Abstract
Recently we reported an unusual multicellular organization in yeast that we termed stalk-like structures. These structures are tall (0.5 to 3 cm long) and narrow (1 to 3 mm in diameter). They are formed in response to UV radiation of cultures spread on high agar concentrations. Here we present an anatomical analysis of the stalks. Microscopic inspection of cross sections taken from stalks revealed that stalks are composed of an inner core in which cells are dense and vital and a layer of cells (four to six rows) that surrounds the core. This outer layer is physically separated from the core and contains many dead cells. The outer layer may form a protective shell for the core cells. Through electron microscopy analysis we observed three types of cells within the stalk population: (i) cells containing many unusual vesicles, which might be undergoing some kind of cell death; (ii) cells containing spores (usually one or two spores only); and (iii) familiar rounded cells. We suggest that stalk cells are not only spatially organized but may undergo processes that induce a certain degree of cell specialization. We also show that high agar concentration alone, although not sufficient to induce stalk formation, induces dramatic changes in a colony's morphology. Most striking among the agar effects is the induction of growth into the agar, forming peg-like structures. Colonies grown on 4% agar or higher are reminiscent of stalks in some aspects. The agar concentration effects are mediated in part by the Ras pathway and are related to the invasive-growth phenomenon.
Some unicellular organisms are capable of forming cooperative multicellular structures, mainly in response to stress conditions, such as starvation or dryness. For example, cells of the bacterium Bacillus subtilis change their colony organization in order to reach potential food sources at distant sites (2, 18). Filamentous fungi are capable of forming hyphae for the same purpose (29, 31). Other organisms form specialized fruiting bodies and even highly differentiated stalks in order to produce and disperse spores (e.g., myxobacteria [4, 13] and the social amoeba Dictyostelium discoideum [12]). Such developed and differentiated structures have not been observed in the yeast Saccharomyces cerevisiae. Yet, in this organism too, there are developmental switches and modifications in colony organization in response to environmental stresses. In response to nitrogen starvation, diploid yeast cells form filamentous structures known as pseudohyphae (10, 21). This phenomenon is genetically related to another phenotype, known as invasive growth, that is observed in haploid yeast cells (24). When grown on solid agar supplemented with rich media, haploid cells penetrate the agar and are not washed away when placed under a water current. Both invasive growth and filamentous growth require an intact Ras/cyclic AMP (cAMP) cascade and Ras/Ste11/Kss1 cascade (19–21, 24, 26, 30). These Ras pathways are required for activation of specific invasive/pseudohyphal genes, such as Flo11 (15, 21, 26), and for suppression of stress-related genes, targets of the Msn2/4 system (30). Interestingly, some laboratory strains, such as ∑1278b, are capable of showing the pseudohyphal/invasive phenotypes, whereas other strains, such as S288C, W303, and SP1, are either completely incapable of invasiveness or show inefficient invasiveness (14, 30). In the SP1 genetic background, invasive growth can be obtained through constitutive activation of the Ras pathway (e.g., through the RAS2Val19 and bcy1Δ mutations). The basis for the differences in invasive capabilities of the different genetic backgrounds is not fully understood. Some strains seem to harbor mutations in genes essential for invasiveness (14). Another explanation is the constitutive suppression of the stress response in the ∑1278b background. Cells of this genetic background contain high cAMP levels; consequently, their Msn2/4/STRE machinery cannot properly respond to stress (30).
Recently, yet another multicellular organization of S. cerevisiae cells was reported. Engelberg et al. reported stalk-like structures that are formed when cultures are spread on high agar concentrations and are subsequently exposed to UV radiation (see details in reference 6) (see examples in Fig. 3 below of this paper). The average length of a stalk is 1 cm (varying between 0.5 and 3 cm) and consists of 0.5 × 106 to 3 × 106 cells. A large variety of yeast genetic backgrounds as well as various mutants were tested for their ability to form stalks, and all were found capable (6; data not shown). Notably, these mutants also included strains that are defective in invasive/pseudohyphal growth. Furthermore, other types of yeast (Candida albicans and Schizosaccharomyces pombe) and even the bacterium Escherichia coli were found capable of forming stalks under similar conditions. We suggested therefore that stalk formation might not be genetically regulated and proposed a mechanical/environmental model to explain the formation of stalks (Fig. 3 in reference 6). Briefly, the model assumes that when a dense layer of cells is spread on a plate, some cells fall into tiny pits in the agar. Most of the cells on the surface die under intensive UV radiation, but cells in the pits, which are shaded, or covered by other cells have better chances of survival. Surviving cells proliferate, but when the pit is filled, only cells in the bottom are in contact with nutrients and continue to divide. Dividing cells continue to fill the already crowded pit and thereby extrude other cells of the colony out of the pit just as toothpaste is squeezed out of the tube. The continued extrusion forms the stalk (Fig. 3 in reference 6). It is not fully understood why dividing cells in the bottom do not penetrate the agar. It seems, however, that the geometry of the pit, the concentration of the agar, and its dryness are the key factors that induce stalk formation and may prevent penetration. This model suggests that a stalk is simply a pile of unorganized cells, passively extruded from the pit by dividing cells in the bottom.
FIG. 3.
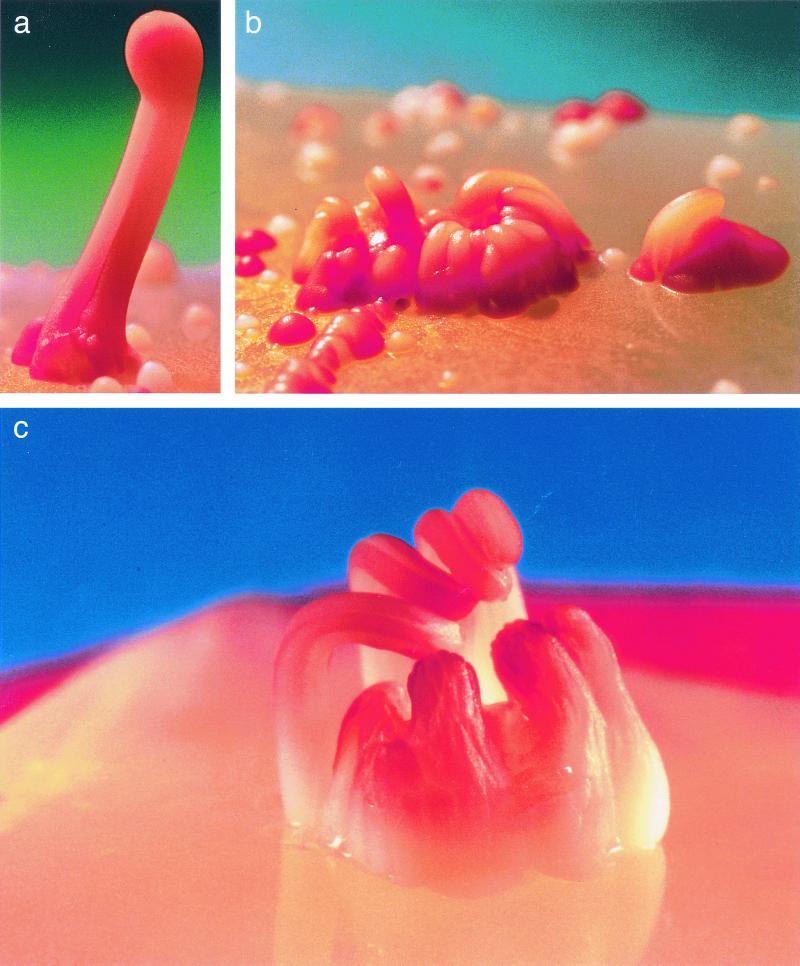
Stalks and minute stalks tend to aggregate. (a) Two stalks merging into one. (b) Aggregation of minute stalks, on a plate supplemented with 4% agar (see text for details). (c) Eight stalks originating from one colony. Pictures were taken by David Darom. The 419M strain was used.
In this report we present further investigation of the yeast stalk phenomenon. Surprisingly, microscopic inspection of cross sections suggested that a stalk is not composed of randomly piled cells but is rather an organized, multicellular structure. We report that a stalk is composed of an inner core in which cells are dense and seem vital. The core is surrounded by an outer layer of cells (composed of four to six rows of cells). The outer layer is physically separated from the core, thereby forming a protective shell for the core cells. Further, within the population of stalk cells we observed at least three types of cells distinct in their morphology: (a) familiar rounded cells; (b) dying or dead cells containing a large number of vesicles; and (c) cells containing spores. We suggest therefore that stalk cells are not only spatially organized but manifest some degree of specialization.
In addition to analyzing stalk anatomy, we attempted to dissect the roles of UV radiation and high agar concentrations on the formation of stalks. We report that UV radiation alone does not affect colony organization, nor does it induce stalk formation. High agar concentration, on the other hand, has a dramatic effect on the size, height, and shape of the colonies. We further report that high agar concentrations induce invasive growth. Rather strikingly, cells of a colony, unlike cells plated to form a lawn, do not invade homogenously. Cells in the center of the colony invade more deeply, forming a peg-like structure. We show that the agar effect on formation of peg-like structures is mediated through the Ras pathway, suggesting a linkage between invasive growth and multicellular organization of yeast colonies.
MATERIALS AND METHODS
Yeast strains and media.
Cells were plated on yeast-peptone-dextrose (YPD) medium (2% glucose, 1% yeast extract, and 2% Bacto Peptone). The agar concentration used for stalk formation was 4%. Agar concentrations in other experiments are described for each experiment. The strains used in this study are listed in Table 1.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| SP1 | MATahis3 leu2 ura3 trp1 ade8 Can | 32 |
| TK161R2V (SP1RAS2Val19) | Isogenic to SP1 but RAS2Val19::HIS3 | 32 |
| SP1ras2Δ | Isogenic to SP1 but ras2::LEU2 | 30 |
| SP1ras2Δyap1Δ | Isogenic to SP1 but ras2::LEU2 yap1::HIS3 | 30 |
| ΣL5527 | MAT ura3 trp1 | 30 |
| Σras2Δ | Isogenic to L5527LH but ras2::LEU2 | 30 |
| L5585 (Σste20Δ) | MATa ste20::TRP1 trp1::hisG ura3-52 | 24 |
| SR599 (Σira1Δ) | MATaira1::LEU2 leu2::hisG, his3::hisG ura3-52 | 26 |
| 419M | MATa/MATα ade2/ade2-R8 ura3-52/ura3-52 his4-912/HIS4 lys2-201/lys2 trp5-d/TRP5 leu2-3,112/LEU2 his7/HIS7 metX/MET can1/CAN | G. Simchen's stock |
| W303 | MATacan1-100 ade2-1 his3-11, 15 leu2-3 trp1-1 ura3-1 | Yeast Genetic Stock Center (Berkeley, Calif.) |
| YPH102 | MATaura3-52 lys2-80 ade2-101 leu2-1 his3-200 | 28 |
Stalk formation.
Stalks were formed as previously described (6).
Invasive-growth assay.
Cells were plated on YPD supplemented with the agar concentrations described in each experiment. Plates were washed under a water current as described (24).
Fixation for microscopic analysis.
As stalks were found to disaggregate readily when incubated in solution (data not shown), standard fixation protocols could not be applied. The following protocols were therefore developed and used: stalks were cut out from the plates together with the piece of agar on which they grew. They were then placed horizontally on 1% agar drops that were kept on the verge of solidifying temperature (40 to 42°C). Stalks were immediately covered with similar agar drops at the same temperature. Agar drops solidified readily upon contact with stalks. This treatment embedded intact stalks inside agar cubes. In most cases stalks were not affected by this treatment. The agar cubes that were containing stalks were incubated in fixation solution (2.5% paraformaldehyde and 2% glutaraldehyde in 0.1 M cacodylic acid) in room temperature for 1 h and were further incubated at 4°C for 48 h. Then fixation solution was diluted (1:2) in cacodylic acid, and the cubes were incubated at 4°C for 1 week. The cubes were washed in the following order: 0.1 M cacodylic acid (10 min, three to five times), double distilled water (DDW) (10 to 15 min, three times), metaperiodate (2% in DDW; 40 to 60 min), DDW (10 to 15 min, two times), and cacodylic acid (10 to 15 min, three times). The cubes were then incubated in osmium solution (1% OsO4, 0.1 M cacodylic acid buffer, and 1% potassium ferricyanide) for 2 h and were further washed with 0.1 M cacodylic acid (four to five times for 1 min and three times for 15 min), ethanol (30, 50, 70, 90, and 95% for 15 min each and 100% for 30 min, three to five times), and propylene oxide (10 min, two times). The cubes were then incubated with agar 100 resin diluted in propylene oxide in the following order: 1:3 dilution for 24 h, 1:1 dilution for 24 h, 3:1 dilution for 24 h, and an undiluted solution for 24 h two times. Finally the cubes were incubated at 60°C overnight.
Preparation of cross sections.
For light microscopy analysis, blocks were sliced in a microtome (thickness, 4 to 7 μm) by using glass knives. The slices were placed on slides, dried, and covered by cover glass. For electron microscopy analysis, blocks were sliced in a microtome (thickness, 700 Å) by using diamond knives.
RESULTS AND DISCUSSION
Cross sections of stalks reveal a core and shell organization.
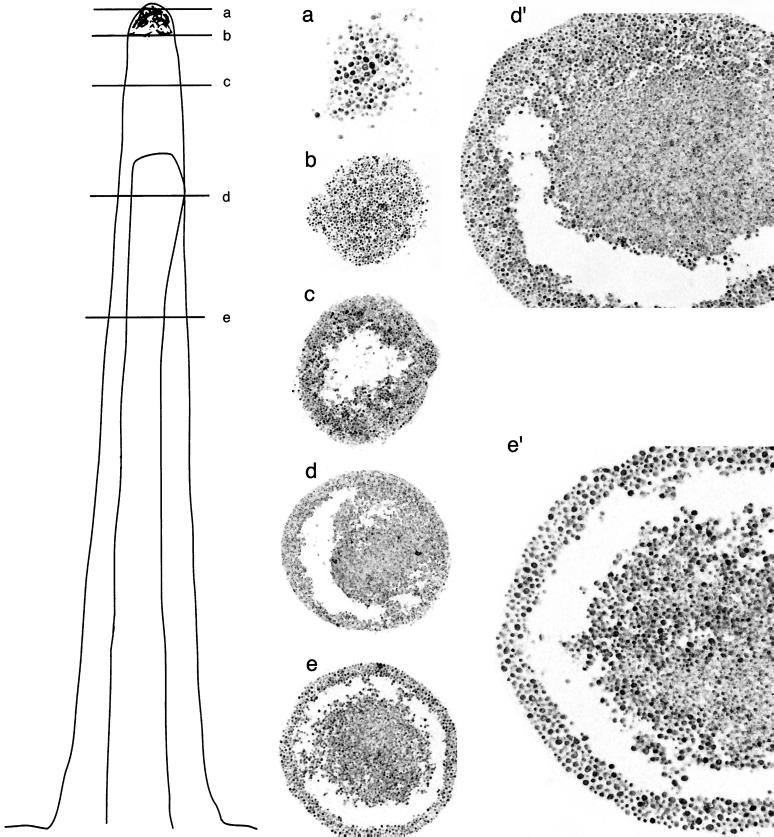
The present model describing stalk formation is a mechanical/environmental model (6). According to this model, a stalk is a pile of unorganized, nondividing, similar cells that were extruded out of a pit in the agar. To test whether stalks are indeed composed of randomly localized, unorganized, similar cells, we prepared cross sections (thickness, 4 to 7 μm; see Materials and Methods) from different locations along the stalk and inspected them under the microscope (Fig. 1). A cross section of the stalk's tip showed, as expected, a layer of cells (Fig. 1a and b). Strikingly, however, a cross section taken from several millimeters below the tip showed that in this area there are no cells in the center of the stalk (Fig. 1c). Cross sections made further below revealed an even more unexpected picture (Fig. 1d and e). These cross sections revealed an inner core that is composed of a large number of dense cells. Surrounding the core is an outer layer, physically separated from the core. The layer is composed of four to six rows of cells. Cells of the outer layer seem less dense and are usually bigger than core cells (Fig. 1d and e). Closer inspection of the outer layer cells showed that some of them are transparent, maybe lacking vital cytoplasm and organelles (Fig. 1d′ and e′ and 2a). Many of the cells in the outer layer may actually be dead cells that form a protective shell around the stalk (see below).
FIG. 1.
Stalks are composed of a core and shell structure. Light microscopy inspection of cross sections (thickness, 4 to 7 μm) prepared from different locations along a stalk. Locations from which cross sections were taken are indicated in the schematic illustration on the left. Stalks were prepared from the strain 419M. Cross sections were prepared from three different stalks, and a similar pattern of organization was observed. Pictures were taken using the following lens objectives (note that additional enlargements were made during printing processes): (a) 40×; (b and c) 20×; (d) 16×; (d′) same cross section as for panel d but with a 20× lens; (e) 20×; (e′) same cross section as for panel e but with a 40× lens.
In summary, the analysis of cross sections at the light microscopy level strongly suggests that a stalk is an organized structure and not a random pile of cells. More importantly, these observations suggest that there are morphological differences between cells in different areas of the stalk. Do these results invalidate the mechanical model for stalk formation? We believe that stalks are formed through the mechanism suggested by this model, but spatial organization, cell death, and (perhaps) specialization take place in a later phase, after the stalks have been formed (see below).
Some cells in stalk contain many unusual vesicles, while others contain spores.
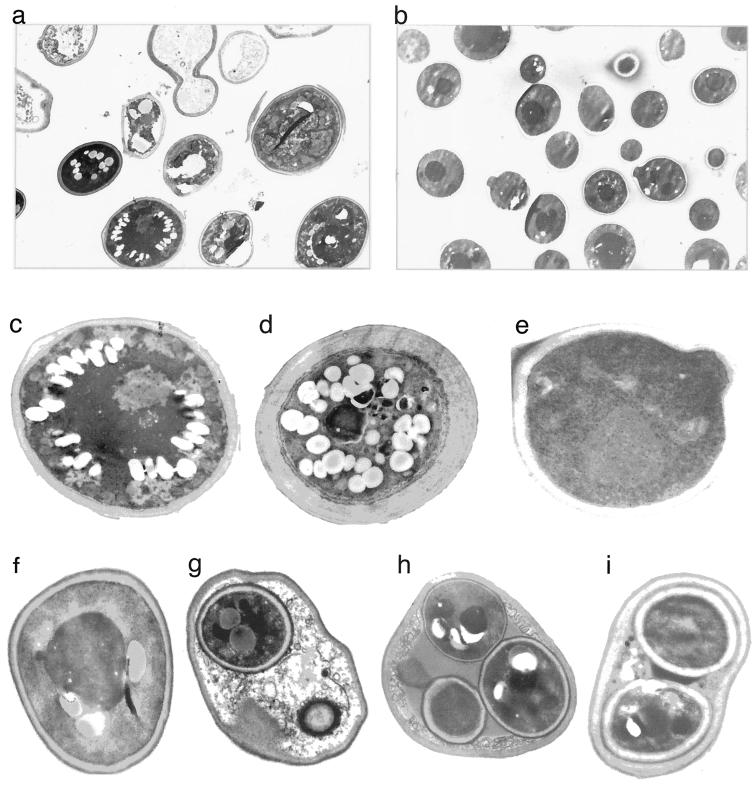
To further test the notion suggested by the analysis of cross sections that morphological differentiation does exist between cells in a stalk, we examined stalk cells using electron microscopy. This analysis revealed significant differences in the morphological structure of different cells in the stalk. Many stalk cells show an aberrant (very wide) cell wall and possess many vesicles and in many cases unidentifiable nuclei and ribosomes (Fig. 2a, c, and d). Many other stalk cells seem more like familiar yeast cells (Fig. 2f), but even these cells contain several vesicles or fat bodies and are somewhat different from cells taken from regular colonies (Fig. 2e). The vesicles observed could be autophagic bodies, suggesting that many cells are undergoing autophagy (1). To verify that the aberrant appearance of many cells was not the consequence of fixation and staining protocols, we looked at cells taken from a normal colony that had been treated in parallel to a stalk with the same technical procedures. These cells showed a familiar phenotype (Fig. 2b and e), supporting the idea that the aberrant morphology of some stalk cells is not a technical artifact. Interestingly, all stalk cells inspected, including those with aberrant cytoplasm, organelles, and cell wall, maintained cell shape. Namely, although unusually wide, in all cases a complete cell wall surrounded the cell. Only intracellular components were affected and ultimately destroyed, rendering the cells transparent to the electron beam (Fig. 2a). This situation is reminiscent of cells from higher eukaryotes when they are undergoing programmed cell death (11, 22). We tested stalk cells for apoptotic markers but could observe neither the classical chromatin condensation nor DNA ladder (data not shown). We therefore cannot define the process responsible for cell death in the stalk. It is possible that many of the dying cells reside in the perimeter of the stalks, an area that is exposed to the environment and is less protected from dryness. As a result, the cells may be simply drying out.
FIG. 2.
Stalks are composed of dying and dead cells, spore-containing cells, and familiar cells. Electron microscopy inspection of cross sections (thickness, 700 Å) of different locations within a stalk. Cross sections of stalks and regular colonies were prepared as described in Materials and Methods. The 419M strain (an a/α diploid) was used in all cases. Electron microscopy analysis was performed on two stalks and two colonies. (a) Cells of a stalk. (b) Cells of a regular colony, used as control. (c and d) Cells of a stalk that contain many vesicles (that could be fat bodies or autophagic bodies), a wide cell wall, and unidentifiable nucleus and ribosomes (these cells may be dying). (e) Cell of a regular colony, used as control. (f) Intact cell of a stalk. (g to i) Cells of a stalk that contain spores.
Another type of cell found in the stalk was those that contain spores (Fig. 2g to i). Interestingly, in most cases inspected by us, those cells were not classical asci and contained only one or two spores (Fig. 2g and i). In fewer cases three spores were observed (Fig. 2h), and only in very rare cases did we see an ascus containing four spores, as is the common case of cultures exposed to sporulation medium (8). We do not know why most spore-containing cells contain less than four spores. Curiously, in mammals and plants, meiosis of an egg results in a single gamete, containing the cytoplasm of the other three meiosis products, rather than four gametes (9).
These findings show that stalks are composed of at least three morphologically distinct types of cells. Combining the analysis of cross sections (Fig. 1) with the electron microscopy studies, it seems that dying cells are located mostly in the outer layers of the stalk, whereas most of the spore-containing cells, as well as rounded, familiar cells, are located in the inner core. Previously it was reported that asci are localized to the upper parts of the stalk (6). Thus, cell specialization is observed both along the longitude and latitude of stalks.
High agar concentration but not UV irradiation affects morphology of yeast colonies.
High agar concentrations and UV irradiation are both essential for stalk formation (6), but each of these treatments alone is not sufficient to induce stalks. It is still possible, however, that these treatments have some effect on the morphology of yeast colonies. To test whether UV irradiation per se has any effect on colony organization, yeast cells were plated (106 cells/plate) on YPD with different agar concentrations (1 to 6%) and were subsequently UV irradiated (∼40 J/m2) as described previously (6). All surviving colonies were inspected carefully for any unusual morphology. Also, the number of stalks formed on each agar concentration was counted. As shown in Table 2, no stalks were formed on plates containing less than 2% agar. Stalks were reproducibly observed on plates supplemented with agar concentrations higher than 4%. We noted that in some cases stalks aggregated in small areas on the plate, suggesting that particular, localized properties of the agar are beneficial for stalk formation. In some cases, several stalks originated from a single colony (Fig. 3c). Finally, we noted that on plates supplemented with high agar concentrations, even colonies that were not considered stalks acquired a particular appearance and tended to aggregate (Fig. 3b). However, no unusual morphology of colonies was observed on plates containing less than 2% agar (and lacking stalks), showing that UV irradiation alone does not induce any unusual form of multicellular organization.
TABLE 2.
Number of stalks obtained with different agar concentrations
| Agar concn (%)a |
No. of stalks per plate |
|---|---|
| 1 | 0 |
| 1.5 | 0 |
| 2 | 0.66 |
| 3 | 2.33 |
| 4 | 1 |
| 5 | 3.33 |
| 6 | 9 |
Cells of the 419M strain were plated on YPD medium, supplemented with the indicated agar concentrations, and subsequently UV irradiated (∼40 J/m2). The number of stalks formed on each agar concentration was counted, and the average number of stalks per plate (three plates of each concentration) is shown.
High agar concentration alone induces dramatic changes in a colony's morphology.
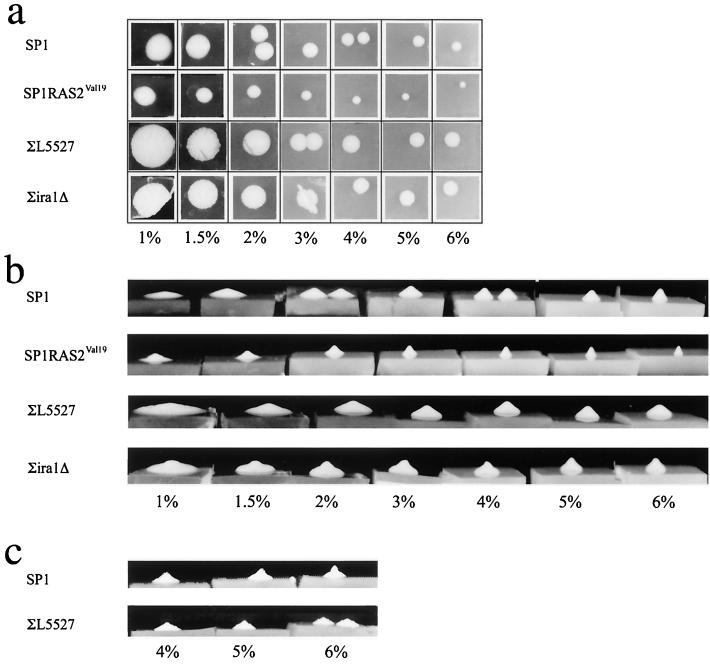
Next, we tested the effect of agar concentration alone on the morphology of yeast colonies. We plated yeast cells (about 50 cells of the wild-type strain SP1 per plate) on YPD media with different agar concentrations (1 to 6%) and allowed colonies to develop without any exposure to UV radiation. We found that the agar concentration dramatically affected the size and shape of the colonies. As the agar concentration increased from 1 to 4%, the diameter of the colonies decreased logarithmically (Fig. 4a, upper row). At agar concentrations higher than 4%, the colonies' diameter decreased only slightly. The decrease in the size of colonies was associated with a decrease in the total number of cells per colony (data not shown). Corresponding to the decrease in diameter observed at high agar concentrations, the height of the colonies increased. This effect can be seen in Fig. 4b (upper row), which shows a side view of the same colonies shown in Fig. 4a. Colonies formed at low agar concentrations (<1.5%) acquired the shape of a large, flat disk. At high agar concentrations (>3%), colonies became slim and relatively tall (1 to 3 mm) and acquired a cone shape (Fig. 4b and c, upper rows). In all strains tested (see below), colonies appeared on the plates and acquired their typical morphology within 24 to 72 h after plating. Yet we noticed that colonies grown at high agar concentrations undergo changes in their morphology if further incubated for 2 to 3 weeks at room temperature. As shown in Fig. 4c, 3 weeks after plating, the colonies lose the cone shape and the upper part of the colony becomes narrower. Some of the 3-week-old colonies are reminiscent of stalks in their overall appearance (although significantly shorter) and were therefore termed “minute stalks.” The appearance of minute stalks after 3 weeks suggests that colonies are dynamic, multicellular structures in which morphological and structural changes take place after they have been formed.
FIG. 4.
Agar concentration, incubation time, and the Ras pathway affect the colony's morphology. The indicated strains were grown on YPD supplemented with different agar concentrations (1 to 6%) as indicated in the bottom of each panel. (a) Photographs of colonies taken from above, 1 week after plating. (b) Photographs taken from a side view, 1 week after plating. Same colonies are shown in panels a and b. (c) Photographs taken from a side view, 3 weeks after plating.
Although minute stalks were observed, it should be stressed that on all agar concentrations tested, not a single stalk was observed.
In summary, agar concentration alone, unlike UV, did affect the morphology of the colonies but could not give rise to well-developed stalks. High agar concentrations induce formation of minute stalks, which are morphologically different from the disk-shaped colonies formed on low agar concentrations. Namely, just as with stalk morphology, the structure of a common colony is also regulated, at least partially, by mechanical and environmental factors.
Formation of minute stalks is regulated both environmentally and genetically.
The results described above show that the agar concentration affects the morphology of yeast colonies of the strain SP1. To verify that the phenomenon is general, we tested the effect of agar concentrations on strains of different genetic backgrounds. Cells of the haploid strains W303, YPH102 (isogenic to S288C), and ∑L5527 (isogenic to ∑1278b) and the diploid strain 419M were plated on YPD media with different agar concentrations (1 to 6%) and were allowed to grow without any exposure to UV irradiation. All strains showed a similar phenotype. Namely, as the agar concentration increased, the colony's shape changed from a flat disk to a cone or to a minute stalk (data not shown). In spite of the overall similar pattern, we observed some morphological differences between colonies of the different strains. For example, colonies of the ∑1278b background (the ∑L5527 strain in Fig. 4a and b) are larger than SP1 colonies (Fig. 4). Also, SP1 colonies are smooth, while ∑L5527 colonies are furrowed (Fig. 4a and c and 6). These differences between strains suggest that the structure of colonies, unlike that of stalks, might be genetically controlled in combination with the environmental control. This notion is in agreement with the study of Reynolds and Fink, who showed that a combination of low agar concentration with the activity of the FLO8 and FLO11 genes is required for biofilm formation in S. cerevisiae (23).
Which genes may affect the shape and morphology of the colonies? One of the differences between SP1 and ∑1278b genetic backgrounds is the more active Ras pathway in ∑1278b. Cells of the ∑1278b genetic background were shown to contain higher cAMP levels, to induce constitutive Gcn4 activity, and to suppress the cellular stress response (16, 30). All those characteristics are known to be associated with an active Ras pathway (3, 5, 7, 17, 25, 27). To test whether the Ras cascade is involved in determining colony morphology, we used mutants in the Ras pathway and plated them on YPD medium with different agar concentrations (1 to 6%). We found that mutants carrying a constitutively active Ras cascade formed unusual colonies compared to the corresponding wild-type strains. As shown in Fig. 4b, colonies of the SP1RAS2Val19 strain did not form a flat disk on low agar concentrations and acquired a conical shape even at 1% agar. Additionally, on high agar concentrations, this strain formed colonies of very small diameter (∼3 mm), reminiscent in structure but not in size of stalks. Activation of the Ras pathway in the ∑1278b background (by deleting the IRA1 gene) had a similar but not identical effect (Fig. 4b). Similar to SP1RAS2Val19 colonies, colonies of the ∑ira1Δ strain did not form a flat disk on low agar concentration but rather formed a bulge at their center. This bulge grew more significant as agar concentrations increased (Fig. 4b). These results support the notion that a combination of environmental and genetic factors determines the colony's morphology. They point at the Ras pathway as an important element in the machinery that controls the colony's morphology.
The same components affect a colony's morphology and invasive growth.
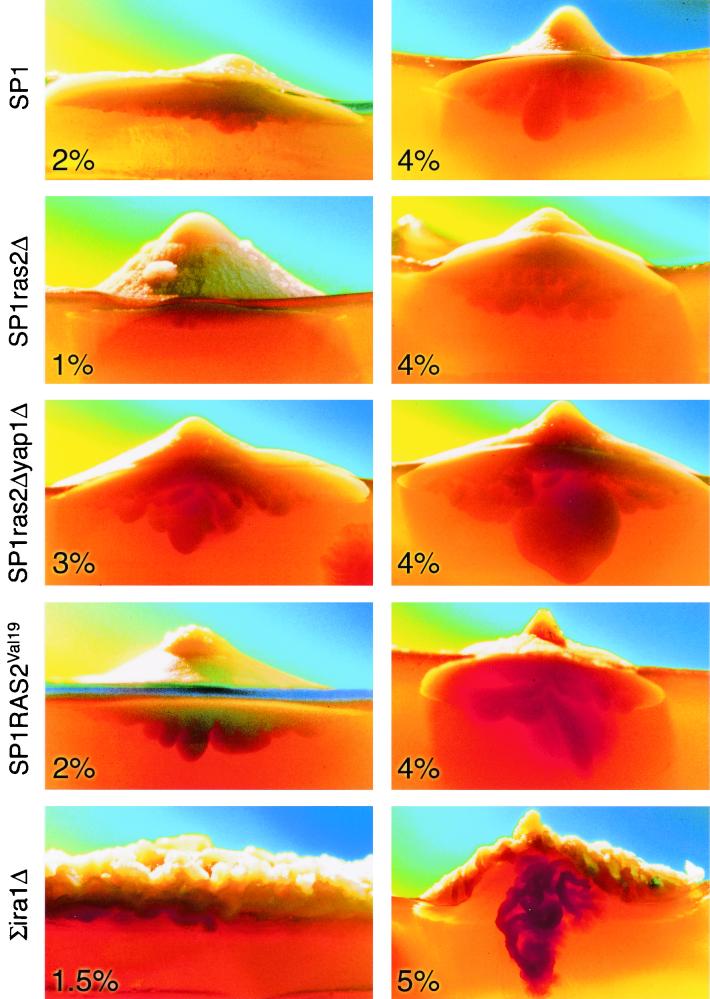
During the course of growing colonies at high agar concentrations, we noticed that unusual morphological changes occur not only above agar surface but also inside the agar under the visible colony. In fact, some of the colonies developed structures inside the agar, large enough to be clearly observed through the agar (Fig. 5). In most cases “under-agar” structures originated from the center of the colony and formed a structure reminiscent of a peg or even a short root (Fig. 5). The tendency to grow into the agar was clearly affected by agar concentrations. Colonies of the SP1 strain, for example, developed shallow peg-like structures when grown on 2% agar, whereas deep structures were developed on 4% agar (Fig. 5, top row). Yet formation of under-agar structures is also genetically controlled. Colonies of the SP1ras2Δ strain did not produce significant peg-like structures on 2 or 4% agar (Fig. 5, second row), whereas SP1RAS2Val19 colonies developed such structures on both low and high agar concentrations (Fig. 5, fourth row). The most unusual under-agar growth was observed in colonies of the ∑ira1Δ strain. This strain manifested its unusual under-agar growth at high agar concentrations but not at low concentrations (Fig. 5, fifth row). Thus, also in the ∑1278b genetic background, a combination of environmental and genetic components is required for formation of peg-like structures. In addition to their well-developed under-agar growth, the ∑ira1Δ colonies had an unusual, furrowed appearance (Fig. 5, fifth row, and 6). It is clear that the Ras cascade has a strong impact not only on colony size and height (Fig. 4a and b), but also on the development of an unusual growth pattern under the colony.
FIG. 5.
High agar concentrations in combination with an active Ras pathway induce formation of organized structures under the agar (peg-like structures). The indicated strains were grown on YPD supplemented with the indicated agar concentrations. Photographs were taken from side view, usually 3 weeks after plating. Colonies were illuminated from the back, using a high-intensity lamp, to allow photographing the structures formed inside the agar. Pictures were taken by David Darom.
It seems that the colony's size, height, and under-agar growth are related phenotypes and are all controlled by the same biochemical activity, seemingly that of the Ras pathway. The Ras pathway has been previously shown to control invasive-growth activity through the cAMP pathway and the Kss1 mitogen-activated protein kinase pathway (19–21, 24, 26, 30). As formation of peg-like structures requires agar invasiveness, it is not surprising that ras2Δ colonies cannot form fully developed pegs and that the RAS2Val19 and ira1Δ strains develop deep pegs (Fig. 5). The idea that formation of pegs is part of the invasive-growth phenomenon is further supported by the fact that the SP1ras2Δyap1Δ strain produced deeper and more developed peg-like structures than did SP1ras2Δ colonies (compare second and third rows of Fig. 5). It was previously shown that while ras2Δ cells cannot invade, they regain some invasiveness capability if their YAP1 gene is disrupted, thereby suppressing their stress response (30).
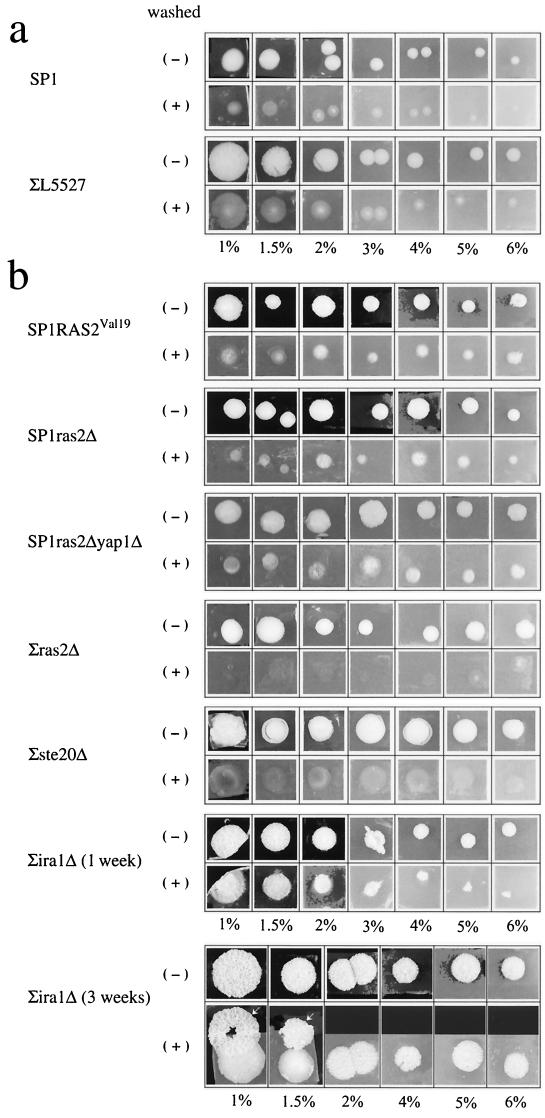
To directly test the linkage between classical invasive growth and formation of peg-like structures, we tested the invasive-growth capability of the various mutants. As expected, strains that developed deep pegs (RAS2Val19, ira1Δ, and ras2Δyap1Δ strains; Fig. 5) showed strong invasive activity (Fig. 6). In contrast, strains that did not develop deep pegs (ras2Δ strain [Fig. 5] and ste20Δ strain [data not shown]) showed very low invasive activity, supporting the idea that the phenotypes are linked. Strikingly, however, some of the strains that do not invade well (e.g., SP1ras2Δ strain ) did invade if plated on high agar concentrations (Fig. 6), in particular if incubated for long periods (2 to 3 weeks). Three-week-old colonies are attached to the agar much more strongly than 1-week old colonies (Fig. 6b; compare colonies of the ∑iraΔ strain). Thus, time plays an important role in invasive growth. Perhaps the increasing dryness and reduction in nutrient availability are the driving forces for time-dependent increase in invasiveness. This result strengthens the notion that colonies are dynamic moieties that change their properties in time. This experiment also revealed that the pattern of invasiveness of colonies is different from that of patches. As was shown in numerous studies (19, 24, 26, 30), patches invade the agar homogeneously. Colonies, on the other hand, seem to invade differentially. Cells in the perimeter do not invade the agar deeply, whereas cells in the center invade deeply and form peg-like structures (Fig. 6).
FIG. 6.
Formation of structures inside the agar is linked to classical invasive growth. The indicated strains were grown on YPD plates supplemented with different agar concentrations (1 to 6%, as indicated in the bottom of each panel). Colonies were photographed before washing (−), washed under a water current, and photographed again (+). (a) Colonies were washed 1 week after plating. (b) Colonies were washed 3 weeks after plating. For the ∑ira1Δ strain the 1-week-old colonies are also shown. Note that a complete layer of cells was washed off the 3-week- old ∑ira1Δ colonies. These layers lay above the colony and are indicated by arrows.
Colonies may be forming a core and shell structure just like stalks.
While washing the colonies, we noticed that in many strains, even invasive ones, cells of the upper layer of the colony were easily washed off the colony by a water current. As this upper layer was most fragile and usually peeled off in many small fragments, only in rare cases were we able to remove it in one piece for demonstration (Fig. 6, colonies of the ∑ira1Δ strain grown on 1% and 1.5% agar). Such a composition of an outer layer covering a core colony is reminiscent of stalks' spatial organization reported above (Fig. 1). To further verify that colonies are similar to stalks in this respect, we prepared cross sections of a regular colony for microscopic inspection. Figure 7 shows that, similarly to stalks, colonies are also composed of an outer layer and a core. Yet some differences between colonies and stalks are apparent. First, the outer layer of the colony is not well separated from the core as was shown for stalks (compare Fig. 1 and 7). In addition, cells in the outer layer of the colony seem denser than cells in the core of the colony, whereas in stalks the phenotypes are opposite (Fig. 1). It could be that separation of outer layer and further differentiation of this layer (perhaps involving cell death) are slow processes in colonies and may be observed in colonies that are several months old. This idea is supported by the fact that the outer layer is easily peeled off only in older colonies. Clearly many more experiments are required to determine the spatial and temporal organization of yeast colonies. Yet based on our initial analysis, we suggest that many morphological characteristics of stalks are found in regular colonies. A stalk could therefore be considered a colony in which, as a result of particular conditions, many properties are extended or emphasized. It must be noted, however, that although from the morphological point of view, stalks could be considered a type of regular colony, the mechanism and regulation of stalk formation are still a puzzle. Clearly, environmental conditions affect the morphology (size, height, invasiveness, and formation of peg-like structures) of both colonies and stalks of S. cerevisiae. Yet while those properties observed in colonies are also genetically controlled, we could not measure any genetic control on stalk formation or morphology. Namely, all mutants tested, including a large variety of mutants of the Ras cascade, were capable of forming fully developed stalks (6; data not shown). Thus, stalk formation is a robust property, which seems to be controlled exclusively by environmental components. Further spatial organization and temporal differentiation of the stalk may be controlled genetically. The biological relevance of cell organization within the stalk is not clear. The possibility remains that these structures are formed only in the laboratory, in response to a most unique set of conditions. Nevertheless, it is clear from the present study that both stalks and regular colonies are organized and dynamic multicellular moieties in which active processes continuously take place a long time after the formation of the colony or stalk.
FIG. 7.
A colony is composed of a core and an outer layer. Light microscopy inspection of a vertical cross section prepared from a regular colony of the 419M strain. The picture was taken using a 16× lens. Additional enlargements were made during the printing processes. Part of the colony is shown.
ACKNOWLEDGMENTS
We thank David Darom, head of the scientific photography unit of our institute, for taking many of the photos for this paper and for advice and help throughout the project. We thank Gerald R. Fink for yeast strains. We thank Alan Bar-Sinai, Michal Bell, Ricardo Capone, Melanie Grably, Irit Marbach, Ayelet Oppenheim, Giora Simchen, Ariel Stanhill, and Gilad Yaacov for critically reading the manuscript.
REFERENCES
- 1.Abeliovich H, Dunn W A, Jr, Kim J, Klionsky D J. Dissection of autophagosome biogenesis into distinct nucleation and expansion steps. J Cell Biol. 2000;151:1025–1034. doi: 10.1083/jcb.151.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Jacob E, Cohen I, Gutnick D L. Cooperative organization of bacterial colonies: from genotype to morphotype. Annu Rev Microbiol. 1998;52:779–806. doi: 10.1146/annurev.micro.52.1.779. [DOI] [PubMed] [Google Scholar]
- 3.Broach J R, Deschenes R J. The function of ras genes in Saccharomyces cerevisiae. Adv Cancer Res. 1990;54:79–139. doi: 10.1016/s0065-230x(08)60809-x. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin M. The Myxobacterales [(fruiting) Myxobacterales] 2nd ed. Vol. 1. Cleveland, Ohio: CRC Press; 1997. [Google Scholar]
- 5.Engelberg D, Klein C, Martinetto H, Struhl K, Karin M. The UV response involving the Ras signaling pathway and AP-1 transcription factors is conserved between yeast and mammals. Cell. 1994;77:381–390. doi: 10.1016/0092-8674(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 6.Engelberg D, Mimran A, Martinetto H, Otto J, Simchen G, Karin M, Fink G R. Multicellular stalk-like structures in Saccharomyces cerevisiae. J Bacteriol. 1998;180:3992–3996. doi: 10.1128/jb.180.15.3992-3996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelberg D, Zandi E, Parker C S, Karin M. The yeast and mammalian Ras pathways control transcription of heat shock genes independently of heat shock transcription factor. Mol Cell Biol. 1994;14:4929–4937. doi: 10.1128/mcb.14.7.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito R E, Klapholz S. Meiosis and ascospore development. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1981. [Google Scholar]
- 9.Gilbert S F. Developmental biology. 5th ed. Sunderland, Mass: Sinauer Associates; 1997. [Google Scholar]
- 10.Gimeno C J, Ljungdahl P O, Styles C A, Fink G R. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell. 1992;68:1077–1090. doi: 10.1016/0092-8674(92)90079-r. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson M D, Weil M, Raff M C. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 12.Kessin R H, Campagne M M V-L. The development of social amoeba. Am Sci. 1992;80:556–565. [Google Scholar]
- 13.Kim S K, Kaiser D, Kuspa A. Control of cell density and pattern by intercellular signaling in Myxococcus development. Annu Rev Microbiol. 1992;46:117–139. doi: 10.1146/annurev.mi.46.100192.001001. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Styles C A, Fink G R. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo W S, Dranginis A M. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:161–171. doi: 10.1091/mbc.9.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marbach I, Licht R, Frohnmeyer H, Engelberg D. Gcn2 mediates Gcn4 activation in response to glucose stimulation or UV radiation not via GCN4 translation. J Biol Chem. 2001;276:16944–16951. doi: 10.1074/jbc.M100383200. [DOI] [PubMed] [Google Scholar]
- 17.Marchler G, Schuller C, Adam G, Ruis H. A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 1993;12:1997–2003. doi: 10.1002/j.1460-2075.1993.tb05849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendelson N H, Salhi B. Patterns of reporter gene expression in the phase diagram of Bacillus subtilis colony forms. J Bacteriol. 1996;178:1980–1989. doi: 10.1128/jb.178.7.1980-1989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosch H U, Kubler E, Krappmann S, Fink G R, Braus G H. Crosstalk between the Ras2p-controlled mitogen-activated protein kinase and cAMP pathways during invasive growth of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:1325–1335. doi: 10.1091/mbc.10.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosch H U, Roberts R L, Fink G R. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5352–5356. doi: 10.1073/pnas.93.11.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan X, Harashima T, Heitman J. Signal transduction cascades regulating pseudohyphal differentiation of Saccharomyces cerevisiae. Curr Opin Microbiol. 2000;3:567–572. doi: 10.1016/s1369-5274(00)00142-9. [DOI] [PubMed] [Google Scholar]
- 22.Raff M C. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds T B, Fink G R. Bakers' yeast, a model for fungal biofilm formation. Science. 2001;291:878–881. doi: 10.1126/science.291.5505.878. [DOI] [PubMed] [Google Scholar]
- 24.Roberts R L, Fink G R. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- 25.Ruis H, Schuller C. Stress signaling in yeast. Bioessays. 1995;17:959–965. doi: 10.1002/bies.950171109. [DOI] [PubMed] [Google Scholar]
- 26.Rupp S, Summers E, Lo H J, Madhani H, Fink G. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 1999;18:1257–1269. doi: 10.1093/emboj/18.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin D Y, Matsumoto K, Iida H, Uno I, Ishikawa T. Heat shock response of Saccharomyces cerevisiae mutants altered in cyclic AMP-dependent protein phosphorylation. Mol Cell Biol. 1987;7:244–250. doi: 10.1128/mcb.7.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Springer M L, Yanofsky C. A morphological and genetic analysis of conidiophore development in Neurospora crassa. Genes Dev. 1989;3:559–571. doi: 10.1101/gad.3.4.559. [DOI] [PubMed] [Google Scholar]
- 30.Stanhill A, Schick N, Engelberg D. The yeast Ras/cyclic AMP pathway induces invasive growth by suppressing the cellular stress response. Mol Cell Biol. 1999;19:7529–7538. doi: 10.1128/mcb.19.11.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timberlake W E. Molecular genetics of Aspergillus development. Annu Rev Genet. 1990;24:5–36. doi: 10.1146/annurev.ge.24.120190.000253. [DOI] [PubMed] [Google Scholar]
- 32.Toda T, Uno I, Ishikawa T, Powers S, Kataoka T, Broek D, Cameron S, Broach J, Matsumoto K, Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985;40:27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]