Abstract
Micro RNAs (MiRNAs) act as a key regulator participating in various biological process, and the roles of that play in chronic obstructive pulmonary disease (COPD) are discovered. However, recent pharmacological treatment for COPD focus on alleviating symptoms and reducing the risk events. The heterogeneous COPD causes variable responses to pharmacological interventions. COPD treatment has gradually developed into precision medicine, integrating clinical and biomarker information to optimize personalized therapy. Thus, targeting miRNAs represents a promising strategy for COPD individual therapy. Twelve COPD patients, 7 community-acquired pneumonia and 4 normal people were recruited. Total RNAs were collected from the bronch alveolar lavage cells and peripheral blood plasma of each participant. miRNAs were profiled by microarray and systematically compared between patients with different groups. Bioinformatic analysis identified pathways relevant to the pathogenesis of COPD. Next, the target pathway networks were mapped. Compared different groups, we obtain differential expression of miRNAs (Q value (Adjusted P value) < .05 and |log2FC| >2). Gene ontology enrichment analyses showed that differentially expressed miRNAs function as regulators in different modules of cellular component, molecular function and biological process. Kyoto Encyclopedia of Genes and Genomes enrichment analyses suggested that signals, such as MAPK signaling pathway, Ras signaling pathway, FoxO signaling pathway and oxidative stress may participate in the pathogenesis of COPD. In the miRNAs target pathway networks, novel-hsa-miR26-3p or hsa-miR-3529-3p/CDC42/MAPK signaling pathway may play a role in regulating COPD. Our findings demonstrate critical roles of the miRNAs in COPD molecular pathology. The data support a plausible mechanism that miRNAs may be involved in the development of COPD by affecting the inflammatory and oxidative stress. Moreover, hsa-miR-4748/CDC42/MAPK signaling pathway may contribute to the pathogenesis of COPD, providing a potential novel therapeutic strategy in COPD.
Keywords: bioinformatic analysis, chronic obstructive pulmonary disease, differential expression, miRNAs, second-generation sequencing
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by incompletely reversible airway obstruction. The statistical data from World health organization show that its morbidity and mortality rates are still on the rise, and it is estimated to be the third leading cause of death in the world by 2030.[1] Previous studies on COPD pathogenesis are mainly concentrated in imbalance between proteolysis and antiproteolysis, inflammatory cell infiltration in the lung, oxidative stress and apoptotic disorders, cellular senescence, etc.[2–5] With the development of genomics and proteomics, the biological effects of micro RNA (miRNAs) are gradually understood in the COPD pathophysiology.
MiRNAs are a class of endogenous and noncoding single-stranded ribonucleic acid (RNA) at length of 19 to 25 nucleotides, through combining the 3’-end nontranslation zone of the purpose mRNAs to inhibit mRNAs translation or promote its complete degradation, acting as a key regulator participating in cell proliferation, differentiation, development and apoptosis.[6–8] The roles of miRNAs play in COPD are gradually discovered. However, recent pharmacological treatment for COPD focus on alleviating symptoms and reducing the risk events.[9] The heterogeneous COPD causes variable responses to pharmacological interventions.[10] COPD treatment has gradually developed into precision medicine, integrating clinical and biomarker information to optimize personalized therapy.[11–13] Thus, targeting miRNAs represents a promising strategy for COPD individual therapy.
In our study, we applied second-generation sequencing method to explore differential expression profiles of miRNAs in bronchoalveolar lavage fluid (BALF) and plasma from patients with COPD and conduct bioinformatic analysis, to providing a potential novel therapeutic strategy in COPD.
2. Methods
2.1. Subjects selection
Twelve COPD patients hospitalized from November 2020 to April 2021 in Wuhan Fourth Hospital were included in the treatment group (Subjects were classified in the COPD group if they had a post bronchodilator forced vital capacity rate of 1 second of less than 0.70). Seven community-acquired pneumonia (CAP) patients hospitalized at the same period and 4 health people were included in control group, whose lung function were normal. This study was approved by the ethics committee of medical research in Wuhan Fourth Hospital (No. KY2020-137-01). Written informed consent was obtained from participants prior to their enrollment in the study. The demographic characteristics of each group were collected. According to the requirement of repeated analysis and biology, the 3 samples of BALF and 9 samples of plasma of each group were collected. Three samples of BALF from COPD and no-COPD patients were collected, named BALF group and BALF-control group. Nine samples of peripheral blood plasma were collected, named COPD group and control group.
This study needed to collect BALF cells, which is an invasive operation with certain risks and pains. For medical ethics, people with CAP was selected as the research object to collect BALF cells.
2.2. Sample collection and miRNA extraction
For the samples from BALF, the patient was locally anesthetized with 1% lidocaine injection at oropharynx, and administrated 5 mg of midazolam intravenously. Then, patients were maintained at supine position and Implemented oxygen, ECG, blood pressure monitoring. Apply fiber bronchoscope to conduct broncholaveolar lavage. A total of 60 mL of physiological saline heating to 37°C was infused, recovered and placed in 15 mL RNase-free tube, stored at 4°C and centrifuged at 8000 g for10 minutes. The supernatant was discard. The cell pellet was transferred to a 1.5 mL RNase-free eppendorf tube and frozened at −80°C. RNA was extracted within 1 week.
For the samples from peripheral blood plasma 2 mL of venous blood was drawed from elbow vein and placed in ethylene diamine tetraacetic acid tube. The sample were centrifuged at 3000 g for 8 minutes. The upper plasma was transfered to 1.5 mL RNase-free eppendorf tube and frozened at −80°C. RNA was extracted within 1 week.
The miRNA was extracted by BGI Biotechnology Corporation (Wuhan, China) in accordance with standard miRNA extraction methods. The total RNA was qualified and quantified using a Nano Drop and Agilent 2100 bioanalyzer (Thermo Fisher Scientific, MA, USA).
2.3. MiRNA library construction and microarray
The miRNAs that met the sequencing criteria were further included in the library and sequenced by MGISEQ2000 platform (MGI-Shenzhen, China) and differentially expressed genes were obtained by DEseq2 method. Hierarchical clustering analysis of differential miRNAs was performed using heatmap function in R software (R Foundation for Statistical Computing, Vienna, Austria). The target gene prediction was conducted by comparing the reference gene with target gene prediction software (RNAhybrid, miRanda and TargetScan).
The expression profiles were assessed by microarray according to the recommended protocols provided by BGI Biotechnology Corporation (Wuhan, China).
2.4. Functional analysis and miRNA target pathway network
Gene Ontology (GO) analyses and subsequent Kyoto Encyclopedia of Genes and Genomes (KEGG) molecular pathway enrichment analyses were performed based on the predicted results of target genes. GO analysis was conducted by GO: TermFinder software (Stanford University, Stanford, CA) (http://www.yeastgenome.org/help/analyze/go-term-finder).
The target genes of miRNA were predicted based on miRanda software (V3.3a; www.microrna.org) and Targetscan (v7.2; www.targetscan.org). And then top5 mRNAs for each miRNA (sorted by free energy) were selected and Cytoscape software (V3.7.1, the U.S. National Institute of General Medical Sciences, Maryland) was used to map the network.
2.5. Statistical analysis
Statistical analyses were conducted with the Statistical Package for Social Sciences software package (SPSS, version 22.0 [IBM Corporation, Armonk, NY]). The patients indicators comply with the normal distribution. The average ± standard deviation is used to describe the variance. Then the homogeneity test of variances is used. If equal variance, t test is applied; if not, adjusted t test is applied. An adjusted Q value (Adjusted P value) < .05 and |log2FC| >2 were set as the threshold for identifying differentially expressed miRNAs. Other analyses were set at Q value < .05.
3. Results
3.1. The clinical characteristics of subjects
A total of 12 COPD patients, 7 CAP patients and 4 normal people were included in our study. The general information of 2 groups was showed in Table 1.
Table 1.
Characteristics and respiratory parameters of COPD patients and controls.
| COPD group (n = 12) | Control group (n = 11) | P | |
|---|---|---|---|
| Age | 60.75 ± 13.00 | 51.82 ± 21.58 | .251 |
| FVC (% pred) | 80.00 ± 27.46 | 91.64 ± 20.91 | .269 |
| FEV1/FVC, % | 43.00 ± 12.02 | 79.09 ± 5.43 | .001 |
COPD = chronic obstructive pulmonary disease, FVC = forced vital capacity, FVC% pred = percentage of FVC in the predicted value, FEV1/FVC = forced vital capacity rate of 1 second.
3.2. The results of sequencing
A total of 24 samples were sequenced on DNBSEQ platform, with an average yield of 33.87 M reads per sample. This BioProject accession number is PRJNA847376. The average alignment ratio of the sample comparison genome was 78.22%. A total of 1,215 miRNAs were detected, including known-miRNAs 989 and novel-miRNAs 226, whereas 13 did not have expressions.
3.3. MiRNA microarray
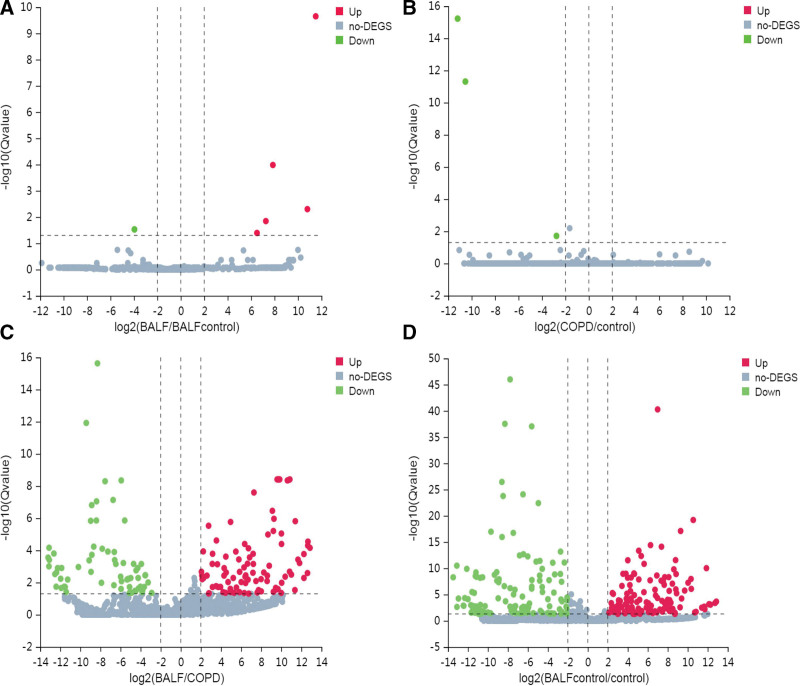
Then, screened miRNAs were conducted by microarray. The parameter was set as Q value (Adjusted P value) < .05 and |log2FC| >2. The results showed that, compared with BALF control group, there was 5 up-regulated miRNAs and 1 up-regulated miRNA in BALF group (Table 2). Compared with control group, there was 3 down-regulated miRNAs in COPD group, and no miRNAs upregulation (Table 2). We also compared differential expressed miRNAs (DEmiRNAs) in BALF and plasma of the COPD patients, and obtained 93 up-regulated miRNAs and 66 down-regulated miRNAs, among which there was 7 novel up-regulated and 6 novel down-regulated miRNAs, the top 10 of which was listed in Table 2. The Volcano plots of DEmiRNAs in 4 groups were made (Fig. 1A–D).
Table 2.
DEmiRNAs between BALF and BALFcontrol, COPD group and control, and the top ten DEmiRNAs between BALF group and COPD group.
| Gene ID | log2 (BALF/BALFcontrol) | Q value (BALF/BALFcontrol) |
|---|---|---|
| hsa-miR-129-5p | 6.502317431 | 0.040359584 |
| hsa-miR-3529-3p | 7.857561109 | 1.04E-04 |
| hsa-miR-365b-3p | 11.5137276 | 2.23E-10 |
| hsa-miR-6503-5p | 10.80251637 | 0.004993365 |
| novel-hsa-miR26-3p | 7.260079439 | 0.014240805 |
| hsa-miR-34b-5p | −3.95506095 | 0.029557731 |
| Gene ID | log2 (COPD/control) | Q value (COPD/control) |
| hsa-miR-4748 | −10.53527538 | 4.91E-12 |
| hsa-miR-491-5p | −2.764433491 | 0.019400655 |
| novel-hsa-miR158-3p | −11.19414124 | 6.00E-16 |
| Gene ID | log2(BALF/ COPD) | Q value (BALF/ COPD) |
| hsa-miR-1227-3p | −13.21112782 | 2.80E-04 |
| hsa-miR-4435 | −13.12686577 | 9.90E-04 |
| novel-hsa-miR220-3p | −13.11618149 | 6.90E-05 |
| novel-hsa-miR254-3p | −13.09308739 | 3.92E-04 |
| hsa-miR-1260b | −12.6329952 | 1.57E-04 |
| hsa-miR-6749-3p | −12.52747701 | 0.002990671 |
| hsa-miR-2115-5p | 12.61332905 | 0.002581809 |
| hsa-miR-449c-5p | 12.64205169 | 5.04E-05 |
| novel-hsa-miR54-5p | 12.65575431 | 2.85E-05 |
| hsa-miR-7704 | 12.86069903 | 6.98E-05 |
BALF = bronchoalveolar lavage fluid, COPD = chronic obstructive pulmonary disease, DEmiRNAs = differential expressed miRNAs.
Figure 1.
MiRNA expression profile of different group. Volcano plots were used to distinguish DEmiRNAs. Red, green and gray indicate up-regulation, down-regulation and no DEGS, respectively. DEGS = differential expressed genes, DEmiRNAs = differential expressed miRNAs, MiRNAs = micro RNAs.
3.4. The differences in miRNAs expression levels
We also detected miRNAs expression quantity between groups. Compared with control group, there is 141 DEmiRNAs in BALF group and 124 DEmiRNAs in COPD group. There was 12 miRNAs expressing both in the COPD group and the BALF instead of 2 control groups, they are hsa-miR-2276-3p, hsa-miR-550a-3-5p, hsa-miR-616-5p, hsa-miR-5684, hsa-miR-6501-3p, hsa-miR-4795-5p, hsa-miR-320e, hsa-miR-1268b, hsa-miR-301b-3p, hsa-miR-4634, hsa-miR-136-5p and hsa-miR-4787-3p.
3.5. The cluster heatmaps of DEmiRNAs
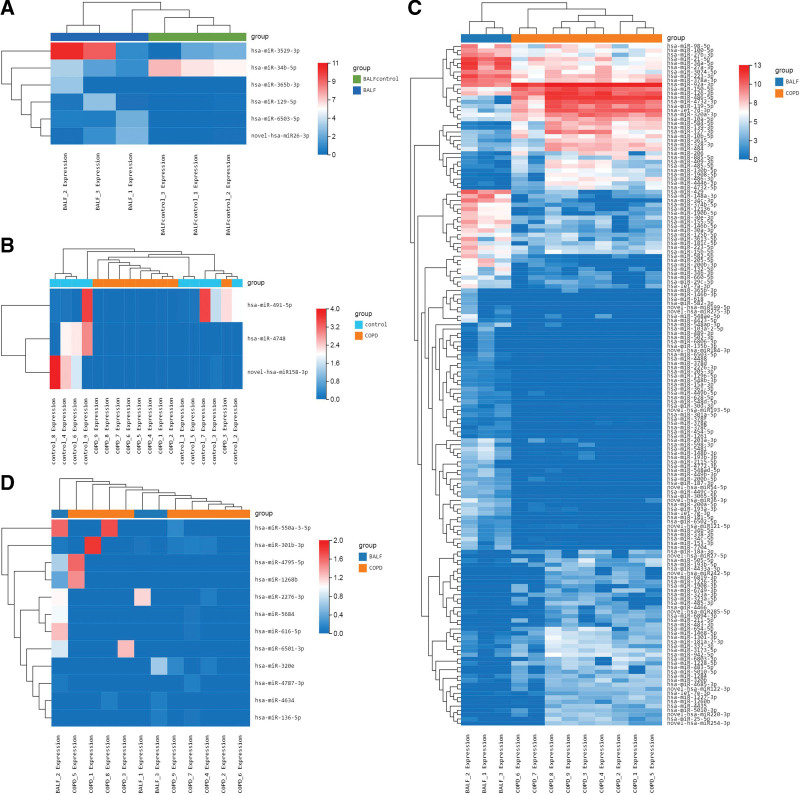
The cluster heatmaps of 6 DEmiRNAs in BALF group and BALF control group was showed in Figure 2A, and that of 3 DEmiRNAs in COPD group and control group was showed in Figure 2B, and that of 159 DEmiRNAs in COPD group and BALF group was showed in Figure 2C. There is no difference between COPD group and control group. As showed in Figure 2D, there is 12 DEmiRNAs expressing only in BALF and plasma from patients. The color box displays the levels of DEmiRNAs.
Figure 2.
The cluster heatmaps of DEmiRNAs from different group. (A–C) The cluster heatmaps of BALF control-vs-BALF group, control-vs-COPD group and COPD-vs-BALF group. (D) The cluster heatmaps of 12 DEmiRNAs both expressed in BALF and plasma. BALF = bronchoalveolar lavage fluid, COPD = chronic obstructive pulmonary disease, DEmiRNAs = differential expressed miRNAs, MiRNAs = micro RNAs.
3.6. GO enrichment analyses
The GO enrichment analyses were performed in DEmiRNAs from BALFcontrol-vs-BALF group, control-vs-COPD group and COPD-vs-BALF group, which consisting cellular component (C), molecular function (F) and biological process (P). The 6 DEmiRNAs in BALF group mainly enriched in the function of cell junction, kinase activity, etc. The 3 DEmiRNAs in plasma from COPD were concentrated on adherens junction, Wnt signaling pathway, etc. The 159 DEmiRNAs were mainly focused on cell adhesion, intracellular signal transduction, etc. The 12 DEmiRNAs both expressed in BALF and COPD group were discovered in intracellular signal transduction, cell adhesion, etc.
3.7. KEGG analyses of target genes
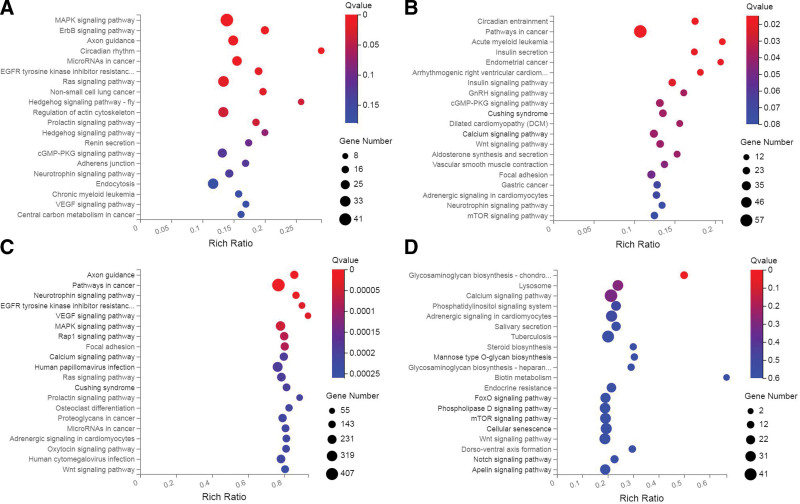
KEGG analyses were conducted in BALFcontrol-vs-BALF group, control-vs-COPD group and COPD-vs-BALF group (Fig. 3A–C). The 6 DEmiRNAs in BALF group were predicted to regulate 1,405 mRNA, 309 pathways were enriched by KEGG, significant enrichment top 20 pathways was showed in Figure 3A. Among them, the pathways related to COPD was MAPK signaling pathway, ErbB signaling pathway, Ras signaling pathway, etc. The 3 DEmiRNAs in COPD group were predicted to regulate 1,308 mRNA, 299 pathways were enriched by KEGG, significant enrichment top 20 pathways was showed in Figure 3B. Among them, the pathways related to COPD was Pathways in cancer, Wnt signaling pathway, Focal adhesion, etc. Compared COPD group and BALF group, the 159 DEmiRNAs in were predicted to regulate 12,474 mRNA, 343 pathways were enriched by KEGG, significant enrichment, top 20 pathways was showed in Figure 3C. Among them, the pathways related to COPD was VEGF signaling pathway, MAPK signaling pathway, Ras signaling pathway, Oxidative phosphorylation, etc. The 12 DEmiRNAs both expressed in BALF and COPD group instead in 2 control group were analyzed and discovered in FoxO signaling pathway, Wnt signaling pathway, Ras signaling pathway, etc (Fig. 3D).
Figure 3.
KEGG analyses of target genes of different group. (A–C) KEGG analyses in BALF control-vs-BALF group, control-vs-COPD group and COPD-vs-BALF group. (D) KEGG analyses of 12 DEmiRNAs both expressed in BALF and COPD group instead in 2 control group. BALF = bronchoalveolar lavage fluid, COPD = chronic obstructive pulmonary disease, DEmiRNAs = differential expressed miRNAs, KEGG = Kyoto encyclopedia of genes and genomes, MiRNAs = micro RNAs.
3.8. Target prediction and network analysis.
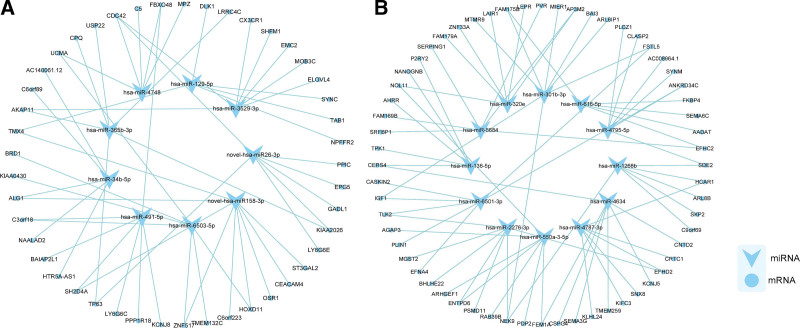
To predict the possible functions of the DEmiRNAs, the miRNA-RNA target pathway networks were mapped. We chose the 6 DEmiRNAs relatively down-regulated in BALF group and 3 DEmiRNAs relatively down-regulated in COPD group to construct the network. As showed in Figure 4A, novel-hsa-miR26-3p or hsa-miR-3529-3p/CDC42/MAPK signaling pathway may play a role in the regulation of COPD. In addition, hsa-miR-129-5p were predicted to target TAB1, which was in connection to MAPK signaling pathway. The 12 DEmiRNAs expressed both in BALF and plasma from patients was chosen to map the network, and found that hsa-miR-1268b was forecast to target SKP2, which was related to FoxO signaling pathway and hsa-miR-6501-3p was predicted to target EFNA4, which was also associated with MAPK signaling pathway (Fig. 4B).
Figure 4.
The networks of miRNA. (A) The network constructed on 6 DEmiRNAs relatively down-regulated in BALF group and 3 DEmiRNAs relatively down-regulated in COPD group. (B) The network constructed on the 12 DEmiRNAs expressed both in BALF and plasma from patients. BALF = bronchoalveolar lavage fluid, COPD = chronic obstructive pulmonary disease, DEmiRNAs = differential expressed miRNAs, MiRNAs = micro RNAs.
4. Discussion
The diagnosis of COPD currently mainly relies on clinical symptoms and pulmonary function tests. However, patients with mild and moderate COPD usually lack the typical symptoms, resulting that they do not receive effective attention and miss the opportunity for early diagnosis and timely intervention. In recent decades, with the development of genomics and proteomics, miRNAs have been found to be involved in the pathological development of many lung diseases such as COPD, lung cancer, pulmonary fibrosis, bronchitis, asthma, etc. The miRNAs present in serum and plasma, namely circulating miRNAs, may become early diagnostic markers for various diseases.
It has been reported that some differentially expressed miRNAs were obtained from sputum, plasma and lung tissue of COPD patients. Van Pottelberge GR et al applied reverse transcription-PCR to detect the differences in the expression of 627 known miRNAs in the induced sputum of smoking patients with COPD and nonsmoking normal subjects and found that the expression levels of let-7c and miR-125b were significantly down-regulated in COPD group, and the expression of let-7c target gene TNFR-II and its protein also showed corresponding changes.[14] Akbas F et al detected 72 miRNAs in peripheral serum of COPD patients by quantitative reverse transcription PCR array, and found that the expression levels of miR-20a, miR-28-3p, miR-34c-5p, and miR-100 were down-regulated, while the expression level of miR-7 was up-regulated.[15] Cao et al detected increased expression of miR-183, miR-200b and miR-200c in the peripheral blood of COPD patients.[16] Kara et al noticed miR-29c and miR-126 expressions showed significant differences in stage III and only miR-126 different in stage IV in peripheral blood.[17] Lacedonia D et al discovered miRNA-338 is higher in the supernatant than in peripheral blood.[18] Ezzie ME et al found that miR-15b, miR-223, miR-1274a, and miR-424 were significantly up-regulated in lung tissue of COPD patients and were associated with inflammatory pathways or signaling molecules such as TGF-β, Wnt, and SMAD7.[19] Gu et alreported miR-195 was significantly up-regulated in the lung tissues and regulates Akt signaling by suppressing PH domain and leucine rich repeat protein phosphatases 2 expression.[20] Shi et al found that the expression of miR-181a, miR-203, miR-338, miR-1 and miR-199a was altered in the lung biopsies and only miR-203 was higher in the blood.[21] Molina-Pinelo S et al found that around 50% miRNAs were no detected in both plasma and bronchoalveolar cell fraction and only 20% of miRNAs showed similar expression in both samples, like miR-17, miR-19b, miR-195 and miR-20b.[22]
These studies rarely reach consistent conclusions, some of which may be contributed to different detection methods, and the other part is also related to different sample sources. Given that the differences between animal models or cell experiments and the real and complex pathophysiological processes of the human body, the suitable sample to obtain for studying the pathogenesis of COPD is cells from the BALF. However, such samples also have a small number of cells and a low amount of miRNA extraction, which are precious samples, and the detection by RT-PCR and miRNA is incomplete. In our study, next-generation sequencing was applied to detect differentially expressed miRNAs in BALF samples, making sure that the detection of miRNAs in precious samples was more comprehensive. The plasma samples from patients were selected for comparative analysis to clarify the differences in the expression of miRNAs in BALF and plasma, and it is expected to discover the specificity of expression in diseased tissues.
In our experiments, differentially expressed miRNAs from BALF and plasma were obtained. It has been reported that these miRNAs play a role in angiogenesis, metastasis and recurrence of various tumors. For instance, the miR-34b family is a well-known cancer suppressor.[23] hsa-miR-129-5p is down-regulated in lung cancer tissues.[24] The down-regulation of PART1 can hinder the occurrence and development of liver cancer by targeting the hsa-miR-3529-3p/FOXC2 axis.[25] The hsa-miR-365b-3p has been identified as a novel miRNA described in various types of tumor including non-small cell lung cancer[26] and hepatocellular carcinoma.[27] miR-491-5p inhibits the proliferation and migration of A549 cells by forkhead box P4.[28]
The signaling pathways revealed by KEGG enrichment analysis and GO enrichment analysis from the 6 differentially expressed miRNAs in BALF group and 3 down-regulated differentially expressed miRNAs partly participate in inflammation and oxidative stress, which play a role in the pathogenesis of COPD. The results were consist with the studies of Renda T,[29] Gaffey K,[30] He S[31] and Malhotra D.[32] The other part is related to tumor, which is also reported.[23–28] Further prediction analysis of target genes found that these differentially expressed miRNAs may be involved in the regulation of COPD by regulating mRNAs. These require further study to understand their roles in the pathogenesis of COPD. In addition, the roles of the 12 differentially expressed miRNAs both expressing in BALF and plasma instead in 2 control group, as well as the 159 differentially expressed miRNAs from comparing COPD group and BALF group required further exploration.
The deficiency of our study is that we did not further expand the population of differentially expressed differentially expressed miRNAs, nor did it directly measure the mRNA of COPD patients, and could not conduct miRNA-mRNA target gene pairing analysis. These will be further explored in subsequent studies.
5. Conclusions
Our findings demonstrate critical roles of the miRNAs in COPD molecular pathology. The data support a plausible mechanism that miRNAs may be involved in the development of COPD by affecting the inflammatory and oxidative stress. Moreover, hsa-miR-4748/CDC42/MAPK signaling pathway may contribute to the pathogenesis of COPD, providing a potential novel therapeutic strategy in COPD.
Author contributions
Conceptualization: Qiaofa Lu.
Data curation: Weina Wang.
Formal analysis: Weina Wang, Lifen Du, Tian Qin.
Funding acquisition: Jianwu Hu.
Investigation: Qiaofa Lu.
Methodology: Jianwu Hu, Weina Wang, Tian Qin.
Project administration: Jianwu Hu.
Resources: Jianwu Hu, Lifen Du.
Software: Lifen Du, Tian Qin.
Supervision: Qiaofa Lu.
Writing – original draft: Jianwu Hu, Weina Wang, Lifen Du.
Writing – review & editing: Jianwu,Hu,Qiaofa Lu, Tian Qin.
Abbreviations:
- BALF =
- bronchoalveolar lavage fluid
- CAP =
- community-acquired pneumonia
- COPD =
- chronic obstructive pulmonary disease
- DEGS =
- differential expressed genes
- DEmiRNAs =
- differential expressed miRNAs
- GO =
- gene ontology
- KEGG =
- Kyoto encyclopedia of genes and genomes
- MiRNAs =
- micro RNAs,
- PHLPP2
- = PH domain and leucine rich repeat protein phosphatases 2,
- RNA
- = ribonucleic acid,
- WHO
- = World health organization
The datasets generated during and/or analyzed during the current study are publicly available.
JH and WW contributed equally to this work.
The authors have no conflicts of interest to disclose.
This research is supported by the Scientific Research Subject of the Health and Family Planning Commission of Wuhan Municipality, China [grant numbers WX16B11].
How to cite this article: Hu J, Wang W, Lu Q, Du L, Qin T. Differential expression of miRNAs in bronchoalveolar lavage fluid and plasma from patients with chronic obstructive pulmonary disease. Medicine 2022;101:40(e30969).
Contributor Information
Jianwu Hu, Email: hu-jianwu@163.com.
Weina Wang, Email: weinawang1225@163.com.
Qiaofa Lu, Email: luqiaofa@163.com.
References
- [1].World Health Organization. Global surveillance, prevention and control of chronic respiratory disease: a comprehensive approach. Geneva, Switzerland: World Health Organization, 2007. Available at: http://www.who.int/gard/publications/GARD_Manual/en/ [access date March 11, 2014]. [Google Scholar]
- [2].Hikichi M, Mizumura K, Maruoka S, et al. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J Thorac Dis. 2019;11(Suppl 17):S2129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang YJ, Xu JY, Meng YQ, et al. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adam MG, Elizabeth S. Oxidative stress in COPD: sources, markers, and potential mechanisms. J Clin Med. 2017;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Barnes PJ, Baker J, Donnelly LE. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med. 2019;200:556–64. [DOI] [PubMed] [Google Scholar]
- [6].Chu M, Wu S, Wang W, et al. miRNA sequencing reveals miRNA-4508 from peripheral blood lymphocytes as potential diagnostic biomarker for silica-related pulmonary fibrosis: a multistage study. Respirology. 2020;25:511–7. [DOI] [PubMed] [Google Scholar]
- [7].O’Farrell HE, Yang IA. Extracellular vesicles in chronic obstructive pulmonary disease (COPD). J Thorac Dis. 2019;11:S2141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Salimian J, Mirzaei H, Moridikia A, et al. Chronic obstructive pulmonary disease: MicroRNAs and exosomes as new diagnostic and therapeutic biomarkers. J Res Med Sci. 2018;23:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49:1700214. [DOI] [PubMed] [Google Scholar]
- [10].Singh D, Roche N, Halpin D, et al. Current controversies in the pharmacological treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:541–9. [DOI] [PubMed] [Google Scholar]
- [11].Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47:410–9. [DOI] [PubMed] [Google Scholar]
- [12].Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD Science Committee report 2019. Eur Respir J. 2019;53:1900164. [DOI] [PubMed] [Google Scholar]
- [13].Stockley RA, Halpin DMG, Celli BR, et al. Chronic obstructive pulmonary disease biomarkers and their interpretation. Am J Respir Crit Care Med. 2019;199:1195–204. [DOI] [PubMed] [Google Scholar]
- [14].Van Pottelberge GR, Mestdagh P, Bracke KR, et al. MicroRNA expression in induced sputum of smokers and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:898–906. [DOI] [PubMed] [Google Scholar]
- [15].Akbas F, Coskunpinar E, Aynaci E, et al. Analysis of serum micro-RNAs as potential biomarker in chronic obstructive pulmonary disease. Exp Lung Res. 2012;38:286–94. [DOI] [PubMed] [Google Scholar]
- [16].Cao Z, Zhang N, Lou T, et al. microRNA-183 down-regulates the expression of BKCaβ1 protein that is related to the severity of chronic obstructive pulmonary disease. Hippokratia. 2014;18:328–32. [PMC free article] [PubMed] [Google Scholar]
- [17].Kara M, Kirkil G, Kalemci S. Differential expression of MicroRNAs in chronic obstructive pulmonary disease. Adv Clin Exp Med. 2016;25:21–6. [DOI] [PubMed] [Google Scholar]
- [18].Donato L, Palladino GP, Foschino Barbaro MP, et al. Expression profiling of miRNA-145 and miRNA-338 in serum and sputum of patients with COPD, asthma, and asthma-COPD overlap syndrome phenotype. Int J Chron Obstruct Pulmon Dis. 2017;12:1811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ezzie ME, Crawford M, Cho JH, et al. Gene expression networks in COPD: microRNA and mRNA regulation. Thorax. 2012;67:122–31. [DOI] [PubMed] [Google Scholar]
- [20].Gu W, Yuan Y, Yang H, et al. Role of miR-195 in cigarette smoke-induced chronic obstructive pulmonary disease. Int Immunopharmacol. 2018;55:49–54. [DOI] [PubMed] [Google Scholar]
- [21].Shi L, Xin Q, Chai R, et al. Ectopic expressed miR-203 contributes to chronic obstructive pulmonary disease via targeting TAK1 and PIK3CA. Int J Clin Exp Pathol. 2015;8:10662–70. [PMC free article] [PubMed] [Google Scholar]
- [22].Molina-Pinelo S, Suárez R, Pastor MD, et al. Association between the miRNA signatures in plasma and bronchoalveolar fluid in respiratory pathologies. Dis Markers. 2012;32:221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–9. [DOI] [PubMed] [Google Scholar]
- [24].Xu C, Du Z, Ren S. MiR-129-5p sensitization of lung cancer cells to etoposide-induced apoptosis by reducing YWHAB. J Cancer. 2020;11:858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Weng Z, Peng J, Wu W, et al. Downregulation of PART1 inhibits proliferation and differentiation of Hep3B cells by targeting hsa-miR-3529-3p/FOXC2 axis. J Oncol. 2021;2021:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang X, Wang J, Pan Y, et al. MicroRNA-365b-3p represses the proliferation and promotes the apoptosis of non-small cell lung cancer cells by targeting PPP5C. Oncol Lett. 2021;21:389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [27].Tian Q, Sun HF, Wang WJ, et al. miRNA-365b promotes hepatocellular carcinoma cell migration and invasion by downregulating SGTB. Future Oncol. 2019;15:2019–28. [DOI] [PubMed] [Google Scholar]
- [28].Wu F, Ji A, Zhang Z, et al. miR-491-5p inhibits the proliferation and migration of A549 cells by FOXP4. Exp Ther Med. 2021;21:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Renda T, Baraldo S, Pelaia G, et al. Increased activation of p38 MAPK in COPD. Eur Respir J. 2008;31:62–9. [DOI] [PubMed] [Google Scholar]
- [30].Gaffey K, Reynolds S, Plumb J, et al. Increased phosphorylated p38 mitogen-activated protein kinase in COPD lungs. Eur Respir J. 2013;42:28–41. [DOI] [PubMed] [Google Scholar]
- [31].He S, Sun S, Lu J, et al. The effects of the miR-21/SMAD7/TGF-β pathway on Th17 cell differentiation in COPD. Sci Rep. 2021;11:6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Malhotra D, Thimmulappa R, Neeraj V, et al. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med. 2009;180:1196–207. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]