Abstract
Electrodiagnostic (EDx) studies are helpful in diagnosing and subtyping of Guillain‐Barré syndrome (GBS). Published criteria for differentiation into GBS subtypes focus on cutoff values, but other items receive less attention, although they may influence EDx subtyping: (a) extensiveness of EDx testing, (b) nerve‐specific considerations, (c) distal compound muscle action potential (CMAP)‐amplitude requirements, (d) criteria for conduction block and temporal dispersion. The aims of this study were to investigate how these aspects were approached by neuromuscular EDx experts in practice and how this was done in previously published EDx criteria for GBS. A completed questionnaire was returned by 24 (of 49) members of the electrophysiology expertise group from the International GBS Outcome Study. Six published EDx criteria for GBS subtyping were compared regarding these aspects. The indicated minimal number of motor nerves to study varied among respondents and tended to be more extensive in equivocal than normal studies. Respondents varied considerably regarding usage of compression sites for subtyping (median/wrist, ulnar/elbow, peroneal/fibular head): 29% used all variables from all sites, 13% excluded all sites, and 58% used only some sites and/or variables. Thirty‐eight percent of respondents required a minimal distal CMAP amplitude to classify distal motor latency as demyelinating, and 58% did for motor conduction velocity. For proximal/distal CMAP‐amplitude ratio and F‐wave latency, a requisite minimal CMAP amplitude was more often required (79%). Also, the various published criteria sets showed differences on all items. Practical use of EDx criteria for subtyping GBS vary extensively across respondents, potentially lowering the reproducibility of GBS subtyping.
Keywords: electrodiagnostic criteria, electromyography, Guillain‐Barré syndrome, nerve conduction studies, variation
1. INTRODUCTION
Electrodiagnostic (EDx) studies are used in the diagnosis, subtyping and prediction of outcome in the Guillain‐Barré syndrome (GBS). Several authors have provided criteria for subtyping the EDx results of GBS patients into axonal or demyelinating subtypes. 1 , 2 , 3 , 4 , 5 , 6 According to the Brighton Collaboration GBS Working Group, 7 EDx findings consistent with GBS are necessary to fulfill the criteria for level 1 diagnostic certainty. In general, classification sets for subtyping focus on cutoff values for axonal and demyelinating subtypes, but differ in requisites to be fulfilled before EDx criteria can be applied.
The four EDx related items of our concern, because of their difference between criteria sets and their possible impact on EDx subtyping, were: (a) Extensiveness of EDx testing, specifically the minimal number of nerves to be studied and the extensiveness of testing within the nerve, that is, proximal nerve segments. According to some authors, 3 , 4 , 5 , 6 detection of axonal or demyelinating features in two nerves is sufficient to subtype these EDx study results as axonal or demyelinating forms of GBS. On the other hand, the other EDx categories (equivocal, inexcitable and normal) were not based on a specified number of nerves with corresponding EDx features. (b) Nerve‐specific considerations. Classical entrapment neuropathies, that is, median nerve at the carpal tunnel, ulnar nerve at the elbow and peroneal nerve at the fibular head, can show EDx features that might also fulfill GBS EDx criteria. This is relevant, because of the high prevalence of compression neuropathies in the general population. For example, carpal tunnel syndrome (CTS) has an estimated prevalence of up to 5%. 8 In EDx subtyping the tibial nerve often received special attention. Due to phase cancelation and submaximal stimulation, proximal compound muscle action potential (CMAP)‐amplitude reduction in the tibial nerve might occur, 9 a finding fulfilling criteria for conduction block (CB). (c) Requirements for the distal CMAP amplitude, that is, should the distal CMAP amplitude be taken into consideration, before conclusions about demyelinating EDx features can be drawn. A low distal CMAP amplitude can represent severe axonal loss, which might be accompanied by selective loss of the fastest motor nerves and a regenerating process with growth of slowly conducting sprouts. 10 Therefore, conduction slowing can happen in an axonal illness, and can mimic a demyelinating process. (d) Criteria for CB and temporal dispersion (TD). Historically, CB was only considered a demyelinating feature, but later studies in GBS showed this might also represent axonal (nodal‐paranodal) pathology, that is, reversible conduction failure. 11 CMAP‐duration prolongation, that is, abnormal TD, was described as a key feature to differentiate axonal from demyelinating CB. 11 The duration and desynchronization (ie, number of turns) of the CMAP in GBS was extensively studied by Clouston et al, 12 and reference values were provided. Others have indicated reference values for distal CMAP‐duration stratified for different low frequency filter settings. 13 Nevertheless, most criteria sets lacked strict criteria for TD 1 , 3 , 4 , 5 and this might induce variation in EDx subtyping.
The aim of this study was to get insight into how neuromuscular EDx experts in the field of GBS approach these different aspects of EDx subtyping. This was inventoried by sending a questionnaire to the members of the EDx expertise group of the International GBS Outcome Study (IGOS). Also, an overview will be presented on how these aspects were addressed in the past, by the authors from previously published criteria sets. 1 , 2 , 3 , 4 , 5 , 6
2. MATERIALS AND METHODS
A questionnaire was sent in June 2021 to the 49 members of the electrophysiology expertise group from the IGOS. See supplement for the full questionnaire. This questionnaire was developed by four authors (S.A., B.C.J., D.R.C., J.D.) and contained 21 questions about four topics: (a) requirements for the extensiveness of EDX testing. Questions were related to minimal number of nerves to be studied and EDx of proximal nerve segments and roots, (b) nerve‐specific criteria. Questions were related to classical compression neuropathies and the tibial nerve, (c) requirements for the distal CMAP amplitude and (d) criteria for CB and TD.
For a historical overview of literature on how these four aspects were approached in the past, the six most commonly used criteria sets were analyzed. 1 , 2 , 3 , 4 , 5 , 6
3. RESULTS
A total of 49 questionnaires were sent and the response rate was 49% (N = 24). Of the respondents, 91% perform or supervise EDx studies at least every week.
3.1. Extensiveness of EDx in GBS
For an overview of literature 1 , 2 , 3 , 4 , 5 , 6 on the most commonly used criteria sets, concerning the different aspects of EDx in GBS, see Table 1.
TABLE 1.
Overview of six different published GBS EDx criteria sets, concerning the different aspects of this study
| Albers | Asbury | Hadden | Ho | Rajabally | Uncini | ||
|---|---|---|---|---|---|---|---|
| Timing EDx | Days | NS | <21 | <15 | <14 | <21 | <24 |
| Subtypes in EDx criteria | Yes/no | N | N | Y | Y | Y | Y |
| Follow‐up EDx study in criteria | N | N | N | N | N | Y | |
| Extensiveness EDx protocol | Minimum number motor nerves | NS | 3 | 4 a | NS | 4 | 3 |
| Sensory conduction advised (yes/no) | Y | N | Y | N | Y | Y | |
| Criteria for sensory conduction (yes/no) | N | N | N | N | N | Y | |
| Minimum number sensory nerves | 2 | NS | NS | N | 2 | 3 | |
| Proximal NCS | Proximal stimulation median nerve advised | N | N | N | N | N | N |
| Proximal stimulation ulnar nerve (at elbow) advised | N | N | N | N | N | N | |
| Proximal stimulation ulnar nerve (above elbow) advised | N | N | N | N | N | N | |
| F‐wave latency criteria | Y | Y | Y | Y | Y | Y | |
| F‐wave absence criteria | N | Y | N | N | Y | Y | |
| F waves—minimal number of trials | 10 | 10 | NS | NS | NS | 16 | |
| Myography (yes/no) advised | Y | N | N | N | N | N | |
| Nerve‐specific considerations | Median—carpal tunnel excluded (yes/no) | Y | N | N | N | N | N |
| Ulnar—sulcus excluded (yes/no) | Y | Y | N | N | N | N | |
| Peroneal—fibular head excluded (yes/no) | Y | Y | N | N | N | N | |
| Tibial—excluded (yes/no/partially) | N | Y | N | N | Y | Y | |
| Distal CMAP amplitude requirements | In distal motor latency | Y | Y | Y | Y | N | N |
| In motor nerve conduction velocity | Y | Y | Y | Y | N | N | |
| In proximal/distal CMAP‐amplitude/area/duration ratio | N | N | Y | NA | N | N | |
| In F wave latency | N | Y | N | N | Y | N | |
| In F wave absence | NA | N | NA | NA | Y | Y | |
| Criterion temporal dispersion | Yes/no | Y | Y | N | Y | N | Y |
| Criterion conduction block | Yes/no | Y | Y | Y | N | Y | Y |
| CB criteria based on: area, amplitude or both | Am | B | Am | NA | NS | Am |
Abbreviations: Am, amplitude; B, both amplitude and area; CB, conduction block; CMAP, compound muscle action potential; EDx, electrodiagnostics; GBS, Guillain‐Barré syndrome; N, no; NA, not applicable; NCS, nerve conduction studies; NS, not specified; Y, yes.
Intended to study four motor nerves, but mean number tested in cohort was 3.7 (SD 1.4).
3.1.1. Number of motor nerves
Regarding the extensiveness of EDx, published criteria sets differed in indicated minimal number of nerves to be studied. Albers et al 1 and Ho et al 3 did not recommend a specified minimal number of motor nerves to be studied, Asbury and Cornblath 2 and Uncini et al 6 recommended to study at least three motor nerves and Hadden et al 4 and Rajabally et al 5 recommended testing at least four motor nerves.
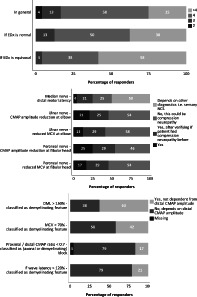
Figure 1 presents the minimum number of motor nerves to study in GBS patients according to the respondents, in general and in case an equivocal or normal study is assumed. According to 58% of respondents, in general an EDx study of four motor nerves was required. If during the examination the EDx results turned out to be abnormal but did not fulfill demyelinating or axonal criteria (equivocal), this number increased to greater than four motor nerves according to 58% of respondents.
FIGURE 1.
Minimal number of motor nerves to be studied in a GBS patient in general (upper), if EDx is normal (middle) and if EDx is equivocal (lower), according to respondents (%). GBS, Guillain‐Barré syndrome
3.1.2. Proximal nerve conduction
None of the published criteria sets 1 , 2 , 3 , 4 , 5 , 6 advised to study proximal nerve segments by stimulation at upper arm and/or Erb's point. All sets provided cutoff values for minimal F‐wave latencies, but some lacked criteria for F‐wave absence. 1 , 3 , 4 Prolonged F‐wave latency can be caused by proximal conduction slowing but also by reduced distal conduction velocity and/or prolonged distal latency. None of the existing criteria provided ways on how to take distal conduction slowing into account in the interpretation of prolonged F‐wave latency.
According to 96% of respondents, evaluation of proximal segments of arm nerve(s) is indicated, either by F waves (58%) or by nerve stimulation in the upper arm and at Erb's point (38%). In case of a significant CMAP‐amplitude reduction at Erb's point, 64% of respondents will not use this for EDx classification, because of the risk of submaximal stimulation. Prolonged F‐wave latency was considered a demyelinating feature irrespective of distal conduction slowing, according to 50% of respondents. Yet, 17% did not use this as independent feature of proximal demyelinating feature because prolonged F‐wave latency could also be attributed to distal demyelination.
3.2. Nerve‐specific considerations
Albers et al 1 advised to exclude the classical compression sites for the median nerve (wrist), ulnar nerve (elbow) and peroneal nerve (fibular head). Asbury and Cornblath 2 excluded both the ulnar nerve (elbow) and the peroneal nerve (fibular head) for the criterion of a CB, but did not mention the carpal tunnel. On the other hand, EDx findings compatible with classical compression neuropathies were not excluded by others. 3 , 4 , 5 , 6
The tibial nerve is known for its pronounced proximal CMAP‐amplitude reduction and prolonged duration due to phase cancelation and submaximal stimulation. 14 As this finding could often fulfill EDx GBS criteria, the tibial nerve was excluded for EDx subtyping in GBS patients in multiple EDx criteria sets. 2 , 5 , 6
3.2.1. Median nerve
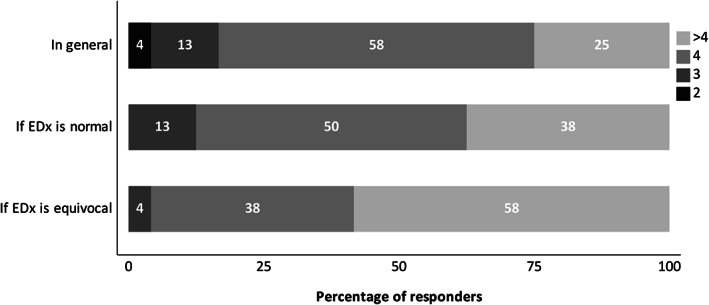
Opinions on how a prolonged distal motor latency of the median nerve should be used in EDx subtyping varied considerably: 25% excluded this variable from subtyping because of possible confusion with a CTS, while 71% only used this if they could verify that CTS was not the case, either clinically (signs or diagnosis of CTS before) or by additional electrophysiological testing (Figure 2).
FIGURE 2.
Respondent opinions on whether EDx variables at classical compression sites should be used for EDx subtyping in GBS. GBS, Guillain‐Barré syndrome
3.2.2. Ulnar nerve
Slowing of motor conduction velocity (MCV) and reduction of CMAP amplitude across the elbow can be found both in ulnar neuropathy at the elbow and in GBS. In 88% of respondents, both variables were used in the same manner: in 13% both were used as indicative of AIDP, in 21% both as supportive of AIDP but only after verifying the presence of a co‐existing ulnar neuropathy (signs or diagnosis of ulnar neuropathy before) and in 54% both variables were excluded for subtyping because of possible confusion with ulnar neuropathy. In the remaining 12% of respondents, both variables were used in different ways: CMAP amplitude reduction was used as supportive of AIDP, but low MCV was not (4%) or vice versa (8%) (Figure 2).
3.2.3. Peroneal nerve
EDx GBS subtyping could be influenced by co‐existing peroneal neuropathy at the fibular head, that is, low MCV and CMAP‐amplitude reduction at fibular head could wrongly be assigned to AIDP. In 83% of respondents, both variables were used in the same manner: in 17% both were used as indicative of AIDP, in 20.8% both as supportive of AIDP but only after verifying the presence of a co‐existing peroneal neuropathy at the fibular head and in 46% both variables were excluded for subtyping because of possible confusion with a compression neuropathy at the fibular head. In the remaining 17% of respondents, both variables were used in different ways: CMAP‐amplitude reduction was used as supportive of AIDP, but low MCV was not (8%) or vice versa (8%) (Figure 2).
3.2.4. Tibial nerve
In 29% of the respondents, the tibial nerve was used for subtyping, but in 4% the tibial nerve was completely excluded. The remaining majority of 67% used some but not all tibial nerve EDx variables. A proximal CMAP‐amplitude reduction of >50 (ratio <0.5) was used for subtyping in 35%, and this was increased up to 61% if cutoff ratio of proximal CMAP‐amplitude reduction was >70% (ratio <0.3). If EDx criteria were used, in which it was advised to exclude the tibial nerve, still 58% of respondents were willing to use this proximal CMAP‐amplitude reduction for subtyping.
3.3. Distal CMAP amplitude
Criteria sets differed for which EDx parameters minimal distal CMAP‐amplitude requisites were provided. All criteria sets provided at least a minimal distal CMAP amplitude for one of the EDx parameters, but none required minimal amplitudes for all variables (CMAP ratio, DML, F wave, MCV).
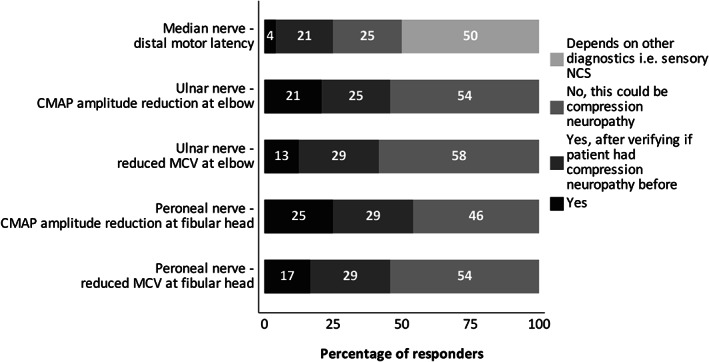
The use of a criterion for minimal distal CMAP amplitude was explored, irrespective of the definition of this minimal amplitude. A minimal CMAP‐amplitude criterion was required for CMAP ratio and F‐wave latency according to 79% of respondents, but only in 38% for DML and 58% for MCV (Figure 3). How the minimal distal CMAP‐amplitude criterion was defined, was not part of this study.
FIGURE 3.
The distal CMAP amplitude as prerequisite before classification of specified EDx variables as demyelinating, according to respondents. CMAP, compound muscle action potential
3.4. CB and TD
Criteria for CB were present in most criteria sets (Table 1). 1 , 2 , 4 , 5 , 6 These were based on CMAP‐amplitude reduction, 1 , 4 , 6 both CMAP amplitude and area reduction 2 or described as proximal to distal CMAP ratio, without further specification 5 (amplitude and/or area). In some criteria sets abnormal TD was strictly defined, 2 , 6 others used a more general definition (defined as: unequivocal TD) 1 , 3 or did not provide criteria for TD. 4 , 5
Despite the lack of criteria for TD in some criteria sets, 88% of respondents would use TD as a demyelinating feature. Also, in the deliberation of a CB to be axonal (reversible conduction failure) or demyelinating, 77% of respondents would take TD into account if EDx criteria do not provide clear rules.
4. DISCUSSION
Classification of EDx study results in GBS patients into axonal and demyelinating subtypes, according to various published EDx criteria sets may seem straightforward due to the use of clearly defined cutoffs. Despite the focus on cutoffs, our study shows that there are differences between respondents in the application and interpretation of various other aspects of the EDx GBS criteria. This diversity might influence the results of the EDx subtype classification, although this was not part of our analysis. To our knowledge, this is the first study studying and reporting variation in the application and interpretation of the EDx criteria for subtyping in GBS.
The most commonly used criteria sets for subtyping all showed differences in approaching the various items of concern, that is, the number of motor nerves to be studied, evaluation of proximal nerve conduction, evaluation of EDx parameters at sites of compression and parameters of the tibial nerve, the role of distal CMAP amplitude, TD and CB.
According to the respondents, the extensiveness of motor nerve conduction studies was dependent on the findings observed during the EDx procedure. If during EDx study the results were equivocal, respondents would proceed to test more motor nerves than typically recommended. On the other hand, according to EDx criteria for demyelinating subtype, testing two motor nerves will suffice in case both show demyelinating features. A fixed number of motor nerves to study was impossible to define. Future EDx criteria should define minimal number of motor nerves to study, specified for the different EDx subtypes.
In accordance with the literature, 15 respondents agreed on the need for evaluation of proximal nerves and roots. The way to study this, either by F waves or by nerve stimulation at proximal sites (upper arm, Erb's point), differed and no recommendations were provided by the analyzed EDx criteria sets. Both techniques have their shortcomings. Proximal stimulation is a complex technique: 64% of respondents reported to not use CMAP‐amplitude reduction at Erb's point in the EDx subtyping because of possible technical issues. Also, the roots (nerve segment proximal to Erb's point) cannot easily be evaluated, because direct stimulation of roots is complex and not routinely performed. Although F waves are generally performed to evaluate proximal nerve segments and roots, F wave latencies can also become abnormally prolonged or F waves can be absent when distal conduction slowing or blocking is present, mimicking proximal nerve conduction abnormalities. From the survey, respondents were variable on whether prolonged F‐wave latency should be used in EDx subtyping in case of clear distal conduction slowing, with 50% of respondents not taking distal conduction slowing or blocking into account in the assessment of F waves.
The issue on EDx findings at the typical sites of entrapment is complex. Entrapment neuropathies, in particular, median nerve entrapment across the wrist, are common but they also represent sites that are vulnerable to conduction slowing in GBS. 2 Most EDx classification sets lack recommendations as to whether or not to use these compression sites for subtype classification. 3 , 4 , 5 , 6 This study showed differences among respondents in excluding variables from sites of entrapment in GBS EDx subtyping, underlining the need for uniformity. One way to partially overcome this issue, might be to check whether patient had clinical signs of pre‐existing entrapment neuropathies, that is, before start of GBS symptoms.
Reduction of conduction velocity in case of severe axonal loss has been described, for example in patients with amyotrophic lateral sclerosis. 9 In the literature, a required minimal distal CMAP amplitude is often used, and supposed to prevent erroneously classification of low MCV as demyelinating feature in case of severe axonal loss. Respondents required a minimal distal CMAP amplitude for CMAP ratio and F‐wave latency in 79%, whereas only in 20% of EDx criteria sets (for CMAP ratio) and 33% (for F‐wave latency) a minimal distal CMAP amplitude was defined. On the other hand, only 38% of respondents use a minimal distal CMAP amplitude for DML, while EDx criteria require these in 67% of sets. This underlines that there is a gap between the EDx criteria and their application in practice.
We showed that CB was defined in multiple ways by the different EDx criteria, with differences in cutoffs but also in definition (area vs amplitude). Asbury and Cornblath 2 and Uncini et al 6 provided cutoffs for abnormal TD, while others did not or these were not clearly defined. 1 , 3 , 4 , 5 In both mentioned EDx criteria sets CMAP amplitude and duration were combined, 2 , 6 into criteria for CB and TD. Uncini et al introduced criteria for axonal CB. Most respondents (88%) were using abnormal TD as a demyelinating feature and 77% used TD to classify CB as axonal or demyelinating, although this was not in accordance to the published EDx criteria used. This suggests that the interpretation of TD as an axonal or demyelinating feature was left to the individual's discretion.
This study has several limitations. First, although we showed extensive variation between respondents in how EDx aspects of this study were handled in practice, we did not investigate to what extent this variation will influence the result of EDx subtyping in practice. A prospective study in a mixed population of patients with GBS would be required to define the effect of this variation. Second, the results presented in this paper represent opinions of neuromuscular specialists mostly from specialized academic centers, which may differ from the practice in other hospitals. Third, our survey did not state the timing of the EDx study in their questions. The timing might especially be relevant for the assessment of the CMAP‐amplitude reduction, as reversible conduction failure is dependent on the timing of the study.
5. CONCLUSION
The EDx classification of patients with GBS has been used extensively in previous research, including research that relates to the type of preceding infection, anti‐ganglioside antibodies, geographic variation and evaluation of subgroups in therapeutic trials. These studies may have been influenced by the application and interpretation of clinical neurophysiologists who have conducted the EDx in their patients. Yet we expect that in most if not all of these studies, this diversity as studied in this paper was not reported as a possible confounding factor. At present it is unknown to what extent this diversity may have influenced the interpretation of the EDx findings in these studies.
Various sets of EDx criteria to classify EDx into demyelinating and axonal subtypes of GBS have been proposed. In addition, some studies have indicated the importance of conducting a second EDx as EDx subtyping might not be possible in the very early dynamic state of GBS and the classifications may change. 16 Others showed no effect of serial EDx on GBS subtyping. 17 None of these criteria or recommendations have specified how to deal with all four issues investigated in the current study, probably contributing to the reported diversity. In an attempt to reach uniformity in clinical practice, there is a need to reach consensus among experts on the application and interpretation of GBS EDx criteria. Therefore, we recommend that future GBS studies using EDx subtyping should specify EDx criteria used and include the additional issues raised here:
The minimum number of nerves to be studied for each EDx subtype separately, that is, axonal, equivocal, demyelinating, inexcitable and normal.
The way proximal nerve and root segments are evaluated: by F waves, direct proximal stimulation or both.
Classification of F‐wave abnormalities, if there is distal conduction slowing, blocking or axonal loss.
Inclusion/exclusion criteria of the typical sites of entrapment.
Requirements for the distal CMAP amplitude for all evaluated EDx variables.
Clear cutoffs for CB and TD and how abnormalities are interpreted (axonal or demyelinating) if not specified in the applied EDx criteria.
CONFLICT OF INTEREST
T.H. Brannagan received consulting fees from Argenix and Grifols and honoraria from CSL Behring for giving lectures. D.R. Cornblath is member of the IGOS steering committee. M. Dimachkie received research and/or educational grants from Alexion, Alnylam Pharmaceuticals, Amicus, Biomarin, Bristol‐Myers Squibb, Catalyst, Corbus, CSL Behring, FDA/OOPD, GlaxoSmithKline, Genentech, Grifols, Kezar, Mitsubishi Tanabe Pharma, MDA, NIH, Novartis, Octapharma, Orphazyme, RaPharma/UCB, Sanofi Genzyme, Sarepta Therapeutics, Shire Takeda, Spark Therapeutics, The Myositis Association, UCB Biopharma/RaPharma, Viromed/Healixmith and TMA. He serves or recently served as consultant for Amazentis, Argenx, Catalyst, Cello, Covance/Labcorp, CSL Behring, EcoR1, Janssen, Kezar, Medlink, Momenta, NuFactor, Octapharma, RaPharma/UCB, Roivant Sciences Inc., Sanofi Genzyme, Shire Takeda, Scholar Rock, Spark Therapeutics, Abata/Third Rock, UCB Biopharma, UpToDate. He received honoraria for lectures and/or presentations at AAN, AANEM and Academic Institutions and participates in a data safety monitoring or advisory board for Covance/Labcorp. B.C. Jacobs received unrestricted research grants for work outside the current study from Baxalta, Grifols, CSL Behring, Annexon, Hansa Biopharma, Roche, Prinses Beatrix Spierfonds, GBS‐CIDP Foundation International and Horizon 2020. He received consulting fees from Roche for activities outside current study. Payments made to the Erasmus Medical Center. He is the chair of steering committee of the International GBS Outcome Study (IGOS). H. Katzberg received grants from Takaeda and CIHR for research support and consulting fees from Akcea, Alnylam, CSL Behring, Merz Pharma and Takaeda, he participates in a monitoring or advisory board for Alexion, Octapharma and UCB Pharma and has stock(options) for Romtech, H.C. Lehmann received honoraria for lectures and presentations from Akcea, Alnylam, Biogen, Celgene, CSL Behring, Grifols, Gruenenthal, LFB Pharma, Takeda, UCB. Y. Péréon received honoraria from Natus for lectures or presentations. R. Reisin received honoraria for lectures or presentations from Takeda, PTC, CSL Behring. He has patents planned, issued or pending by CSL Behring. He participates in data safety monitoring or advisory board of Takeda. He received a donation from PTC. The following authors declared no conflict of interest: S. Arends, P. van den Bergh, D. Binda, C. Casasnovas, D.R. Cornblath, J. Drenthen, E. Fulgenzi, G. Gutiérrez Gutiérrez, S.T. Hsieh, L. Kiers, G.A. Marfia, G. Mataluni, J. Pardo, Y. Sekiguchi, N. Shahrizaila, A. Uncini, C. Verhamme, W. Waheed, M. Wang.
PARTICIPATING MEMBER OF IGOS ELECTROPHYSIOLOGY EXPERTISE GROUP
1.
Peter Van den Bergh3, Belgium; Davide Binda18, Italy; Thomas H. Brannagan19, USA; Carlos Casasnovas20, Spain; Mazen M. Dimachkie6, USA; Judith Drenthen1, The Netherlands; Robert D.M. Hadden4, United Kingdom; Ernesto A. Fulgenzi21, Argentina; Gerardo Gutiérrez Gutiérrez7, Spain; Sung‐Tsang Hsieh22, Taiwan; Hans Katzberg8, Canada; Lynette Kiers9, Australia; Helmar C. Lehmann10, Germany; Girolama A. Marfia23, Italy; Giorgia Mataluni23, Italy; Julio Pardo24, Spain; Yann Péréon11, France; Ricardo C. Reisin12, Argentina; Yukari Sekiguchi25, Japan; Nortina Shahrizaila5, Malaysia; Antonino Uncini13, Italy; Camiel Verhamme14, The Netherlands; Min Wang26, China; Wagar Waheed15, USA; David R. Cornblath16, USA
Department of Neurology, Erasmus University Medical Center, Rotterdam, The Netherlands
Department of Neurology, Haga Teaching Hospital The Hague, The Hague, The Netherlands
Department of Neurology, University Hospital St‐Luc, Brussels, Belgium
Department of Neurology, King's College Hospital, London, United Kingdom
Department of Neurology, University of Malaya, Kuala Lumpur, Malaysia
Department of Neurology, The University of Kansas Medical Center, Kansas City, USA
Department of Neurology, Hospital Universitario Infanta Sofia, San Sebastian de los Reyes, Spain
Department of Neurology, Toronto General Hospital, University Health Network, Toronto, Canada
Clinical Neurophysiology, Department of Neurology, The Royal Melbourne Hospital, Parkville, Australia
Department of Neurology, University Hospital of Cologne, Cologne, Germany
Department of Clinical Neurophysiology, Reference Centre for Neuromuscular Disorders AOC, Filnemus, Euro‐NMD, University of Nantes, Nantes, France
Department of Neurology, Hospital Británico, Buenos Aires, Argentina
Department of Neuroscience, Imaging and Clinical Sciences, University “G. D'Annunzio”, Chieti, Italy
Department of Neurology, Amsterdam Neuroscience, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, The Netherlands
Department of Neurology, University of Vermont Medical Centre, Burlington, USA
Department of Neurology, Johns Hopkins University, Maryland, Baltimore, USA
Department of Immunology, Erasmus University Medical Center, Rotterdam, The Netherlands
Department of Neurology, Valduce Hospital, Como, Italy, davide.binda@gmail.com
Department of Neurology, Columbia University, New York City, USA, tb2325@cumc.columbia.edu
Department of Neurology, Hospital Universitari de Bellvitge‐IDIBELL and CIBERER, Barcelona, Spain, carloscasasnovas@bellvitgehospital.cat
Department of Neurology, Hospital Cesar Milstein, Buenos Aires, Argentina, efulgenzi@intramed.net
Department of Neurology, National Taiwan University Hospital, Taipei, Taiwan, shsieh@ntu.edu.tw
Department of System Medicine, Dysimmune Neuropathies Unit, Policlinico Tor Vergata, Roma, Italy, marfia@uniroma2.it; giorgia.mataluni@gmail.com
Department of Neurology, Complejo Hospitalario Universitario de Santiago, Santiago de Compostela, Spain, julio.pardo.fernandez@sergas.es
Department of Neurology, Chiba University Hospital, Chiba, Japan, syukari536@chiba-u.jp
Department of Neurology, Affiliated Hospital of Jining Medical University, Jining, Shandong, China, emgwangmin@126.com
Arends S, Drenthen J, Van den Bergh PY, et al. Electrodiagnostic subtyping in Guillain‐Barré syndrome: Use of criteria in practice based on a survey study in IGOS . J Peripher Nerv Syst. 2022;27(3):197‐205. doi: 10.1111/jns.12504
Members of IGOS Electrophysiology Expertise Group are provided in Appendix.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author (S.A.) upon reasonable request.
REFERENCES
- 1. Albers JW, Donofrio PD, McGonagle TK. Sequential electrodiagnostic abnormalities in acute inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 1985;8:528‐539. doi: 10.1002/mus.880080609 [DOI] [PubMed] [Google Scholar]
- 2. Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain‐Barré syndrome. Ann Neurol. 1990;27(suppl):21‐24. doi: 10.1002/ana.410270707 [DOI] [PubMed] [Google Scholar]
- 3. Ho TW, Mishu B, Li CY, et al. Guillain‐Barré syndrome in northern China. Relationship to campylobacter jejuni infection and anti‐glycolipid antibodies. Brain. 1995;118:597‐605. [DOI] [PubMed] [Google Scholar]
- 4. Hadden RDM, Cornblath DR, Hughes RAC, et al. The plasma exchange/sandoglobulin Guillain‐Barré syndrome trial group. Electrophysiological classification of Guillain‐Barré syndrome: clinical associations and outcome. Ann Neurol. 1998;44:780‐788. doi: 10.1002/ana.410440512 [DOI] [PubMed] [Google Scholar]
- 5. Rajabally YA, Durand MC, Mitchell J, Orlikowski D, Nicolas G. Electrophysiological diagnosis of Guillain‐Barré syndrome subtype: could a single study suffice? J Neurol Neurosurg Psychiatry. 2015;86:115‐119. doi: 10.1136/jnnp-2014-307815 [DOI] [PubMed] [Google Scholar]
- 6. Uncini A, Ippoliti L, Shahrizaila N, Sekiguchi Y, Kuwabara S. Optimizing the electrodiagnostic accuracy in Guillain‐Barré syndrome subtypes: criteria sets and sparse linear discriminant analysis. Clin Neurophysiol. 2017;128:1176‐1183. doi: 10.1016/j.clinph.2017.03.048 [DOI] [PubMed] [Google Scholar]
- 7. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain‐Barré syndrome and fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599‐612. doi: 10.1016/j.vaccine.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 8. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282(2):153. [DOI] [PubMed] [Google Scholar]
- 9. Barkhaus PE, Kincaid JC, Nandedkar SD. Tibial motor nerve conduction studies: an investigation into the mechanism for amplitude drop of the proximal evoked response. Muscle Nerve. 2011;44(5):776‐782. [DOI] [PubMed] [Google Scholar]
- 10. Behnia M, Kelly JJ. Role of electromyography in amyotrophic lateral sclerosis. Muscle Nerve. 1991;14:1236‐1241. [DOI] [PubMed] [Google Scholar]
- 11. Kuwabara S, Yuki N, Koga M, et al. IgG anti‐GM1 antibody is associated with reversible conduction failure and axonal degeneration in Guillain‐Barré syndrome. Ann Neurol. 1998;44:202‐208. doi: 10.1002/ana.410440210 [DOI] [PubMed] [Google Scholar]
- 12. Clouston PD, Kiers L, Zuniga G, Cros D. Quantitative analysis of the compound muscle action potential in early acute inflammatory demyelinating polyneuropathy. Electroencephalogr Clin Neurophysiol. 1994;93:245‐254. doi: 10.1016/0168-5597(94)90026-4 [DOI] [PubMed] [Google Scholar]
- 13. Mitsuma S, Van den Bergh P, Rajabally Y, et al. Effects of low frequency filtering on distal compound muscle action potential duration for diagnosis of CIDP: a Japanese‐European multicenter prospective study. Clin Neurophysiol. 2015;126:1805‐1810. doi: 10.1016/j.clinph.2014.11.027 [DOI] [PubMed] [Google Scholar]
- 14. Buschbacher RM. Tibial nerve motor conduction to the abductor hallucis. Am J Phys Med Rehabil. 1999;78(6):s15‐s20. doi: 10.1097/00002060-199911001-00004 [DOI] [PubMed] [Google Scholar]
- 15. Berciano J, Sedano MJ, Pelayo‐Negro AL, et al. Proximal nerve lesions in early Guillain‐Barré syndrome: implications for pathogenesis and disease classification. J Neurol. 2017;264(2):221‐236. doi: 10.1007/s00415-016-8204-2 [DOI] [PubMed] [Google Scholar]
- 16. Nedkova V, Gutiérrez‐Gutiérrez G, Navacerrada‐Barrero FJ, Berciano J, Casasnovas C. Re‐evaluating the accuracy of optimized electrodiagnostic criteria in very early Guillain‐Barré syndrome: a sequential study. Acta Neurol Belg. 2021;121:1141‐1150. doi: 10.1007/s13760-021-01603-7 [DOI] [PubMed] [Google Scholar]
- 17. Van den Bergh PYK, Piret F, Woodard JL, et al. Guillain‐Barré syndrome subtype diagnosis: a prospective multicentric European study. Muscle Nerve. 2018;58:23‐28. doi: 10.1002/mus.26056 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (S.A.) upon reasonable request.


