Abstract
Background
Ventricular arrhythmias (VAs) are observed in 25%–50% of continuous‐flow left ventricular assist device (CF‐LVAD) recipients, but their role on mortality is debated.
Methods
Sixty‐nine consecutive patients with a CF‐LVAD were retrospectively analyzed. Study endpoints were death and occurrence of first episode of VAs post CF‐LVAD implantation. Early VAs were defined as VAs in the first month after CF‐LVAD implantation.
Results
During a median follow‐up of 29.0 months, 19 patients (27.5%) died and 18 patients (26.1%) experienced VAs. Three patients experienced early VAs, and one of them died. Patients with cardiac resynchronization therapy (CRT‐D) showed a trend toward more VAs (p = 0.076), compared to patients without CRT‐D; no significant difference in mortality was found between patients with and without CRT‐D (p = 0.63). Patients with biventricular (BiV) pacing ≥98% experienced more frequently VAs (p = 0.046), with no difference in mortality (p = 0.56), compared to patients experiencing BiV pacing <98%. There was no difference in mortality among patients with or without VAs after CF‐LVAD [5 patients (27.8%) vs. 14 patients (27.5%), p = 0.18)], and patients with or without previous history of VAs (p = 0.95). Also, there was no difference in mortality among patients with a different timing of implant of implantable cardioverter‐defibrillator (ICD), before and after CF‐LVAD (p = 0.11).
Conclusions
VAs in CF‐LVAD are a common clinical problem, but they do not impact mortality. Timing of ICD implantation does not have a significant impact on patients' survival. Patients with BiV pacing ≥98% experienced more frequently VAs.
Keywords: heart failure, left ventricular assist devices, mortality, ventricular arrhythmias
We reported a retrospective study in which we analyzed 69 consecutive CF‐LVAD‐carrier patients. In these patients, ventricular arrhythmias are a common clinical problem, but they do not impact mortality. Timing of ICD implantation does not have a significant impact on patients' survival. Patients with biventricular pacing for ≥98% of the time experienced more frequently VAs.

1. INTRODUCTION
Continuous‐flow left ventricular assist devices (CF‐LVADs) were developed as a bridge to transplant in patients needing mechanical circulatory support for end‐stage heart failure (HF). Due to the increased incidence of advanced HF, the relative paucity of available organ donors, and the significant technological devices improvements, destination therapy (DT) has become a further indication. 1
Advanced HF is characterized by an increased arrhythmic burden. Ventricular arrhythmias (VAs) are a negative prognostic marker, but it is not ascertained if they directly impact disease progression, or they are a marker of an end‐stage disease. Proposed mechanisms of VAs in CF‐LVAD carriers vary by patient population and duration of CF‐LVAD support; they include ischemia, fibrosis, underlying cardiomyopathy substrate, inotropic and pressor therapies, mechanical origin from the inflow cannula or suction events. 1 , 2 , 3
Contrasting results are available regarding the effects of ICDs and CRT‐D in patients with CF‐LVADs; it is also not known whether ICD implantation is associated with better prognosis. 4
In this single‐center retrospective observational study, we sought to investigate the clinical characteristics of VAs, the role of ICD and CRT‐D, and mortality in CF‐LVAD recipients.
2. MATERIALS AND METHODS
In the present single‐center, retrospective study, we enrolled all consecutive patients who underwent CF‐LVAD implantation at San Raffaele University Hospital in Milan from 2011 to 2019. With the approval of our local Ethical Committee, we collected data on patients with ICD who were implanted an LVAD at our institution from 2011 to 2019; data were anonymized prior to insertion in the electronic database.
According to the most recent HF guidelines, 5 we selected for CF‐LVAD implantation only patients with Stage D HF with reduced ejection fraction and reduced functional capacity, as measured by a maximal oxygen consumption VO2 < 14 mg/kg/min. Final careful multidisciplinary evaluation of patients' candidacy was essential before implantation. Lavare Cycle and suction alarms were active in all HeartWare patients. ICD or CRT‐D were implanted following current guidelines on sudden cardiac death (SCD) prevention and HF management. 6 , 7 CRT‐D was programmed with biventricular pacing (BiV) ON and left ventricular lead ON.
The CF‐LVAD controller monitor was checked during every clinical visit, according to state‐of‐the‐art standard of care for CF‐LVAD recipients management. ICD interrogation was performed every 6 months. Clinical follow‐up was also provided by in‐person visits, in‐patient and outpatient medical records, and by phone interview.
Variables collected during patient visits were ICD programming parameters, VAs occurrence, CF‐LVAD parameters, and laboratory parameters including hematologic and biochemical values. Vas were defined as sustained ventricular tachycardia (VT) or ventricular fibrillation (VF). VAs were classified according to their time of occurrence: early VAs occurring within 30 days after CFLVAD implantation and late VAs occurring after 30 days. CF‐LVAD parameters collected were pump flow (PF) (L/min), pump power (PP) (W), pulsatility index (PI). Study outcomes were death and occurrence of first episode of VAs post LVAD implantation.
2.1. Statistical methods
Descriptive statistics were expressed in terms of median and interquartile range (IQR) for continuous variables, while frequency distribution and percentage were reported for categorical variables. Since normality distribution assumption within groups was not met for most of the variables, Mann–Whitney test or Fisher's exact test was applied to compare patient groups, respectively, in presence of continuous or categorical variables. Kaplan–Meier's curves were drawn to describe the patients' freedom from death and VAs during the follow‐up period. The alpha level was set at the conventional 0.05 level. Analyses were performed using R statistical software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Patients' characteristics
Figure 1 illustrates the flow diagram of the study design.
FIGURE 1.
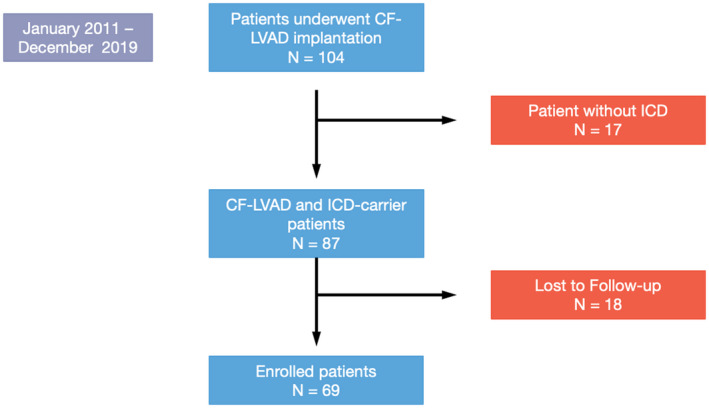
Flow diagram of the study design
One hundred and four patients underwent CF‐LVAD implantation procedure at San Raffaele Hospital from 2011 to 2019. Seventeen patients did not have any implanted ICD; 18 patients performed ICD interrogations in other centers; therefore, their ICD reports were not available for analysis. A total of 69 were retrospectively analyzed. Median age at implantation was 66.0 years (IQR: 63.0–71.0), 65 (94.2%) were males; median age at follow‐up was 70.0 years (IQR 65.0–73.0). In 43 patients (62.3%), indication to implant was end‐stage ischemic cardiomyopathy, and in 19 patients (27.5%), nonischemic dilated cardiomyopathy; other indications and clinical characteristics at implantation are summarized in Tables 1 and S1. The implanted device was HeartMate II in 1 patient (1.4%), HeartMate III in 33 patients (47.8%), and Heartware HVAD in 35 patients (50.8%).
TABLE 1.
Characteristics of patients implanted with CF‐LVAD and comparisons between patients with versus without post‐implant VAs occurrence
| No‐VAs group (N = 51) | VAs group (N = 18) | Total (N = 69) | p value | |
|---|---|---|---|---|
| Age at implantation (years) | 66.0 (62.5–71.0) | 66.5 (65.0–69.0) | 66.0 (63.0–71.0) | 0.89 |
| Gender (male) | 48 (94.1) | 17 (94.4) | 65 (94.2) | 0.99 |
| Diagnosis | 0.35 | |||
| ACM | 1 (2.0) | 0 (0.0) | 1 (1.4) | |
| HCM | 1 (2.0) | 1 (5.6) | 2 (2.8) | |
| ICM | 34 (66.7) | 10 (55.6) | 44 (63.8) | |
| NIDCM | 12 (23.5) | 7 (38.9) | 19 (27.5) | |
| Radiation‐related CM | 1 (2.0) | 0 (0.0) | 1 (1.4) | |
| Chemotherapy‐related CM | 1 (2.0) | 0 (0.0) | 1 (1.4) | |
| Valvular heart disease | 1 (2.0) | 0 (0.0) | 1 (1.4) | |
| Hypertension | 22 (43.1) | 8 (44.4) | 30 (43.5) | 0.99 |
| COPD | 15 (29.4) | 5 (27.8) | 20 (29.0) | 0.99 |
| CABG | 6 (11.8) | 2 (11.1) | 8 (11.6) | 0.99 |
| PTCA | 22 (43.1) | 8 (44.4) | 30 (43.5) | 0.99 |
| Diabetes | 16 (31.4) | 2 (11.1) | 18 (26.1) | 0.12 |
| CKD | 26 (51.0) | 9 (50.0) | 35 (50.7) | 0.99 |
| AF history | 16 (31.4) | 8 (44.4) | 24 (34.8) | 0.39 |
| VAs history | 26 (51.0) | 13 (72.2) | 39 (56.5) | 0.17 |
| Cardiac surgery | 19 (37.3) | 6 (33.3) | 25 (36.2) | 0.99 |
| Anti RAAs | 24 (47.1) | 4 (22.2) | 28 (40.6) | 0.09 |
| BBs | 47 (92.2) | 18 (100.0) | 65 (94.2) | 0.57 |
| AADs | 14 (28.0) | 9 (50.0) | 23 (33.8) | 0.14 |
| ICD implanted after CF‐LVAD | 7 (13.7%) | 3 (16.7%) | 10 (14.5%) | 0.71 |
| Echo LV EDV (ml) | 202.5 (184.8–220.2) | 244.0 (217.5–291.0) | 238.0 (191.0–244.0) | 0.25 |
| Echo LV EDD (mm) | 60.5 (57.2–66.0) | 61.5 (57.0–65.0) | 61.0 (57.0–66.0) | 0.67 |
| Echo LV ejection fraction (%) | 20.0 (18.0–20.0) | 20.0 (17.0–23.0) | 20.0 (19.0–22.0) | 0.31 |
| Echo RV TAPSE (mm) | 15.0 (13.0–16.0) | 16.0 (15.0–17.0) | 14.5 (13.0–16.0) | 0.22 |
| Echo RV S′ TDI (cm/s) | 9.0 (8.0–10.0) | 10.0 (8.1–10.0) | 9.0 (8.0–10.0) | 0.20 |
| Echo MR | 25 (49.0) | 6 (33.3) | 31 (44.9) | 0.28 |
| Echo AR | 4 (7.8) | 3 (16.7) | 7 (10.1) | 0.37 |
| Pump speed (rpm) | ||||
| HeartMate III | 5400.0 (5275.0–5550.0) | 5300.0 (5200.0–5400.0) | 5300.0 (5200.0–5400.0) | 0.17 |
| HeartWare HVAD | 2720.0 (2610.0–2800.0) | 2600.0 (2500.0–2655.0) | 2640.0 (2585.0–2785.0) | 0.12 |
| Pulse flow (L/min) | 4.4 (3.8–5.2) | 4.2 (3.8–4.6) | 4.3 (3.8–5.0) | 0.21 |
| Pulse power (W) | 4.2 (3.8–4.8) | 4.2 (3.6–5.0) | 4.2 (3.7–5.0) | 0.55 |
| Pulse index | 4.2 (3.3–5.2) | 3.8 (3.2–3.9) | 3.8 (3.3–4.8) | 0.23 |
Note: Results are reported as n (%) for categorical variables and median (interquartile range) for continuous variables.
Abbreviations: AADs, anti arrhythmic drugs; ACM, arrhythmogenic cardiomyopathy; AF, atrial fibrillation; AR, aortic regurgitation at least moderate; BBs, Beta blockers; CABG, coronary artery bypass graft; CF‐LVAD, continuous‐flow left ventricular assist device; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EDD, end‐diastolic diameter; EDV, end‐diastolic volume; HCM, hypertrophic cardiomyopathy; ICM, ischemic cardiomyopathy; MR, mitral regurgitation at least moderate; NIDCM, nonischemic dilated cardiomyopathy; PTCA, percutaneous transluminal coronary angioplasty; RAAS, Renin–angiotensin system inhibitors; TDI, tissue doppler imaging; VAs, ventricular arrhythmias.
3.2. Ventricular arrhythmias and mortality
All analyzed patients had an implanted ICD. Fifty‐nine patients (85.5%) received an ICD before CF‐LVAD implant, and 10 patients (14.5%) after CF‐LVAD. These 10 patients received CF‐LVAD as bridge‐to‐recovery therapy; however, after 8.6 months (IQR 7.5–9.3) from CF‐LVAD implant, refractory hemodynamic instability persisted, and an ICD was implanted in primary prevention.
Despite expected real‐world practice‐related heterogeneity, ICD detection and therapy settings showed limited inter‐individual variability: VF zone was programmed ON in all patients for median heart rate of 210.0 bpm (200.0–212.0); 54 patients (78.3%) had 1 VT detection zone and 12 patients (17.4%) 2 VT detection zones. VT1 detection zone was programmed ON in 66 patients (95.7%) for heart rate of 167.0 bpm (161.0–171.0), and VT2 zone was ON in 12 patients (17.4%) for heart rate of 183.0 bpm (181.8–184.0).
At the median follow‐up of 29.0 months (18.0–44.0) after CF‐LVAD implantation, 18 patients (26.1%) experienced VAs; all VAs were appropriately detected and treated by ICD shock. The median time from implantation to first VA occurrence was 9.5 months (4.2–13.5). Three patients experienced early VAs, and one of them died. No electromagnetic interference episodes were observed. Among CF‐LVAD parameters, PP was lower in patients that experienced VAs [3.8 W (3.5–4.1) vs. 4.3 W (3.8–5.2), p = 0.004]; no significant differences among patients with and without VA regarding PF [4.2 L/min (3.8–4.6) vs. 4.4 L/min (3.8–5.2), p = 0.21] and PI [3.8 (3.2–3.9) vs. 4.2 (3.3–5.2), p = 0.22] were found. No significant differences regarding time to first VA occurrence among patients receiving ICD before or after CF‐LVAD implantation were found [15 patients (25.4%) vs. 3 patients (30.0%), LogRank p = 0.92]. No significant differences were found between LVAD model in mortality (LogRank p = 0.6), first VA occurrence (LogRank p = 0.48), and VAs history (LogRank p = 0.85).
At the median follow‐up, 19 patients (27.5%) died. Causes of death were: cerebral hemorrhage in 9 patients (47.4%), septic shock in 7 patients (36.8%), and end‐stage respiratory failure in 3 patients (15.8%). There was no death due to VAs. Among CF‐LVAD parameters, PP was higher in patients that experienced death [5.0 W (4.2–5.5) vs. 4.1 W (3.6–4.4), p = 0.003], with no significant differences regarding PF [4.8 L/min (3.9–5.4) vs. 4.2 L/min (3.7–4.6), p = 0.076], and PI [4.2 (3.1–5.0) vs. 3.8 (3.3–4.7), p = 0.94].
There was no difference in mortality among patients with and without VAs after CF‐LVAD [5 patients (27.8%) vs. 14 patients (27.5%), LogRank p = 0.18], and patients with or without previous history of VAs [11 patients (28.2%) vs. 8 patients (26.7%), LogRank p = 0.95]. Also, Kaplan–Maier analysis did not show significant differences in mortality among patients with ICD implanted before and after CF‐LVAD [16 patients (27.1%) vs. 3 patients (30.0%), LogRank p = 0.11] (Figure 2).
FIGURE 2.
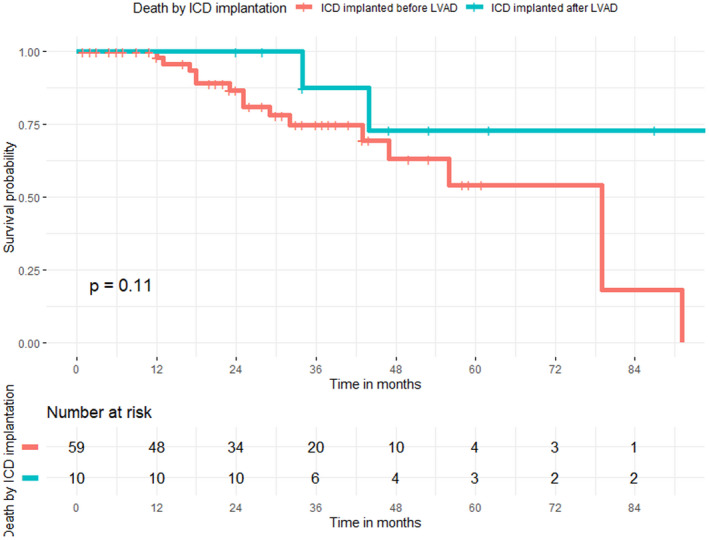
Kaplan–Meier curve for death in patients with CF‐LVAD stratified by ICD implantation
Thirty‐two patients (46.4%) received a CRT‐D, 28 patients (40.6%) before, and 4 patients (5.8%) after CF‐LVAD implantation. Twenty‐seven patients (39.1%) received BiV pacing for ≥98% of the time. Within the group of patients experiencing VAs after LVAD implantation, patients with CRT‐D showed a trend toward more VAs [12 patients with CRT‐D (38.7%) vs. 6 patients without CRT‐D (16.2%), LogRank p = 0.076] and, moreover, patients with BiV pacing ≥98% more frequently experienced a VA episode [10 patients (37.0%) vs. 8 patients (19.0%), LogRank p = 0.046]. Regarding mortality rate, no significant differences were found between patients with and without CRT‐D [11 patients (35.5%) vs. 8 patients (21.6%), LogRank p = 0.63], and between patients with BiV pacing ≥98% and < 98% [8 patients (29.6%) vs. 11 patients (26.2%), LogRank p = 0.56], (Figure 3). There were no significant differences regarding the time to first VA occurrence among patients with ischemic, nonischemic dilated, and other cardiomyopathies (LogRank p = 0.5).
FIGURE 3.
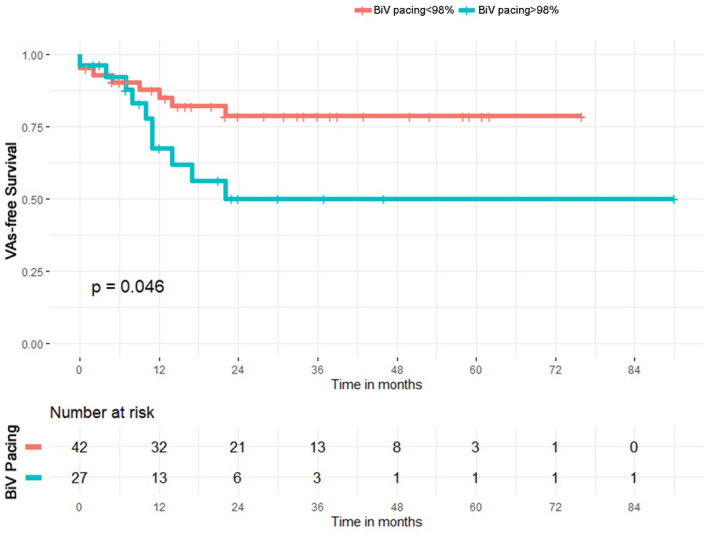
Kaplan–Meier curve for first VA occurrence in patients with CF‐LVAD stratified by biventricular pacing ≥ or ≤ 98%
4. DISCUSSION
In a retrospective cohort of CF‐LVAD patients, this study demonstrated that early VAs are a common phenomenon; higher BiV pacing percentages were associated with increased VA rate, with no apparent impact on mortality. No differences on deaths were observed among patients with ICD implanted before and after CF‐LVAD.
4.1. Clinical role of VAs in CF‐LVAD
VAs were previously reported in 25%–50% of CF‐LVAD recipients 8 , 9 ; that observation is concordant with our findings. The impact of VAs on early and total mortality in this subset of patients is still a matter of debate. Only one study demonstrated that early VAs within 30 days after CF‐LVAD implantation are associated with an increased risk for death. 8 Of note, the authors reported that early VAs occurrence trended to reduction in recent years (2008 to 2015), concomitant with the increase in the HeartMate II and HeartWare implantation. 8 In our study, most patients received HeartMate III and Heartware HVAD; we found a low number of early VAs, which might be related to technological improvements of devices.
History of previous VA and new VA occurrence was not associated with increased mortality in our cohort; this might be related to the relatively low number of early VAs observed. A meta‐analysis reported that post‐CF‐LVAD VAs were associated with an increased risk of all‐cause mortality. 10 However, these results should be interpreted in the context of a low SCD risk population. 11 Furthermore, in an analysis from the INTERMACS registry, VAs were not found to be a predictor of mortality, 12 and the incidence of post‐CF‐LVAD VAs has been decreasing over the past decade. 8 However, in the absence of randomized trials, ICD may have a role in improving outcomes in LVAD recipients.
Our data are consistent with previous study from Gordon et al. that reported 37% of patients experiencing a new onset VA after CF‐LVAD implantation, with a mortality of 21% at a comparable mean follow‐up. Of note, the reported mean time to first VA was 42 days, which is shorter than the current study (9 months); this can be explained by the indication of CF‐LVAD as bridge to transplant, which was higher in the study from Gordon et al. (59%) with a younger and more “acute” population. 13
4.2. Implantable cardioverter‐defibrillators and cardiac resynchronization therapy in CF‐LVAD patients
The role of the implantable cardioverter‐defibrillator (ICD) for primary prevention of SCD in patients with HF has been well established in multiple large randomized trials. 14 , 15 However, there have been reports of CF‐LVAD recipients surviving long time despite ventricular fibrillation occurrence, thus questioning the indication to ICD implant in these patients. 16
Garan et al. 17 reported their single‐center experience with CF‐LVADs and VAs in 94 patients; 17 of them did not have any ICDs; no patient discharged from the hospital without an ICD died during follow‐up. 17 These results are consistent with Lee et al., 18 who reported no significant benefit from ICD therapy in patients with a CF‐LVAD (p = 0.56), and no patients with SCD. Building upon these premises, in our study we observed no SCD and no difference in mortality among patients who were implanted with an ICD before or after CF‐LVAD.
We found that patients with BiV pacing <98% were more ischemic, but the difference is not statistically significant (p = 0.06). However, it is in line with previous findings, in which high PVC count (≥200 PVC/h) is mostly associated with ischemic cardiomyopathy and with a reduction of BiV pacing percentage and of clinical response. 19
Cardiac resynchronization therapy has been associated with several clinical effects in patients with HF, left ventricular ejection fraction ≤35%, and a wide QRS, including improvement in left ventricular dimensions, functional status, quality of life in patients, and survival. 20 , 21 However, CF‐LVAD recipients were not included in clinical trials, and the benefit of CRT in these patients remains unclear. In the ASSIST‐ICD study, CRT in CF‐LVAD was a predictor of late VAs at univariate analysis, with a hazard ratio of 2.21. 9 However, observational studies showed a possible decrease in VAs in patients with CRT, without overall survival benefit. 22 , 23
A randomized single‐center study and various retrospective studies reported no changes between CRT‐D ON and OFF in mortality, inappropriate shocks, arrhythmic hospitalizations, and hospitalizations for HF. 23 , 24 , 25 However, in two of these studies, the reported percent of BiV pacing was 96%, and no separate analysis was performed for higher and low percent of biventricular pacing. In our study, CRT‐D was associated with increased VAs only if BiV was ≥98%, with no effect on mortality. Previous studies suggested that BiV pacing might hesitate into a pro‐arrhythmic effect, due to reversal of myocardial depolarization through epicardial stimulation via the coronary sinus, or by pacing within re‐entry circuit; it might also promote SCD. 26 , 27 , 28 In the CARE‐HF trial, despite a significant decrease in all‐cause mortality in CRT group, the percentage of SCDs was higher (35.4% vs. 31.7%). 21 Finally, the CRT group and the subgroup with higher BiV pacing percentage do not have clinical features of greater severity than the other patients (Table S2). In our opinion, in this group of patients, BiV pacing may worsen a labile arrhythmic compensation picture by increasing pro‐arrhythmic factors such as QTc intervals and PVC daily burden: this could increase the occurrence of PVC and VAs; despite all, it may not cause any effect on overall mortality.
4.3. Study limitations
This study was a retrospective single‐center study from of a referral University Hospital. The main limitation of this study is the limited number of patients included. Moreover, this study evaluated only ICD‐detected VAs in CF‐LVAD recipients; in fact, patients who did not receive any ICD were excluded from the study. As for the Kaplan–Meier curves, the reduced number of patients at risk after the second year did not allow to reach unbiased conclusions about long‐term follow‐up analyses. The occurrence of pre‐ and post‐operative VAs was evaluated as a dichotomous variable, not allowing the evaluation of differences between patients experiencing single or multiple episodes. Finally, although no statistically significant difference is reached regarding LVEDV in patients with and without VAs history, it cannot be excluded that this is due to the fact that this study is underpowered for these findings; larger, long‐term prospective trials could overcome this limitation, expanding and completing the presented preliminary results.
5. CONCLUSIONS
VAs in CF‐LVAD are a clinical problem that might be increased by biventricular pacing, without significant impact on mortality. Furthermore, timing of ICD implantation may not have an impact on patients' survival.
CONFLICT OF INTEREST
The author(s) declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTIONS
Simone Gulletta, Anna Mara Scandroglio, Luigi Pannone, Giulio Falasconi, and Pasquale Vergara: conception and design; analysis and interpretation of data; revising the manuscript critically for important intellectual content; final approval of the manuscript submitted. Giulio Melisurgo, Silvia Ajello, Giuseppe D'Angelo, Lorenzo Gigli, Felicia Lipartiti, Eustachio Agricola, Elisabetta Lapenna, Alessandro Castiglioni, Michele De Bonis, Giovanni Landoni, Paolo Della Bella, and Alberto Zangrillo: analysis and interpretation of data; drafting of the manuscript; final approval of the manuscript submitted.
ETHICS STATEMENT
The study complied with the Declaration of Helsinki; the research has been approved by the institutional committee on human research.
Supporting information
Table S1
Gulletta S, Scandroglio AM, Pannone L, Falasconi G, Melisurgo G, Ajello S, Clinical characteristics and outcomes of patients with ventricular arrhythmias after continuous‐flow left ventricular assist device implant. Artif Organs. 2022;46:1608–1615. 10.1111/aor.14234
Simone Gulletta and Anna Mara Scandroglio equally contributed to the study and should be considered as shared first Authors.
Alberto Zangrillo and Pasquale Vergara should be considered as shared last Authors.
Contributor Information
Giulio Falasconi, @DrFalasconi.
Pasquale Vergara, Email: pasqualevergara@hotmail.com, P_Vergara_MD.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request to the first author for purposes of results reproducibility.
REFERENCES
- 1. Bedi M, Kormos R, Winowich S, McNamara DM, Mathier MA, Murali S. Ventricular arrhythmias during left ventricular assist device support. Am J Cardiol. 2007;99:1151–3. [DOI] [PubMed] [Google Scholar]
- 2. Cantillon DJ, Bianco C, Wazni OM, Kanj M, Smedira NG, Wilkoff BL, et al. Electrophysiologic characteristics and catheter ablation of ventricular tachyarrhythmias among patients with heart failure on ventricular assist device support. Heart Rhythm. 2012;9:859–64. [DOI] [PubMed] [Google Scholar]
- 3. Vollkron M, Voitl P, Ta J, Wieselthaler G, Schima H. Suction events during left ventricular support and ventricular arrhythmias. J Heart Lung Transplant. 2007;26:819–25. [DOI] [PubMed] [Google Scholar]
- 4. Gopinathannair R, Cornwell WK, Dukes JW, Ellis CR, Hickey KT, Joglar JA, et al. Device therapy and arrhythmia management in left ventricular assist device recipients: a scientific statement from the American Heart Association. Circulation. 2019;139:e967–89. [DOI] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128:1810–52. [DOI] [PubMed] [Google Scholar]
- 6. Priori SG, Blomstrom‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the Management of Patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
- 7. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 8. Greet BD, Pujara D, Burkland D, Pollet M, Sudhakar D, Rojas F, et al. Incidence, predictors, and significance of ventricular arrhythmias in patients with continuous‐flow left ventricular assist devices: a 15‐year institutional experience. JACC Clin Electrophysiol. 2018;4:257–64. [DOI] [PubMed] [Google Scholar]
- 9. Galand V, Flecher E, Auffret V, Boule S, Vincentelli A, Dambrin C, et al. Predictors and clinical impact of late ventricular arrhythmias in patients with continuous‐flow left ventricular assist devices. JACC Clin Electrophysiol. 2018;4:1166–75. [DOI] [PubMed] [Google Scholar]
- 10. Makki N, Mesubi O, Steyers C, Olshansky B, Abraham WT. Meta‐analysis of the relation of ventricular arrhythmias to all‐cause mortality after implantation of a left ventricular assist device. Am J Cardiol. 2015;116:1385–90. [DOI] [PubMed] [Google Scholar]
- 11. Kadado AJ, Akar JG, Hummel JP. Arrhythmias after left ventricular assist device implantation: incidence and management. Trends Cardiovasc Med. 2018;28:41–50. [DOI] [PubMed] [Google Scholar]
- 12. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Seventh INTERMACS annual report: 15 000 patients and counting. J Heart Lung Transplant. 2015;34:1495–504. [DOI] [PubMed] [Google Scholar]
- 13. Gordon JS, Maynes EJ, Choi JH, Wood CT, Weber MP, Morris RJ, et al. Ventricular arrhythmias following continuous‐flow left ventricular assist device implantation: a systematic review. Artif Organs. 2020;44:E313–25. [DOI] [PubMed] [Google Scholar]
- 14. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. [DOI] [PubMed] [Google Scholar]
- 15. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 16. Oz MC, Rose EA, Slater J, Kuiper JJ, Catanese KA, Levin HR. Malignant ventricular arrhythmias are well tolerated in patients receiving long‐term left ventricular assist devices. J Am Coll Cardiol. 1994;24:1688–91. [DOI] [PubMed] [Google Scholar]
- 17. Garan AR, Yuzefpolskaya M, Colombo PC, Morrow JP, Te‐Frey R, Dano D, et al. Ventricular arrhythmias and implantable cardioverter‐defibrillator therapy in patients with continuous‐flow left ventricular assist devices: need for primary prevention? J Am Coll Cardiol. 2013;61:2542–50. [DOI] [PubMed] [Google Scholar]
- 18. Lee W, Tay A, Subbiah RN, Walker BD, Kuchar DL, Muthiah K, et al. Impact of implantable cardioverter defibrillators on survival of patients with centrifugal left ventricular assist devices. Pacing Clin Electrophysiol. 2015;38:925–33. [DOI] [PubMed] [Google Scholar]
- 19. Akerstrom F, Pachon M, Martinez‐Ferrer JB, Alzueta J, Perez L, Fernandez Lozano I, et al. Premature ventricular contractions in patients with an implantable cardioverter defibrillator cardiac resynchronization therapy device: results from the UMBRELLA registry. Indian Pacing Electrophysiol J. 2020;20:91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, et al. Cardiac‐resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–50. [DOI] [PubMed] [Google Scholar]
- 21. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. [DOI] [PubMed] [Google Scholar]
- 22. Schleifer JW, Mookadam F, Kransdorf EP, Nanda U, Adams JC, Cha S, et al. Effect of continued cardiac resynchronization therapy on ventricular arrhythmias after left ventricular assist device implantation. Am J Cardiol. 2016;118:556–9. [DOI] [PubMed] [Google Scholar]
- 23. Richardson TD, Hale L, Arteaga C, Xu M, Keebler M, Schlendorf K, et al. Prospective randomized evaluation of implantable cardioverter‐defibrillator programming in patients with a left ventricular assist device. J Am Heart Assoc. 2018;7:e007748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gopinathannair R, Roukoz H, Bhan A, Ravichandran A, Ahmed MM, Familtsev D, et al. Cardiac resynchronization therapy and clinical outcomes in continuous flow left ventricular assist device recipients. J Am Heart Assoc. 2018;7:e009091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roukoz H, Bhan A, Ravichandran A, Ahmed MM, Bhat G, Cowger J, et al. Continued versus suspended cardiac resynchronization therapy after left ventricular assist device implantation. Sci Rep. 2020;10:2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fish JM, Brugada J, Antzelevitch C. Potential proarrhythmic effects of biventricular pacing. J Am Coll Cardiol. 2005;46:2340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Medina‐Ravell VA, Lankipalli RS, Yan GX, Antzelevitch C, Medina‐Malpica NA, Medina‐Malpica OA, et al. Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization: does resynchronization therapy pose a risk for patients predisposed to long QT or torsade de pointes? Circulation. 2003;107:740–6. [DOI] [PubMed] [Google Scholar]
- 28. Tayeh O, Farouk W, Elazab A, Khald H, Curnis A. Potential pro‐arrhythmic effect of cardiac resynchronization therapy. J Saudi Heart Assoc. 2013;25:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data are available upon reasonable request to the first author for purposes of results reproducibility.


