Background:
Lymphovenous anastomosis (LVA) is a microsurgical treatment for lymphedema of the lower extremity (LEL). This study systematically reviews the most recent data on outcomes of various LVA techniques for LEL in diverse patients.
Methods:
A comprehensive literature search was conducted in the Ovid MEDLINE, Ovid EMBASE, and Scopus databases to extract articles published through June 2021. Studies reporting data on objective postoperative improvement in lymphedema and/or subjective improvement in quality of life for patients with LEL were included. Extracted data comprised demographics, number of patients and lower limbs, duration of symptoms before LVA, surgical technique, duration of follow-up, and objective and subjective outcomes.
Results:
A total of 303 articles were identified and evaluated, of which 74 were ultimately deemed eligible for inclusion in this study, representing 6260 patients and 2554 lower limbs. The average patient age ranged from 22.6 to 76.14 years. The duration of lymphedema before LVA ranged from 12 months to 11.4 years. Objective rates of improvement in lymphedema ranged from 23.3% to 100%, with the greatest degree of improvement seen in patients with early-stage LEL.
Conclusions:
LVA is a safe and effective technique for the treatment of LEL of all stages. Several emerging techniques and variations may lead to improved patient outcomes.
Takeaways
Question: How effective is lymphovenous anastomosis (LVA) in the treatment of lower extremity lymphedema?
Findings: A systematic review of the literature was performed highlighting recent outcomes and best practices with respect to the treatment of lower extremity lymphedema with LVA.
Meaning: LVA is a safe and effective technique for the treatment of lower extremity lymphedema of all stages.
Introduction
Lymphedema is a progressive, debilitating state of lymphatic dysfunction that causes regional accumulation of interstitial fluid and tissue swelling.1 Associated chronic fluid stasis triggers inflammation, adipose hypertrophy, fat deposition, and fibrotic changes that gradually degrade subcutaneous tissue from a soft, swollen state to more indurated one (Fig. 1).2 Primary lymphedema (PL) is rare and likely caused by genetic mutations, leading to hypoplastic, impaired lymphatic vessels.1,3 Secondary lymphedema (SL) is more common, frequently occurring due to cancer treatment.4 However, SL may arise following traumatic, infectious, or neoplastic events that damage or obstruct normal lymphatics.5 In the lower extremity (LE), SL often follows gynecologic and urologic malignancy and treatment, particularly after inguinal lymph node dissection and radiation therapy. Incidence of cancer-related lymphedema is approximately 25% to 70% in select populations.6,7
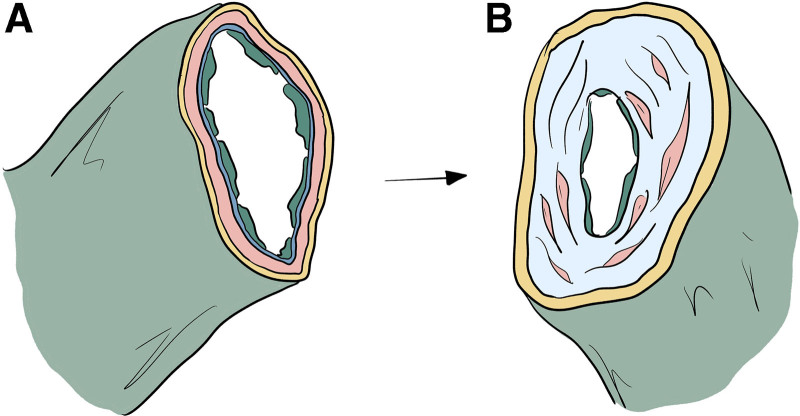
Fig. 1.
Fibrosis, adipose hypertrophy, and adipose deposition in lymphatic vessels associated with lymphedema. A: Transected healthy lymphatic vessel showing intact endothelium, smooth muscle, and adventitia. B: Cross section of a sclerotic vessel with significant collagen deposition and reduced lumen size characteristic of later stage lymphedema. LVA is dependent on the functional capacity of vessels, which tends to be diminished with increasing severity of disease.
In general, lymphedema is initially managed conservatively with wrapping, inelastic bandages, and controlled compression therapy. Complete decongestive therapy employs a combination of manual lymphatic drainage, daily compression bandaging, elastic garments, and therapeutic exercises.8 Surgical intervention is considered after failure of conservative measures.9 Debulking and liposuction procedures are employed occasionally, but do not address the underlying pathophysiology and are associated with significant complications.6 Recently, minimally invasive microsurgical techniques that facilitate bypass of obstructed lymphatics have gained popularity, including lymphovenous anastomosis (LVA), lympholymphatic bypass, and vascularized lymph node transfer (VLNT).10
LVA has become a first-line microsurgical intervention in lymphedema treatment.11,12 It involves one or more anastomoses of lymphatic vessels in the affected region to nearby venules, facilitating bypass of obstructed channels.13 A growing body of literature illustrates the superiority of LVA for extremity lymphedema,12 but few studies report objective and subjective measures following LE LVA, and consensus regarding ideal perioperative treatment and optimal operative technique is limited.14,15 This article reviews recent findings regarding lower extremity lymphedema (LEL) and details perioperative and operative advances with LVA.
Methods
Literature Review and Search Criteria
Three databases were used during the literature search for this scoping review: Ovid MEDLINE, Ovid EMBASE, and Scopus. A combination of Medical Subject Headings (MeSH) and keywords were used to complete the search. MeSH terms included lymphedema; lower extremity; anastamosis, surgical; and lymphatic vessels. Keywords included lymphedema; lower extremity*; lower limb*; feet, toes, lymphovenous anastomosis, lymphovenous bypass, and lymphatic surgery. MeSH terms were combined with their counterpart keywords using the Boolean operator “OR” followed by combining concepts with the Boolean operator “AND” to complete the search. Initial results included 450 titles. After removal of duplicates, 303 references were reviewed for inclusion/exclusion criteria (*indicates truncation of word or phrase).
Inclusion and Exclusion Criteria
Included studies represented those in which LVA was performed for primary or secondary LEL with results on objective improvement in limb volume or subjective improvement in patient symptoms and quality of life. Only human studies written in English were considered. Excluded articles included review articles, one- and two-patient case reports, studies with fewer than five patients, conference summaries, abstracts, descriptive or narrative studies, studies of lymphedema prevention, studies reporting outcomes on lymphatic flow without clinical correlation, and studies published before 1990.
Data Extraction and Outcome Measures
Study screening and data extraction were performed by two independent reviewers (E.M.V. and L.A.K.) according to eligibility criteria. In the case of discrepancy, a third reviewer (C.M.T.) determined inclusion or exclusion of the article. For included studies, extracted data included mean patient age, mean patient body mass index (BMI), study type, year of publication, number of patients, number of limbs, type of lymphedema, mean duration of lymphedema symptoms before LVA, operative characteristics, including type and number of anastomoses, pre- and postoperative interventions and diagnostic procedures, objective and subjective outcomes, and mean follow-up. Only lower extremity data were included. If a study included data on multiple treatment groups, weighted averages of extracted data were calculated to provide an accurate overall assessment of outcomes.
RESULTS
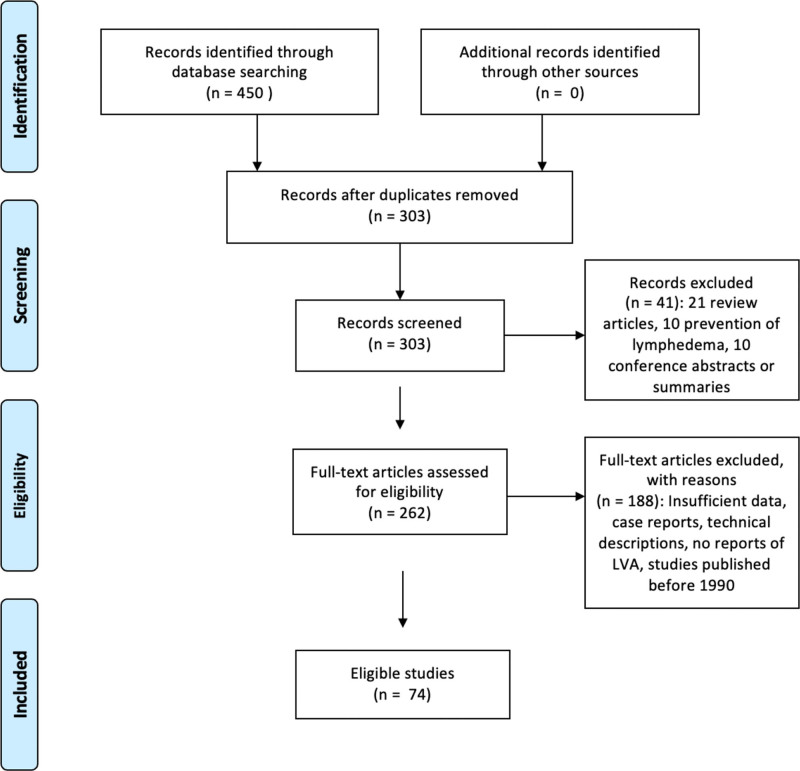
Of the 303 articles initially identified, 74 met criteria for inclusion (Fig. 2), representing a total of 6260 patients and 2554 lower extremities (See table, Supplemental Digital Content 1, which displays key characteristics of included articles. http://links.lww.com/PRSGO/C228).16–89 Fifteen articles reported only the number of patients undergoing LVA, and did not specify the number of limbs.17,20,38,43,44,49,52,55,56,62,67,72,78,87 Studies were published between 199689 and 2021 (Table 1).24 The age range of patients undergoing LVA was 22.641 to 76.1 years (Table 2),23 with a mean age of 54.13 years. In those studies that reported it (43%), mean BMI ranged from 22 kg/m2 65 to 29.3 kg/m2.17 The mean BMI of all patients was 24.57 kg/m2.
Fig. 2.
PRISMA flow diagram.
Table 1.
Results
| Authors (y) | Study Size | Type of Lymphedema (PL or SL) | Duration of Lymphedema before LVA | Intervention | Follow-up | Objective Improvement in % Patients | Subjective Improvement in % Patients |
|---|---|---|---|---|---|---|---|
| Yang et al24 | 26 | PL and SL | 6 y | LVA (+conservative therapy) | 6 mo | ||
| Cha et al16 | 42 | PL and SL | 8.8 y | LVA (+conservative therapy) | Minimum of 8 mo | 100 | 100 |
| Yoshida et al26 | 74 | PL | 6.1 y | LVA (+conservative therapy) | 6 mo | ||
| Scaglioni et al22 | 7 | PL and SL | Superficial and deep LVA | 9.43 mo | 100 | 100 | |
| Yoshida et al26 | 28 | PL | 3 y | LVA, great saphenous vein stripping (+conservative therapy) | |||
| Onoda et al20 | 21 | PL and SL | Multisite LVA (+complex decongestive physiotherapy) | 31 mo | 85.7 | ||
| Pak et al21 | 160 | SL | LVA, lymph node to vein anastomosis | 23.3 mo | |||
| Yoshida et al26 | 50 | SL | LVA (+conservative therapy) | 6 mo | 100 | ||
| Kim et al19 | 69 | PL and SL | 5. 26 y | LVA | 11.2 mo | 69.9 | |
| Drobot et al17 | 39 | PL and SL | 6.69 y | LVA | 7.26 mo | 100 | |
| Hara et al18 | 34 | PL and SL | 7.5 y | LVA | 8.6 mo | 83.3 | |
| Kristiansen et al30 | 12 | PL and SL | 4 y | End-to-end LVA | 12 mo | 42 | |
| Tsai et al32 | 100 | PL and SL | LVA | 9.8 mo | |||
| Yang et al33 | 100 | PL and SL | 4.8 y | LVA (+conservative therapy) | 6 mo | ||
| Bianchi et al28 | 12 | SL | LVA | 9 mo | 100 | ||
| Akita et al27 | 106 | LVA, venoplasty | |||||
| Yoshida et al34 | 12 | PL and SL | 1.66 y | LVA (+conservative therapy) | 14.5 mo | 100 | |
| Qui et al31 | 15 | PL and SL | End-to-end and end-to side LVA | 25 mo | 46.7 | 84 | |
| Cheng et al29 | 10 | PL and SL | 1 y | Side-to-end LVA (+conservative therapy) | 37.5 mo | 100 | |
| Yoshida et al34 | 113 | PL and SL | 6.4 y | LVA (+conservative therapy) | 20.6 mo |
Table 2.
Patient Demographics
| Authors (y) | No. of Patients | No. of Lower Limbs | Mean/Median Age (y) | Mean/Median BMI (kg/m2) |
|---|---|---|---|---|
| Yang et al24 | 26 | 26 | 59.6 | 25.8 |
| Cha et al16 | 42 | 50 | 53.8 | 26.9 |
| Yoshida et al34 | 74 | 136 | 73.6 | 26 |
| Scaglioni et al22 | 7 | 7 | 56.4 | |
| Yoshida et al26 | 28 | 51 | 76.14 | 25.7 |
| Onoda et al20 | 21 | 65.5 | ||
| Pak et al21 | 160 | 160 | 62.5 | |
| Yoshida et al34 | 50 | 50 | ||
| Kim et al19 | 69 | 69 | 55.34 | 23.38 |
| Drobot et al17 | 39 | 48.8 | 29.3 | |
| Hara et al18 | 34 | 42 | 56.4 | |
| Kristiansen et al30 | 12 | 14 | 51 | |
| Tsai et al32 | 100 | 103 | 58.6 | |
| Yang et al33 | 100 | 100 | 58.4 | 25.46 |
| Bianchi et al28 | 12 | 12 | 57.6 | 25.48 |
| Akita et al27 | 106 | 129 | 53.6 | 23.4 |
| Yoshida et al34 | 12 | 16 | 61.6 | |
| Qiu et al31 | 15 | 15 | 57.1 | 26.3 |
| Cheng et al29 | 10 | 10 | 63 | 25.9 |
| Yoshida et al34 | 113 | 185 | 61.1 | 25.1 |
Studies were conducted retrospectively, other than 14 performed prospectively.22,28–31,42,47,52,54–56,59,66,84 No randomized control trials (RCTs) were identified. Mean duration of lymphedema symptoms before treatment ranged from 12 months29,65 to 11.4 years.86 Follow-up duration ranged from 1 month61 to 5.7 years.86. Notably, one study reported follow-up for 40 years.72 Four studies reported exclusively on primary lymphedema,23,25,39,60 24 studies reported on secondary lymphedema21,26,28,37,38,40,42,47–49,53,62–65,69,70,73,74,77,79,83,86,87, and five studies did not specify lymphedema etiology.27,66,68,71,80 The remaining 41 studies included patients with either primary or secondary lymphedema.16–20,22,24,29–36,41,43–46,50–52,54–59,61,67,72,75,76,78,81,82,84,85,88,89
Baseline and post-surgical lymphedema objective assessment were performed in various ways, including circumferential limb measurements in 34 studies, magnetic resonance volumetry in three studies, body weight in one study, body composition analysis (used to determine changes in extracellular fluid (ECF) volume) in one study, perometer limb volumetry in two studies, bioelectrical impedance in one study, computed tomography measurement of subcutaneous fat thickness in one study, lymphoscintigraphy in one study, ICG lymphography in three studies, and LEL index in 24 studies. In total, 68 studies reported objective improvements in lymphedema following LVA. The fraction of patients with objective improvement after LVA ranged from 23.3%58 to 100%, with a 100% improvement rate reported in 18 studies.16,22,26,29,34,37,43,47,48,59,63,64,69,70,74,79,82,88 Many studies quantified objective improvement with reports of improvements as substantial as a mean 40.5% decrease in limb volume,24 an average 860 mL decrease in edematous limb volume,46 a 63.8% mean reduction in lymphedema,56 and an average 22.67 point decrease in LEL index.59 Overall average reduction in limb volume was 22.67%, and reduction in excess volume or lymphedema was 45.52%. Additionally, there were reports of complete resolution of lymphorrhea in patients after LVA.34,45
Regarding surgical technique, Aljindan et al36 compared side-to-end to end-to-end LVA, noting improved results in the side-to-end group. Scaglioni et al22 compared prior LVA of superficial lymphatics with LVA of deep lymphatics, noting significantly better outcomes in the latter group. Other surgical techniques investigated included lymph node to vein anastomosis,21 a line production technique to shorten operative time,47 “pi-shaped” anastomosis,66 and side-to-end LVA using temporary lymphatic expansion.75 Several alternative methods resulted in equivalent or superior outcomes compared with traditional LVA. The range of number of anastomoses per patient was 136 to 9.3.82, with a mean of 3.9. While most studies evaluated only variations of LVA and its primary outcomes, several investigated the outcomes of the so-called stacked procedures, or LVA in addition to another intervention. These additional procedures included superficial and deep LVA,22 great saphenous vein stripping,23 lymph node to vein anastomosis,21 venoplasty,27vascularized lymph node transplantation,29,58 vein grafting,64 and lymphovenous implantation.84 Intraoperatively, nearly 75% of studies used of indocyanine green (ICG) lymphography for detection of superficial lymphatics and to assess lymphatic function. Preoperatively, lymphatic functional evaluation was mainly performed with lymphoscintigraphy,17,19,21,22,37–39,45,52,57,61,79,83 patent blue dye,40,46,83,84 indigo carmine dye,54,88 ultrasonographic methods,16,18,28,38,44,49,50,80,81 and magnetic resonance lymphangiography (MRL).16,51 Of note, there was inconsistent evidence that the number of anastomoses is correlated with an improved outcome.
Overall, surgical complications were rare, and included a postoperative pelvic recurrence of lymphedema (one of 10 patients),29 postoperative infection (two of 12 patients),30 subcutaneous ecchymoses (six total patients across all studies),32,71,78 cellulitis (a finding that is frequently seen in lymphedema patients before and after surgery),67 lymphangitis (one of 26 patients),84 and failed anastomoses (nine individual failed anastomoses in 48 patients and 80 total anastomoses).75 While cellulitis appears to be the most common complication of LVA, followed distantly by postoperative ecchymosis, there were no reports whatsoever of any major complications such as donor site lymphedema, emergent need to return patients to the operating room, need for surgical revision, or patient death.
Fifteen of the eligible studies noted either partial or complete reductions in episodes of cellulitis postoperatively.16,25,27,29,31,35,36,51,54,56,57,61,67,84,86 Of particular importance, Cha et al16 reported that even in patients with advanced stage lymphedema with a paucity of functional lymphatics, the incidence of cellulitis per year decreased from 0.84 to 0.07 after LVA. Yoshida et al35 reported that of the 31 patients in a 113-patient cohort who experienced episodes of cellulitis preoperatively, none experienced cellulitis after LVA. Mihara et al67 evaluated episodes of cellulitis as a primary outcome of LVA and reported a mean 1.28 episode decrease in the year after surgery compared with the year before. In earlier stage patients, Ito et al56 reported a mean decrease in the number of cellulitis episodes of 1.4 over the course of the follow-up period.
Regarding subjective improvement, patient reporting and quality-of-life (QOL) measures were detailed in a third of studies. Four studies used validated QOL instruments, including the LYMQoL survey,29,42 the Lymph-ICF QOL questionnaire,31 and the McGill Pain Questionnaire.72 Other employed techniques included clinical assessment, subjective evaluation, clinical photography, non-validated QOL questionnaires,37,52 and patient self-reporting. The proportion of patients who reported subjective improvement overall was 89.87%. Factors most strongly associated with subjective improvements were reductions in pain, improved sense of comfort, reduced sense of limb heaviness, ability to better fit in clothing, reduced sense of anxiety related to going in public, and increased self-confidence and self-esteem.
DISCUSSION
This study systematically reviewed the findings of 74 articles in which investigators reported use of LVA for the treatment of primary or secondary lower extremity lymphedema. Comprehensive data analysis was carried out on the duration and severity of symptoms before microsurgical intervention, treatment protocol (including surgical technique and perioperative measures), and the outcomes of surgery with particular attention to measurable reductions in lymphedema and subjective measures of patients’ sense of pain, comfort, functionality, and tissue quality. Each included study reported positive outcomes of LVA microsurgery, and the percentages of patients in study cohorts experiencing objective improvements in lymphedema ranged from at least 23.3% of patients to as many as 100% of patients. A total of 18 studies reported that 100% of patients benefited from some degree of objectively measurable improvement.16,22,26,29,34,37,43,47,48,59,63,64,69,70,74,79,82,88
Among the most crucially important aspects of LVA for LEL is identification of appropriate lymphatic vessels and veins for anastomosis. ICG lymphography (injection of ICG tracers visualized by a handheld near-infrared camera) is well described as a gold standard technique for the real time evaluation of lymphatic function,90 preoperative planning, intraoperative lymphatic visualization,56 and prediction of outcome.85 However, it has several disadvantages. The technique cannot show lymphatic vessels deeper than 1.5 cm, and dermal backflow patterns can obscure otherwise functional lymphatic vessels.28 Importantly, some findings show that ICG-negative lymphatics can be used for anastomosis to produce good outcomes,43 and it has been noted that ICG lymphographic findings are not always consistent with a patient’s lymphedema clinically.17 As such, some alternative techniques have been used to expand the number of patients who are eligible for LVA. Ultrasonographic visualization of both lymphatics and veins allows for selection of deeper vessels for anastomosis, which can lead to improved surgical outcomes, especially among more advanced stage patients whose disease is often characterized by nonfunctional superficial lymphatics and deposition of fibrous and adipose tissue that makes localization of lymphatics difficult.16,49 Magnetic resonance lymphography (MRL) can similarly be utilized to locate vessels that are invisible to ICG with superior spatial resolution,91 and single-photon emission computed tomography (SPECT) fused with co-registered CT scan can be used for superior localization of lymph nodes and allow for greater understanding of a patient’s lymphodynamics.38,61 Lastly, lymphoscintigraphy can be used for initial diagnosis and assessment as well as help to predict rates of edematous reduction following surgery.19
Given the existence of many preoperative imaging modalities, it is important to highlight the imperative need to localize functional lymphatics for LVA. The ideal preoperative method for visualizing lymphatics should be individualized for patients depending on the stage of their lymphedema. For example, earlier stage patients are more likely to have healthy, functional lymphatics in superficial distributions that are easily visualized by ICG lymphography. Conversely, patients with more severe lymphedema are more likely to benefit from methods that reveal deeper lymphatics, such as MR lymphangiography or ultrasonography. Individual patients’ lymphatics will then influence the ideal techniques and anastomotic arrangements that will optimize individual outcomes.
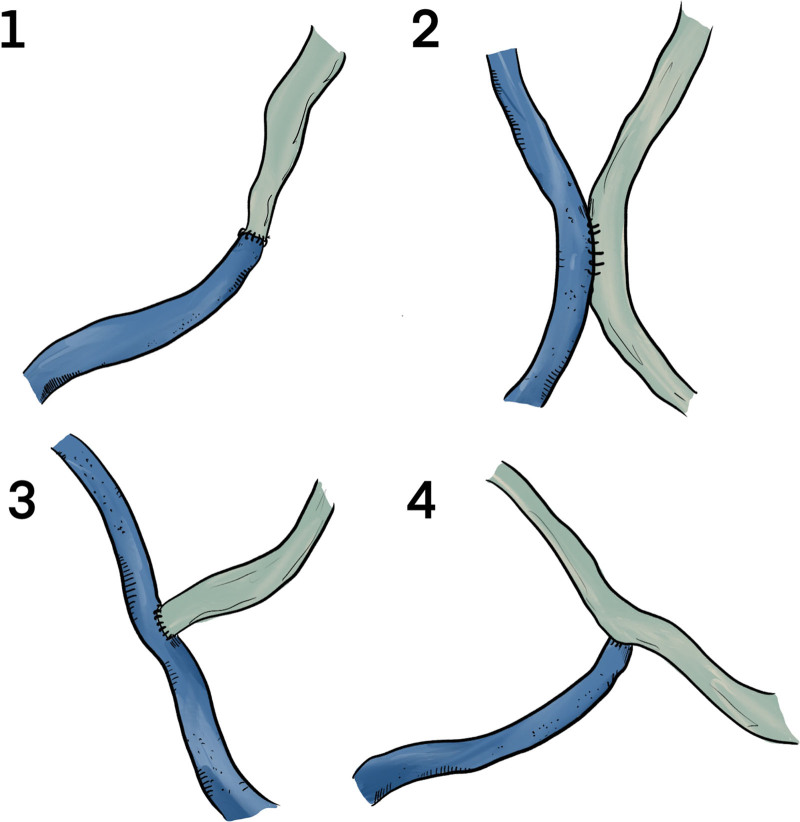
The studies included in this review report a wide variety of surgical techniques and operative protocols. Broadly speaking, veins and lymphatics can be anastomosed in the end-to-side (ES), end-to-end (EE), and side-to-side arrangements (SS). In addition, the side-to-end (SE) configuration, where the end of a recipient vein is anastomosed to the side of a lymphatic, is another possibility, considered by some to be the most efficient possible bypass, allowing for bidirectional lymph flow into a recipient vein (draining both proximally and distally) while preserving the native lymph flow of the vessels and preventing damage to existing vessels (Fig. 3).29 This technique is also the most technically demanding, commonly only carried out by more experienced microsurgeons.63 While some authors believe that SE anastomosis is superior, many others do not so explicitly favor a single configuration over others. More complex still, anastomotic configurations are often limited by the available vessels found in individual patients, forcing surgeons to improvise based on a patient’s unique lymphovascular network. For example, EE anastomosis requires that veins and lymphatics be very nearly the same size, but the vein and lymphatic vessel can be found some distance apart; ES anastomosis requires that vessels be found close together, but one vein can capacitate several anastomoses; SE anastomosis also requires proximity, but allows for bidirectional drainage.32 A number of novel surgical techniques have been reported to aid in negotiating the technical difficulty of SE anastomosis. Other techniques seek to optimize lymphatic flow and drainage, maximize durability and patency of anastomoses, and prevent reflux of fluid from the venous system into lymphatics.
Fig. 3.
Anastomotic configurations possible with LVA. 1, end-to-end anastomosis. 2, side-to-side anastomosis. 3, end-to-side anastomosis. 4, side-to-end anastomosis. While SE anastomosis is the most technically difficult to execute, many contend that it allows for the best possible results by preserving physiologic lymph flow and allowing for bidirectional drainage.
To facilitate SE anastomosis, microsurgeons have used the “parachute technique,”63 leaving all sutures untied until each is placed, promoting greater visibility of the lymphatic vessel. Yamamoto et al82 and Narushima et al92 (the latter not included for analysis as lower limb data were not extractable) both report on intravascular stenting methods that help simplify the surgical technique required for successful anastomosis and decrease the surgeon’s risk of damaging vessel lumens. Another technique involves clamping lymphatics distally and manually massaging lymph nearer the area of anastomosis to temporarily expand the vessel, facilitating anastomosis.75 Then, venous reflux and ecchymosis are well described as complications that portend poorer outcomes.27,87 Several techniques seek to reduce these complications, including external valvuloplasty,71 venoplasty,27 and “peripheral venous angle plasty,”80 all of which make use of veins with fully intact valves (as confirmed most commonly by the “retrograde milking test”). These investigations note a complete absence of venous reflux in the study groups and markedly improved reductions in lymphedema when compared with patients treated with LVA only. Consensus has yet to emerge on whether the number or the quality of anastomoses is more important to produce optimal outcomes. Some contend that the number of anastomoses is directly proportional to the improvement that an individual patient can expect from the procedure.59 Others assert that the quality of anastomosis (that is, anastomosing the most quality veins and lymphatics, ensuring patency and lack of reflux, etc.) is exceedingly important and should not be overlooked in favor of greater numbers.48 One notable technique that aims to improve anastomotic quality is the “superior edge-of-the-knee incision (SEKI) method,” where lymph is cyclically propelled through anastomoses in the thigh region by the patient’s walking motion. Patients treated with the SEKI method showed very significantly improved outcomes compared to patients in the control group treated only with traditional LVA.62
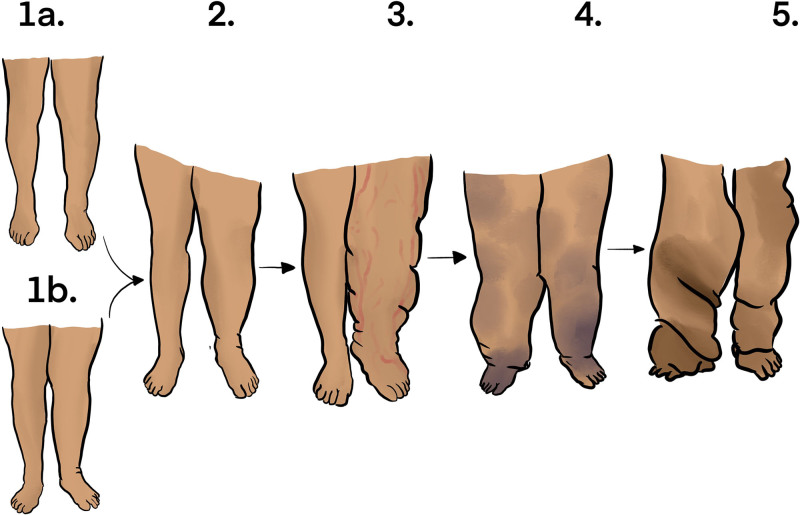
While patients with lymphedema of all Campisi93 stages (Fig. 4) were included in this study, nine investigations specifically evaluated surgical outcomes among patients with later and more advanced stage disease,16,18,20,25,33,39,50,58,87 and four studies reported primarily on early stage patients.53,56,65,79 In a study by Yang et al,33 moderate to advanced stage lymphedema patients (Campisi stage 2 or stage 3) experienced a 36.4% decrease in lower limb volume after LVA, which was not statistically different from the 43.8% average limb volume reduction seen in patients with less advanced disease (Campisi stage 0 or stage 1). Overall, however, included studies establish that LVA is more effective in patients with less severe lymphedema who have experienced symptoms for a shorter period.40 Yet the question remains whether LVA is the optimal treatment even in patients with more severe LEL. Cha et al16 report durable improvements in limb volume in a cohort of 42 patients (50 lower limbs), all of whom had advanced lymphedema, some with significant deposits of fibrous and adipose tissue. Using ultrasonography and MRL, investigators located functional lymphatics for anastomosis. While encouraging for later-stage patients, the authors note that the presence of functional lymphatics, although difficult to locate for many patients, is a crucial precondition for LVA success. Because LVA is possibly less effective in more severe cases, alternative options have been described including VLNT, in which functional lymph nodes are microsurgically transplanted into the extremity to improve lymphatic function.94 Akita et al58 directly compared the efficacy of LVA and VLNT, and among patients with more advanced disease, VLNT led to significantly better results. Although VLNT may produce better outcomes, it should be noted that the procedure is associated with an increased risk of complication, including a risk of lymphedema at the donor site. At the same time, LVA is a promising treatment for lymphorrhea, which is a severely debilitating complication of very advanced lymphedema that involves extrusion of lymph from the skin.95 Two studies included in this review report on using LVA for lymphorrhea with great success, achieving complete resolution in many cases.34,45
Fig. 4.
Campisi staging of lymphedema. 1a, impaired lymphatic function without evidence of gross lymphedema. 1b, appearance of limb swelling reducible with elevation of the limb. 2, marked swelling that does not completely reduce with limb elevation. 3, increased volume of swelling with the appearance of lymphangitis. 4, fibrosis of lymphatics accompanied by warts. 5, elephantiasis.
LVA is a highly advanced, technically difficult, time-consuming technique. In general, shorter operative times correlate well with the attending surgeon’s level of experience,51 though times can be reduced when multiple surgeons work with several microscopes.64 Because of its difficulty, some note that LVA involves techniques only to be executed by these experienced surgeons.63 There are many proposed technical simplifications that lead to shorter operative times; however, another innovative solution to this problem is the “line production system” proposed by Yoshida et al,47 in which one novice surgeon or trainee dissects vessels in one operative field before an expert surgeon later anastomoses vessels in that area. This method proved to be much faster, resulted in more successful and patent anastomoses, and yielded superior postoperative outcomes at follow-up. More research is necessary to determine the methods and techniques that minimize costs, use of resources, and operative times while optimizing patient outcomes.
This review article has many strengths. First, to our knowledge, this is the most recent and comprehensive review of the outcomes of LVA for LEL, and it characterizes several important recent developments and shifting trends for the treatment of this condition. Great efforts were made to include all reports of LVA for LEL in the literature, and particular attention was paid to the immense variety of techniques, anastomotic variations, and innovative practices that are available to microsurgeons. In addition, this review acknowledges the ongoing debate on the efficacy of LVA for later stage lymphedema patients and highlights recent findings that LVA may be a perfectly viable option, even for those who have Campisi stage III and IV lymphedema. Despite these strengths, many questions remain unresolved.
The limitations of this study should also be noted. Most importantly, there is some degree of heterogeneity in the literature regarding perioperative protocol, surgical technique, diagnostic method, and measurement of the severity of lymphedema at baseline and improvement in lymphedema resulting from LVA. Because of this nonuniformity, it is difficult to generalize findings, and robust statistical analysis is significantly hindered. Most investigations on LVA have been conducted in a retrospective fashion, and randomized controlled trials (RCTs) are essentially nonexistent in the literature. With mostly heterogenous retrospective data, there is a risk of interpretation bias. With regard for subjective outcomes, it is important to recognize the additional risk of selective reporting bias. Overall, there is a need to more rigorously characterize and define optimal perioperative protocols, surgical techniques, and objective and subjective outcomes associated with LVA for LEL, and to do this, more uniform data and prospective analyses are needed.
CONCLUSIONS
The results of this systematic review demonstrate that LVA is an effective, safe, and versatile technique for the treatment of primary and secondary LEL. Although the best results can likely be expected in patients with earlier stage and less advanced lymphedema, LVA should not be discounted as a viable option for later-stage patients with more severe disease. There are numerous innovative and emerging techniques that may possibly lead to better, more durable outcomes depending on individual patient needs. Although consensus has yet to emerge regarding the optimal treatment plan for the best possible patient outcomes, we postulate that surgeons should strive to carefully select appropriate and quality lymphatics and veins for anastomosis (not excluding deep vessels), increase the number and quality of anastomoses to the extent possible, and make use of the most effective bypass methods when allowed by individual patient anatomy.
Supplementary Material
Footnotes
Published online 7 October 2022.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77:1009–1020. [DOI] [PubMed] [Google Scholar]
- 2.Warren AG, Brorson H, Borud LJ, et al. Lymphedema: a comprehensive review. Ann Plast Surg. 2007;59:464–472. [DOI] [PubMed] [Google Scholar]
- 3.Brouillard P, Boon LM, Vikkula M, et al. Genetics of lymphatic anomalies. J Clin Invest. 2014;124:898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrell RM, Halyard MY, Schild SE, et al. Breast cancer-related lymphedema. Mayo Clin Proc. 2005;80:1480–1484. [DOI] [PubMed] [Google Scholar]
- 5.Maclellan RA, Greene AK. Lymphedema. Semin Pediatr Surg. 2014;23:191–7. [DOI] [PubMed] [Google Scholar]
- 6.Ciudad P, Sabbagh MD, Agko M, et al. Surgical management of lower extremity lymphedema: a comprehensive review. Indian J Plast Surg. 2019;52:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dessources K, Aviki E, Leitao MM, Jr. Lower extremity lymphedema in patients with gynecologic malignancies. Int J Gynecol Cancer. 2020;30:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang DW, Masia J, Garza R, et al. Lymphedema: surgical and medical therapy. Plast Reconstr Surg. 2016;138:209S–218S. [DOI] [PubMed] [Google Scholar]
- 9.Manrique OJ, Bustos SS, Ciudad P, et al. Overview of lymphedema for physicians and other clinicians: a review of fundamental concepts. Mayo Clin Proc. 2020:S0025-6196:30033–1. [DOI] [PubMed] [Google Scholar]
- 10.Cormier JN, Rourke L, Armer J, et al. The surgical treatment of lymphedema: a systematic review of the contemporary literature (2004–2010). Ann Surg Oncol. 2012;19:642–51. [DOI] [PubMed] [Google Scholar]
- 11.Nielubowicz J, Olszewski W. Experimental lymphovenous anastomosis. Br J Surg. 1968;55:449–51. [DOI] [PubMed] [Google Scholar]
- 12.Scaglioni MF, Fontein DBY, Arvanitakis M, et al. Systematic review of lymphovenous anastomosis (LVA) for the treatment of lymphedema. Microsurgery. 2017;37:947–953. [DOI] [PubMed] [Google Scholar]
- 13.Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg. 2000;16:437–42. [DOI] [PubMed] [Google Scholar]
- 14.Coriddi M, Dayan J, Sobti N, et al. Systematic review of patient-reported outcomes following surgical treatment of lymphedema. Cancers (Basel). 2020;12(3):565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winters H, Tielemans HJ, Sprangers PN, et al. Peri-operative care for patients undergoing lymphaticovenular anastomosis: a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:178–188. [DOI] [PubMed] [Google Scholar]
- 16.Cha HG, Oh TM, Cho MJ, et al. Changing the paradigm: lymphovenous anastomosis in advanced stage lower extremity lymphedema. Plast Reconstr Surg. 2021;147:199–207. [DOI] [PubMed] [Google Scholar]
- 17.Drobot A, Bez M, Abu Shakra I, et al. Microsurgery for management of primary and secondary lymphedema. J Vasc Surg Venous Lymphat Disord. 2021;9:226–233.e1. [DOI] [PubMed] [Google Scholar]
- 18.Hara HA-O, Mihara MA-O. Lymphaticovenous anastomosis for advanced-stage lower limb lymphedema. J Clin Med. 2021;10:1540. [DOI] [PubMed] [Google Scholar]
- 19.Kim HO, Woo KJ, Kim BS, et al. Lymphoscintigraphic findings as indicators of lymphaticovenous anastomosis outcome in patients with extremity lymphedema: a retrospective cohort study. Clin Nucl Med. 2021;46:549–555. [DOI] [PubMed] [Google Scholar]
- 20.Onoda S, Nishimon K. The utility of surgical and conservative combination therapy for advanced stage lymphedema. J Vasc Surg Venous Lymphat Disord. 2021;9:234–241. [DOI] [PubMed] [Google Scholar]
- 21.Pak CS, Suh HP, Kwon JG, et al. Lymph Node to Vein Anastomosis (LNVA) for lower extremity lymphedema. J Plast Reconstr Aesthet Surg. 2021;74:2059–2067. [DOI] [PubMed] [Google Scholar]
- 22.Scaglioni MF, Meroni M, Fritsche E. Combining superficial and deep lymphovenous anastomosis for lymphedema treatment: preliminary results. Microsurgery. 2022;42:22–31. [DOI] [PubMed] [Google Scholar]
- 23.Yoshida S, Koshima I, Imai H, et al. Combined lymphovenous anastomosis and great saphenous vein stripping for comorbid lymphedema and varicose veins. Lymphat Res Biol. 2022;20:213–219. [DOI] [PubMed] [Google Scholar]
- 24.Yang JA-O, Huang LH, Wu SA-O, et al. Lymphaticovenous anastomosis supermicrosurgery decreases oxidative stress and increases antioxidant capacity in the serum of lymphedema patients. J Clin Med. 2021;10:1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshida S, Koshima I, Imai H, et al. Characteristics and outcomes of lymphaticovenular anastomosis in older patients with bilateral involvement versus younger patients with unilateral involvement in lower extremity lymphedema. J Vasc Surg Venous Lymphat Disord. 2020;8:646–657. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida S, Koshima I, Imai H, et al. Modified intraoperative distal compression method for lymphaticovenous anastomosis with high success and a low venous reflux rates . J Plast Reconstr Aesthet Surg. 2021;74:2050–2058. [DOI] [PubMed] [Google Scholar]
- 27.Akita S, Yamaji Y, Tokumoto H, et al. Prevention of venous reflux with full utilization of venoplasty in lymphaticovenular anastomosis. J Plast Reconstr Aesthet Surg. 2020;73:537–543. [DOI] [PubMed] [Google Scholar]
- 28.Bianchi A, Visconti G, Hayashi A, et al. Ultra-High frequency ultrasound imaging of lymphatic channels correlates with their histological features: a step forward in lymphatic surgery.s J Plast Reconstr Aesthet Surg. 2020;73:1622–1629. [DOI] [PubMed] [Google Scholar]
- 29.Cheng MH, Tee R, Chen C, et al. Simultaneous ipsilateral vascularized lymph node transplantation and contralateral lymphovenous anastomosis in bilateral extremity lymphedema with different severities. Ann Surg Oncol. 2020;27:5267–5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristiansen M, Halle M, Pignatti M, et al. Evaluation and selection of lower limb lymphedema patients for lymphaticovenular anastomosis: a prospective study. Injury. 2020;51(4):S108–S113. [DOI] [PubMed] [Google Scholar]
- 31.Qiu SS, Pruimboom T, Cornelissen AJM, et al. Outcomes following lymphaticovenous anastomosis (LVA) for 100 cases of lymphedema: results over 24-months follow-up. Breast Cancer Res Treat. 2020;184:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai PL, Wu SC, Lin WC, et al. Determining factors in relation to lymphovascular characteristics and anastomotic configuration in supermicrosurgical lymphaticovenous anastomosis – a retrospective cohort study. Int J Surg. 2020;81:39–46. [DOI] [PubMed] [Google Scholar]
- 33.Yang JC, Wu SC, Lin WC, et al. Supermicrosurgical lymphaticovenous anastomosis as alternative treatment option for moderate-to-severe lower limb lymphedema. J Am Coll Surg. 2020;230:216–227. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida S, Hamada Y, Koshima I, et al. Role of lymphatico venular anastomosis for treatment of lymphorrhea in lower limbs. J Plast Reconstr Aesthet Surg. 2020;73(7):1357–1404. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida S, Koshima I, Imai H, et al. Lymphovenous anastomosis for morbidly obese patients with lymphedema. Plast Reconstr Surg Glob Open. 2020;8:e2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AlJindan FK, Lin CY, Cheng MH. Comparison of outcomes between side-to-end and end-to-end lymphovenous anastomoses for early-grade extremity lymphedema. Plast Reconstr Surg. 2019;144:486–496. [DOI] [PubMed] [Google Scholar]
- 37.Chung JH, Baek SO, Park HJ, et al. Efficacy and patient satisfaction regarding lymphovenous bypass with sleeve-in anastomosis for extremity lymphedema. Arch Plast Surg. 2019;46:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gentileschi S, Albanese R, Pino V, et al. SPECT/CT and fusion ultrasound to target the efferent groin lymph node for lymphatic surgery. Microsurgery. 2019;39:605–612. [DOI] [PubMed] [Google Scholar]
- 39.Hadiwattage S, Watawana L, Manjula Peiris SP, et al. Impact of lymphovenous anastomosis on limb circumference in patients with lymphoedema tarda. J. Lymphoedema. 2019;14:29–31. [Google Scholar]
- 40.Klingelhoefer E, Hesse K, Taeger CD, et al. Factors affecting outcomes after supermicrosurgical lymphovenous anastomosis in a defined patient population. Clin Hemorheol Microcirc. 2019;73:53–63. [DOI] [PubMed] [Google Scholar]
- 41.Koshima I, Yoshida S, Nagamatsu S, et al. Effect of pregnancy on lower limb lymphedema in patients treated with multisite lymphaticovenular anastomoses. Lymphology. 2019;52:187–193. [PubMed] [Google Scholar]
- 42.Phillips GSA, Gore S, Ramsden A, et al. Lymphaticovenular anastomosis in the treatment of secondary lymphoedema of the legs after cancer treatment. J Plast Reconstr Aesthet Surg. 2019;72:1184–1192. [DOI] [PubMed] [Google Scholar]
- 43.Scaglioni Mf, Uyulmaz S, Arvanitakis M, Intraoperatively detected but previously indocyanine green-negative lymphatic vessels may have misprized potentials and should not be neglected in lymphaticovenous bypass surgery. Ann Plast Surg. 2019;83:69–72. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki Y, Sakuma H, Yamazaki S. Comparison of patency rates of lymphaticovenous anastomoses at different sites for lower extremity lymphedema. J Vasc Surg Venous Lymphat Disord. 2019;7:222–227. [DOI] [PubMed] [Google Scholar]
- 45.Yang JC, Yen YH, Wu SC, et al. Supermicrosurgical lymphaticovenous anastomosis as an alternative treatment option for patients with lymphorrhea. Plast Reconstr Surg. 2019;144:1214–1224. [DOI] [PubMed] [Google Scholar]
- 46.Yasunaga Y, Yanagisawa D, Ohata E, et al. Bioelectrical impedance analysis of water reduction in lower-limb lymphedema by lymphaticovenular anastomosis. J Reconstr Microsurg. 2019;35:306–314. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida S, Koshima I, Imai H, et al. Line production system for multiple lymphaticovenular anastomoses. J Plast Reconstr Aesthet Surg. 2019;72:1334–1339. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida S, Koshima I, Sasaki A, et al. Mechanical dilation using nylon monofilament aids multisite lymphaticovenous anastomosis through improving the quality of anastomosis. Ann Plast Surg. 2019;82:201–206. [DOI] [PubMed] [Google Scholar]
- 49.Hayashi AA-O, Hayashi N, Yoshimatsu H, Effective and efficient lymphaticovenular anastomosis using preoperative ultrasound detection technique of lymphatic vessels in lower extremity lymphedema. J Surg Oncol. 2018;117:290– 298. [DOI] [PubMed] [Google Scholar]
- 50.Mihara M, Hara H, Kawakami Y, et al. Multi-site lymphatic venous anastomosis using echography to detect suitable subcutaneous vein in severe lymphedema patients. J Plast Reconstr Aesthet Surg. 2018;71:e1–e7. [DOI] [PubMed] [Google Scholar]
- 51.Pereira N, Lee YH, Suh Y, et al. Cumulative experience in lymphovenous anastomosis for lymphedema treatment: the learning curve effect on the overall outcome. J Reconstr Microsurg. 2018;34:735–741. [DOI] [PubMed] [Google Scholar]
- 52.Salgarello M, Mangialardi ML, Pino V, et al. A Prospective evaluation of health-related quality of life following lymphaticovenular anastomosis for upper and lower extremities lymphedema. J Reconstr Microsurg. 2018;34:701–707. [DOI] [PubMed] [Google Scholar]
- 53.Akita SA-O, Ogata F, Manabe I, et al. Noninvasive screening test for detecting early stage lymphedema using follow-up computed tomography imaging after cancer treatment and results of treatment with lymphaticovenular anastomosis. Microsurgery. 2017;37:910–916. [DOI] [PubMed] [Google Scholar]
- 54.Lee KA-O, Park JA-O, Mun GA-O. Serial two-year follow-up after lymphaticovenular anastomosis for the treatment of lymphedema. Microsurgery. 2017;37:763–770. [DOI] [PubMed] [Google Scholar]
- 55.Campisi CC, Ryan M, Boccardo F, et al. A single-site technique of multiple lymphatic-venous anastomoses for the treatment of peripheral lymphedema: long-term clinical outcome. J Reconstr Microsurg. 2016;32:42–9. [DOI] [PubMed] [Google Scholar]
- 56.Ito R, Wu CT, Lin MC, et al. Successful treatment of early-stage lower extremity lymphedema with side-to-end lymphovenous anastomosis with indocyanine green lymphography assisted. Microsurgery. 2016;36:310–5. [DOI] [PubMed] [Google Scholar]
- 57.Mihara M, Hara H, Tange S, et al. Multisite lymphaticovenular bypass using supermicrosurgery technique for lymphedema management in lower lymphedema cases. rces plast reconstr surg. 2016;138:262–272. [DOI] [PubMed] [Google Scholar]
- 58.Akita S, Mitsukawa N, Kuriyama M, et al. Comparison of vascularized supraclavicular lymph node transfer and lymphaticovenular anastomosis for advanced stage lower extremity lymphedema. Ann Plast Surg. 2015;74:573–9. [DOI] [PubMed] [Google Scholar]
- 59.Chen WF, Yamamoto T, Fisher M, et al. The “Octopus” lymphaticovenular anastomosis: Evolving beyond the standard supermicrosurgical technique. J Reconstr Microsurg. 2015;31:450–7. [DOI] [PubMed] [Google Scholar]
- 60.Hara H, Narushima M, Koshima I, et al. Indication of lymphaticovenous anastomosis for lower limb primary lymphedema. Plast Reconstr Surg. 2015;136:883–893. [DOI] [PubMed] [Google Scholar]
- 61.Iimura T, Fukushima Y, Kumita S, et al. Estimating lymphodynamic conditions and lymphovenous anastomosis efficacy using (99m)tc-phytate lymphoscintigraphy with SPECT-CT in patients with lower-limb lymphedema. Plast Reconstr Surg Glob Open. 2015;3:e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seki Y, Yamamoto T, Yoshimatsu H, et al. The superior-edge-of-the-knee incision method in lymphaticovenular anastomosis for lower extremity lymphedema. Plast Reconstr Surg. 2015;136:665e–675e. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto T, Tashiro K, Koshima I, et al. Technical simplification of the supermicrosurgical side-to-end lymphaticovenular anastomosis using the parachute technique. Microsurgery. 2015;35:129–34. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto T, Yoshimatsu H, Yamamoto N, et al. Multisite lymphaticovenular anastomosis using vein graft for uterine cancer-related lymphedema after pelvic lymphadenectomy. Vasc Endovascular Surg. 2015;49:195–200. [DOI] [PubMed] [Google Scholar]
- 65.Akita S, Mitsukawa N, Kuriyama M, et al. Suitable therapy options for sub-clinical and early-stage lymphoedema patients. J Plast Reconstr Aesthet Surg. 2014;67:520–5. [DOI] [PubMed] [Google Scholar]
- 66.Ayestaray B, Bekara F. π-shaped lymphaticovenular anastomosis: the venous flow sparing technique for the treatment of peripheral lymphedema. J Reconstr Microsurg. 2014;30:551–560. [DOI] [PubMed] [Google Scholar]
- 67.Mihara M, ara H, Furniss D, et al. Lymphaticovenular anastomosis to prevent cellulitis associated with lymphoedema. Br J Surg. 2014;101:1391–6. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto T, Narushima M, Yoshimatsu H, et al. Minimally invasive lymphatic supermicrosurgery (MILS): indocyanine green lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann Plast Surg. 2014;72:67–70. [DOI] [PubMed] [Google Scholar]
- 69.Yamamoto T, Yamamoto N, Azuma S, et al. Near-infrared illumination system-integrated microscope for supermicrosurgical lymphaticovenular anastomosis. Microsurgery. 2014;34:23–7. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto T, Yoshimatsu H, Narushima M, et al. Sequential anastomosis for lymphatic supermicrosurgery: multiple lymphaticovenular anastomoses on 1 venule. Ann Plast Surg. 2014;73:46–9. [DOI] [PubMed] [Google Scholar]
- 71.Akita S, Mitsukawa N, Kuriyama M, et al. External valvuloplasty for subcutaneous small veins to prevent venous reflux in lymphaticovenular anastomosis for lower extremity lymphedema. Plast Reconstr Surg. 2013;132:1008–1014. [DOI] [PubMed] [Google Scholar]
- 72.Olszewski WL. Lymphovenous microsurgical shunts in treatment of lymphedema of lower limbs: a 45-year experience of one surgeon/one center. Eur J Vasc Endovasc Surg. 2013;45:282–90. [DOI] [PubMed] [Google Scholar]
- 73.Yamamoto T, Yoshimatsu H, Narushima M, et al. A modified side-to-end lymphaticovenular anastomosis. Microsurgery. 2013;33:130–3. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto T, Yoshimatsu H, Narushima M, et al. Split intravascular stents for side-to-end lymphaticovenular anastomosis. Ann Plast Surg. 2013;71:538–40. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto T, Yoshimatsu H, Koshima I, et al. Side-to-end Lymphaticovenular anastomosis through temporary lymphatic expansion. PLoS One. 2013;8:e59523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Auba C, Marre D, Rodríguez-Losada G, et al. Lymphaticovenular anastomoses for lymphedema treatment: 18 months postoperative outcomes. Microsurgery. 2012;32:261–268. [DOI] [PubMed] [Google Scholar]
- 77.Maegawa J, Hosono M, Iwai T, et al. Net effect of lymphaticovenous anastomosis on volume reduction of peripheral lymphoedema after complex decongestive physiotherapy. Eur J Vasc Endovasc Surg. 2012;43(5):602– 60–8.. [DOI] [PubMed] [Google Scholar]
- 78.Maegawa J, Tomoeda H, Hosono M, et al. Outcomes of lymphaticovenous side-to-end anastomosis in peripheral lymphedema. J Vasc Surg. 2012;55:753–760. [DOI] [PubMed] [Google Scholar]
- 79.Mihara M, Hara H, Kikuchi K, et al. Scarless lymphatic venous anastomosis for latent and early-stage lymphoedema using indocyanine green lymphography and non-invasive instruments for visualising subcutaneous vein. J Plast Reconstr Aesthet Surg. 2012; 65:1551– 155–8.. [DOI] [PubMed] [Google Scholar]
- 80.Yamaguchi K, Kimata Y, Suami H, et al. Peripheral venous angle plasty: a new lymphovenous anastomosis technique for lower extremity lymphedema. Plast Reconstr Surg. 2012;130:233e–235e. [DOI] [PubMed] [Google Scholar]
- 81.Mihara M, Hayashi Y, Murai N, et al. Regional diagnosis of lymphoedema and selection of sites for lymphaticovenular anastomosis using elastography. Clin Radiol. 2011;66:715–19. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto T, Narushima M, Kikuchi K, et al. Lambda-shaped anastomosis with intravascular stenting method for safe and effective lymphaticovenular anastomosis. Plast Reconstr Surg. 2011;127:1987–1992. [DOI] [PubMed] [Google Scholar]
- 83.Maegawa J, Mikami T, Kobayashi S, et al.Types of lymphoscintigraphy and indications for lymphaticovenous anastomosis. Microsurgery. 2010;30:437–42. [DOI] [PubMed] [Google Scholar]
- 84.Demirtas Y, Ozturk N, Topalan M, et al. Supermicrosurgical lymphaticovenular anastomosis and lymphaticovenous implantation for treatment of unilateral lower extremity lymphedema. Microsurgery. 2009;29:609–618. [DOI] [PubMed] [Google Scholar]
- 85.Demirtas Y, Ozturk N, Topalan M, et al. Comparison of primary and secondary lower-extremity lymphedema treated with supermicrosurgical lymphaticovenous anastomosis and lymphaticovenous implantation. J Reconstr Microsurg. 2010;26:137–43. [DOI] [PubMed] [Google Scholar]
- 86.Matsubara S, Sakuda H, Nakaema M, et al. Long-term results of microscopic lymphatic vessel-isolated vein anastomosis for secondary lymphedema of the lower extremities. Surg Today. 2006;36:859–864. [DOI] [PubMed] [Google Scholar]
- 87.Koshima I, Nanba Y, Takahashi Y, et al. Minimal invasive lymphaticovenular anastomosis under local anesthesia for leg lymphedema: is it effective for stage III and IV? Annals of Plastic Surgery: 2004;53:261–266. [DOI] [PubMed] [Google Scholar]
- 88.Koshima I, Nanba Y, Itoh S, et al. Long-term follow-up after lymphaticovenular anastomosis for lymphedema in the leg. BMC Surg. 2019;19:177. [DOI] [PubMed] [Google Scholar]
- 89.Koshima I, Moriguchi T, Kajiwara Y, et al. Ultrastructural observations of lymphatic vessels in lymphedema in human extremities. Plast Reconstr Surg. 1996;97:397–405; [DOI] [PubMed] [Google Scholar]
- 90.Yamamoto T, Narushima M, Oshima A, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;127:1979–1986. [DOI] [PubMed] [Google Scholar]
- 91.Yasunaga Y, Nakajima Y, Mimura S, et al. Magnetic resonance lymphography as three-dimensional navigation for lymphaticovenular anastomosis in patients with leg lymphedema. J Plast Reconstr Aesthet Surg. 2021;74:1253–1260. [DOI] [PubMed] [Google Scholar]
- 92.Narushima M, Yamamoto Y, Mundinger GS, et al. The intravascular stenting method for treatment of extremity lymphedema with multiconfiguration lymphaticovenous anastomoses. Plast Reconstr Surg. 2010;125:935–943. [DOI] [PubMed] [Google Scholar]
- 93.Campisi C, Boccardo F. Lymphedema and microsurgery. Microsurgery. 2002;22:74–80. [DOI] [PubMed] [Google Scholar]
- 94.Schaverien MV, Badash I, Patel KM, et al. Vascularized lymph node transfer for lymphedema. Semin Plast Surg. 2018;32:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Inoue M, Nakatsuka S, Yashiro H, et al. Lymphatic intervention for various types of lymphorrhea: access and treatment. Radiographics. 2016;36:2199–2211 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.