Abstract
Objectives
The FlowTriever All‐Comer Registry for Patient Safety and Hemodynamics (FLASH) is a prospective multi‐center registry evaluating the safety and effectiveness of percutaneous mechanical thrombectomy for treatment of pulmonary embolism (PE) in a real‐world patient population (NCT03761173). This interim analysis reports outcomes for the first 250 patients enrolled in FLASH.
Background
High‐ and intermediate‐risk PEs are characterized by high mortality rates, frequent readmissions, and long‐term sequelae. Mechanical thrombectomy is emerging as a front‐line therapy for PE that enables immediate thrombus reduction while avoiding the bleeding risks inherent with thrombolytics.
Methods
The primary endpoint is a composite of major adverse events (MAE) including device‐related death, major bleeding, and intraprocedural device‐ or procedure‐related adverse events at 48 h. Secondary endpoints include on‐table changes in hemodynamics and longer‐term measures including dyspnea, heart rate, and cardiac function.
Results
Patients were predominantly intermediate‐risk per ESC guidelines (6.8% high‐risk, 93.2% intermediate‐risk). There were three MAEs (1.2%), all of which were major bleeds that resolved without sequelae, with no device‐related injuries, clinical deteriorations, or deaths at 48 h. All‐cause mortality was 0.4% at 30 days, with a single death that was unrelated to PE. Significant on‐table improvements in hemodynamics were noted, including an average reduction in mean pulmonary artery pressure of 7.1 mmHg (22.2%, p < 0.001). Patient symptoms and cardiac function improved through follow‐up.
Conclusions
These interim results provide preliminary evidence of excellent safety in a real‐world PE population. Reported outcomes suggest that mechanical thrombectomy can result in immediate hemodynamic improvements, symptom reduction, and cardiac function recovery.
Keywords: hemodynamics, mechanical thrombectomy, percutaneous intervention, pulmonary embolism
1. INTRODUCTION
Pulmonary embolism (PE) has been characterized by high acute mortality rates and unsatisfactory long‐term outcomes. 1 PE represents the third leading cause of death from cardiovascular disease, 2 with the clinical impact of PE predicated by the degree of acute right ventricular (RV) dysfunction. Contemporary registries report 30‐day mortality rates up to 30% in high‐risk PE and 15% in intermediate‐risk PE. 3 Furthermore, patients who survive the acute episode often experience long‐term complications including pulmonary hypertension, 1 exercise intolerance, dyspnea, and reduced quality of life. 4 , 5 , 6
The ideal treatment for PE patients at risk for progressive decompensation would allow for safe, rapid debulking of the pulmonary arteries (PA) to improve acute right heart strain and prevent long term consequences of pulmonary vascular obstruction. Historically, systemic and catheter‐directed thrombolytics have represented the dominant therapies to improve acute RV failure. In the landmark PEITHO trial, intermediate‐risk PE patients treated early with thrombolytics had less hemodynamic deterioration compared to anticoagulation alone. 7 These results parallel earlier thrombolytic trials which included both intermediate‐risk and high‐risk PE patients. 8 The consistent trade‐off with thrombolytic therapy in these trials, however, was an increased major bleeding rate of up to 10%, 2 , 9 including an intracerebral hemorrhage rate of up to 2%. 2 , 8 , 10 Therefore, the need for careful consideration of thrombolysis risks versus benefits has been paramount when considering advanced treatment.
The FlowTriever System (Inari Medical) is the first FDA‐cleared mechanical thrombectomy device for the treatment of PE. The large‐bore system combines aspiration and mechanical thrombus extraction to obviate the need for thrombolytics and their associated bleeding risk. The safety and effectiveness of FlowTriever thrombectomy in treating intermediate‐risk PE was first demonstrated in the FLARE trial. 11 After single‐session thrombectomy, patients showed significant improvement in right ventricle/left ventricle (RV/LV) ratio and a low rate of major adverse events, with only 1.9% of patients receiving adjunctive thrombolytics. While these initial results were encouraging, the FLARE study was performed using the first‐generation FlowTriever System, the patient cohort was restricted to intermediate‐risk PE patients, and outcome measures were limited to surrogate measures of clinical outcomes. Therefore, the FlowTriever All‐Comer Registry for Patient Safety and Hemodynamics (FLASH) was designed to evaluate the safety and effectiveness of the current FlowTriever System in a broader real‐world setting, including both intermediate‐risk and high‐risk PE patients and collecting direct measures of patient hemodynamic status following thrombectomy as well as longer‐term clinical outcomes. This report represents the first publication of results from the initial 250 patients enrolled in the FLASH registry.
2. MATERIALS AND METHODS
2.1. Study design
FLASH is a prospective, multi‐center registry (ClinicalTrials.gov identifier: NCT03761173) to evaluate real‐world outcomes in PE patients treated with the FlowTriever System. Investigators obtained Institutional Review Board approval at each site before enrolling patients, and all patients provided written informed consent.
Inclusion and exclusion criteria were selected to allow an assessment of the FlowTriever System in an all‐comers population to mimic real‐world clinical practice. Inclusion criteria were patients over 18 years old who had clinical signs and symptoms of acute PE with evidence of proximal filling defect in at least one main or lobar PA and who were undergoing PE treatment with the FlowTriever System per the investigator's discretion. Exclusion criteria were limited only to patients unable to be anticoagulated, known sensitivity to radiographic contrast that cannot be adequately pretreated, evidence that the patient was not an appropriate candidate for mechanical thrombectomy, life expectancy less than 30 days, or current participation in another investigational drug or device treatment study that would interfere with participation in FLASH. Enrolled patients were classified as high‐risk or intermediate‐risk (including intermediate‐high and intermediate‐low subclasses) according to the criteria specified in the current ESC guidelines for diagnosis and management of acute PE. 12 After treatment with the FlowTriever System, follow‐up assessments occurred at 48 h, 30 days, and 6 months.
2.2. Primary endpoint
The primary endpoint of FLASH is a composite of major adverse events (MAE) within 48 h of the index procedure consisting of device‐related death, major bleeding, and device‐ or procedure‐related adverse events. Major bleeding was defined based on BARC Type 3b or greater. 13 Intraprocedural device‐ or procedure‐related adverse events were specified as clinical deterioration defined by hemodynamic or respiratory worsening meeting specific thresholds described in the study protocol, device‐related pulmonary vascular injury, and device‐related cardiac injury.
2.3. Secondary endpoints
Secondary safety endpoints include the individual components of the MAE composite endpoint, major access site complications requiring open surgical or endovascular intervention or blood transfusion, all‐cause mortality through 30 days, and device‐related serious adverse events (SAE) within 30 days. Secondary effectiveness endpoints include on‐table changes in hemodynamics and vitals during the procedure, as well as markers of cardiac size and function at follow‐up as measured by echocardiography. The baseline RV/LV ratio is a composite of computed tomography (CT) and echocardiography assessments, with CT prioritized if both were available. To exclude bias due to differences in imaging techniques, the longitudinal analysis of RV/LV ratio was exclusively based on paired echocardiography data, with the latest follow‐up prioritized if multiple were available.
Additional secondary endpoints include utility measures such as thrombectomy time (calculated from when the Triever aspiration catheter enters the vasculature until final removal), estimated blood loss, lengths of postprocedure hospital and intensive care unit (ICU) stays, and dyspnea as measured on the modified Medical Research Council (mMRC) dyspnea scale from 0 to 4.
2.4. Hemodynamic calculations
Invasive hemodynamic assessment was performed per protocol before the procedure and 5 min following removal of the Triever catheter. Standard hemodynamic variables were collected, including right atrial pressure and systolic, diastolic, and mean PA pressures. Other derived hemodynamic endpoints including cardiac index, total pulmonary vascular resistance, stroke volume index, RV stroke work index, and PA pulsatility index were calculated using standard formulae.
2.5. Data collection and analysis
Patients were considered to be enrolled when the FlowTriever System entered the vasculature, with signed informed consent allowed before or after the procedure to provide investigators flexibility in enrolling a real‐world PE population. This interim analysis focused on assessment of acute and 48‐h safety and effectiveness along with additional longer‐term safety and clinical outcomes where available, including serious adverse events, hospital readmissions, heart rate, and dyspnea scores at up to 30 days and RV size and function at the latest point available up through study exit. An independent medical monitor adjudicated adverse events and determined device‐ and procedure‐relatedness. Data are presented either as numbers (%), mean ± standard deviation, or median [interquartile range]. p values for hypothesis testing were calculated by Wilcoxon signed‐rank and McNemar's tests for continuous and categorical outcomes, respectively, using available pairwise values.
3. RESULTS
3.1. Patient population
From December 2018 to July 2020, 250 patients with acute high‐risk (massive) or intermediate‐risk (submassive) PE were enrolled at 19 US sites. The majority of patients were male (52.4%), and the average age was 60.9 ± 13.9 years. Concomitant deep vein thrombosis (DVT) was present in most patients (68.4%) and almost a quarter of patients (23.6%) presented with a history of cancer, including 8.0% with active cancer. Nearly 15% of patients had undergone recent surgery, and 40% had a contraindication to thrombolytics. Most (93.2%) patients had intermediate‐risk PE and 6.8% had high‐risk PE per current ESC guidelines. Most (84.3%) patients had an sPESI score ≥1. Additional patient demographics and PE‐relevant variables are outlined in Table 1.
Table 1.
Baseline demographics and clinical characteristics of acute PE
| Characteristic | n (%) or mean ± SD |
|---|---|
| Age (Years) | 60.9 ± 13.9 |
| Male sex | 131 (52.4%) |
| History of DVT | 53 (21.2%) |
| History of PE | 31 (12.4%) |
| History of pulmonary hypertension | 36 (14.5%) |
| Concomitant DVT | 169 (68.4%) |
| Active bleed | 6 (2.4%) |
| History of cancer | 59 (23.6%) |
| Active cancer | 20 (8.0%) |
| Recent surgery | 35 (14.0%) |
| Thrombolytics contraindication | 100 (40.0%) |
| Intermediate‐risk (Submassive) | 233 (93.2%) |
| Intermediate‐high | 200 (85.8%) |
| Intermediate‐low | 15 (6.4%) |
| Unclassified | 18 (7.7%) |
| High‐risk (massive) | 17 (6.8%) |
| sPESI | 1.6 ± 1.1 |
| 0 | 37 (15.7%) |
| ≥1 | 198 (84.3%) |
| Positive biomarkers (troponin and/or BNP) | 226 (96.2%) |
| RV/LV ratio (CT or echocardiogram) | 1.5 ± 0.5 |
| Saddle PE | 97 (38.8%) |
| Unilateral PE | 29 (11.6%) |
| Bilateral PE | 124 (49.6%) |
Note: Numbers vary from 233 to 250 patients for the different variables.
Abbreviations: numbers (n), standard deviation (SD), deep vein thrombosis (DVT), pulmonary embolism (PE), simplified pulmonary embolism severity index (sPESI), B‐type natriuretic peptide (BNP), right ventricle (RV), left ventricle (LV), computed tomography (CT).
3.2. Procedural characteristics and hospital stay
A representative FLASH case, including pulmonary angiograms and extracted clot burden, is shown in Figure 1. The median thrombectomy time reported was 46.0 [29.0–70.0] min, and the median estimated blood loss was 255.0 [100.0–425.0] ml. Twelve patients (4.8%) were given adjunctive therapy, including 11 patients (4.4%) who received thrombolytics. There was only one (0.4%) access site complication, a hematoma which occurred in a patient who had received intraprocedural thrombolytics. Most patients (56.8%) did not require an ICU stay following thrombectomy. Further procedural results are shown in Table 2.
Figure 1.
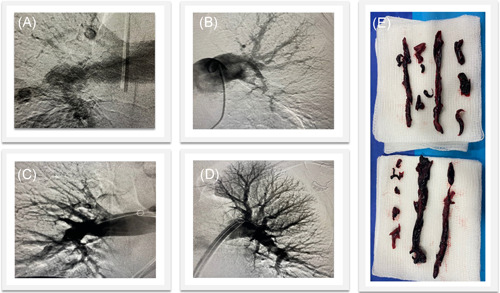
Representative FLASH case of a high‐risk PE patient treated with the FlowTriever System. Pre‐thrombectomy pulmonary angiograms showed filling defects in the right (A) and left (B) PAs which resolved following treatment in (C) and (D). Extracted thrombus is shown in (E). Case images provided by Dr. Hriday Shah (St. Joseph Mercy, Ann Arbor, MI). PA, pulmonary arteries; PE, pulmonary embolism [Color figure can be viewed at wileyonlinelibrary.com]
Table 2.
Procedural characteristics
| Characteristic | n (%) or median [IQR] |
|---|---|
| Access site | Femoral: 247 (98.8%) |
| Jugular: 3 (1.2%) | |
| Access site complications | 1 (0.4%) |
| Thrombectomy time (min) | 46.0 [29.0–70.0] |
| Estimated blood loss (ml) | 255.0 [100.0–425.0] |
| Patients receiving adjunctive therapy | 12 (4.8%) |
| Hospital length of stay (days postprocedure) | 3.0 [2.0–5.0] |
| ICU length of stay (days postprocedure) | 0.0 [0.0–1.0] |
| Patients in ICU (postprocedure) | 108 (43.2%) |
Note: Numbers vary from 220 to 250 patients for the different variables.
Abbreviations: ICU, intensive care unit; IQR, interquartile range; n, numbers.
3.3. Primary endpoint and safety
The major safety outcomes are outlined in Table 3. Within 48 h postprocedure, MAEs included in the primary endpoint occurred in 3 (1.2%) patients, all of which were considered SAEs but none of which were device‐related or involved intracerebral hemorrhage. All three MAEs were major bleeds that occurred in patients who recently underwent other interventions or received thrombolytics. All three patients recovered following a transfusion of two units of blood without further sequelae. There were no other intraprocedural device‐ or procedure‐related adverse events, including no clinical deteriorations, device‐related pulmonary vascular injuries, or device‐related cardiac injuries. There were also no patient deaths (0.0%) at 48 h.
Table 3.
Safety endpoints following treatment with the FlowTriever System
| Safety endpoints | n (%) |
|---|---|
| 48‐h all‐cause mortality | 0 (0%) |
| 30‐day all‐cause mortality | 1 (0.4%) |
| 48‐h MAE composite | 3 (1.2%) |
| Device‐related death | 0 (0%) |
| Major bleeds (none were intracerebral hemorrhages) | 3 (1.2%) |
| Intraprocedural device‐ or procedure‐related adverse events | 0 (0%) |
| Clinical deterioration | 0 (0%) |
| Device‐related pulmonary vascular injuries | 0 (0%) |
| Device‐related cardiac injuries | 0 (0%) |
| 30‐day SAE (device‐related) | 0 (0%) |
Note: Numbers vary from 247 for 48‐h data to 242 for 30‐day data.
Abbreviations: numbers (n), major adverse events (MAE), serious adverse events (SAE).
Among 242 patients with 30‐day safety data available, there was one patient death (0.4%) through 30 days, an 80‐year‐old woman who experienced septic shock and ischemic bowel 12 days postprocedure. In addition to the three MAEs, there were 12 other non‐device‐related SAEs reported through 30 days, each of which occurred in a single patient. A list of adjudicated SAEs is provided in Table 4.
Table 4.
Serious adverse events (not including MAEs) observed within 30 days and relation to the FlowTriever System
| Preferred MedDRA term | Device‐related? | Number of occurrences |
|---|---|---|
| Anemia | No | 1 |
| Bradycardia | No | 1 |
| Cardiac failure | No | 1 |
| Cerebrovascular accident | No | 1 |
| Deep vein thrombosis | No | 1 |
| Hemoptysis | No | 1 |
| Hypotension | No | 1 |
| Hypovolemic shock | No | 1 |
| Intestinal perforation | No | 1 |
| Retroperitoneal hematoma | No | 1 |
| Shock hemorrhagic | No | 1 |
| Ventricular tachycardia | No | 1 |
Note: These events do not include the three MAEs reported in the primary endpoint results, all of which were also SAEs.
Abbreviations: MAE, major adverse event; MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
3.4. Acute hemodynamics and vitals
A summary of acute changes in hemodynamics and vitals is shown in Table 5. In all 248 patients with mean PA pressures available pre‐ and post‐thrombectomy, mean PA pressures improved significantly by 7.1 mmHg on average (22.2%, p < 0.001) from 31.9 ± 8.3 mmHg to 24.8 ± 8.6 mmHg (Figure 2). Furthermore, in the subset of patients with evidence of pulmonary hypertension at baseline (mean PA pressure ≥25 mmHg; n = 199, 80.2%), there was also a significant on‐table reduction in mean PA pressure of 7.6 mmHg (22.0%, p < 0.001) from 34.6 ± 7.0 mmHg to 27.0 ± 8.0 mmHg. In all 202 patients with cardiac index (CI) available pre‐ and post‐thrombectomy, there was no significant change in CI (2.7 ± 1.0 vs. 2.8 ± 1.7 l/min/m2 (p = 0.881)). However, in the subset of patients with a low baseline CI (<2.0 l/min/m2; n = 42, 20.8%), CI significantly improved by 13.3% on‐table from 1.7 ± 0.2 to 1.9 ± 0.4 l/min/m2 (p = 0.005). The overall decrease in PA pressure and increased cardiac output resulted in a significant decrease in the total pulmonary vascular resistance from 6.1 ± 2.5 to 4.6 ± 2.0 Wood units (p < 0.001).
Table 5.
On‐table changes in hemodynamics and vitals following treatment with the FlowTriever System
| Hemodynamic/vital value |
Pre‐FT (mean ± SD) |
Post‐FT (mean ± SD) |
Difference (% change) |
p value |
|---|---|---|---|---|
| Systolic PA pressure (mmHg) |
51.9 ± 12.4 n = 248 |
39.8 ± 12.8 n = 246 |
−12.3 (−23.7%) n = 245 |
<0.001 |
| Mean PA pressure (mmHg) |
31.9 ± 8.3 n = 249 |
24.8 ± 8.6 n = 248 |
−7.1 (−22.2%) n = 248 |
<0.001 |
| Mean PA pressure (baseline ≥ 25 mmHg) |
34.6 ± 7.0 n = 200 |
27.0 ± 8.0 n = 199 |
−7.6 (−22.0%) n = 199 |
<0.001 |
| Mean PA pressure (baseline < 25 mmHg) |
21.1 ± 2.8 n = 49 |
16.1 ± 4.3 n = 49 |
−5.1 (−22.8%) n = 49 |
<0.001 |
| Right atrial pressure (mmHg) |
11.1 ± 5.9 n = 229 |
8.9 ± 5.5 n = 208 |
−2.3 (−18.1%) n = 206 |
<0.001 |
| Heart rate (bpm) |
101.4 ± 15.0 n = 250 |
87.9 ± 13.3 n = 250 |
−13.5 (−12.6%) n = 250 |
<0.001 |
| Systolic blood pressure (mmHg) |
126.3 ± 20.8 n = 248 |
124.8 ± 22.5 n = 247 |
−1.7 (−0.5%) n = 247 |
0.185 |
| CI (l/min/m2) |
2.7 ± 1.0 n = 220 |
2.8 ± 1.7 n = 207 |
0.1 (3.9%) n = 202 |
0.881 |
| CI (baseline < 2.0 l/min/m2) |
1.7 ± 0.2 n = 47 |
1.9 ± 0.4 n = 42 |
0.2 (13.3%) n = 42 |
0.005 |
| CI (baseline ≥ 2.0 l/min/m2) |
2.9 ± 0.9 n = 173 |
3.0 ± 1.9 n = 160 |
0.0 (1.4%) n = 160 |
0.207 |
| Total pulmonary vascular resistance (mmHg/l/min) |
6.1 ± 2.5 n = 220 |
4.6 ± 2.0 n = 207 |
−1.5 (−20.4%) n = 202 |
<0.001 |
|
Stroke volume index (baseline < 33 ml/m2/beat) |
23.5 ± 5.6 n = 147 |
25.9 ± 7.9 n = 134 |
2.3 (12.0%) n = 134 |
0.001 |
| RV stroke work index (g·m/m2) |
8.4 ± 4.9 n = 208 |
7.0 ± 8.3 n = 184 |
−1.3 (−15.5%) n = 178 |
<0.001 |
| PA pulsatility index |
4.0 ± 4.6 n = 225 |
4.6 ± 5.8 n = 201 |
0.7 (14.4%) n = 199 |
0.816 |
Note: All hemodynamic values, with the exception of heart rate, were assessed on‐table, immediately before and after the procedure. Heart rate was assessed as the in‐hospital pre‐procedure average and the in‐hospital postprocedure average. Mean PA pressure is reported separately in patients with elevated baseline values, physiologically normal baseline values, and in all patients with data. CI is reported separately in patients with low baseline values, physiologically normal baseline values, and in all patients with data. Stroke volume index is exclusively reported in patients with low baseline values. All p values are based on available paired assessments using Wilcoxon signed‐rank tests.
Abbreviations: bpm, beats per minute; CI, cardiac index; FT, FlowTriever; PA, pulmonary artery; RV, right ventricular; SD, standard deviation.
Figure 2.
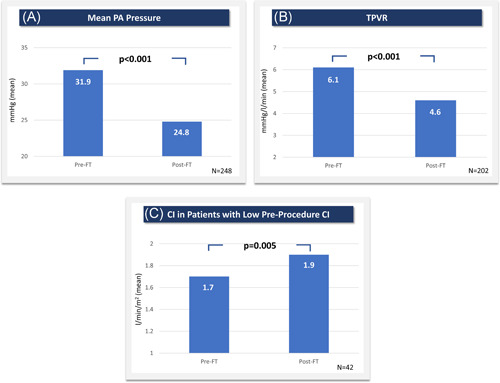
On‐table hemodynamic improvements. (A) Mean PA pressure, (B) total pulmonary vascular resistance, and (C) cardiac index in patients with baseline values below 2.0 l/min/m2 were all significantly improved immediately following FlowTriever thrombectomy. CI, cardiac index; FT, FlowTriever; PA, pulmonary artery; TPVR, total pulmonary vascular resistance. All p values are based on available paired assessments using Wilcoxon signed‐rank tests [Color figure can be viewed at wileyonlinelibrary.com]
Heart rate significantly decreased during hospitalization by 13.5 bpm on average (12.6%, p < 0.001) from a pre‐procedure average of 101.4 ± 15.0 bpm to a postprocedure average of 87.9 ± 13.3 bpm. Heart rate continued to significantly improve at 30 days (80.7 ± 14.6 bpm, p < 0.001) compared to the in‐hospital pre‐procedure average (Figure 3). There was also a significant decrease in the percentage of tachycardic (>100 bpm) patients from 49.2% pre‐procedure to 17.2% during postprocedure in‐hospital monitoring (p < 0.001) and to 11.3% at 30 days (p < 0.001).
Figure 3.
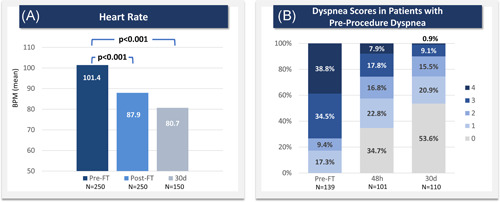
Heart rate and dyspnea improvements through 30 days. (A) Heart rate is reported as the in‐hospital pre‐procedure average (“Pre‐FT”), the in‐hospital post‐procedure average (“Post‐FT”), and the value at the 30‐day visit (“30d”). p values are based on Wilcoxon signed‐rank tests for follow‐up values paired with Pre‐FT values. (B) Dyspnea severity is reported for patients who were dyspneic before treatment, showing dyspnea scores pre‐procedure and at 48 h and 30 days postprocedure based on the modified Medical Research Council (mMRC) dyspnea scale. BPM, beats per minute; FT, FlowTriever [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Dyspnea scores
Among patients who were dyspneic before thrombectomy (score > 0 on the mMRC scale), dyspnea scores decreased significantly from 2.9 ± 1.1 preprocedure to 1.4 ± 1.3 at 48 h (p < 0.001). The average dyspnea score showed further significant improvement to 0.8 ± 1.1 at 30 days (p < 0.001) in patients with paired assessments, with an 86.4% reduction in the percentage of patients with a dyspnea score of 3 or 4 as compared to pre‐procedure (Figure 3).
3.6. RV size and function
RV size and function were assessed using multiple echocardiographic variables at follow‐up times ranging from 48 h to 6 months after thrombectomy. Each patient's latest follow‐up measurement was prioritized if more than one was available, with the median follow‐up time ranging between 32.0 and 33.5 days depending on the variable. In 86 patients with paired measurements, the mean RV/LV ratio normalized at follow‐up, significantly decreasing by 0.36 ± 0.76 (28.3%) from 1.27 ± 0.26 pre‐procedure to 0.91 ± 0.75 at follow‐up (p < 0.001). In 61 patients with paired measurements, RV systolic pressure also significantly decreased by 19.1 ± 15.6 mmHg (35.8%) from 53.3 ± 14.2 mmHg pre‐procedure to 33.5 ± 11.9 mmHg at follow‐up (p < 0.001). RV systolic dysfunction and dilatation also significantly improved (p < 0.001), with 90.9% of patients having no or mild RV systolic dysfunction and 84.2% having no or mild RV dilatation at follow‐up. All available data for RV size and function improvements are summarized in Figure 4.
Figure 4.
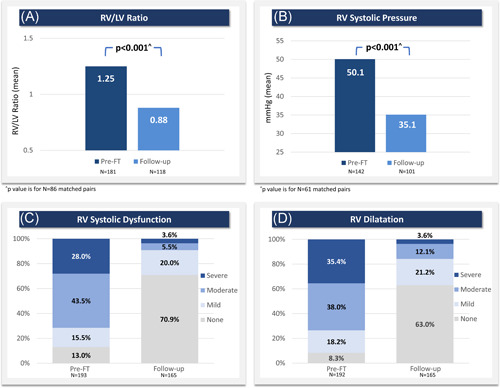
Improved RV size and function assessed via echocardiography. (A) Mean RV/LV ratio, (B) mean RV systolic pressure, (C) RV systolic dysfunction grades, and (D) RV dilatation grades each showed significant improvement at follow‐up, which represents a composite of the latest assessment available for each subject. Median follow‐up time was 32.0–33.5 days, depending on the variable. In (A) and (B), all available preprocedure and follow‐up data are shown, though statistical comparisons are limited to patients with paired assessments (RV/LV ratio: n = 86 with mean difference of −0.36; RV systolic pressure: n = 61 with mean difference of −19.1 mmHg). p values are calculated from Wilcoxon signed‐rank tests. FT, FlowTriever; LV, left ventricle; RV, right ventricle [Color figure can be viewed at wileyonlinelibrary.com]
3.7. 30‐day readmissions
A total of 13/216 (6.0%) patients were readmitted to the hospital through 30 days. Only one (0.5%) of these readmissions was related to the patient's acute PE, involving a patient who had experienced minor hemoptysis during the procedure and was re‐admitted for another occurrence of hemoptysis which resolved without sequelae.
4. DISCUSSION
Interim results from the first 250 patients enrolled in the FLASH registry indicate that mechanical thrombectomy with the FlowTriever System acutely improves clinically relevant hemodynamic parameters in high‐risk and intermediate‐risk PE patients. Safety outcomes in this real‐world population were favorable, with a low 1.2% MAE rate and no deaths through 48 h. At 30 days, mortality and incidence of serious adverse events remained low, while patients showed continuous improvement in heart rate and dyspnea. RV size and function also showed improvement through the latest follow‐up in those patients who had paired assessments available.
Though a current limitation of this technology is the potential blood loss associated with aspiration thrombectomy, which may be clinically relevant in patients with anemia at baseline or low filling pressures, the low rate of major bleeding events (1.2%) reported is encouraging as it is similar to that observed with anticoagulation alone in randomized trials 7 and registries. 3 , 14 Moreover, although the FlowTriever System is a large‐bore system, a low access site complication rate of 0.4% was observed and no device‐related cardiac or pulmonary injuries or clinical deteriorations were reported.
The patient population studied in FLASH was notably sicker than previous thrombectomy studies, 11 , 15 with 6.8% of patients having high‐risk PE and the large majority of remaining patients having intermediate‐high‐risk PE (85.8%). Furthermore, 40.0% of patients were contraindicated for thrombolytic therapy, a patient population commonly excluded from PE trials due to increased bleeding risk. Nearly 25% of patients in this study had a history of cancer, 8.0% had active cancer, 14.0% had recent surgery, and 68.4% had concomitant DVT, a known predictor of mortality. 12 Despite the elevated risk profile of the real‐world patients enrolled in FLASH, there were no patient deaths through 48 h and only one mortality (0.4%) through 30 days. The mortality rate in FLASH compares favorably to data from the national PERT registry, which shows 30‐day mortality in acute PE patients of up to 30% in high‐risk PE patients and up to 15% for intermediate‐risk PE patients. 3
While consensus is still developing among interventionalists regarding which specific clinical indications should prompt the use of mechanical thrombectomy for PE, the patient profile in this study suggests that large clot burden (saddle or bilateral PE present in 88.4% of patients), elevated cardiac biomarkers (present in 96.2% of patients), and elevated RV/LV ratio (1.5 ± 0.5) may be factors influencing the decision to pursue mechanical thrombectomy to rapidly remove thrombus and normalize RV function.
In this analysis, FlowTriever thrombectomy resulted in significant on‐table decreases in both mean and systolic PA pressures and total pulmonary vascular resistance. RV size and function showed evidence of recovery in those patients who had paired assessments available, and a favorable impact on CI was observed in patients with low pre‐procedure CI. Interestingly, while only 6.8% of patients were categorized as having high‐risk PE, 21.4% of patients in whom pre‐procedure CI was measured presented with a pre‐procedure CI below 2.0 l/min/m2, indicating that some seemingly stable patients have significant hemodynamic compromise, similar to prior reports. 16 While the hemodynamic improvements observed in the FLASH registry are similar to data reported for catheter‐directed thrombolysis at the completion of the drug infusion, 16 , 17 a key difference in the FLASH data is that these improvements were achieved much more quickly on‐table and without the associated bleeding risk of thrombolytics. Even with newer generation hybrid catheter‐directed thrombolysis technology, the improvement in PA pressure and cardiac output is negligible at the completion of the procedure. 18
In addition to providing evidence supporting the safety and effectiveness of the FlowTriever System in real‐world PE patients, these interim results also suggest the ability of mechanical thrombectomy to reduce hospital resource use in the treatment of acute PE. Most patients were able to avoid the ICU postprocedure and had shorter hospital stays than previously reported, with a median hospital stay of 3.0 days postprocedure as compared to a mean 8.8 days in SEATTLE II. 17 The ICU benefit was largely due to lack of thrombolytic infusions, but it also points to the fact that most patients had on‐table hemodynamic improvements and were less critical postprocedure. All patients were treated in a single session, avoiding the need to schedule multiple procedures in the angiography suite, and the 30‐day readmission rate was low (6.0%), with only one patient being readmitted to the hospital for a cause related to the acute PE or treatment. This readmission rate in particular compares favorably to the nearly 20% readmission rate cited in the literature for venous thromboembolism and PE patients. 14 , 19
4.1. Study limitations
There are several limitations of this interim registry analysis, the primary of which is the lack of a comparator arm. Though a comparator arm is not part of the FLASH registry design, the next phase of the registry will include the addition of a contextual arm for patients who receive anticoagulation alone. Given the practical limitations of consenting some PE patients, especially those unable to sign informed consent before treatment, the outcomes reported may be impacted by selection bias. A minority of patients (6%) had intermediate‐low risk for mortality when risk‐stratified using mainstay clinical guidelines. 12 The inclusion of these intermediate‐low‐risk patients in this interim analysis may have slightly insulated the overall safety profile and mortality rate, though any potential skewing effect is expected to be minimal due to the low numbers of intermediate‐low‐risk patients enrolled. In addition, most of the present analysis is limited to 30‐day follow‐up, tempering what conclusions can be drawn about longer‐term outcomes in these patients. This limitation will be addressed with longer‐term follow‐up and additional functional data collection as the registry continues, which will allow further assessment of the clinical benefits of FlowTriever thrombectomy over time. Finally, due to the nature of a registry, investigators are given minimal guidance on treatment approaches and thus certain sites follow established workflows for their PE patients which may involve ICU stays and adjunctive therapies, including thrombolytics.
5. CONCLUSION
The FLASH registry is designed to study a broad range of acute and longer‐term outcomes in a real‐world PE patient population following mechanical thrombectomy with the FlowTriever System. Primary results from the first 250 patients enrolled in FLASH underscore the favorable safety profile of FlowTriever thrombectomy, with 0.4% all‐cause mortality within 30 days in an intermediate‐ and high‐risk patient population. In addition, these data suggest that FlowTriever thrombectomy can result in significant immediate improvements in hemodynamics as well as dyspnea resolution and cardiac function recovery. Further data collection out to 6 months will provide additional insights on safety and effectiveness of mechanical thrombectomy as a frontline therapy for PE and provide a platform for designing future definitive studies.
CONFLICT OF INTERESTS
Catalin Toma received advisory board funds from Phillips/Volcano. Matthew C. Bunte received research support from Inari Medical. Wissam A. Jaber received small consultation fees from Inari Medical, advisory board funds from Medtronic, and proctorship fees from Abbott Vascular. Jeffrey Chambers and Herman Kado are consultants for Inari Medical. Brian Stegman is a speaker/proctor for Edwards Lifesciences, a proctor and consultant for Medtronic, and a consultant for Cardionomics. Daniel A. Leung received speaker/consultant fees from Boston Scientific. Mitchell Weinberg is a consultant for Magneto Thrombectomy Solutions, Boston Scientific, Medtronic, Terumo Microvention, and Neptune Medical. Robert E. Beasley has received consultant fees and/or grant or research support from Abbott, BSCI, Cardinal Health/Cordis, Centerline BioMedical, Cook Medical, CR BARD/Becton Dickinson, CSI, Endologix, Inari Medical, Medtronic, MicroMedical Solutions, Penumbra, Philips/Volcano/Spectranetics, Terumo/Bolton, and WL Gore. Michael A. Brown received small speakers' fees from Inari Medical.
ACKNOWLEDGMENTS
The authors acknowledge data analysis and editorial support provided by Craig Markovitz, PhD and Jessica Parsons, PhD.
Toma C, Bunte MC, Cho KH, et al. Percutaneous mechanical thrombectomy in a real‐world pulmonary embolism population: interim results of the FLASH registry. Catheter Cardiovasc Interv. 2022;99:1345‐1355. 10.1002/ccd.30091
DATA AVAILABILITY STATEMENT
This study is an interim analysis of an actively enrolling registry, data will not be made available. However, analytical methods supporting the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Tapson VF, Platt DM, Xia F, Teal SA, de la Orden M, Divers CH, Satler SA, Joish VN, Channick RN. Monitoring for pulmonary hypertension following pulmonary embolism: the INFORM study. Am J Med. 2016;129(9):978‐985. [DOI] [PubMed] [Google Scholar]
- 2. Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani J, Desai ND, Piazza G, Gladwin MT, Chatterjee S, Kobayashi T, Kabrhel C, Barnes G. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation. 2019; 140:e774‐e801. [DOI] [PubMed] [Google Scholar]
- 3. Schultz J, Giordano N, Zheng H, Parry BA, Barnes GD, Heresi GA, Jaber W, Wood T, Todoran T, Courtney DM, Naydenov S, Khandhar S, Green P, Kabrhel C. EXPRESS: a multidisciplinary pulmonary embolism response ream (PERT)—experience from a national multicenter consortium. Pulm Circ. 2019;9(3):2045894018824563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sista AK, Klok FA. Late outcomes of pulmonary embolism: the post‐PE syndrome. Thromb Res. 2018; 164:157‐162. [DOI] [PubMed] [Google Scholar]
- 5. Sista AK, Miller LE, Kahn SR, Kline JA. Persistent right ventricular dysfunction, functional capacity limitation, exercise intolerance, and quality of life impairment following pulmonary embolism: systematic review with meta‐analysis. Vasc Med. 2017; 22:37‐43. [DOI] [PubMed] [Google Scholar]
- 6. Tavoly M, Utne KK, Jelsness‐Jorgensen LP, Wik HS, Klok FA, Sandset PM, Ghanima W. Health‐related quality of life after pulmonary embolism: a cross‐sectional study. BMJ Open. 2016; 6:e013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meyer G, Vicaut E, Danays T, Agnelli G, Becattini C, Beyer‐Westendorf J, Bluhmkim E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galie N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV. Fibrinolysis for patients with intermediate‐risk pulmonary embolism. N Engl J Med. 2014; 370:1402‐1411. [DOI] [PubMed] [Google Scholar]
- 8. Marti C, John G, Konstantinides S, Combescure C, Sanchez O, Lankeit M, Meyer G, Perrier A. Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta‐analysis. Eur Heart J. 2015; 36:605‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, Kumbhani DJ, Mukherjee D, Jaff MR, Giri J. Thrombolysis for pulmonary embolism and risk of all‐cause mortality, major bleeding, and intracranial hemorrhage: a meta‐analysis. JAMA. 2014; 311:2414‐2421. [DOI] [PubMed] [Google Scholar]
- 10. Geller BJ, Adusumalli S, Pugliese SC, Khatana SAM, Nathan A, Weinberg I, Jaff MR, Kobayashi T, Mazurek JA, Khandhar S, Yang L, Groeneveld PW, Giri JS. Outcomes of catheter‐directed versus systemic thrombolysis for the treatment of pulmonary embolism: a real‐world analysis of national administrative claims. Vasc Med. 2020; 25(4): 334‐340. [DOI] [PubMed] [Google Scholar]
- 11. Tu T, Toma C, Tapson VF, Adams C, Jaber WA, Silver M, Khandhar S, Amin R, Weinberg M, Engelhardt T, Hunter M, Holmes D, Hoots G, Hamdalla H, Maholic RL, Lilly SM, Ouriel K, Rosenfield K. A prospective, single‐arm, multicenter trial of catheter‐directed mechanical thrombectomy for intermediate‐risk acute pulmonary embolism: the FLARE study. J Am Coll Cardiol Intv. 2019; 12:859‐869. [DOI] [PubMed] [Google Scholar]
- 12. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing G‐J, Harjola V‐P, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Ainle FN, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL. 2019. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020; 41:543‐603. [DOI] [PubMed] [Google Scholar]
- 13. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736‐2747. [DOI] [PubMed] [Google Scholar]
- 14. Secemsky E, Chang Y, Jain CC, Beckman JA, Giri J, Jaff MR, Rosenfield K, Rosovsky R, Kabrhel C, Weinberg I. Contemporary management and outcomes of patients with massive and submassive pulmonary embolism. Am J Med. 2018;131(12):1506‐1514. [DOI] [PubMed] [Google Scholar]
- 15. Sista AK, Horowitz JM, Tapson VF, Rosenberg M, Elder MD, Schiro BJ, Dohad S, Amoroso NE, Dexter DJ, Loh CT, Leung DA, Bieneman BK, Perkowski PE, Chuang ML, Benenati JF. Indigo Aspiration System for treatment of pulmonary embolism: results of the EXTRACT‐PE trial. J Am Coll Cardiol Intv. 2021; 14:319‐329. [DOI] [PubMed] [Google Scholar]
- 16. Khandhar SJ, Mehta M, Cilia L, Palevsky H, Matthai W, Rivera‐Lebron B, Toma C. Invasive hemodynamic assessment of patients with submassive pulmonary embolism. Catheter Cardiovasc Interv. 2020; 95:13‐18. [DOI] [PubMed] [Google Scholar]
- 17. Piazza G, Hohlfelder B, Jaff MR, Ouriel K, Engelhardt TC, Sterling KM, Jones NJ, Gurley JC, Bhatheja R, Kennedy RJ, Goswami N, Natarajan K, Rundback J, Sadiq IR, Liu SK, Bhalla N, Raja ML, Weinstock BS, Cynamon J, Elmasri FF, Garcia MJ, Kumar M, Ayerdi J, Soukas P, Kuo W, Liu P‐Y, Goldhaber SZ. A prospective, single‐arm, multicenter trial of ultrasound‐facilitated, catheter‐directed, low‐dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. J Am Coll Cardiol Intv. 2015;8:1382‐1392. [DOI] [PubMed] [Google Scholar]
- 18. Sista AK, Bhatheja R, Rali P, Natarajan K, Green P, Piazza G, Comerota AJ, Parikh SA, Lakhter V, Bashir R, Rosenfield K. First‐in‐human study to assess the safety and feasibility of the Bashir Endovascular Catheter for the treatment of acute intermediate‐risk pulmonary embolism. Circ Cardiovasc Interv. 2021; 14:e009611. [DOI] [PubMed] [Google Scholar]
- 19. Secemsky EA, Rosenfield K, Kennedy KF, Jaff M, Yeh RW. High burden of 30‐day readmissions after acute venous thromboembolism in the United States. J Am Heart Assoc. 2018; 7(13):e009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study is an interim analysis of an actively enrolling registry, data will not be made available. However, analytical methods supporting the findings of this study are available from the corresponding author upon reasonable request.


