 b,c | 29 c,d | 16 d | 16 d | |
| Texture | Sticky | 45 a | 48 a | 21 b | 27 b | 22 b | 26 b |
| Springy | 53 a | 44 a,b | 36 b | 29 b,c | 18 c | 9 d | |
| Crunchy | 54 a | 45 a,b | 35 b,c | 28 c,d | 16 d,e | 11 e | |
| Hardeness | 52 a | 43 a,b | 38 a,b | 29 b,c | 15 c,d | 12 d | |
| Cohesiveness | 52 a | 43 a,b | 38a,b,c | 29 b,c,d | 14 d,e | 13 e | |
| Cartilaginous | 52 a | 43 a,b | 36 b,c | 31 b,c | 15 d | 12 d | |
| Residual sensation | Tooth adhesion | 12 d | 15 d | 32 b,c | 35 a,b,c | 45 a,b | 50 a |
| Bitter | 14 d,e | 13 e | 29 c,d | 34 b,c | 48 a,b | 51 a | |
| Mineral | 26 a | 26 a | 36 a | 35 a | 35 a | 31 a |
Different superscripts in the same row indicate significant differences
Sum of rankings of the nine panelists (higher values mean more intensity)
Nomenclature: DH hydrated D. antarctica, DC cooked D. antarctica, PH hydrated Pyropia spp., PC cooked Pyropia spp, UH hydrated U. lactuca, UC cooked U. lactuca
Evaluation of sensory attributes
Color and appearance
Color is an important visual trait appreciated by seaweed consumers (Zhu et al. 2022). Independent of treatment, all three seaweed species showed different L*, a* and b*color parameters (Table 5). The a* value of U. lactuca varied significantly (p = 0.05) between the hydrated and cooked samples, meaning that it shifted from bright green to a more reddish color (Table 5). In the case of Pyropia spp., the cooked sample was significantly different in all parameters from the hydrated seaweed, and visually it became darker. The color of D. antarctica, did not change significantly after cooking (Table 5).
Table 5.
Color parameters of hydrated and cooked seaweeds
| Sample | DH | DC | PH | PC | UH | UC |
|---|---|---|---|---|---|---|
| L* | 26.98 ± 0.61 a | 27.05 ± 1.64 a | 50.86 ± 2.02 b | 40.93 ± 3.10 c | 49.83 ± 1.39 d | 47.36 ± 2.32 d |
| a* | 3.06 ± 0.35 a | 3.09 ± 0.17 a | 4.60 ± 0.77 b | 9.05 ± 2.60 c | -7.27 ± 0.78 d | -0.77 ± 0.34 e |
| b* | 4.41 ± 0.5 a | 4.17 ± 0.63 a | 12.19 ± 12.19 b | 3.43 ± 0.78 c | 30.29 ± 1.48 e | 24.33 ± 1.71 e |
Different superscripts in the same row indicate significant differences between treatments in each seaweed. Values are expressed as mean ± standard deviation (n = 10)
Nomenclature: L* lightness, a* redness, b* yellowness, DH hydrated D. antarctica, DC cooked D. antarctica, PH hydrated Pyropia spp., PC cooked Pyropia spp, UH hydrated U. lactuca, UC cooked U. lactuca
Taste and aroma
Regarding the taste and aroma, the hydrated U. lactuca showed more herbal notes and earthy/mouldy aroma than other seaweeds (Table 4 and Fig. 1). Pyropia spp., showed a high marine aroma and a sweet, salty and umami taste (Table 4). Finally, D. antarctica was characterized by a caramelized marine aroma, a sweet and umami taste and had the lowest value in the ranking for the salty descriptor (Table 4). Mineral aroma and residual mineral flavor were not significantly different between seaweeds independent of treatments.
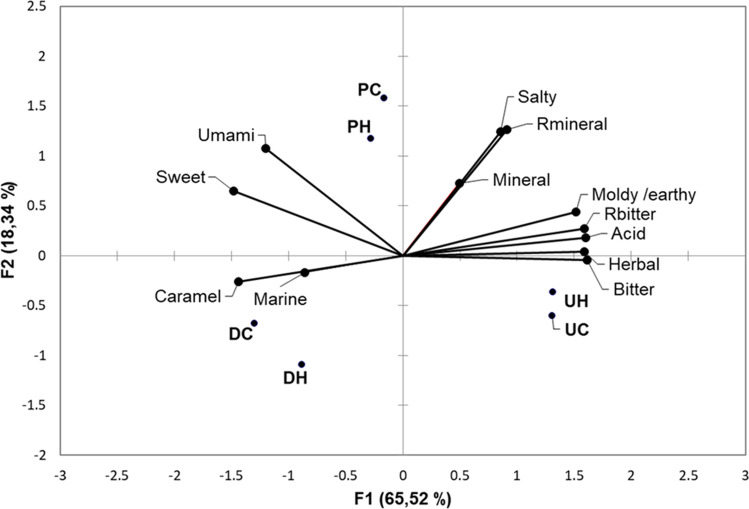
Fig. 1.
Generalized Procrustes Analysis (GPA) plot of descriptors for taste, aroma and residual flavour obtained by Ranking Descriptive Analysis. Rbitter and Rmineral refer to residual sensations. Nomenclature: DH: hydrated D. antarctica; DC: cooked D. antarctica; PH: hydrated Pyropia spp.; PC: cooked Pyropia spp; UH: hydrated U. lactuca; UC: cooked U. lactuca
A significant correlation (p = 0.05) was observed between flavor descriptors and some chemical components. There was a strong positive correlation between umami taste and sweet taste perceived by the panel (r = 0.829), and a positive correlation between the sweet taste and the caramel aroma (r = 0.771) of D. antarctica and Pyropia spp.
Umami compounds
The content of umami compounds varied between seaweeds. The seaweed with the highest content of L-Glu was Pyropia spp., between 310.06 (hydrated) and 324.27 (cooked) mg (100 g) −1 d.w. Instead, the seaweed with the lowest value was U. lactuca with values ranging between 37.07 (hydrated) and 35.60 (cooked) mg (100 g) −1 d.w. (Table 6). The seaweed that obtained the highest content of L-Asp was D. antarctica with values between 71.09 (hydrated) and 78.57 (cooked) mg (100 g)−1 d.w. Regarding free nucleotides, the seaweed with the highest content of nucleotides was Pyropia spp., followed by D. antarctica. No nucleotides were identified for cooked or hydrated U. lactuca.
Table 6.
Amino acid and 5′-nucleotides content in hydrated and cooked seaweed, and equivalent umami concentration EUC
| Sample | DH | DC | PH | PC | UH | UC |
|---|---|---|---|---|---|---|
| L-Asp a | 71.09 ± 2.15 | 78.57 ± 2.07 | 51.45 ± 0.86 | 58.54 ± 1.89 | 53.10 ± 3.19 | 36.81 ± 0.40 |
| L-Glu a | 209.09 ± 6.98 | 209.98 ± 2.07 | 310.06 ± 4.89 | 324.27 ± 11.75 | 37.07 ± 0.31 | 35.60 ± 0.61 |
| IMP a | 0.963 ± 0.01 | 1.328 ± 0.04 | 7.809 ± 0.07 | 7.333 ± 0.76 | N.D | N.D |
| GMP a | 0.014 ± 0.15 | 0.044 ± 0.14 | 0.071 ± 0.05 | 0.764 ± 0.19 | N.D | N.D |
| EUC b | 3.19 | 3.06 | 4.60 | 5.34 | 0.04 | 0.04 |
Values are expressed as mean ± standard deviation (n = 3)
Nomenclature: DH hydrated D. antarctica, DC cooked D. antarctica, PH hydrated Pyropia spp., PC cooked Pyropia spp, UH hydrated U. lactuca, UC cooked U. Lactuca, L-Asp Aspartic acid, L-Glu Glutamic acid, N.D. not detected
amg (100 g)−1 d.w
bgMSG (100 g)−1 d.w
EUC varied between seaweed species, but not between cooking conditions (Table 6). The seaweed with the highest EUC value was Pyropia spp., 4.60 and 5.34 (g MSG (100 g)−1 d.w.) for hydrated and cooked samples, respectively, followed by D. antarctica (4.60 and 5.34 g MSG (100 g)−1 d.w., respectively). The lowest EUC value corresponded to U. lactuca for both sample treatments 0.04 (g MSG (100 g)−1 d.w.). These results are consistent with those reported by the sensory panel (Table 4). The correlation analysis using Spearman's bivariate method, showed a significant (p = 0.05) and positive correlation (r = 0.943) between the umami taste perceived by the sensory panel and the EUC values.
Texture analysis
The sensory panel evaluated the difference in textural properties. The hardness of all the seaweeds decreased with cooking. Hydrated D. antarctica and Pyropia spp. decreased their hardness by approximately 10 points with respect to their respective cooked samples, and the hydrated U. lactuca only decreased by 3 points with respect to the cooked sample. Other important parameters were crunchy, cartilaginous and springy sensation that together with hardness decreased with cooking. The only parameter that seemed to increase with cooking was stickiness. The seaweed with highest values for all texture parameters was D. antarctica and the lowest score was obtained by U. lactuca.
Texture profile analysis
Cooking of U. lactuca generated significant differences (p < 0.05) in all texture parameters except for hardness (Table 7). In the case Pyropia spp., the descriptors cohesiveness and resilience presented a significant difference between uncooked and cooked samples. For D. antarctica, significant differences were detected in hardness, chewiness, and resilience. Since the physical testing method was different for D. antarctica (i.e., compression rather than puncture) comparisons with the other two seaweed species cannot be made, however, cooking induced textural changes to diverse extents for the three seaweeds.
Table 7.
Texture parameters determined by the TPA method
| Sample | DH | DC | PH | PC | UH | UC |
|---|---|---|---|---|---|---|
| Hardness(N) | 10.20 ± 1.52 a | 2.72 ± 1.24 b | 1.01 ± 0.22 c | 0.91 ± 0.13 c | 2.43 ± 0.77 d | 1.85 ± 0.78 d |
| Adhesiveness (g*sec) | –19.11 ± 9.19 a | –25.83 ± 16.23 a | –4.53 ± 2.68 b | –7.39 ± 4.73 b | –3.61 ± 2.35 c | –9.08 ± 5.62 d |
| Springiness | 0.95 ± 0.08 a | 0.98 ± 0.07 a | 0.98 ± 0.01 b | 1.00 ± 0.07 b | 0.95 ± 0.03 c | 0.89 ± 0.06 d |
| Cohesiveness | 0.90 ± 0.10 a | 0.92 ± 0.04 a | 0.84 ± 0.35b | 0.79 ± 0.09 c | 0.83 ± 0.05 c | 0.74 ± 0.08 d |
| Chewiness | 8.83 ± 2.30 a | 2.45 ± 1.17 b | 0.83 ± 0.18 c | 0.71 ± 0.10 c | 1.91 ± 0.60 c | 1.23 ± 0.56 d |
| Resilience | 0.77 ± 0.10 a | 0.45 ± 0.03 b | 0.32 ± 0.02 b | 0.26 ± 0.02 c | 0.24 ± 0.03 c | 0.18 ± 0.04 d |
Different superscripts in the same row indicate significant differences between treatments in each seaweed. Values are expressed as mean ± standard deviation (n = 10)
Nomenclature: DH hydrated D. antarctica, DC cooked D. antarctica, PH hydrated Pyropia spp., PC cooked Pyropia spp, UH hydrated U. lactuca, UC cooked U. Lactuca
Discussion
Chemical characterization of seaweeds
The protein content of seaweeds varies with the species. High contents of proteins are reported for green and red seaweeds, and some of these species can reach up to 40% of their dry weight in protein (Holdt and Kraan 2011). Results of protein content for Pyropia spp. (23.3%) and U. lactuca (19.6%) were similar to those reported by Cian et al. (2013), 24.61 g (100 g)−1 d.w. for the red seaweed Porphyra columbina while Ortiz et al. (2006) and Rasyid (2017) reported a protein content for U. lactuca ranging between 13.6 and 27.2 g (100 g)−1 d.w. The protein content in brown seaweeds in generally low; D. antarctica was the seaweed with the lowest protein content, about 7% of its dry weigh, a value similar to that of Mateluna et al. (2020). Regarding lipids, seaweeds have low contents of triglycerides that vary between species, season, and environmental factors. Our results agree with previous studies (Anantharaman et al. 2013; Pirian et al. 2020), confirming that green seaweeds, in general, contain higher concentrations of lipids than red and brown seaweeds. The values obtained for Pyropia spp. and D. antarctica were in accordance with those reported by Cian et al. (2013) and Mateluna et al. (2020).
Carbohydrates, particularly polysaccharides, are in high concentration in seaweed as they fulfil important structural, storage and functional functions (Quitral et al. 2012). Values for Pyropia spp. and D. antarctica were similar to those obtained by Cian et al. (2013) and Ortiz et al. (2006). In the case of U. lactuca, Rasyid (2017) and Ortiz et al. (2006), reported a higher amount of carbohydrates, a difference that could be due to environmental conditions or growth stage of the seaweed.
Mineral content in this studio differs from those reported by Ortiz et al. (2006), who found that D. antarctica contained a higher ash content (17.9 g (100 g)−1 d.w.) than U. lactuca (11.0 g (100 g)−1 d.w.). In the case of Pyropia spp., the ash content (18.8 g (100 g)−1 d.w.) was higher than the value reported by Cian et al. (2013). Seaweeds vary greatly in their ash and mineral content due to factors such as geographical origin and seasonal, environmental, and physiological variations (Rupérez 2002).
In summary, results of the proximate analysis of seaweeds were consistent with those of other authors and any differences may be attributed to factors such as environmental conditions, geographical location, stage of development and morphological characteristics of seaweeds, among others (Circuncisão et al. 2018; Figueroa et al. 2021).
Development of a sensory vocabulary and definition of descriptors
A common way to develop a sensory vocabulary is to start by asking members of a panel to write down an attribute list for a food and then the panel leader promotes a group discussion to agree on the definitions (Mc Donnell et al. 2001). Thus, a preliminary sensory vocabulary is open to improvements and validation by expert panelists and it may lead to the creation of formal lexicons for specific foods or beverages (Baker et al. 2014). In our case, with the aid of a vocabulary, research chefs and consumers will be able to speak the same language when it comes to describing the sensory characteristics of seaweeds, seaweed products, and dishes. In fact, a detailed analysis of research articles suggesting the incorporation of seaweeds in any form (i.e., flours, extracts, etc.) into traditional food products (breads, pasta, meat products, etc.) show that addition of seaweed ingredients is limited at around 10% by undesirable sensory effects (Birch et al. 2018; Prager 2020; Figueroa et al. 2021).
Evaluation of sensory attributes
Color and appearance
The three seaweeds species are very different in terms of appearance and color. Morphologically, U. lactuca is a foliose seaweed with a laminar thallus with blades around 15 cm in size, of light green to dark green color; Pyropia spp. is a foliose seaweed with translucent fronds of a color that varies between pink and purple, and having blades approximately 10 cm in size; D. antarctica, on the other hand, is a brown seaweed with cylindrical fronds that can measure up to 15 m in length. In cross-section, the outer part consists of a hard cortex and the interior looks like a honeycomb (Mateluna et al. 2020). Its color varies from dark brown to greenish brown (Santelices 1989).
Cooking modifies the structure of seaweeds, and this change is more evident in some species than in others (Chen and Roca 2018). Panelists did not recognize major differences in the appearance of raw and cooked seaweeds (Table 4). In the case of D. antarctica its tubular shape became flattened after cooking and the alteration was expressed by the sensory panel as being less turgid (Table 4). These changes may be attributed to the solubilization of structural polysaccharides in hot water leading to collapse of the honeycomb inner structure (Mouritsen 2013; Bruhn et al. 2019).
Color was different between seaweeds and treatments. These color changes after cooking are probably due to degradation of pigments (e.g., chlorophylls, xanthophylls, and carotenes) leading to the formation of secondary-colored substances (Stévant et al. 2018). Pina et al. (2014) and Amorim et al. (2012) pointed out that the presence of β-carotene and lutein increased in the red seaweed Chondrus crispus, the brown seaweed Undaria pinnatifida (Wakame), and Laminaria spp. (Kombu) after hot culinary treatments, probably by their release from an obliterated cellular matrix.
Taste and aroma
Flavor of seaweeds, or the sensory impression determined by the chemical senses of taste and smell, depends on the species, geographical origin, time of harvest, and the processing method, among other factors (López-Pérez et al. 2017). The green seaweed U. lactuca had more herbal notes and earthy/mouldy aroma among seaweeds because it contains high levels of dimethyl sulfide (DMS) which provides "cabbage sulfur" and "seaside fresh" aromas (López-Pérez et al. 2017; Francezon et al. 2021). Sugisawa et al. (1990), pointed out that the herbaceous aroma was mainly due to the high content of aldehydes that are perceived as herbaceous, green and cucumber aromas. Regarding red seaweeds, Porphyra spp., belonging to the same family as Pyropia spp., have high contents of DMS and other sulfur compounds that provide a strong marine flavor in the seaweed (Francezon et al. 2021). Furthermore, this seaweed contains high amounts of free glutamate and 5' ribonucleotides that enhance the umami flavor in some dishes such as sushi (Mouritsen et al. 2012). In addition, it contains a great diversity and abundance of halogenated compounds that contribute to marine and shellfish aromas (Francezon et al. 2021). These compounds could be responsible for the marine aromas and the sweet, salty and umami taste. In the case of D. antarctica, there is limited information on the volatile composition and flavor compounds. Moraes et al. (2021) found that D. antarctica contained a large amount of 1-octen-3-ol, a mushroom-like aroma.
Mineral and residual sensations were not different between treatments. Regarding residual sensations, Sanchez-García et al. (2021), point out that cooked samples of Ulva rigida exhibited low values of several aroma descriptors, including seaside and seaweed notes. The earthy/mouldy aroma and bitter taste of U. lactuca remained after cooking. Pyropia spp., however, experienced noticeable changes in flavor, particularly, in marine aroma. The caramel and marine aromas changed significantly for D. antarctica after cooking. It should be stressed at this point that sensory perceptions depend on the origin of samples and cannot be generalized to the particular species.
Figure 1 shows how samples and descriptors distributed in the different quadrants of the GPA graph. D. antarctica exhibited most of the desirable descriptors, such as caramel, umami and marine aromas while U. lactuca turned out to have undesirable bitter and earthy/mouldy descriptors. Pyropia spp. was almost equidistant from the other two samples where umami and salty were the closest descriptors. Also evident from Fig. 1 is that cooking changed only slightly the position of samples in the graph.
There is an interesting correlation between the enhancement of sweetness perception and umami taste (Woskow 1969). This relationship is evidenced in the results obtained. Furthermore, these results stimulate further research on the content of sugars and umami components of seaweeds and harvesting conditions that optimize a positive sensory perceptions by consumers.
Umami compounds
There is limited information on the content of amino acids responsible for the umami taste of most edible seaweeds (Mouritsen et al. 2019). Often these free amino acids precipitate forming a white layer on the surface of the dried seaweeds (Mouritsen et al. 2012). Umami taste in seaweeds is mainly a result of the synergistic presence between glutamic acid and aspartic acid with 5’ ribonucleotides (Yamaguchi 1991). Milinovic et al. (2020) reported that green seaweed, in general, has a low content of free umami compounds compared to other edible macroalgae, which is consistent with results obtained in this study. Moreover, they pointed out that of twelve different species of seaweeds, the Rhodophyta containd the highest concentration of L-Glu and L-Asp. The authors also suggested that red seaweeds are a good option to introduce the umami taste in culinary recipes. Kawashima et al. (2018) identified the components of the umami taste of nori (Porphyra spp.) and reported concentrations of the free amino acids L-Glu and L-Asp of 261 mg (100 g) −1 and 56 mg (100 g) −1, respectively, similar to those of Pyropia spp. in Table 6. Regarding brown seaweed, the glutamate content is highly variable depending on the species. Mouritsen et al. (2019) studied the MSG content in dashi broth from 20 different species of brown seaweed and found out that it ranged from 0.015 to 37 mg mL−1.
Umami components and nucleotides were determined in seaweed extracts (as described in Sect. 2.5.1) so they correspond to the free form of these compounds (Peinado et al. 2014; Kawashima et al. 2018). Thus, the reported concentrations may not represent the actual contents in hydrated and cooked seaweeds used in sensory evaluation, but they are a good proxy of the free forms of compounds in solution inside the hydrated and cooked seaweeds. Regarding the nucleotide content of seaweeds, some studies indicate that the presence of 5'GMP and 5'IMP is very low, often imperceptible (Milinovic et al. 2020). Values in Table 6 show that the total concentration of the nucleotides 5'IMP and 5'GMP are low, ranging from 0.97 to 8.0 mg (100 g) −1 d.w. and not detectable in U. lactuca. Tashiro et al. (1991), reported 5'IMP concentrations in the range of 9 to 10 mg (100 g) −1 in dried nori On the other hand, Peinado et al. (2014) reported total nucleotide concentrations similar to those existing in tomatoes, potatoes and fungi (of the order of 500 mg (100 g) −1) in Pelvetia canaliculata and Fucus vesiculosus. Further work should be undertaken regarding the nucleotide content of seaweeds and their variability between species.
Texture analysis
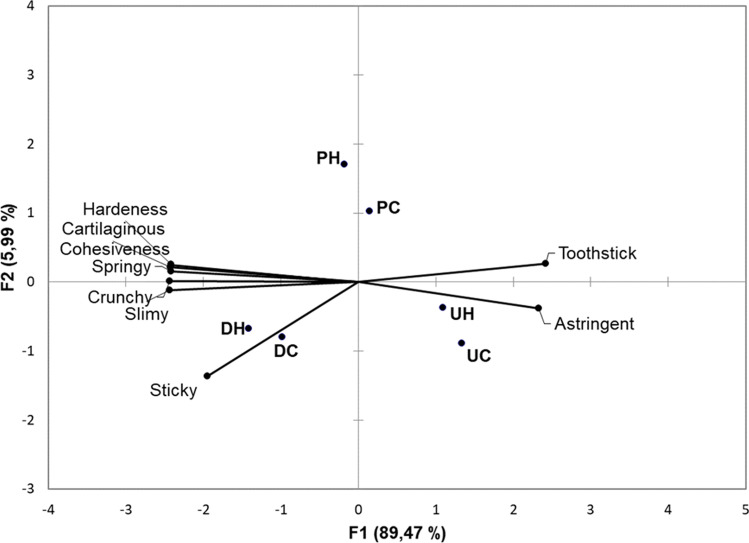
Texture is a critical attribute in the acceptance of seaweeds. Table 4, shows that the three seaweeds were different in terms of stickiness, elasticity, crispness, hardness, cohesiveness, and cartilaginous sensation. Ulva lactuca obtained the lowest values in the RDA, which means that it was the softest seaweed, the least sticky, elastic, crispy, cartilaginous, and cohesive. In the case of Pyropia spp., the values obtained from RDA are intermediate between those U. lactuca and D. antarctica, which means that texture is not a distinctive parameter for this seaweed. Finally, D. antarctica obtained the highest RDA values in all attributes and was perceived as hard elastic, cohesive and cartilaginous. The texture difference between the three seaweeds is also appreciated in the GPA graph in Fig. 2. D. antarctica and U. lactuca are in opposite quadrants along the X-axis, meaning that they possessed contrasting textural characteristics.
Fig. 2.
Generalized Procrustes Analysis (GPA) plot of descriptors for texture obtained by Ranking Descriptive Analysis. Nomenclature: DH: hydrated D. antarctica; DC: cooked D. antarctica; PH: hydrated Pyropia spp.; PC: cooked Pyropia spp; UH: hydrated U. lactuca; UC: cooked U. lactuca
Panelists did not find much difference in textural descriptors between hydrated and cooked samples of the same species (Table 4 and Fig. 2). For U. lactuca, the perception of springiness decreased significantly with cooking. Results are similar to those obtained by Sanchez-García et al. (2021), in that the descriptors of hardness, stickiness and elasticity did not change significantly for Ulva rigida. In the case of Pyropia spp. and D. antarctica, the elastic, crunchy, hardness, cohesiveness, and cartilaginous descriptors tend to decrease when the seaweeds are cooked. Bruhn et al. (2019) found that cooking decreases the viscous appearance of Saccharina japonica due to the dissolution or washing in water of polysaccharides such as laminarin or fucoidan. In this study, just enough water was used to hydrate, therefore an increase in viscosity is perceived in the treated seaweed. Therefore, one option to remove this often unpleasant characteristic is to wash the seaweed, but unfortunately some flavor compounds such as umami compounds will be lost in the broth.
Texture profile analysis
Seaweeds have tough and elastic cell walls reinforced with polysaccharides (Mouritsen 2013). When these polysaccharides contact the hot water, some of them dissolve and weaken the cell structures, generating changes in texture, a phenomenon observed in Table 7 that also occurs in land plants. This change in texture was demonstrated by Mateluna et al. (2020), who studied the microstructural and textural changes of D. antarctica after cooking. According to Vervoort et al. (2012), hydrothermal processing of carrots generates a loss of turgor that translates into textural softening. Adhesiveness increases when the seaweeds are cooked at 100 °C (Table 7) but the effect is not significant. Alginates, carrageenans and ulvans are the main structural components of cell walls of brown, red and green seaweeds, respectively, and these polysaccharides are solubilized by hot water (Xu et al. 2017). This may explain that upon cooking the cell walls break down releasing polymers that generate a viscous or slippery sensation in the mouth.
Correlation between sensory analysis and TPA
Instrumental analysis of foods saves time and costs while providing a guide to design and implement sensory trials (Ross 2009). In general, only a few correlations exist between sensory and instrumental textural measurements by TPA. This is attributed to the complexity of the chewing process compared to mechanical tests (Saldaña et al. 2015). However, some correlations existed between data from the sensory panel and the TPA. The hardness and cartilaginous descriptors obtained from the sensory analysis correlated with the hardness measured by TPA (r = 0.543). The linear correlation is statistically not very strong, but it was sensed by the panel, and it may be due to the fact that the ranking method does not reveal the magnitude of the differences between descriptors of samples. Another correlated parameter was sensory stickiness and TPA adhesiveness (r = -0.886). Meullenet et al. (1998) studied 21 foods from various origins by sensory analysis and TPA and found acceptable linear correlations only for hardness and springiness. We agree with their conclusion that the instrumental testing conditions should closely represent phenomena perceived during sensory evaluation.
Conclusions
This work reports on the development of a preliminary vocabulary to evaluate the sensory properties of local seaweeds consumed in Chile. It provides a basic terminology that can be adapted and complemented to other seaweed species and locations. The chemical composition of the three edible seaweeds in this study (D. antarctica, Pyropia spp., and U. lactuca) was comparable to that of similar seaweeds reported in the literature. The nine-member sensory panel agreed after several sessions on a 25-descriptor vocabulary. During sensory analysis of raw and cooked samples, these descriptors managed to differentiate the sensory properties of the seaweeds with the Ranking Descriptive Analysis (RDA) method as demonstrated by Generalized Procrustes Analysis (GPA). Although it was not possible to obtain the true intensity of the descriptors (as would be achieved using Quantitative Descriptive Analysis), a ranking profile of the main characteristic descriptors for each seaweed was obtained. Ulva lactuca was characterized as a bitter seaweed, with an herbaceous aroma and the softest of the three seaweeds. Pyropia spp., was the most umami seaweed although it did not have a distinctive texture and cooking increased the aroma of caramel, and earthy/mouldy and the umami taste. Durvillea antarctica was the least salty, with a caramel aroma and the hardest, and most cartilaginous and sticky seaweed. In general, cooking did not greatly modify the sensory attributes of seaweeds. Physical and chemical analyses corroborated the sensations perceived by the panel in terms of umami taste and texture. Part of the sensory differences observed between the samples investigated may be attributed to their different morphologies, microstructures, and chemical composition.
This work should inspire further research that accurately defines the meanings of the descriptors by an expert panel and the determination of their quantitative sensory values on a numerical scale, superseding the ranking method used in this work. Another aspect requiring further study is the implementation of instrumental testing conditions in TPA that closely relate to phenomena perceived during the sensory evaluation of seaweeds.
Acknowledgements
Authors acknowledge financial support from grant 1180082 of the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT), and a grant from the Technological Centers of Excellence with Basal Financing ANID-Chile to the Cape Horn International Center (CHIC- ANID PIA/BASAL PFB210018).
Author contributions
Conceptualization: Valentina Figueroa, José Miguel Aguilera; Methodology: Valentina Figueroa, Andrea Bunger; Investigation: Valentina Figueroa; Formal analysis: Valentina Figueroa, Andrea Bunger, Jaime Ortiz; Writing—Original Draft: Valentina Figueroa; Supervision: José Miguel Aguilera and Jaime Ortiz; Writing—Review & Editing: José Miguel Aguilera.
Funding
This work was supported by the Fondo Nacional de Desarrollo Científico y Tecnológico FONDECYT [1180082], and partly (JMA) by a grant from the Technological Centers of Excellence with Basal Financing ANID-Chile to the Cape Horn International Center (CHIC- ANID PIA/BASAL PFB210018).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguilera JM. Seaweeds from Chile on the Table. Santiago: Pontificia Universidad Católica; 2021. [Google Scholar]
- Aguirre ME, Owens CM, Miller RK, Alvarado CZ. Descriptive sensory and instrumental texture profile analysis of woody breast in marinated chicken. Poult Sci. 2018;97:1456–1461. doi: 10.3382/ps/pex428. [DOI] [PubMed] [Google Scholar]
- Amorim K, Lage-Yusty M, López-Hernandez J. Changes in bioactive compounds content and antioxidant activity of seaweed after cooking processing. CYTA J Food. 2012;10:321–324. doi: 10.1080/19476337.2012.658871. [DOI] [Google Scholar]
- Anantharaman P, Parthiban C, Saranya C, Girija K, Hemalatha A, Suresh M. Biochemical composition of some selected seaweeds from Tuticorin coast. Adv Appl Sci Res. 2013;4:362–366. [Google Scholar]
- Ansari S, Maftoon-Azad N, Farahnaky A, Hosseini E, Badii F. Effect of moisture content on textural attributes of dried figs. Int Agrophysics. 2014;28:403–412. doi: 10.2478/intag-2014-0031. [DOI] [Google Scholar]
- AOAC . Official Methods of Analysis. Gaithersburg: AOAC; 2012. [Google Scholar]
- Baker AK, Vixie B, Rasco BA, Ovissipour M, Ross CF. Development of a lexicon for caviar and its usefulness for determining consumer preference. J Food Sci. 2014;79:2533–2541. doi: 10.1111/1750-3841.12703. [DOI] [PubMed] [Google Scholar]
- Birch D, Skallerud K, Paul NA. Who are the future seaweed consumers in a Western society? Insights from Australia. Br Food J. 2018;121:603–615. doi: 10.1108/BFJ-03-2018-0189. [DOI] [Google Scholar]
- Bruhn A, Brynning G, Johansen A, Lindegaard MS, Sveigaard HH, Aarup B, Fonager L, Andersen LL, Rasmussen MB, Larsen MM. Fermentation of sugar kelp (Saccharina latissima) - Effects on sensory properties, and content of minerals and metals. J Appl Phycol. 2019;31:3175–3187. doi: 10.1007/s10811-019-01827-4. [DOI] [Google Scholar]
- Bueno de Godoy RC, Chambers E, Yang G (2020) Development of a preliminary sensory lexicon for mate tea. J Sens Stud 35:e12570
- Chapman A, Stévant P, Larssen WE (2015) Food or fad? Challenges and opportunities for including seaweeds in a Nordic diet. Bot Mar 58:423–433
- Chen K, Roca M. Cooking effects on chlorophyll profile of the main edible seaweeds. Food Chem. 2018;15:368–374. doi: 10.1016/j.foodchem.2018.06.040. [DOI] [PubMed] [Google Scholar]
- Chen DW, Zhang M. Non-volatile taste active compounds in the meat of Chinese mitten crab (Eriocheir sinensis) Food Chem. 2007;104:1200–1205. doi: 10.1016/j.foodchem.2007.01.042. [DOI] [Google Scholar]
- Chizoti TS, Cruz MF, Benassi MT, Brugnaro C, de Medeiros SDS, Bordi PL Jr, Verruma-Bernardi MR (2018) Sensory analysis of chocolate milk for college students. J Obesity Overweight 4:107
- Chun S, Chambers E, Han I (2020) Development of a sensory flavor lexicon for mushrooms and subsequent characterization of fresh and dried mushrooms. Foods 9:980 [DOI] [PMC free article] [PubMed]
- Cian RE, Fajardo MA, Alaiz M, Vioque J, González RJ, Drago SR. Chemical composition, nutritional and antioxidant properties of the red edible seaweed Porphyra columbina. Int J Food Sci Nutr. 2013;65:299–305. doi: 10.3109/09637486.2013.854746. [DOI] [PubMed] [Google Scholar]
- Circuncisão AR, Catarino MD, Cardoso SM, Silva AMS (2018) Minerals from macroalgae origin: Health benefits and risks for consumers. Mar Drugs 16:400 [DOI] [PMC free article] [PubMed]
- FAO (1999) Guidelines for the sensory evaluation of fish and shellfish in laboratories. https://www.fao.org/faowhocodexalimentarius/shproxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B31-1999%252FCXG_031e.pdf. Accessed 27 Dec 2021
- Figueroa V, Farfán M, Aguilera JM (2021) Seaweeds as novel foods and source of culinary flavors. Food Rev Int. 10.1080/87559129.2021.1892749
- Francezon N, Tremblay A, Mouget JL, Pasetto P, Beaulieu L. Algae as a source of natural flavors in innovative foods. J Agric Food Chem. 2021;69:11753–11772. doi: 10.1021/acs.jafc.1c04409. [DOI] [PubMed] [Google Scholar]
- Galán-Soldevilla H, Ruiz-Pérez-Cacho MP, Serrano S, Jodral M, Bentabol A. Development of a preliminary sensory lexicon for floral honey. Food Qual Prefer. 2005;16:71–77. doi: 10.1016/j.foodqual.2004.02.001. [DOI] [Google Scholar]
- Giboreau A, Dacremont C, Egoroff C, Guerrand S, Urdapilleta I, Candel D, Dubois D. Defining sensory descriptors: Towards writing guidelines based on terminology. Food Qual Prefer. 2007;18:265–274. doi: 10.1016/j.foodqual.2005.12.003. [DOI] [Google Scholar]
- Guerrero I, Romero A, Tous J. Importance of Generalised Procrustes Analysis in sensory characterisation of virgin olive oil. Food Qual Prefer. 2001;12:515–520. doi: 10.1016/S0950-3293(01)00046-5. [DOI] [Google Scholar]
- Hayakawa F, Kazami Y, Wakayama H, Oboshi R. Sensory lexicon of brewed coffee for Japanese consumers, untrained coffee professionals and trained coffee tasters. J Sens Stud. 2010;25:917–939. doi: 10.1111/j.1745-459X.2010.00313.x. [DOI] [Google Scholar]
- Holdt SL, Kraan S. Bioactive compounds in seaweed: Functional food applications and legislation. J Appl Phycol. 2011;23:543–597. doi: 10.1007/s10811-010-9632-5. [DOI] [Google Scholar]
- ISO (2008) Sensory analysis-vocabulary. https://www.iso.org/obp/ui#iso:std:iso:5492:ed-2:v1:en. Accessed 23 Dec 2021
- Jowitt R. The terminology of food texture. J Texture Stud. 1974;5:351–358. doi: 10.1111/j.1745-4603.1974.tb01441.x. [DOI] [Google Scholar]
- Kato K, Hayashi M, Umene S, Masunaga HA. Novel method for producing softened edible seaweed kombu. Food Sci Technol. 2015;65:618–623. [Google Scholar]
- Kawashima T, Shirai T, Matsuda H, Osako K, Okazaki E. Identification and roles of the taste-active components of dried nori. Japan J Food Eng. 2018;19:121–127. doi: 10.11301/jsfe.18515. [DOI] [Google Scholar]
- Kreischer L, Schuttelaar M. Ocean greens: Explore the world of edible seaweed and sea vegetables. New York: The Experiment; 2016. [Google Scholar]
- Lawless LJ, Civille GV. Developing lexicons: A review. J Sens Stud. 2013;28:270–281. doi: 10.1111/joss.12050. [DOI] [Google Scholar]
- López-Pérez O, Picon A, Nuñez M. Volatile compounds and odour characteristics of seven species of dehydrated edible seaweeds. Food Res Int. 2017;99:1002–1010. doi: 10.1016/j.foodres.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Luna MP, Aguilera JM. Kinetics of colour development of molten glucose, fructose and sucrose at high temperatures. Food Biophys. 2014;9:61–68. doi: 10.1007/s11483-013-9317-0. [DOI] [Google Scholar]
- Mamede MEO, Benassi MT. Efficiency assessment of Flash Profiling and Ranking Descriptive Analysis: a comparative study with star fruit-powdered flavored drink. Food Sci Technol. 2016;36:195–203. doi: 10.1590/1678-457X.0003. [DOI] [Google Scholar]
- Mansilla A, González L, Astorga MS, Ávila M, Ojeda J, Rosenfeld S, Marambio J. Uso de algas marinas en la gastronomía Magallánica. Punta Arenas: Universidad de Magallanes; 2012. [Google Scholar]
- Mateluna C, Figueroa V, Ortiz J, Aguilera JM. Effect of processing on texture and microstructure of the seaweed Durvillaea antarctica. J Appl Phycol. 2020;32:4211–4219. doi: 10.1007/s10811-020-02259-1. [DOI] [Google Scholar]
- Mc Donnell E, Hulin-Bertaud S, Sheehan E, Delahunty C. Development and learning process of a sensory vocabulary for the odor evaluation of selected distilled beverages using descriptive analysis. J Sens Stud. 2001;16:425–445. doi: 10.1111/j.1745-459X.2001.tb00311.x. [DOI] [Google Scholar]
- Meilgaard MC, Civille GV, Carr BT. Sensory evaluation techniques. Boca Raton: CRC Press; 2016. [Google Scholar]
- Meullenet JF, Lyon BG, Carpenter JA, Lyon CE. Relationship between sensory and instrumental texture profile attributes. J Sens Stud. 1998;13:77–93. doi: 10.1111/j.1745-459X.1998.tb00076.x. [DOI] [Google Scholar]
- Milinovic J, Campos B, Mata P, Diniz M, Noronha JP. Umami free amino acids in edible green, red, and brown seaweeds from the Portuguese seashore. J Appl Phycol. 2020;32:3331–3339. doi: 10.1007/s10811-020-02169-2. [DOI] [Google Scholar]
- Moraes L, Carapina C, Ferrerira L, Mansilla A, Ziemann MA, Pereira CM. Evaluation of volatile organic compounds in brown and red sub-Antarctic macroalgae. Rev Bras Bot. 2021;44:79–84. doi: 10.1007/s40415-020-00684-7. [DOI] [Google Scholar]
- Mouritsen OG. Seaweeds: Edible, Available, and Sustainable. London: The University of Chicago Press; 2013. [Google Scholar]
- Mouritsen OG, Williams L, Bjerregaard R, Duelund L (2012) Seaweeds for umami flavour in the new Nordic cuisine. Flavour 1:4
- Mouritsen OG, Duelund L, Petersen MA, Hartmann AL, Frøst MB. Umami taste, free amino acid composition, and volatile compounds of brown seaweeds. J Appl Phycol. 2019;31:1213–1232. doi: 10.1007/s10811-018-1632-x. [DOI] [Google Scholar]
- Munoz IL, Díaz NF (2022) Minerals in edible seaweed: Health benefits and food safety issues. Crit Rev Food Sci Nutr 62:1592–1697 [DOI] [PubMed]
- O’Connor K. Seaweed a global history. London: Reaktion Books; 2017. [Google Scholar]
- Ortiz J, Romero N, Robert P, Araya J, López-Hernández J, Bozzo C, Navarrete E, Osorio A, Rios A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chem. 2006;99:108–104. doi: 10.1016/j.foodchem.2005.07.027. [DOI] [Google Scholar]
- Osborne DR, Voogt P. Análisis de los Nutrientes de los Alimentos. Zaragoza: Acribia; 1986. [Google Scholar]
- Peinado I, Girón J, Koutsidis G, Ames JM. Chemical composition, antioxidant activity and sensory evaluation of five different species of brown edible seaweeds. Food Res Int. 2014;66:36–44. doi: 10.1016/j.foodres.2014.08.035. [DOI] [Google Scholar]
- Pérez-Lloréns JL, Hernández I, Vergara JJ, Brun FG, León A (2017) ¿Las Algas se Comen? Un Periplo por la Biología, la Historia, las Curiosidades y la Gastronomía. Editorial UCA, Cádiz
- Pina AL, Costa AR, Lage-Yusty MA, López-Hernandez J. An evaluation of edible red seaweed (Chondrus crispus) components and their modification during the cooking process. Food Sci Technol. 2014;56:175–180. [Google Scholar]
- Pirian K, Jeliani ZZ, Arman M, Sohrabipour J, Yousefzadi M. Proximate analysis of selected macroalgal species from the persian gulf as a nutritional resource. Trop Life Sci Res. 2020;31:17. doi: 10.21315/tlsr2020.31.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto-Muiños (2021) Porto-Muiños.www.portomuinos.com. Accessed 23 Dec 2021
- Prager HR (2020) What can be done to increase acceptance of seaweed into the western diet. University of Science and Technology, Norway. https://www.ntnu.edu/documents/139799/1273574286/TPD4505.Henry.Prager.pdf/bcb465ea-79e3-45c0-b1d2-1775b3d1852f. Accessed 20 Dec 2021
- Quitral V, Morales C, Sepúlveda M, Schwartz MM. Nutritional and health properties of seaweeds and its potential as a functional ingredient. Rev Chil Nutr. 2012;39:196–202. [Google Scholar]
- Rasyid A. Evaluation of nutritonal composition of the dried seaweed Ulva lactuca from Pameungpeuk Waters. Indonesia Trop Life Sci Res. 2017;28:119–125. doi: 10.21315/tlsr2017.28.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter VB, Avancini de Almeida TC, Prudencio SH, Benassi M. Proposing a ranking descriptive sensory method. Food Qual Prefer. 2010;21:611–620. doi: 10.1016/j.foodqual.2010.03.011. [DOI] [Google Scholar]
- Ross CF. Sensory science at the human–machine interface. Trends Food Sci Technol. 2009;20:63–72. doi: 10.1016/j.tifs.2008.11.004. [DOI] [Google Scholar]
- Rupérez P. Mineral content of edible marine seaweeds. Food Chem. 2002;79:23–26. doi: 10.1016/S0308-8146(02)00171-1. [DOI] [Google Scholar]
- Saldaña E, Behrens JH, Serrano JS, Ribeiro F, de Almeida MA, Contreras-Castillo CJ. Microstructure, texture profile and descriptive analysis of texture for traditional and light mortadella. Food Struct. 2015;6:13–20. doi: 10.1016/j.foostr.2015.09.001. [DOI] [Google Scholar]
- Sanchez-García F, Mirzayeva A, Roldán A, Castro R, Palacios V, Barroso CG, Durán-Gerruero E. Effect of different cooking methods on sea lettuce (Ulva rigida) volatile compounds and sensory properties. J Sci Food Agric. 2021;101:970–980. doi: 10.1002/jsfa.10705. [DOI] [PubMed] [Google Scholar]
- Santelices B. Algas marinas de Chile: Distribución, ecología, utilización y diversidad. Santiago: Ediciones Universidad Católica de Chile; 1989. [Google Scholar]
- Santos MT, Narendra N. Volatile components in seaweeds. Oceanogr Mar Biol. 2018;2:195–201. [Google Scholar]
- Sato H, Koizumi R, Nakazawa Y, Yamazaki M, Itoyama R, Ichisawa M, Negishi J, Sakuma R, Furusho T, Sagane Y, Takano K. Data on the sensory evaluation of potatoes (Solanum tuberosum) from different areas of Hokkaido, Japan, performed by untrained young adults. Data Brief. 2017;15:397–400. doi: 10.1016/j.dib.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura-Campos M, Ruiz-Ruiz J, Betancur-Ancora D, López-Rodríguez K, Chel-Guerrero L, Alaiz-Barragan M. Implementación y validación de un método de análisis de aminoácidos por cromatografía de líquidos de alta resolución. Rev Facult Ing Quím. 2011;51:32–40. [Google Scholar]
- Sharma C, Chambers E, Jayanty SS, Rajakalyan VS, Holm DG, Talavera M. Development of a lexicon to describe the sensory characteristics of a wide variety of potato cultivars. J Sens Stu. 2020;4:e12577. [Google Scholar]
- Smyth HE, Sanderson JE, Sultanbawa Y. Lexicon for the sensory description of Australian native plant foods and ingredients. J Sens Stud. 2012;27:471–481. doi: 10.1111/joss.12012. [DOI] [Google Scholar]
- Stévant P, Indergård E, Ólafsdóttir A, Marfaing H, Larssen WE, Fleurence J, Roleda MY, Rustad T, Slizyte R, Nordtvedt TS. Effects of drying on the nutrient content and physico-chemical and sensory characteristics of the edible kelp Saccharina latissima. J Appl Phycol. 2018;30:2587–2599. doi: 10.1007/s10811-018-1451-0. [DOI] [Google Scholar]
- Stévant P, Olafsdottir A, Deleris P, Dumay J, Fluence J. Data on the sensory characteristics and chemical composition of the edible red seaweed dulse (Palmaria palmata) after dry and semi-dry storage. Data Brief. 2020;33:1–15. doi: 10.1016/j.dib.2020.106343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisawa H, Nakamura K, Tamura H. The aroma profile of the volatiles in marine green algae (Ulva pertusa) Food Rev Int. 1990;6:573–589. doi: 10.1080/87559129009540893. [DOI] [Google Scholar]
- Suwonsichon S (2019) The importance of sensory lexicons for research and development of food products. Foods 8:27 [DOI] [PMC free article] [PubMed]
- Talavera-Bianchi M, Chambers E, IV, Chambers DH. Lexicon to describe flavor of fresh leafy vegetables. J Sens Stud. 2010;25:163–183. doi: 10.1111/j.1745-459X.2009.00249.x. [DOI] [Google Scholar]
- Tan HSG, Tibboel CJ, Stieger M. Why do unusual novel foods like insects lack sensory appeal? Investigating the underlying sensory perceptions. Food Qual Prefer. 2017;60:48–58. doi: 10.1016/j.foodqual.2017.03.012. [DOI] [Google Scholar]
- Tanaka M. Texture of Japanese foods. Food Rev Int. 1986;2:247–265. doi: 10.1080/87559128609540797. [DOI] [Google Scholar]
- Taniwaki M, Sakurai N. Evaluation of the internal quality of agricultural products using acoustic vibration techniques. J Jpn Soc Hortic Sci. 2010;79:113–128. doi: 10.2503/jjshs1.79.113. [DOI] [Google Scholar]
- Tashiro T, Fujita E, Tamai M, Higashi J. High-performance liquid chromatographic determination of 5’- and 2’(3’)-mononucleotides in seaweeds and fishes (Analysis of nucleic acid related substances by ion- exchange chromatography Part VII) Nippon Shokuhin Kogyo Gakkaishi. 1991;38:1–16. doi: 10.3136/nskkk1962.38.1. [DOI] [Google Scholar]
- Texture Analysis Professionals Blog (2017) Food Texture around the World. https://textureanalysisprofessionals.blogspot.com/2017/10/food-texture-around-world.html
- Tuorila H, Hartmann C. Consumer responses to novel and unfamiliar foods. Curr Opin Food Sci. 2019;33:1–8. [Google Scholar]
- Vervoort L, Van der Placken L, Grauwet T, Verlinde P, Matser AM, Hendrickx A, van Loey A. Thermal versus high pressure processing of carrots: A comparative pilot-scale study on equivalent basis. Food Tech. 2012;15:1–13. [Google Scholar]
- Woskow MH. Selectivity in flavor modification by 5'-ribonucleotides. Food Tech. 1969;23:32–37. [Google Scholar]
- Wu G, Ross CF, Morris CF, Murphy KM. Lexicon development, consumer acceptance, and drivers of liking of quinoa varieties. J Food Sci. 2017;82:993–1005. doi: 10.1111/1750-3841.13677. [DOI] [PubMed] [Google Scholar]
- Xu SY, Huang X, Cheong KL. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar Drugs. 2017;15:388. doi: 10.3390/md15120388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. Basic properties of umami and effects on humans. Physiol Behav. 1991;49:833–841. doi: 10.1016/0031-9384(91)90192-Q. [DOI] [PubMed] [Google Scholar]
- Yang J, Lee J. Application of sensory descriptive analysis and consumer studies to investigate traditional and authentic foods: A review. Foods. 2019;8:54. doi: 10.3390/foods8020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Chambers E, Wang H. Flavor lexicon development (in English and Chinese) and descriptive analysis of Sichuan pepper. J Sens Stud. 2020;26:e12636. [Google Scholar]
- Zhu X, Healy LE, Sevindik O, Sun DW, Selli S, Kelebek H, Tiwari B. Impacts of novel blanching treatments combined with commercial drying methods on the physicochemical properties of Irish brown seaweed Alaria esculenta. Food Chem. 2022;369:1–19. doi: 10.1016/j.foodchem.2021.130949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.