Abstract
Context
This scoping review examines the current research on the effect of cannabis upon pain intensity in spinal cord injury (SCI) pain. Chronic pain is a significant secondary condition following SCI, and traditional treatments (e.g. opioids, NSAIDs) are often criticized for providing inadequate relief. As a result, there is increasing interest in and use of cannabis and cannabinoid-based medications as an alternative means of pain control.
Objective
The purpose of this review was to examine the scientific evidence on the effect of cannabis/cannabinoids upon pain intensity in SCI by mapping the current literature.
Methods
Two hundred and fifty-two studies were identified by searching electronic databases for articles published through February 2020. In addition, reviewers scanned the reference lists of identified articles and examined clinicaltrials.gov for unpublished data in this area. Title, abstract, and full-text reviews were completed by two independent reviewers. Data extraction was performed by a single reviewer and verified by a second reviewer.
Results
Six articles covering five treatment studies were included. Studies yielded mixed findings likely due to large variability in methodology, including lack of standardized dosing paradigms, modes of use, and duration of trial.
Conclusions
The current quality and level of evidence is insufficient to draw reliable conclusions of the efficacy of cannabis upon SCI-related pain itensity. We identify specific limitations of past studies and present guidelines for future research.
Trial registration: ClinicalTrials.gov identifier: Nct01606202..
Keywords: Spinal cord injury, Cannabis, Medical marijuana, Chronic pain, Neuropathic pain
Introduction
Chronic pain is a significant comorbidity for individuals following a spinal cord injury (SCI), affecting approximately two-thirds of this population.1–3 Current guidelines for pain management in this population recommend using opioids and NSAIDs for nociceptive pain, and anticonvulsants and/or antidepressants for neuropathic pain.4 However, both patients and clinicians have expressed dissatisfaction and frustration since conventional treatments relieve only 50% of their pain.5,6 Due to inadequate relief, individuals with SCI often seek out alternative forms of pain control including baclofen, transcutaneous electrical nerve stimulation, and psychological treatments.7
Cannabis is among the more frequently utilized, alternative methods, and is often perceived by patients as effective for relieving chronic SCI-related pain.8,9 Studies of individuals with SCI report that between 22.5% and 30% utilize cannabis for symptom relief.8,9 Many report cannabis to have a good effect on pain and to work better than prescribed medications.10,11 Depending on location and legality of cannabis, studies report daily use in 29% to 67% of current cannabis users with SCI, however other subsets include weekly, monthly, and even yearly use.12 Smoking and vaping are the most common routes of administration, though topical and edible routes are also reported.10,12 A recent survey conducted in Colorado found the state’s SCI community to endorse more medical reasons for cannabis use compared to individuals with traumatic brain injury. Reasons for use included pain and spasticity reduction, reducing depression, stimulating appetite, decreasing nausea, and improving sleep.13
There are now known to be well over 100 distinct cannabinoids in botanical cannabis, as well as associated terpenoid compounds that also appear to be pharmacologically active when ingested. Exogenous cannabinoids, such as tetrahydrocannabinol (THC) or cannabidiol (CBD), mimic endogenous cannabinoids by adhering to the same receptors within the endocannabinoid system (ECS). The ECS is comprised of a cannabinoid receptors (primarily CB1, CB2) found in the central and peripheral nervous system, immune system, and gastrointestinal systems.14 CB1 receptors, abundant in the central nervous system, appear to play an important role in the regulation of pain.15
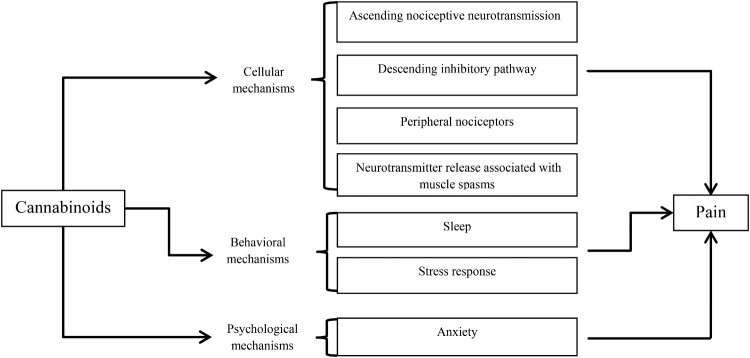
It is not yet fully understood how cannabinoids specifically modulate pain, however the wide distribution of the ECS indicates multiple mechanisms through which cannabinoids can impact pain via cellular, behavioral and psychological levels. These potential mechanisms are schematically represented in Figure 1. At the cellular level, endocannabinoids mediate neurotransmission in the periaqueductal gray (PAG), a structure comprising part of a natural descending inhibitory pain pathway. Post-synaptic cells within the PAG release endocannabinoids which then limit incoming pain signals from pre-synaptic cells, resulting in analgesia.14,16,17 Cannabinoids may also dampen pain signaling by enhancing the activity of gamma amino butyric acid receptors in the spinal cord.18 At the behavioral level, poor sleep quality is associated with worse pain outcomes such as lower pain threshold and increased pain reactivity.19 The ECS seems to play a role in regulating the sleep-wake cycle possibly through the effect of endocannabinoids on rapid-eye movement sleep via the lateral hypothalamus.20 At the psychological level, cannabinoids regulate anxiety and anxious behaviors and thoughts (e.g. avoidance, negativity, and fear) that can exacerbate pain.21–23
Figure 1.
Mechanisms through which cannabinoids can affect pain.
Taken together, self-report data and mechanistic research suggest that cannabis may have some potential to alleviate SCI-related pain. Thus far, there have been no reviews focused exclusively on the effect of cannabis upon pain intensity in spinal cord injury. The purpose of this review was to examine the scientific evidence in SCI by mapping the current literature and identifying gaps in this growing area of research.
Methods
This scoping review was guided by the Arksey and O’Malley framework and included the following stages: (I) study identification, (II) study selection, (III) data extraction, and (IV) gathering and reporting of results.24 An extension of the Preferred Reporting Items for Systematic reviews and Meta-Analysis guideline for scoping reviews was utilized to ensure completeness of this report.25
Research question
This scoping review was led by the following research question: “What is the current level of evidence on the effect of cannabis/cannabinoids upon pain intensity in SCI?”
Information source selection
We searched standard databases including PubMed, Scopus, EMBASE, and CINHAL that house biomedical literature from scholarly journals. In addition, we searched clinicaltrials.gov so as to identify unpublished results. Reference list searches were completed for included articles.
Search strategy
The initial search took place on August 29th 2019 and an updated search was completed on February 5th 2020. No limits on date were placed on the initial database search. The updated search limited publication dates from August 2019 to February 2020 to ensure only new studies were identified. The following databases were searched for relevant studies: PubMed, CINHAL, Scopus, EMBASE, and clinicaltrials.gov. Search syntax and filters varied between databases, therefore our search strategy was adapted slightly for each.
We used the following strategy search for PubMed: “spinal cord injury” OR “spinal cord injuries” OR “Spinal Cord Injuries”[Mesh] OR “Neuralgia”[Mesh]) AND (“medical marijuana” OR “cannabis-based” OR THC OR tetrahydrocannabinol OR dronabinol OR “Dronabinol”[Mesh] OR CBD OR Cannabidiol OR nabilone OR “nabilone” [Supplementary Concept] OR “Medical Marijuana”[Mesh] OR “Cannabis” [Mesh]) AND (pain OR analges* OR “Pain Management”[Mesh] OR Pain[Mesh] OR “Analgesia”[Mesh]).
In order to search clinicaltrials.gov, we entered the following terms: “Condition or disease: Spinal Cord Injuries / Other terms: Cannabis / Study type: All Studies / Study Results: All Studies / Status: Completed”
Inclusion criteria
We identified treatment trials examining cannabis/cannabinoid-based medications for pain related to spinal cord injury (SCI). Eligible studies could include randomized controlled trials (RCTs), controlled trials, prospective open-label studies, and case studies. Studies needed to utilize a cannabinoid preparation, applied by any route of administration or dose, and could involve synthetic cannabinoids (dronabinol, nabilone), whole-plant extracts, isolated or combined cannabinoid preparations (THC only, CBD only, THC-CBD). To be included, studies needed to report on the outcome of pain intensity, and were required to present these findings separately for those with SCI if utilizing a mixed sample. Only studies written in English were included in this review.
Study selection process
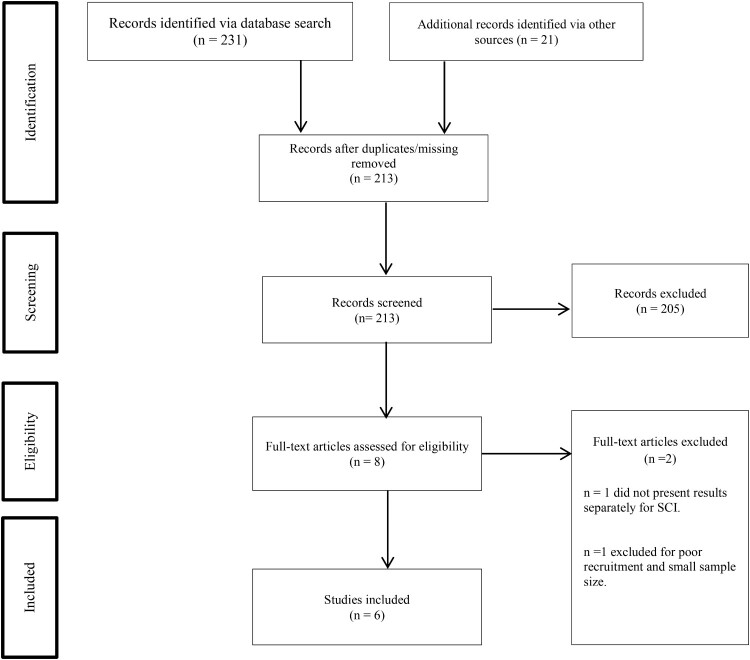
After removing duplicate and missing articles, a single reviewer screened remaining article titles. Two reviewers then independently screened the eligible abstracts. As an additional step, one reviewer screened the methods of articles whose abstracts lacked sufficient information to determine inclusion. Finally, both reviewers independently screened full-text articles. All discrepancies regarding inclusion were resolved through discussion and consensus. See Figure 2 for information on each stage of the selection process.
Figure 2.
Study selection.
Data charting process
A data charting form was developed by the first and second author, this was informed by the Joanna Briggs Institute data extraction template.26 Microsoft Excel was used to chart and store data. A single reviewer extracted data, while another monitored the process to ensure accuracy. We contacted the principal investigators for permissions and data (if not publicly available) for studies identified through clincaltrials.gov.
Data items extracted
(1) Subject demographics & clinical characteristics: numbers of males and females, mean age, numbers of SCI and SCD (spinal cord disease), numbers of participants with paraplegia and tetraplegia, and pain type. For studies listing level of injury, we broadly categorized these as paraplegia or tetraplegia. (2) Form of cannabis: (a) whole-plant cannabis [delta-9-THC], (b) drugs with chemicals from the marijuana plant [nabiximols (SativexR)], (c) drugs with synthetic versions of chemicals found in marijuana [Dronabinol], and (d) drugs with chemicals similar to marijuana, but not found in the plant [Nabilone (Cisamet); Dexanabinol; CT-3 (ajulemic acid), etc.]. (3) Route of administration (oral, oromusocal, pulmonary, and transdermal routes), (4) study design, (5) treatment (dosing frequency, duration), (6) pain measure, (7) pain outcome, (8) effect size, (9) withdrawals, (10) side effects, and (11) adverse events.
Quality assessment
We chose the Physiotherapy Evidence Database (PEDro) scale to assess the quality of included studies.27–29 The scale consists of 11 items created to determine the validity of the methods and statistics utilized when interpreting study findings. Points were awarded only if criterion were obviously satisfied. The PEDro scores are listed in the third column of Table 2, along with study design information.
Table 2.
Study information.
| Article | Subjects | Study design / PEDro score | Treatment | Concomitant medications | Pain measure | Outcome | Effect size |
|---|---|---|---|---|---|---|---|
| Maurer et al.32 | 1 | RCT, double-blind crossover / 5 | 5 mg of oral delta-9-THC vs. active comparator (50 mg codeine p.o) vs. inactive placeb0. Conditions applied 18 times each in a randomized and balanced order over 5 months. | Continued all other concurrent medication as usual (baclofen & clonazepam) | VAS of 50 mm, poles = ‘none’ to ‘very strong’ at four time points: after taking, before falling asleep, at night, & when getting up. | P < 0.05 (THC vs inactive placebo) No differences between THC vs active comparator. | d = 1.13-1.89 (THC vs inactive placebo) d = 0.01-0.88 (THC vs active comparator) |
| Hagenbach et al.35 | 22 | Open label / 1 | 10 mg oral dronabinol. Titrate Up: 10–14 days starting day 2 based on individual preference. Duration: 6 weeks. Washout period: 7 days. Mean daily dose = 31 mg (range: 15–60 mg) | Stopped all spasmolytic medications prior to enrollment; required urine drug screen. | 0=no pain, 1=minimal, 2=minor, 3=moderate, 4=strong, 5=very strong, 6=intolerable. | P = 0.047 | Data unavailable. |
| Rintala et al.34 | 7 | RCT, double-blind, crossover / 6 | 5 mg of oral dronabinol or active placebo (25 mg of diphenhydramine). Titrate Up: 12 days. Stabilization: 7 days. Maintenance: 28 days. Titrate Down: 9 days. Washout period: 7 days. Maximum daily dose: 20 mg of dronabinol and 75 mg of diphenhydramine. | Stopped all pain medications prior to enrollment. Oxycodone-acetaminophen 5-325 mg for breakthrough pain. | BPI pain intensity item: NRS (0 ‘no pain’ to 10 ‘pain as bad as you can imagine’) | P = 0.102 (dronabinol vs active placebo) | Data unavailable. |
| GW Pharmaceuticals Ltd. 2012 | 116 | RCT, quadruple-blind, parallel-group / 10 | Nabiximols or inactive placebo via oromucosal spray. Each puff delivered 100 μl. Subjects self-titrated. Maximum permitted dose: eight puffs in any 3-hour period and 48 puffs in any 24-hour period. Duration: 21–30 days. | Stable medication regime for 4 weeks and stopped all cannabinoids for 7 days prior to enrollment. Acetaminophen for breakthrough pain. | Central Neuropathic Pain NRS (0 = ‘no pain’ and 10 = ‘worst possible pain’) | P = 0.708 (Nabiximols vs inactive placebo) | ±d = 0.039 |
| Wilsey et al.31 A | 42 | RCT, double-blind, crossover / 8 | Vaporized 2.9% or 6.7% delta-9-THC, or inactive placebo applied during 8-hour sessions. Washout period: 3 day minimum. Randomized order of conditions. Cumulative dosing scheme: 4 puffs after baseline; then 4–8 puffs after 240 min. For all conditions, the most frequent number of puffs = 8. | Continued all other concurrent medication as usual. Stopped all cannabis use for 7 days prior to enrollment. | NRS (0 = ‘no pain’ and 10 = ‘worst possible pain’) | P < 0 .05 (THC vs inactive placebo) | *d ≈ 0.7 (2.9% THC vs inactive placebo) *d ≈ 1.0 (6.7% THC vs inactive placebo) |
| Wilsey et al.31 B | 42 | RCT, double-blind, crossover / 8 | Vaporized 2.9% delta-9-THC, 6.7% delta-9-THC, or inactive placebo applied during 8-hour sessions. Washout period: 3 day minimum. Randomized order of conditions. Cumulative dosing scheme: 4 puffs after baseline; then 4–8 puffs after 240 min. The mean range of cannabis vaporized = 45.9 mg (29.9–83.8 mg) during the 2.9% THC sessions and 56.3 mg (15.7–172.9 mg) during the 6.7% THC sessions. | Continued all other concurrent medication as usual. Stopped all cannabis use for 7 days prior to enrollment. | 10-item NPS scale (0 = ‘no pain’ and 10 =‘strongest pain imaginable’) | Greater reduction in itching (P = 0.0174) and burning (P=0.0395) for 6.7% delta-9-THC | Data unavailable. |
PEDro = Physiotherapy Evidence Database scale (0-11). VAS = Visual Analog Scale. BPI: Brief Pain Inventory. NRS = Numerical Rating Scale. NPS = Neuropathic Pain Scale. Bolded items are statistically significant. ±Calculated effect size. *Estimated effect size.
Result reporting process
We report frequencies and percentages of demographics and AEs. Study methods are presented in Table 3. We calculated effect size (Cohen’s d) and percentages for which data is available. We provide estimates of effect size for studies which reported information on number needed to treat (NNT); estimates were calculated using a conversion table.30
Table 3.
Side effects and adverse events.
| Article | Side Effects | Adverse Events | Withdrawals |
|---|---|---|---|
| Maurer et al.32 | |||
| Delta-9-THC | Not reported. | Not reported. | 0 |
| Codeine | Not reported. | Not reported. | |
| Hagenbach et al.35 | |||
| Dronabinol | n = 22∞ | Not reported. | 7 |
| Dry mouth (32%), Sleepiness (36%), Anxiety (32%) | |||
| Rintala et al.34 | |||
| Dronabinol | n = 7 | Not reported. | 2 |
| Dry mouth (71%), Constipation (71%), Fatigue (57%), Drowsiness (57%) | |||
| Diphenhydramine | n = 5 | Not reported. | 0 |
| Fatigue (100%), Dry mouth (60%), Constipation (60%), Drowsiness (60%) | |||
| GW Pharmaceuticals Ltd. 2012 | |||
| Nabiximols | n = 46 | n = 3 | 2 |
| Dizziness (30%), Disgeusia (20%) UTI (17%), Somnolence (15%), Nausea (13%), Headache (11%) | Anemia (33%), Fall (33%), Infections (33%), Tibia fracture (33%), Confusion (33%), Paranoia (33%) | ||
| Placebo | n = 29 | n = 2 | 1 |
| Dizziness (17%), Disgeusia (14%), UTI (14%), Nausea (10%), Oral pain (10%); Alanine aminotransferase increase (10%), Gamma-glutamyltransferase increase (10%) | Fall (50%), Bladder infection (50%), Pneumonia (50%), Upper limb fracture (50%), Dizziness (50%), Contusion (50%) | ||
| Wilsey et al.31 papers A & B | |||
| 2.9% and 6.7% delta-9 THC | Any drug effect, good drug effect, bad drug effect, high, drunk, impaired, stoned, like the drug effect, sedated, confused, nauseous, desired more, hungry, changes perceiving time, changes perceiving space, difficult paying attention, difficulty remembering things.*** |
n = 1 Syncopy (100%) |
0 |
∞ = ‘n’ includes drop-outs. UTI = Urinary Tract Infection. ***Side effects increased with concentrations of delta-9-THC.
Results
A total of 252 articles were identified. After removing missing articles, we screened 213 studies. Articles were excluded if they did not focus on SCI (n = 195), did not assess pain (n = 1); did not present results separately for SCI (n = 7), did not have results available (n = 1), or did not include an intervention (n = 1). Reviewers completed a full-text review of eight papers from which two papers were excluded; one for not presenting results separately for SCI (n = 1) and one for poor recruitment/inability to draw conclusions based on small sample size (n = 1). Ultimately, six articles were included in the scoping review.31–37 Two articles covering the same study were included in the current review because they presented different aspects of the research.
Table 1 displays demographics and clinical characteristics of the included articles. Most articles focused exclusively on spinal cord injury or disease, however two articles used a mixed sample of traumatic SCI, multiple sclerosis, cervical disc disease, spinal cord tumor, arachnoid cysts, syringomyelia, and vertebral artery occlusion.31,33 Disease duration was not reported. Number of years post-injury was reported by two studies and ranged from 13.3 to 21.9 years.34,35 Neuropathic pain was assessed in all studies for which this information was reported (n = 5); Hagenbach primarily focused on spasticity, and did not record pain type. Duration of pain was reported infrequently: Rintala et al. reported pain began within the first two years of their sample’s SCI, and Wilsey papers A & B reported average pain duration to be 11.6 years for their mixed sample. Studies reported including similar numbers of individuals with tetraplegia and paraplegia. However, this information was not available for one study.36 Wilsey et al.31 A and B papers provided information on their subjects’ cannabis history – 40% of the sample were active cannabis users (used cannabis within 30 days of randomization), 50% were ex-users, and 10% had never used cannabis.31,33
Table 1.
Demographics and clinical characteristics.
| Article | Male, n (%) | Age, mean (SD) | SCI or Disease | Paraplegia, n (%) | Tetraplegia, n (%) | Pain type, n (%) |
|---|---|---|---|---|---|---|
| Maurer et al.32 | 1 (100%) | 28 | Disease | 1 (100%) | 0 (0%) | Severe paraesthesias and painful spastic paraparesis. |
| Hagenbach et al.35 | 23 (92%) | 42.6 (12.5) | Injury | 11 (44%) | 14 (56%) | Not reported. |
| Rintala et al.34 | 5 (71.4%) | 50.1 (8.3) | Injury | 4 (57.1%) | 3 (48.9%) | Chronic neuropathic pain at least three levels below the spinal cord lesion. |
| GW Pharmaceuticals Ltd. 2012 | 91 (78.4%) | 48.1 (12.69) | Injury | Not reported. | Not reported. | Central neuropathic pain |
| Wilsey et al.31 A | 29 (69%) | 46.4 (13.6) | 69% Injury, 31% Disease | 20 (48%) | 22 (52%) | Central neuropathic pain. |
| Wilsey et al.31 B | 29 (69%) | 46.4 (13.6) | 69% Injury, 31% Disease | 20 (48%) | 22 (52%) | Central neuropathic pain. |
Treatment
Forms of cannabis/cannabinoid medication were THC (n = 3), followed by dronabinol (n = 2), and nabiximols (n = 1). For studies evaluating THC: The manuscripts by Wilsey and colleagues refer to their treatment as concentrations of ‘delta-9-THC’ derived from whole plant cannabis. Maurer et al. uses the term ‘delta-9-THC’ to describe their treatment (5 mg of THC in the form of impregnated sugar lumps) – however the investigators do not specify if this was synthetic or derived from whole plant materials. Route of administration comprised of oral (n = 3), vaporized (n = 2), and oromucosal spray (n = 1). Treatment method varied between studies with little consistency in titration, maintenance duration, and maximum permitted dose (see Table 2). Three studies did not provide information on treatment adherence and fidelity.32,35,37 Wilsey et al.31 A and B papers refer to a “cued-puff” procedure in their Methods, this suggests that participants were observed during treatment application. Rintala et al. specifies that compliance was monitored in-person at assessment time points and on a weekly basis via telephone. However, no articles provided actual data on adherence.
Study design
Most studies employed a randomized-controlled methodology with blinding (n = 5). The most common design was cross-over (n = 4), followed by parallel-group (n = 1). We included one case study and one open label study.32,35 Sample sizes ranged from 1 to 116 individuals. Both inactive (n = 4) and active (n = 1) placebos were described. One study also included an active comparator.32 Please note: we only discuss pain outcomes from phase 1 (open label dronabinol) of the Hagenbach et al. study since data were unavailable for phase 2 (open label rectal THC-HS) and phase 3 (double-blind, parallel group RCT).
Pain measure
Most studies assessed pain intensity (n = 4) using a 0–10 numerical rating scale, though anchors varied. Two studies used multi-item measures: Wilsey31 B used the validated 10-item Neuropathic Pain Scale and Maurer et al. used a 4-item visual analog scale with the following prompts: “(a) After taking the substance”, “(b) When falling asleep”, “(c) When awaking during the night”, and “(d) When getting up in the morning”.
Quality assessment
Studies scoring low on the PEDro were limited by designs precluding between-group comparisons (n = 5), vague blinding procedures (n = 5), lack of analysis using “intention to treat” (n = 6), lack of allocation concealment (n = 3), presenting outcomes for less than 85% of enrolled participants (n = 2), and not presenting both point measures and measures of variability for the key outcome (n = 2).
Pain outcome & effect size
The included studies present mixed findings regarding the effect of cannabis/cannabinoids on SCI-related pain intensity. The highest quality study found no statistically significant difference between nabiximols and inactive placebo for reducing pain (P = 0.708).36,37 In contrast, lower quality studies reported more statistically significant findings (n = 4), however only one study reported effect size.32 Though all studies reported on the statistical significance of their findings, many did not provide means and standard deviations for the primary outcome at baseline and post-treatment.
Side effects, adverse events, and withdrawals
Side effects were reported for most studies (n = 5), however adverse events were available for only three studies. Only one paper did not report on either parameter.32 Systematic assessments were utilized in two studies,34,37 while the remaining utilized self-report measures.31,33,35 Table 3 presents the percentage of individuals who endorsed specific effects out of the total number of participants reporting side effects/adverse events.
Discussion
Pain is a significant secondary condition accompanying spinal cord injury (SCI). Due to inadequate relief from standard pain treatments, many individuals with SCI report seeking alternatives, such as cannabis. Though self-reports of those with SCI who use cannabis/cannabinoids for pain have mostly been positive, there have been a limited number of treatment trials focused specifically on this area.38 We believe this scoping review is the first to examine the level of evidence available on cannabis for pain management specifically in spinal cord injury.
Overview of evidence
A number of methodological weaknesses limit what can be concluded from the existing body of research. Type, dosage and route of administration of cannabinoids was highly variable across studies. There was a dearth of parallel group designs and studies were underpowered to detect anticipated effects. Pain assessments were often non-standard and inconsistent across investigations. Important procedural elements such as randomization, blinding, and concealment were not adequately described. Participant retention was poor and analyses were not intent-to-treat. Means and standard deviations describing key outcomes at all time points were not reported. The majority of the studies did not report effect size for key findings. There needs to be considerable improvements in the design of future studies. We provide recommendations for the next generation of trials in the next section.
Recommendations
Study design
Previous trials were limited by small sample sizes and crossover study designs, with the latter potentially clouding comparisons through the introduction of carryover effects. Future studies should employ a between-subjects design to more cleanly evaluate the outcome of a treatment, and report the steps taken to establish their sample size to ensure adequate power. Another key issue with previous trials involved use of per-protocol analysis – which can be biased by focusing solely on the outcomes of “ideal” subjects. Future trials should analyze all randomized subjects – including those who withdraw using intention-to-treat analysis. Trials should report effect size to better determine the clinical significance of findings and allow for comparisons across studies. Similarly, future studies should consider reporting 95% Confidence Intervals – statistics which further speak to the clinical utility of treatments. Finally, it is vital to clearly state all precautions taken to uphold blinding of assessors, clinicians, and subjects. Without accurate reports of blinding protocols and allocation concealment, studies run the risk of introducting bias at several stages in the study. In order to ensure all key design elements are addressed, we recommend following standard RCT design guidelines such as the Consolidated Standards of Reporting Trials (CONSORT) 2010 Check-list.39 Investigators can find literature reviews regarding the conduct of RCTs specifically focused on SCI from the International Spinal Cord Injury Society.40–44
Pain assessment
Future research on the potential analgesic effects of cannabis should include more information on pain type. At a minimum, investigators should differentiate the potential effects of cannabis on nociceptive and neuropathic pains. Nociceptive pain includes musculoskeletal pain (accompanying spasms or due overexertion) and visceral pain in the abdominal area. Neuropathic pain can manifest as increased pain sensitivity (hyperalgesia) or pain in response to light touch/harmless stimulation (allydonia). Neuropathic pain can be further sub-classified depending on the location: (i) at-level, (ii) below-level, and (iii) other neuropathic pain.45 Prevalence of neuropathic pain and musculoskeletal pain are similar in SCI (69.3% and 60.9% respectively), though more than 30% of individuals report a combination of both5. The majority of studies included in this review chose to focus on neuropathic pain, however they often utilized inconsistent measures to assess outcomes. Future studies should follow current recommendations for assessing SCI pain type assessment as outlined by the SCI Common Data Element (CDE) Oversight Committee and the National Institute of Neurological Disorders and SCI (NINDS).46–48
Treatment design & safety
Inconsistent treatment procedures comprise a major limitation in the current body of research. Without standard and replicable treatments across studies, it is not feasible to draw overarching conclusions regarding the efficacy of cannabis for SCI-related pain management. Future studies should clearly outline treatment components such as dosing, titration and maintenance, maximum dose, and duration. Large variations in these key factors can skew outcomes and lead to inaccurate inferences of treatment efficacy. Form and route of administration (ROA) are of particular importance as both may influence pharmacodynamics of cannabis/cannabinoids.49,50 Though past studies focused on oral and vaporized ROA, there is self-report evidence that the SCI community also uses edible and topical forms of cannabis/cannabinoids.8,38 Further research is needed to determine what benefits and harms are associated with which ROAs in this population.
Numerous health concerns, such as respiratory dysfunction, autonomic dysreflexia, altered sensation, temperature dysregulation, muscle spasms, and bowel/bladder dysfunction, often accompany SCI. Thus a greater emphasis should be placed on assessing safety and tolerability of cannabis/cannabinoid treatments in this population. Future studies should follow standard guidelines for reporting adverse events and side effects through a combination of structured check-lists and self-report measures.51,52 Another essential consideration is concomitant medication use, especially changes in or interactions with other pain related medications. Individuals with SCI often take many medications, to treat pain and the other health conditions they face that could potentially interact with cannabinoid/cannabis treatments.53 Close monitoring of changes in other medications and any adverse drug–drug interactions is a crucial part of evaluating the safety of cannabinoid pain treatments for spinal cord injury.
Clinical characteristics
Finally, there needs to be consistency in reporting of sociodemographic and clinical characteristics of subjects, with identification of any confounding co-morbidities, including coexistent medical conditions that may impact pain or perception thereof. One such consideration is history of cannabis use. Investigators should consider how prior experience may influence their results – subjects with a history of cannabis use may have a higher tolerance and thus require dosage adjustments or vice versa. We recommend describing prior history of cannabis use in prospective subjects to ensure the sample is as similar at baseline in this domain. One standardized measure to characterize cannabis use is the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (DFAQ-CU).54
Contextual factors
Unfortunately, confusing legal and policy barriers remain that limit our understanding of cannabis effects and inhibit the conduct of high-quality research. The forms of cannabis that most people with SCI use, that is botanical cannabis and plant-derived products such as edibles and tinctures, are designated as schedule one drugs by the Federal United States Drug Enforcement Agency (DEA), through the Controlled Substances Act (CSA).55 These restrictions exist despite the fact that botanical cannabis and products generally contain less than 20% THC, the most psychoactive ingredient in cannabis as well as other potentially therapeutic cannabinoids, including CBD.56 Schedule one classification implies that cannabis has no currently accepted medical use and has high potential for abuse.57 As a schedule one drug it is technically illegal for a health care provider to prescribe cannabis. This also creates significant regulatory and administrative barriers, markedly limiting research. Remarkably the DEA considers cocaine and methamphetamine to be safer than cannabis, placing them in the less restricted schedule two category.55
In contrast, the cannabinoids that can be studied and prescribed (such as dronabinol and nabiximols) are in the less restrictive, schedule two or three categories but may contain up to 100% THC (dronabinol gel capsules), the most psychoactive ingredient in cannabis . For this reason, many patients report finding forms of cannabis such as dronabinol too sedating. The upshot is that the forms of cannabis that can be studied are not the ones that patients use and claim to be helpful, while the forms of cannabis that people use cannot be studied.
Although more than half of the states have legalized cannabis for medicinal purposes, this has been primarily due to political pressures placed on state governments by patients and their advocacy groups.58 Moreover, the laws still differ considerably from state to state, and even among countries, with much ambiguity regarding what constitutes acceptable medical use and guidelines for such usage. The risk of using cannabis can be particularly high for people who rely on federally regulated programs, including Social Security Disability Income (SSDI) and Medicare health insurance.59 They risk losing these federally regulated benefits if they are charged with criminal activity over the use of cannabis.60
The current state of the science maintains that status quo in which health care providers lack knowledge regarding the pharmacology of cannabis and do not have access to educational opportunities to improve their knowledge.61 They remain concerned about a perceived lack of standards for cannabis dispensary staff training as well as apprehension over the consistency of the ingredients and the possible inclusion of unknown ingredients with unknown effects.61,62 When their patients use cannabis, they remain unsure about correct dosing, the potential interactions of cannabis with other medications, and its effect on performance in the workplace.
Limitations
The use of cannabinoids to treat pain in this setting is still an emerging area in medicine, thus the size of the research available is limited. Though we searched standard databases and clinicaltrials.gov in order to identity studies, we did not conduct a gray literature search for unpublished data in this area. Additionally, we limited our search to studies available in English, and are not able to evaluate studies from other nations which have examined the effect of cannabis upon pain intensity in SCI. Further, the extent of methodological weaknesses in the existing literature limit the extent to which the data can be analyzed and compared across studies. This ultimately impedes what can be concluded from the existing body of research.
Conclusion
The current quality and level of evidence is insufficient to draw reliable conclusions for the efficacy of cannabis for SCI-related pain intensity. The available studies provide mixed evidence, primarily due to inconsistent methodologies which makes drawing conclusions difficult. Addressing these methodological issues would allow for higher quality research that is better suited for evaluating overall efficacy of cannabis for SCI-related pain. Yet, changes in regulatory and funding limitations are needed to allow more rigorous research to occur. Without those changes and better research, people with SCI and their health care providers will have little reliable scientific evidence to guide them regarding the actual benefits and harms associated with cannabis use for SCI-related pain.
Disclaimer statements
Contributors None.
Funding None.
Conflicts of interest The authors report no conflict of interest.
Glossary
Acronyms: SCI: spinal cord injury (pg. 2, line 3); NSAIDS: nonsteroidal anti-inflammatories (pg. 2, line 4); ECS: endocannabinoid system (pg. 2, line 25); THC: tetrahydrocannabinol (pg. 2, line 24); CBD: cannabidiol (pg. 2, line 24); RCT: randomized controlled trial (pg. 4, line 19)
References
- 1.Norrbrink Budh C, Lund I, Hultling C, Levi R, Werhagen L, Ertzgaard P, et al. Gender related differences in pain in spinal cord injured individuals. Spinal Cord 2003;41:122–8. [DOI] [PubMed] [Google Scholar]
- 2.Turner JA, Cardenas DD, Warms CA, McClellan CB.. Chronic pain associated with spinal cord injuries: a community survey. Arch Phys Med Rehabil 2001;82:501–9. [DOI] [PubMed] [Google Scholar]
- 3.Siddall PJ, McClelland JM, Rutkowski SB, Cousins MJ.. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 2003;103:249–57. [DOI] [PubMed] [Google Scholar]
- 4.Guy SD, Mehta S, Casalino A, Côté I, Kras-Dupuis A, Moulin DE, et al. The canpain sci clinical practice guidelines for rehabilitation management of neuropathic pain after spinal cord: recommendations for treatment. Spinal Cord 2016;54:S14–23. [DOI] [PubMed] [Google Scholar]
- 5.Heutink M, Post MW, Wollaars MM, van Asbeck FW.. Chronic spinal cord injury pain: pharmacological and non-pharmacological treatments and treatment effectiveness. Disabil Rehabil 2011;33:433–40. [DOI] [PubMed] [Google Scholar]
- 6.Warms CA, Turner JA, Marshall HM, Cardenas DD.. Treatments for chronic pain associated with spinal cord injuries: many are tried, few are helpful. Clin J Pain 2002;18:154–63. [DOI] [PubMed] [Google Scholar]
- 7.Hagen EM, Rekand T.. Management of neuropathic pain associated with spinal cord injury. Pain Ther 2015;4:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drossel C, Forchheimer M, Meade MA.. Characteristics of individuals with spinal cord injury who use cannabis for therapeutic purposes. Top Spinal Cord Inj Rehabil 2016;22(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardenas DD, Jensen MP.. Treatments for chronic pain in persons with spinal cord injury: a survey study. J Spinal Cord Med 2006;29:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce D, Brady JP, Foster E, Shattell M.. Preferences for medical marijuana over prescription medications among persons living with chronic conditions: alternative, complementary, and tapering uses. J Altern Complement Med 2018;24:146–53. [DOI] [PubMed] [Google Scholar]
- 11.Bourke JA, Catherwood VJ, Nunnerley JL, Martin RA, Levack WMM, Thompson BL, et al. Using cannabis for pain management after spinal cord injury: a qualitative study. Spinal Cord Ser Cases 2019;5:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andresen SR, Biering-Sorensen F, Hagen EM, Nielsen JF, Bach FW, Finnerup NB.. Cannabis use in persons with traumatic spinal cord injury in Denmark. J Rehabil Med 2017;49:152–60. [DOI] [PubMed] [Google Scholar]
- 13.Hawley LA, Ketchum JM, Morey C, Collins K, Charlifue S.. Cannabis use in individuals with spinal cord injury or moderate to severe traumatic brain injury in Colorado. Arch Phys Med Rehabil 2018;99:1584–90. [DOI] [PubMed] [Google Scholar]
- 14.Zou S, Kumar U.. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. Int J Mol Sci 2018;19:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology 2018;43:155–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alger BE. Getting high on the endocannabinoid system. Cerebrum 2013;2013:14. [PMC free article] [PubMed] [Google Scholar]
- 17.Chiou LC, Hu SS, Ho YC.. Targeting the cannabinoid system for pain relief? Acta Anaesthesiol Taiwan 2013;51:161–70. [DOI] [PubMed] [Google Scholar]
- 18.Baron EP. Medicinal properties of cannabinoids, terpenes, and flavonoids in cannabis, and benefits in migraine, headache, and pain: an update on current evidence and cannabis science. Headache 2018;58:1139–86. [DOI] [PubMed] [Google Scholar]
- 19.Krause AJ, Prather AA, Wager TD, Lindquist MA, Walker MP.. The pain of sleep loss: a brain characterization in humans. J Neurosci 2019;39:2291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prospéro-García O, Amancio-Belmont O, Becerril Meléndez AL, Ruiz-Contreras AE, Méndez-Díaz M.. Endocannabinoids and sleep. Neurosci Biobehav Rev 2016;71:671–9. [DOI] [PubMed] [Google Scholar]
- 21.Lafenêtre P, Chaouloff F, Marsicano G.. The endocannabinoid system in the processing of anxiety and fear and how cb1 receptors may modulate fear extinction. Pharmacol Res 2007;56:367–81. [DOI] [PubMed] [Google Scholar]
- 22.Lutz B, Marsicano G, Maldonado R, Hillard CJ.. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 2015;16:705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD.. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 2016;17:T70–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arksey H, O'Malley L.. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. [Google Scholar]
- 25.Tricco A, Lillie E, Zarin W, O'Brien K, Colquhoun H, Levac D, et al. Prisma extension for scoping reviews (PRISMA-SCR): checklist and explanation. Ann Intern Med 2018;169:467–73. [DOI] [PubMed] [Google Scholar]
- 26.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-SCR): checklist and explanation. Ann Int Med 2018;169:467–73. [DOI] [PubMed] [Google Scholar]
- 27.Bhogal SK, Teasell RW, Foley NC, Speechley MR.. The pedro scale provides a more comprehensive measure of methodological quality than the jadad scale in stroke rehabilitation literature. J Clin Epidemiol 2005;58:668–73. [DOI] [PubMed] [Google Scholar]
- 28.Pinto RZ, Maher CG, Ferreira ML, Ferreira PH, Hancock M, Oliveira VC, et al. Drugs for relief of pain in patients with sciatica: systematic review and meta-analysis. Br Med J 2012;344:e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamato TP, Maher C, Koes B, Moseley A.. The pedro scale had acceptably high convergent validity, construct validity, and interrater reliability in evaluating methodological quality of pharmaceutical trials. J Clin Epidemiol 2017;86:176–81. [DOI] [PubMed] [Google Scholar]
- 30.Furukawa TA. From effect size into number needed to treat. Lancet 1999;353:1680. [DOI] [PubMed] [Google Scholar]
- 31.Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A.. An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. J Pain 2016;17:982–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurer M, Henn V, Dittrich A, Hofmann A.. Delta-9-tetrahydrocannabinol shows antispastic and analgesic effects in a single case double-blind trial. Eur Arch Psychiatry Clin Neurosci 1990;240:1–4. [DOI] [PubMed] [Google Scholar]
- 33.Wilsey BL, Deutsch R, Samara E, Marcotte TD, Barnes AJ, Huestis MA, Le D.. A preliminary evaluation of the relationship of cannabinoid blood concentrations with the analgesic response to vaporized cannabis. J Pain Res 2016;9:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rintala DH, Fiess RN, Tan G, Holmes SA, Bruel BM.. Effect of dronabinol on central neuropathic pain after spinal cord injury. Am J Phys Med Rehabil 2010;89:840–8. [DOI] [PubMed] [Google Scholar]
- 35.Hagenbach U, Luz S, Ghafoor N, Berger JM, Grotenhermen F, Brenneisen R, et al. The treatment of spasticity with delta(9)-tetrahydrocannabinol in persons with spinal cord injury. Spinal Cord 2007;45:551–62. [DOI] [PubMed] [Google Scholar]
- 36.Berman J, Bosworth T, Guy G, Slott C, Sativex Spinal Cord Injury Study Group . Sativex® in the treatment of central neuropathic pain due to spinal cord injury: A randomised controlled study. British Pain Society, Annual Scientific Meeting; London, England 2007.
- 37.Clinicaltrials.Gov [internet]. Identifier: Nct01606202, a study of cannabis based medicine extracts and placebo in patients with pain due to spinal cord injury [database on the Internet]. National Library of Medicine (US). 2012. Available from: https://clinicaltrials.gov/ct2/show/NCT01606202.
- 38.Stillman M, Capron M, Mallow M, Ransom T, Gustafson K, Bell A, et al. Utilization of medicinal cannabis for pain by individuals with spinal cord injury. Spinal Cord Ser Cases 2019;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulz KF, Altman DG, Moher D, The Consort Group . Consort 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010; 152: 726–32. [DOI] [PubMed] [Google Scholar]
- 40.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 2007;45:190–205. [DOI] [PubMed] [Google Scholar]
- 41.Lammertse D, Tuszynski MH, Steeves JD, Curt A, Fawcett JW, Rask C, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial design. Spinal Cord 2007;45:232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007;45:206–21. [DOI] [PubMed] [Google Scholar]
- 43.Tuszynski MH, Steeves JD, Fawcett JW, Lammertse D, Kalichman M, Rask C, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord 2007;45:222–31. [DOI] [PubMed] [Google Scholar]
- 44.Wu X, Liu J, Tanadini LG, Lammertse DP, Blight AR, Kramer JL, et al. Challenges for defining minimal clinically important difference (mcid) after spinal cord injury. Spinal Cord 2015;53:84–91. [DOI] [PubMed] [Google Scholar]
- 45.Masri R, Keller A.. Chronic pain following spinal cord injury. Adv Exp Med Biol 2012;760:74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Widerström-Noga E, Biering-Sørensen F, Bryce T, Cardenas DD, Finnerup NB, Jensen MP, et al. The international spinal cord injury pain basic data set. Spinal Cord 2008;46:818–23. [DOI] [PubMed] [Google Scholar]
- 47.Widerström-Noga E, Biering-Sørensen F, Bryce TN, Cardenas DD, Finnerup NB, Jensen MP, et al. The international spinal cord injury pain basic data set (version 2.0). Spinal Cord 2014;52:282–6. [DOI] [PubMed] [Google Scholar]
- 48.Widerström-Noga E, Biering-Sørensen F, Bryce TN, Cardenas DD, Finnerup NB, Jensen MP, et al. The international spinal cord injury pain extended data set (version 1.0). Spinal Cord 2016;54:1036–46. [DOI] [PubMed] [Google Scholar]
- 49.Russell C, Rueda S, Room R, Tyndall M, Fischer B.. Routes of administration for cannabis use - basic prevalence and related health outcomes: a scoping review and synthesis. Int J Drug Policy 2018;52:87–96. [DOI] [PubMed] [Google Scholar]
- 50.Lucas CJ, Galettis P, Schneider J.. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol 2018;84:2477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ioannidis JP, Evans SJ, Gøtzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the consort statement. Ann Intern Med 2004;141:781–8. [DOI] [PubMed] [Google Scholar]
- 52.Kelly WN, Arellano FM, Barnes J, Bergman U, Edwards IR, Fernandez AM, et al. Guidelines for submitting adverse event reports for publication. Pharmacoepidemiol Drug Saf 2007;16:581–7. [DOI] [PubMed] [Google Scholar]
- 53.Alsherbiny MA, Li CG.. Medicinal cannabis-potential drug interactions. Medicines (Basel) 2018;6:3. doi: 10.3390/medicines6010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuttler C, Spradlin A.. Measuring cannabis consumption: psychometric properties of the daily sessions, frequency, age of onset, and quantity of cannabis use inventory (DFAQ-CU). PLoS One 2017;12:e0178194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.United states drug enforcement administration [internet]. Drug scheduling.: Department of Justice; [cited 2020 05/26/2020]; Available from: https://www.dea.gov/drug-scheduling.
- 56.Hilton G, Unsworth CA, Stuckey R, Murphy GC.. The experience of seeking, gaining and maintaining employment after traumatic spinal cord injury and the vocational pathways involved. Work 2018;59:67–84. [DOI] [PubMed] [Google Scholar]
- 57.Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD.. Characteristics of patients with chronic pain accessing treatment with medical cannabis in Washington state. J Opioid Manag 2009;5:257–86. [DOI] [PubMed] [Google Scholar]
- 58.Carlini BH, Garrett SB, Carter GT.. Medicinal cannabis: A survey among health care providers in Washington state. Am J Hosp Palliat Care 2017;34:85–91. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal SK, Carter G, Sullivan M, Morrill R, Zumbrunnen C, Mayer J.. Distress, coping, and drug law enforcement in a series of patients using medical cannabis. J Nerv Ment Dis 2013;201:292–303. [DOI] [PubMed] [Google Scholar]
- 60.Carter GT, Javaher SP, Nguyen MH, Garret S, Carlini BH.. Re-branding cannabis: the next generation of chronic pain medicine? Pain Manag 2015;5:13–21. [DOI] [PubMed] [Google Scholar]
- 61.Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD.. Medicinal use of cannabis in the United States: historical perspectives, current trends, and future directions. J Opioid Manag 2009;5:153–68. [DOI] [PubMed] [Google Scholar]
- 62.Valencia CI, Asaolu IO, Ehiri JE, Rosales C.. Structural barriers in access to medical marijuana in the USA-a systematic review protocol. Syst Rev 2017;6:154. doi: 10.1186/s13643-017-0541-4. [DOI] [PMC free article] [PubMed] [Google Scholar]