Abstract
Aim
There is an association between heart failure with preserved ejection fraction (HFpEF) and insulin resistance, but less is known about the diabetic continuum, and in particular about pre‐diabetes, in HFpEF. We examined characteristics and outcomes of participants with diabetes or pre‐diabetes in PARAGON‐HF.
Methods and results
Patients aged ≥50 years with left ventricular ejection fraction ≥45%, structural heart disease and elevated N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) were eligible. Patients were classified according to glycated haemoglobin (HbA1c): (i) normal HbA1c, <6.0%; (ii) pre‐diabetes, 6.0%–6.4%; (iii) diabetes, ≥6.5% or history of diabetes. The primary outcome was a composite of cardiovascular (CV) death and total heart failure hospitalizations (HFH). Of 4796 patients, 50% had diabetes and 18% had pre‐diabetes. Compared to patients with normal HbA1c, patients with pre‐diabetes and diabetes more often were obese, had a history of myocardial infarction and had lower Kansas City Cardiomyopathy Questionnaire scores, while patients with diabetes had more clinical evidence of congestion, but similar NT‐proBNP concentrations. The risks of the primary composite outcome (rate ratio [RR] 1.59, 95% confidence interval [CI] 1.35–1.88), total HFH (RR 1.67, 95% CI 1.39–2.02) and CV death (hazard ratio [HR] 1.35, 95% CI 1.07–1.71) were higher among patients with diabetes, compared to those with normal HbA1c. Patients with pre‐diabetes had a higher risk (which was intermediate between that of patients with diabetes and those with normal HbA1c) of the primary outcome (HR 1.27, 95% CI 1.00–1.60) and HFH (HR 1.35, 95% CI 1.03–1.77), but not of CV death (HR 1.02, 95% CI 0.75–1.40). Patients with diabetes treated with insulin had worse outcomes than those not, and those with ‘lean diabetes’ had similar mortality rates to those with a higher body mass index, but lower rates of HFH.
Conclusion
Pre‐diabetes is common in patients with HFpEF and is associated with worse clinical status and greater risk of HFH. Clinical Trial Registration: ClinicalTrials.gov Identifier NCT01920711.
Keywords: Heart failure, Diabetes, Obesity, Insulin
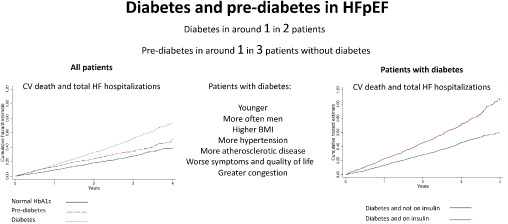
Patient characteristics and outcomes, according to diabetes status, and according to whether patients with diabetes were treated with insulin or not, in PARAGON‐HF. BMI, body mass index; CV, cardiovascular; HbA1c, glycated haemoglobin; HF, heart failure; HFpEF, heart failure with preserved ejection fraction.
Introduction
Diabetes is common in patients with heart failure (HF), whether left ventricular ejection fraction (LVEF) is reduced or preserved. 1 , 2 , 3 , 4 Recently, regional and racial variations in the prevalence of diabetes in patients with HF and reduced ejection fraction (HFrEF) have been highlighted, as have geographic differences in management of diabetes in these patients. 5 , 6 , 7 Similarly, a high prevalence of pre‐diabetic dysglycaemia (‘pre‐diabetes’) has been described in patients with HFrEF, which has also been shown to be associated with worse outcomes, compared with normoglycaemia. 7 , 8 Much less is known about this group of patients with HF and preserved ejection fraction (HFpEF). The PARAGON‐HF (Prospective comparison of ARni with Arb Global Outcomes in heart failure with preserved ejectioN fraction) trial offers a unique opportunity to study the prevalence of diabetes and pre‐diabetes and associated outcomes in a large multinational cohort of patients with HFpEF. 9 , 10 , 11 In this post hoc analysis, we report the prevalence of pre‐diabetes and diabetes in patients with HFpEF and their characteristics, treatment, rates of hospitalization and mortality. In addition, among individuals with diabetes, we looked at those with ‘lean diabetes’ and people treated with insulin, as both these subgroups have been reported to be at higher risk of adverse clinical outcomes. 12 , 13 , 14
Methods
The trial sponsor, Novartis, is committed to sharing access to patient‐level data and supporting clinical documents from eligible studies with qualified external researchers. These requests are reviewed and approved by an independent review panel, based on scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The trial data availability is according to the criteria and process described on www.clinicalstudydatarequest.com.
Study design and patients
The design, baseline characteristics and primary outcomes of PARAGON‐HF are published. 9 , 10 , 11 In brief, 4796 patients in New York Heart Association (NYHA) functional class II–IV with a LVEF ≥45%, elevated N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) level and structural heart disease were enrolled. Key exclusion criteria included body mass index (BMI) >40 kg/m2, known intolerance of angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, a history of angioedema, systolic blood pressure <100 mmHg, estimated glomerular filtration rate (eGFR) <25 ml/min/1.73 m2, or serum potassium >5.4 mmol/L. Patients were randomly assigned to treatment with sacubitril‐valsartan or valsartan. Enrolment in the trial took place from 2014 to 2016, before the routine use of sodium–glucose cotransporter 2 (SGLT2) inhibitors in HF. The trial was approved by Ethics Committees at all participating sites and all patients provided written informed consent.
Definition of glycaemic status
Investigators were asked to state on the trial case report form whether patients had a known diagnosis of diabetes. All patients also had a measurement of glycated haemoglobin (HbA1c) at baseline. Patients were categorized into three groups based on their history of diabetes and baseline HbA1c level using the International Diabetes Expert Committee (World Health Organization criteria): (i) normal HbA1c, <6.0%; (ii) pre‐diabetes, 6.0%–6.4%; and (iii) diabetes, ≥6.5% or a known prior diagnosis of diabetes. 15
Outcomes
The primary outcome for this analysis was that used in PARAGON‐HF, i.e. total HF hospitalizations and cardiovascular death. All‐cause mortality, first HF hospitalization, decline in renal function (eGFR decrease of 50% or more, end‐stage kidney disease, or death due to kidney failure), change from baseline to 8 months in Kansas City Cardiomyopathy Questionnaire clinical summary score (KCCQ‐CSS) and in NYHA class were evaluated as secondary outcomes.
Statistical analyses
Baseline characteristics according to diabetes status were described by use of proportions for categorical variables and means with standard deviations, or medians with quartiles for continuous variables. We also examined two subgroups of patients among those with diabetes: patients with ‘lean diabetes’ (defined as BMI <25 kg/m2) and those treated with insulin. Differences in baseline characteristics according to diabetes status were tested by use of χ2 tests, Wilcoxon rank‐sum tests, t‐tests, or tests for trends where appropriate. The primary composite outcome and total HF hospitalizations were analysed by use of the semiparametric proportional rates method of Lin et al. 16 stratified by geographic region. Cox proportional hazard models stratified by region were used to compare the risk of first events according to diabetes status. Nelson–Aalen and Kaplan–Meier curves were used to illustrate the cumulative recurrent and first events, respectively. Adjusted models included age, sex, race, prior HF hospitalization, HF duration, NYHA class, NT‐proBNP, smoking status (current), LVEF, systolic blood pressure, heart rate, serum creatinine level and history of myocardial infarction, stroke, chronic obstructive pulmonary disease, atrial fibrillation, hypertension, randomly assigned treatment and region (where model not stratified). Treatment effect across the spectrum of HbA1c was modelled using a fractional polynomial and the results were displayed graphically using the mfpi command in STATA. A restricted cubic spline was used to model the rate ratio for the primary composite outcome across the spectrum of HbA1c, referent to HbA1c 5.6%. Change from baseline in KCCQ‐CSS to 8 months was analysed using a multilevel mixed‐effects linear regression model, together with a multilevel mixed‐effects logistic regression model for a 5‐point deterioration. Change from baseline in NYHA class to 8 months was analysed using a multilevel mixed‐effects logistic regression model. All mixed‐effects models included baseline value, randomized treatment and treatment–visit interaction. Analyses were conducted using STATA version 16 (StataCorp LLC, College Station, TX, USA). A p‐value of <0.05 was considered statistically significant.
Results
Of the 4796 patients studied, 2388 (49.8%) had diabetes; 2062 with a prior diagnosis, and 326 without a prior diagnosis, but with a HbA1c ≥6.5% (i.e. with undiagnosed diabetes). Those with undiagnosed diabetes accounted for 7% of all patients, and for 12% of patients without a prior diagnosis of diabetes. A further 874 participants (18% of all patients/36% of those without diabetes [diagnosed or undiagnosed]) had a HbA1c between 6.0% and 6.4% (i.e. pre‐diabetes). Only 1534 participants (32%) had a HbA1c <6.0% (i.e. normal HbA1c) (Table 1 ). The distribution of HbA1c is shown in online supplementary Figure S1 .
Table 1.
Baseline characteristics according to diabetes status: diabetes (prior diagnosis or glycated haemoglobin [HbA1c] ≥6.5%), pre‐diabetes (HbA1c 6.0%–6.4%) or normal HbA1c (<6.0%)
| Normal HbA1c (<6.0%) | Pre‐diabetes (HbA1c 6.0%–6.4%) | Diabetes (prior diagnosis or HbA1c ≥6.5%) | p‐value for trend (all) | p‐value (pre‐diabetes vs. normal HbA1c) | p‐value (diabetes vs. normal HbA1c) | |
|---|---|---|---|---|---|---|
| Patients, n (%) | 1534 (32.0) | 874 (18.2) | 2388 (49.8) | |||
| Age, years, mean (± SD) | 73.2 ± 8.7 | 73.9 ± 8.1 | 72.0 ± 8.3 | <0.001 | 0.057 | <0.001 |
| Women, n (%) | 822 (53.6) | 479 (54.8) | 1178 (49.3) | 0.01 | 0.56 | 0.009 |
| Race, n (%) | 0.18 | 0.54 | 0.34 | |||
| Asian | 196 (12.8) | 95 (10.9) | 316 (13.2) | |||
| Black | 27 (1.8) | 14 (1.6) | 61 (2.6) | |||
| Other | 54 (3.5) | 34 (3.9) | 92 (3.9) | |||
| White | 1257 (81.9) | 731 (83.6) | 1919 (80.4) | |||
| Region, n (%) | 0.002 | 0.012 | <0.001 | |||
| Asia‐Pacific/other | 247 (16.1) | 117 (13.4) | 398 (16.7) | |||
| Central Europe | 489 (31.9) | 339 (38.8) | 887 (37.1) | |||
| Latin America | 136 (8.9) | 77 (8.8) | 157 (6.6) | |||
| North America | 170 (11.1) | 86 (9.8) | 303 (12.7) | |||
| Western Europe | 492 (32.1) | 255 (29.2) | 643 (26.9) | |||
| HbA1c, %, median (Q1–Q3) | 5.6 (5.4–5.8) | 6.1 (6.0–6.3) | 7.0 (6.5–7.9) | <0.001 | <0.001 | <0.001 |
| Non‐ischaemic aetiology, n (%) | 1089 (71.0) | 576 (65.9) | 1407 (58.9) | <0.001 | 0.009 | <0.001 |
| Prior HF hospitalization, n (%) | 675 (44.0) | 383 (43.8) | 1248 (52.3) | <0.001 | 0.93 | <0.001 |
| HF duration, n (%) | 0.001 | 0.40 | 0.026 | |||
| 0–3 months | 256 (16.7) | 155 (17.8) | 362 (15.2) | |||
| 3–6 months | 206 (13.5) | 122 (14.0) | 258 (10.8) | |||
| 6–12 months | 209 (13.7) | 109 (12.5) | 298 (12.5) | |||
| 1–2 years | 210 (13.7) | 129 (14.8) | 340 (14.3) | |||
| 2–5 years | 313 (20.5) | 151 (17.3) | 529 (22.2) | |||
| >5 years | 336 (22.0) | 207 (23.7) | 594 (24.9) | |||
| NYHA class, n (%) | 0.049 | 0.67 | 0.031 | |||
| I | 40 (2.6) | 18 (2.1) | 79 (3.3) | |||
| II | 1216 (79.3) | 694 (79.5) | 1796 (75.2) | |||
| III | 271 (17.7) | 159 (18.2) | 502 (21.0) | |||
| IV | 7 (0.5) | 2 (0.2) | 10 (0.4) | |||
| LVEF, %, mean (± SD) | 58.1 ± 8.0 | 57.4 ± 7.7 | 57.2 ± 7.9 | <0.001 | 0.050 | 0.001 |
| Heart rate, bpm, mean (± SD) | 69.0 ± 11.9 | 70.3 ± 12.5 | 71.4 ± 12.3 | <0.001 | 0.012 | <0.001 |
| SBP, mmHg, mean (± SD) | 130.1 ± 15.6 | 128.3 ± 15.0 | 131.7 ± 15.5 | <0.001 | 0.005 | 0.003 |
| BMI, kg/m2, median (Q1–Q3) | 28.7 (25.6–32.5) | 29.2 (25.8–33.2) | 31.1 (27.4–34.8) | <0.001 | 0.034 | <0.001 |
| BMI, kg/m2, n (%) | <0.001 | 0.073 | <0.001 | |||
| <18 | 7 (0.5) | 0 (0.0) | 2 (0.1) | |||
| 18–24.99 | 315 (20.5) | 164 (18.8) | 268 (11.2) | |||
| 25–29.99 | 597 (38.9) | 327 (37.5) | 758 (31.7) | |||
| ≥30 | 615 (40.1) | 382 (43.8) | 1360 (57.0) | |||
| Waist circumference, cm, median (Q1–Q3) | 101 (92–110) | 103 (93–112) | 107 (97–116) | <0.001 | 0.009 | <0.001 |
| Waist‐hip ratio, mean (± SD) | 0.95 ± 0.12 | 0.96 ± 0.13 | 0.98 ± 0.12 | <0.001 | 0.26 | <0.001 |
| Current smoker, n (%) | 120 (7.9) | 66 (7.6) | 167 (7.0) | 0.31 | 0.82 | 0.32 |
| eGFR, ml/min/1.73 m2, mean (± SD) | 63.4 ± 18.4 | 62.3 ± 18.1 | 62.2 ± 19.9 | 0.008 | 0.16 | 0.066 |
| Serum creatinine, mg/dl, mean (± SD) | 1.06 ± 0.28 | 1.07 ± 0.29 | 1.11 ± 0.33 | <0.001 | 0.33 | <0.001 |
| Potassium, mmol/l, median (Q1–Q3) | 4.5 (4.2–4.7) | 4.5 (4.2–4.8) | 4.5 (4.2–4.8) | <0.001 | 0.096 | <0.001 |
| NT‐proBNP, pg/ml, median (Q1–Q3) without AF | 590 (371–1024) | 618 (393–1058) | 596 (382–1074) | 0.73 | 0.27 | 0.66 |
| NT‐proBNP, pg/ml, median (Q1–Q3) with AF | 1572 (1162–2358) | 1620 (1171–2330) | 1577 (1174–2197) | 0.66 | 0.68 | 0.74 |
| KCCQ‐CSS, mean (± SD) | 74.4 ± 18.1 | 71.7 ± 19.8 | 71.2 ± 19.2 | <0.001 | <0.001 | <0.001 |
| Signs or symptoms, n (%) | ||||||
| Paroxysmal nocturnal dyspnoea | 57 (3.7) | 27 (3.1) | 107 (4.5) | 0.18 | 0.43 | 0.24 |
| Dyspnoea at rest | 41 (2.7) | 22 (2.5) | 76 (3.2) | 0.32 | 0.83 | 0.36 |
| Dyspnoea on effort | 1414 (92.2) | 811 (93.1) | 2199 (92.2) | 0.90 | 0.43 | 0.97 |
| Fatigue | 770 (50.2) | 441 (50.7) | 1226 (51.4) | 0.47 | 0.83 | 0.47 |
| Orthopnoea | 251 (16.4) | 144 (16.5) | 491 (20.6) | 0.001 | 0.92 | 0.001 |
| Third heart sound | 30 (2.0) | 20 (2.3) | 61 (2.6) | 0.22 | 0.58 | 0.22 |
| Jugular venous distention | 210 (13.8) | 110 (12.8) | 335 (14.2) | 0.67 | 0.49 | 0.74 |
| Oedema | 560 (36.5) | 297 (34.1) | 969 (40.6) | 0.005 | 0.24 | 0.010 |
| Rales | 108 (7.0) | 64 (7.3) | 173 (7.3) | 0.82 | 0.78 | 0.80 |
| Medical history, n (%) | ||||||
| Myocardial infarction | 262 (17.1) | 184 (21.1) | 637 (26.7) | <0.001 | 0.016 | <0.001 |
| Atrial flutter/fibrillation a | 455 (29.8) | 329 (37.7) | 768 (32.3) | 0.19 | <0.001 | 0.095 |
| Hypertension | 1452 (94.7) | 823 (94.2) | 2309 (96.7) | 0.001 | 0.61 | 0.002 |
| Stroke | 157 (10.2) | 79 (9.1) | 272 (11.4) | 0.19 | 0.36 | 0.26 |
| COPD | 195 (12.7) | 125 (14.3) | 350 (14.7) | 0.09 | 0.27 | 0.087 |
| Anaemia | 183 (11.9) | 97 (11.1) | 427 (17.9) | <0.001 | 0.54 | <0.001 |
| Lower limb artery stenosis | 18 (1.2) | 16 (1.8) | 72 (3.0) | <0.001 | 0.19 | <0.001 |
| Sleep apnoea | 97 (6.3) | 46 (5.3) | 221 (9.3) | <0.001 | 0.30 | <0.001 |
| Medications, n (%) | ||||||
| Diuretics | 1464 (95.4) | 831 (95.1) | 2290 (95.9) | 0.45 | 0.69 | 0.49 |
| ACE‐inhibitor/ARB | 1317 (85.9) | 753 (86.2) | 2069 (86.6) | 0.48 | 0.84 | 0.48 |
| Beta‐blocker | 1159 (75.6) | 701 (80.2) | 1961 (82.1) | <0.001 | 0.009 | <0.001 |
| MRA | 396 (25.8) | 222 (25.4) | 621 (26.0) | 0.87 | 0.82 | 0.89 |
| Antiplatelets | 170 (11.1) | 87 (10.0) | 378 (15.8) | <0.001 | 0.39 | <0.001 |
| Insulin | 0 (0.0) | 1 (0.1) | 656 (27.5) | – | – | – |
| Oral hypoglycaemic agent | 3 (0.2) | 3 (0.3) | 1476 (61.8) | – | – | – |
| Sulfonylurea | 0 (0.0) | 0 (0.0) | 463 (19.4) | – | – | – |
| Thiazolidinedione | 0 (0.0) | 0 (0.0) | 17 (0.7) | – | – | – |
| Biguanide | 3 (0.2) | 2 (0.2) | 1045 (43.8) | – | – | – |
| GLP‐1 receptor agonist | 0 (0.0) | 0 (0.0) | 20 (0.8) | – | – | – |
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1; HF, heart failure; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire clinical summary score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation.
On electrocardiogram at baseline.
Baseline characteristics
Patients with diabetes were younger and were more often men and from Central/Eastern Europe, compared to those with normal HbA1c (Table 1 ). Individuals with diabetes had a higher BMI, waist circumference and waist hip‐ratio than those with normal HbA1c. Patients with diabetes more commonly had a history of hypertension (and a higher systolic blood pressure) and evidence of atherosclerotic/atherothrombotic disease (e.g. myocardial infarction, stroke, peripheral artery disease), anaemia and sleep apnoea. Patients with diabetes had a worse NYHA class distribution and a worse mean KCCQ‐CSS, compared to those with normal HbA1c. Patients with diabetes had more congestion (reflected in peripheral oedema and orthopnoea) but a similar plasma NT‐proBNP, compared to those with normal HbA1c. In general, participants with pre‐diabetes had a phenotypic picture intermediate between those with normal HbA1c and diabetes (Table 1 ). Some notable exceptions were the proportion of women (54%, 55% and 49% among those with normal HbA1c, pre‐diabetes and diabetes, respectively), and the proportion with anemia (12%, 11%, and 18%, respectively). The KCCQ‐CSS was similarly reduced (i.e. worse) in patients with pre‐diabetes and those with diabetes, compared to patients with normal HbA1c.
Patients with ‘lean diabetes’
Among participants with diabetes, 270 patients (11%) had a BMI <25 kg/m2 (‘lean diabetes’). Patients with a lower BMI were older, had a different racial composition (39% vs. 10% Asian; 58% vs. 83% White) and geographic distribution than patients with BMI ≥25 kg/m2, had a higher (better) mean KCCQ‐CSS but a higher median NT‐proBNP than those with a higher BMI (Table 2 ).
Table 2.
Baseline characteristics according to ‘lean diabetes’ phenotype (versus non‐lean) and insulin use (versus no insulin use) in patients with diabetes
| BMI ≥25 kg/m2 | BMI <25 kg/m2 | p‐value | No insulin | Insulin | p‐value | |
|---|---|---|---|---|---|---|
| Patients, n (%) | 2118 (88.7) | 270 (11.3) | 1732 (72.5) | 656 (27.5) | ||
| Age, years, mean (± SD) | 71.8 ± 8.1 | 74.0 ± 9.2 | <0.001 | 72.7 ± 8.3 | 70.4 ± 8.1 | <0.001 |
| Women, n (%) | 1042 (49.2) | 136 (50.4) | 0.72 | 864 (49.9) | 314 (47.9) | 0.38 |
| Race, n (%) | <0.001 | <0.001 | ||||
| Asian | 211 (10.0) | 105 (38.9) | 235 (13.6) | 81 (12.3) | ||
| Black | 60 (2.8) | 1 (0.4) | 30 (1.7) | 31 (4.7) | ||
| Other | 84 (4.0) | 8 (3.0) | 64 (3.7) | 28 (4.3) | ||
| White | 1763 (83.2) | 156 (57.8) | 1403 (81.0) | 516 (78.7) | ||
| Region, n (%) | <0.001 | <0.001 | ||||
| Asia‐Pacific/other | 289 (13.6) | 109 (40.4) | 296 (17.1) | 102 (15.5) | ||
| Central Europe | 826 (39.0) | 61 (22.6) | 691 (39.9) | 196 (29.9) | ||
| Latin America | 145 (6.8) | 12 (4.4) | 113 (6.5) | 44 (6.7) | ||
| North America | 288 (13.6) | 15 (5.6) | 169 (9.8) | 134 (20.4) | ||
| Western Europe | 570 (26.9) | 73 (27.0) | 463 (26.7) | 180 (27.4) | ||
| HbA1c, %, median (Q1–Q3) | 7.0 (6.5–7.9) | 6.9 (6.5–7.7) | 0.26 | 6.7 (6.3–7.4) | 7.9 (7.1–9.2) | <0.001 |
| Non‐ischaemic aetiology, n (%) | 1272 (60.1) | 135 (50.0) | 0.002 | 1044 (60.3) | 363 (55.3) | 0.03 |
| Prior HF hospitalization, n (%) | 1112 (52.5) | 136 (50.4) | 0.50 | 860 (49.7) | 388 (59.1) | <0.001 |
| HF duration, n (%) | 0.06 | 0.12 | ||||
| 0–3 months | 315 (14.9) | 47 (17.4) | 270 (15.6) | 92 (14.1) | ||
| 3–6 months | 222 (10.5) | 36 (13.3) | 182 (10.5) | 76 (11.6) | ||
| 6–12 months | 254 (12.0) | 44 (16.3) | 230 (13.3) | 68 (10.4) | ||
| 1–2 years | 311 (14.7) | 29 (10.7) | 256 (14.8) | 84 (12.8) | ||
| 2–5 years | 475 (22.5) | 54 (20.0) | 374 (21.7) | 155 (23.7) | ||
| >5 years | 534 (25.3) | 60 (22.2) | 415 (24.0) | 179 (27.4) | ||
| NYHA class, n (%) | 0.17 | 0.048 | ||||
| I | 68 (3.2) | 11 (4.1) | 58 (3.4) | 21 (3.2) | ||
| II | 1583 (74.8) | 213 (78.9) | 1324 (76.5) | 472 (72.0) | ||
| III | 458 (21.6) | 44 (16.3) | 344 (19.9) | 158 (24.1) | ||
| IV | 8 (0.4) | 2 (0.7) | 5 (0.3) | 5 (0.8) | ||
| LVEF, %, mean (± SD) | 57.2 ± 7.9 | 57.1 ± 7.9 | 0.77 | 57.1 ± 7.9 | 57.6 ± 7.9 | 0.20 |
| Heart rate, bpm, mean (± SD) | 71.2 ± 12.1 | 72.7 ± 13.5 | 0.067 | 71.5 ± 12.3 | 71.1 ± 12.3 | 0.50 |
| SBP, mmHg, mean (± SD) | 131.8 ± 15.4 | 130.2 ± 15.6 | 0.11 | 131.1 ± 15.1 | 133.2 ± 16.3 | 0.002 |
| BMI, kg/m2, median (Q1–Q3) | 31.8 (28.6–35.3) | 23.4 (21.9–24.3) | <0.001 | 30.4 (27.3–34.2) | 32.4 (28.6–35.9) | <0.001 |
| Waist circumference, cm, median (Q1–Q3) | 109 (100–118) | 90 (84–96) | <0.001 | 105 (96–115) | 110 (100–119) | <0.001 |
| Waist‐hip ratio, mean (± SD) | 0.99 ± 0.12 | 0.93 ± 0.08 | <0.001 | 0.97 ± 0.11 | 0.99 ± 0.14 | 0.011 |
| Current smoker, n (%) | 142 (6.7) | 25 (9.4) | 0.11 | 122 (7.1) | 45 (6.9) | 0.85 |
| eGFR, ml/min/1.73 m2, mean (± SD) | 62.0 ± 19.8 | 64.0 ± 20.2 | 0.11 | 63.2 ± 19.5 | 59.6 ± 20.5 | <0.001 |
| Serum creatinine, mg/dl, mean (± SD) | 1.12 ± 0.33 | 1.07 ± 0.31 | 0.013 | 1.09 ± 0.32 | 1.18 ± 0.36 | <0.001 |
| Potassium, mmol/l, median (Q1–Q3) | 4.5 (4.2–4.8) | 4.5 (4.2–4.8) | 0.57 | 4.5 (4.2–4.8) | 4.5 (4.2–4.9) | 0.22 |
| NT‐proBNP, pg/ml, median (Q1–Q3) without AF | 572 (375–992) | 879 (483–1584) | <0.001 | 587 (382–1091) | 620 (379–1059) | 0.49 |
| NT‐proBNP, pg/ml, median (Q1–Q3) with AF | 1557 (1159–2168) | 1793 [1370–2465] | 0.009 | 1576 (1192–2204) | 1595 (1136–2178) | 0.62 |
| KCCQ‐CSS, mean (± SD) | 70.5 ± 19.2 | 76.5 ± 18.5 | <0.001 | 71.9 ± 18.8 | 69.2 ± 20.1 | 0.002 |
| Signs or symptoms, n (%) | ||||||
| Paroxysmal nocturnal dyspnoea | 99 (4.7) | 8 (3.0) | 0.20 | 74 (4.3) | 33 (5.0) | 0.42 |
| Dyspnoea at rest | 70 (3.3) | 6 (2.2) | 0.34 | 56 (3.2) | 20 (3.1) | 0.82 |
| Dyspnoea on effort | 1958 (92.6) | 241 (89.3) | 0.05 | 1598 (92.4) | 601 (91.8) | 0.62 |
| Fatigue | 1088 (51.4) | 138 (51.1) | 0.92 | 901 (52.1) | 325 (49.6) | 0.28 |
| Orthopnoea | 453 (21.4) | 38 (14.1) | 0.005 | 314 (18.2) | 177 (27.0) | <0.001 |
| Third heart sound | 55 (2.6) | 6 (2.2) | 0.75 | 51 (3.0) | 10 (1.5) | 0.048 |
| Jugular venous distention | 306 (14.6) | 29 (10.8) | 0.095 | 229 (13.3) | 106 (16.4) | 0.054 |
| Oedema | 889 (42.0) | 80 (29.6) | <0.001 | 672 (38.8) | 297 (45.3) | 0.004 |
| Rales | 157 (7.4) | 16 (5.9) | 0.37 | 127 (7.3) | 46 (7.0) | 0.79 |
| Medical history, n (%) | ||||||
| Myocardial infarction | 556 (26.3) | 81 (30.0) | 0.19 | 429 (24.8) | 208 (31.7) | <0.001 |
| Atrial flutter/fibrillation a | 690 (32.7) | 78 (28.9) | 0.20 | 615 (35.6) | 153 (23.5) | <0.001 |
| Hypertension | 2063 (97.4) | 246 (91.1) | <0.001 | 1671 (96.5) | 638 (97.3) | 0.34 |
| Stroke | 240 (11.3) | 32 (11.9) | 0.79 | 183 (10.6) | 89 (13.6) | 0.04 |
| COPD | 315 (14.9) | 35 (13.0) | 0.40 | 246 (14.2) | 104 (15.9) | 0.30 |
| Anaemia | 381 (18.0) | 46 (17.0) | 0.70 | 265 (15.3) | 162 (24.7) | <0.001 |
| Lower limb artery stenosis | 56 (2.7) | 16 (5.9) | 0.003 | 44 (2.5) | 28 (4.3) | 0.03 |
| Sleep apnoea | 215 (10.2) | 6 (2.2) | <0.001 | 130 (7.5) | 91 (14.0) | <0.001 |
| Medications, n (%) | ||||||
| Diuretics | 2044 (96.5) | 246 (91.1) | <0.001 | 1650 (95.3) | 640 (97.6) | 0.01 |
| ACE‐inhibitor/ARB | 1842 (87.0) | 227 (84.1) | 0.18 | 1506 (87.0) | 563 (85.8) | 0.47 |
| Beta‐blocker | 1757 (83.0) | 204 (75.6) | 0.003 | 1418 (81.9) | 543 (82.8) | 0.61 |
| MRA | 546 (25.8) | 75 (27.8) | 0.48 | 454 (26.2) | 167 (25.5) | 0.71 |
| Antiplatelets | 318 (15.0) | 60 (22.2) | 0.002 | 241 (13.9) | 137 (20.9) | <0.001 |
| Insulin | 591 (27.9) | 65 (24.1) | 0.18 | 0 (0.0) | 656 (100.0) | <0.001 |
| Oral hypoglycaemic agents | 1332 (62.9) | 144 (53.3) | 0.002 | 1096 (63.3) | 380 (57.9) | 0.02 |
| GLP‐1 receptor agonist | 20 (0.9) | 0 (0.0) | 0.11 | 11 (0.6) | 9 (1.4) | 0.08 |
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; GLP‐1, glucagon‐like peptide‐1; HF, heart failure; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire clinical summary score; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SD, standard deviation.
On electrocardiogram at baseline.
Diabetes patients treated with insulin
Amongst participants with diabetes, 656 patients (27%) were treated with insulin, alone or in combination with other glucose‐lowering therapy. Patients treated with insulin were younger, more obese, and a had a higher median HbA1c level, compared to those not treated with insulin (Table 2 ). Insulin‐treated patients had a worse mean KCCQ‐CSS, worse NYHA class distribution, more peripheral oedema and orthopnoea, a higher prevalence of coronary artery disease, anaemia and sleep apnoea, but less atrial fibrillation. Despite this overall worse HF profile, more evidence of congestion and lower eGFR, insulin‐treated patients had similar plasma concentrations of NT‐proBNP compared to those not treated with insulin.
Clinical outcomes according to glycated haemoglobin categories and diabetes status
The risks of the primary composite outcome, its components and all‐cause mortality were highest among those with diabetes, compared to patients with pre‐diabetes and those with normal HbA1c (Table 3 ; Figures 1 and 2 ; online supplementary Figure S2 ). Patients with pre‐diabetes had a higher risk of the primary composite outcome and HF hospitalization, but similar risk of cardiovascular death and all‐cause mortality, compared to patients with normal HbA1c.
Table 3.
Event rates and hazard/rate ratios for all outcomes, according to diabetes status
| No. of events | Crude rate per 100 py | Adjusted HR/RR (95% CI) | p‐value | |
|---|---|---|---|---|
| Primary composite outcome | ||||
| Normal HbA1c | 427 | 9.4 (8.6–10.4) | 1.00 (ref.) | |
| Pre‐diabetes | 295 | 11.8 (10.5–13.2) | 1.27 (1.00–1.60) | 0.05 |
| Diabetes | 1181 | 17.3 (16.3–18.3) | 1.59 (1.35–1.88) | <0.001 |
| Total hospitalizations for HF | ||||
| Normal HbA1c | 320 | 7.1 (6.3–7.9) | 1.00 (ref.) | |
| Pre‐diabetes | 230 | 9.2 (8.1–10.5) | 1.35 (1.03–1.77) | 0.03 |
| Diabetes | 937 | 13.7 (12.9–14.6) | 1.67 (1.39–2.02) | <0.001 |
| CV death | ||||
| Normal HbA1c | 107 | 2.4 (2.0–2.9) | 1.00 (ref.) | |
| Pre‐diabetes | 65 | 2.6 (2.0–3.3) | 1.02 (0.75–1.40) | 0.88 |
| Diabetes | 244 | 3.6 (3.1–4.0) | 1.35 (1.07–1.71) | 0.01 |
| First hospitalization for HF or CV death | ||||
| Normal HbA1c | 278 | 6.6 (5.8–7.4) | 1.00 (ref.) | |
| Pre‐diabetes | 178 | 7.8 (6.7–9.0) | 1.17 (0.96–1.42) | 0.11 |
| Diabetes | 627 | 10.3 (9.5–11.1) | 1.38 (1.19–1.60) | <0.001 |
| First hospitalization for HF | ||||
| Normal HbA1c | 209 | 4.9 (4.3–5.6) | 1.00 (ref.) | |
| Pre‐diabetes | 138 | 6.0 (5.1–7.1) | 1.25 (1.00–1.55) | 0.047 |
| Diabetes | 491 | 8.0 (7.4–8.8) | 1.44 (1.22–1.70) | <0.001 |
| Death from any cause | ||||
| Normal HbA1c | 180 | 4.0 (3.4–4.6) | 1.00 (ref.) | |
| Pre‐diabetes | 106 | 4.2 (3.5–5.1) | 0.98 (0.77–1.25) | 0.87 |
| Diabetes | 405 | 5.9 (5.4–6.5) | 1.40 (1.17–1.68) | <0.001 |
| Renal composite outcome | ||||
| Normal HbA1c | 20 | 0.4 (0.3–0.7) | 1.00 (ref.) | |
| Pre‐diabetes | 10 | 0.4 (0.2–0.7) | 1.01 (0.47–2.17) | 0.97 |
| Diabetes | 67 | 1.0 (0.8–1.3) | 2.28 (1.37–3.79) | 0.002 |
CI, confidence interval; CV, cardiovascular; HbA1c, glycated haemoglobin; HF, heart failure; HR, hazard ratio; py, person‐years; RR, rate ratio.
Adjusted for age, sex, race, New York Heart Association class, smoking status (current), prior HF hospitalization, HF duration, left ventricular ejection fraction, N‐terminal pro‐B‐type natriuretic peptide (log), systolic blood pressure, heart rate, serum creatinine level, history of myocardial infarction, stroke, chronic obstructive pulmonary disease, atrial fibrillation, hypertension, and randomly assigned treatment.
Renal composite outcome = Estimated glomerular filtration rate decrease of 50% or more, end‐stage kidney disease, or death due to kidney failure.
Figure 1.
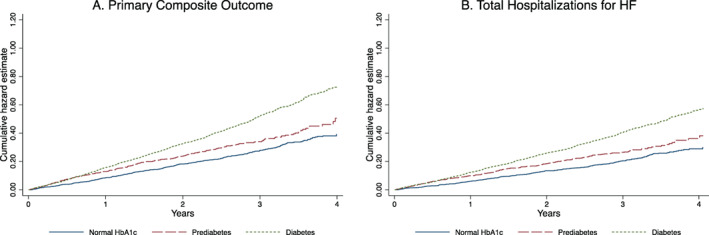
(A) Primary composite outcome of cardiovascular death and total heart failure (HF) hospitalizations (both first and recurrent) and (B) total HF hospitalizations (both first and recurrent), according to diabetes status. HbA1c, glycated haemoglobin.
Figure 2.
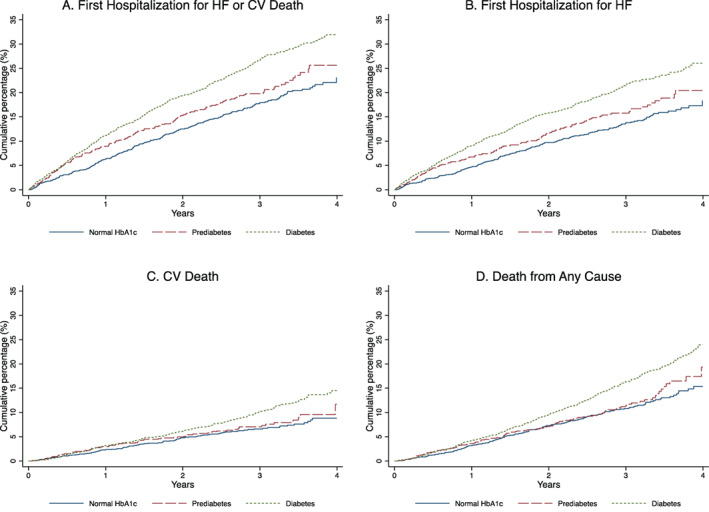
Composite outcome of cardiovascular (CV) death or first heart failure (HF) hospitalization (A), the components of the composite (B, C) and death from any cause (all analysed as time‐to‐first event) (D), according to diabetes status. HbA1c, glycated haemoglobin.
At 8‐month follow‐up, there was no difference in worsening of NYHA class or change in KCCQ‐CSS amongst groups (online supplementary Table S1 ).
The risk of the composite renal outcome was also greater amongst patients with diabetes compared to other groups (Table 2 ).
Clinical outcomes in patients with ‘lean diabetes’
Patients with a BMI <25 kg/m2 had slightly, but not significantly, higher risk of cardiovascular and all‐cause mortality than those with BMI ≥25 kg/m2 (Table 4 ). The rate of first HF hospitalization was similar for patients with BMI <25 or ≥25 kg/m2 but patients with BMI <25 kg/m2 had a lower rate of total HF hospitalizations, compared with patients with a BMI ≥25 kg/m2 (Table 4 and online supplementary Figures S3 and S4 ).
Table 4.
Event rates and hazard/rate ratios for all outcomes according to ‘lean diabetes’ phenotype (versus non‐lean) and insulin use (versus no insulin use) in patients with diabetes
| No. of events | Crude rate per 100 py | Adjusted HR/RR (95% CI) | p‐value | |
|---|---|---|---|---|
| Primary composite outcome | ||||
| BMI ≥25 kg/m2 | 1063 | 17.4 (16.4–18.5) | 1.00 (ref.) | |
| BMI <25 kg/m2 | 118 | 16.3 (13.6–19.5) | 0.86 (0.65–1.14) | 0.29 |
| No insulin | 731 | 14.7 (13.7–15.8) | 1.00 (ref.) | |
| Insulin | 450 | 24.3 (22.1–26.6) | 1.30 (1.06–1.60) | 0.01 |
| Total hospitalizations for HF | ||||
| BMI ≥25 kg/m2 | 858 | 14.1 (13.2–15.0) | 1.00 (ref.) | |
| BMI <25 kg/m2 | 79 | 10.9 (8.7–13.6) | 0.75 (0.55–1.03) | 0.08 |
| No insulin | 564 | 11.3 (10.4–12.3) | 1.00 (ref.) | |
| Insulin | 373 | 20.1 (18.2–22.3) | 1.32 (1.05–1.67) | 0.02 |
| CV death | ||||
| BMI ≥25 kg/m2 | 205 | 3.6 (2.9–3.8) | 1.00 (ref.) | |
| BMI <25 kg/m2 | 39 | 5.4 (3.9–7.4) | 1.25 (0.85–1.84) | 0.25 |
| No insulin | 167 | 3.4 (2.9–3.9) | 1.00 (ref.) | |
| Insulin | 77 | 4.2 (3.3–5.2) | 1.20 (0.90–1.61) | 0.22 |
| First hospitalization for HF or CV death | ||||
| BMI ≥25 kg/m2 | 552 | 10.1 (9.3–11.0) | 1.00 (ref.) | |
| BMI <25 kg/m2 | 75 | 11.3 (9.0–14.2) | 0.98 (0.75–1.27) | 0.87 |
| No insulin | 411 | 9.1 (8.2–10.0) | 1.00 (ref.) | |
| Insulin | 216 | 13.7 (12.0–15.7) | 1.31 (1.10–1.57) | 0.003 |
| First hospitalization for HF | ||||
| BMI ≥25 kg/m2 | 437 | 8.0 (7.3–8.8) | 1.00 (ref.) | |
| BMI <25 kg/m2 | 54 | 8.1 (6.2–10.6) | 0.94 (0.69–1.27) | 0.68 |
| No insulin | 321 | 7.1 (6.4–7.9) | 1.00 (ref.) | |
| Insulin | 170 | 10.8 (9.3–12.6) | 1.27 (1.04–1.56) | 0.02 |
| Death from any cause | ||||
| BMI ≥25 kg/m2 | 349 | 5.7 (5.1–6.3) | 1.00 (ref.) | |
| BMI <25 kg/m2 | 56 | 7.7 (5.9–10.0) | 1.08 (0.79–1.47) | 0.62 |
| No insulin | 274 | 5.5 (4.9–6.2) | 1.00 (ref.) | |
| Insulin | 131 | 7.1 (6.0–8.4) | 1.40 (1.12–1.75) | 0.003 |
| Renal composite outcome | ||||
| BMI ≥25 kg/m2 | 59 | 1.0 (0.8–1.3) | 1.00 (ref.) | |
| BMI <25 kg/m2 | 8 | 1.1 (0.6–2.2) | 0.84 (0.37–1.90) | 0.67 |
| No insulin | 42 | 0.9 (0.6–1.2) | 1.00 (ref.) | |
| Insulin | 25 | 1.4 (0.9–2.0) | 1.30 (0.76–2.22) | 0.34 |
BMI, body mass index; CI, confidence interval; CV, cardiovascular; HF, heart failure; HR, hazard ratio; py, person‐years; RR, rate ratio.
Adjusted for age, sex, race, New York Heart Association class, smoking status (current), prior HF hospitalization, HF duration, left ventricular ejection fraction, N‐terminal pro‐B‐type natriuretic peptide (log), systolic blood pressure, heart rate, serum creatinine level, history of myocardial infarction, stroke, chronic obstructive pulmonary disease, atrial fibrillation, hypertension and randomly assigned treatment.
Renal composite outcome = Estimated glomerular filtration rate decrease of 50% or more, end‐stage kidney disease, or death due to kidney failure.
Clinical outcomes in patients with diabetes according to insulin treatment
Patients treated with insulin had a higher risk of the primary composite outcome, its components and all‐cause mortality (Table 4 and online supplementary Figures S5 and S6 ).
Effect of sacubitril‐valsartan according to diabetes status and glycated haemoglobin level
For the primary composite outcome, we found no significant interaction between the effects of treatment (sacubitril‐valsartan compared to valsartan), and glycaemic subgroup (p interaction = 0.96) (online supplementary Table S2 ) or using HbA1c as a continuous variable (p interaction = 0.32) (Figure 3 ). The same was true for the effect of treatment on the components of the primary outcome across the glycaemic subgroups (online supplementary Table S2 ).
Figure 3.
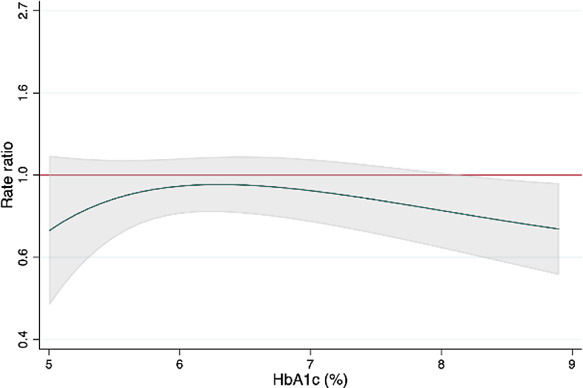
Effect of sacubitril‐valsartan, compared with valsartan, on the primary composite outcome (cardiovascular death and total heart failure hospitalizations) across the range of glycated haemoglobin (HbA1c) 5% to 9%. The green line is the rate ratio; the red line is the line of unity; the grey shaded area represents the 95% confidence interval.
Discussion
In this post‐hoc analysis of the PARAGON‐HF trial, we found that diabetes and pre‐diabetes together affect around two thirds of patients with HFpEF, highlighting the highly dysglycaemic character of this HF phenotype, globally. Patients with each of pre‐diabetes and diabetes had higher rates of HF hospitalization than patients with normal HbA1c, with the risk of patients with pre‐diabetes intermediate between that of patients with diabetes and those with normal HbA1c. The risk of all‐cause mortality and cardiovascular death were significantly higher in patients with diabetes but similar in patients with pre‐diabetes and those with normal HbA1c. Glycaemic status had no significant influence on the effect of sacubitril‐valsartan on outcomes.
In patients with HF and diabetes, with a HFpEF phenotype, there is the development of myocardial hypertrophy, collagen deposition and fibrosis. Such pathological changes are driven by several factors, including hyperglycaemia, hyperinsulinaemia, lipotoxicity and altered cardiac metabolism. 17 These changes can be seen in patients not only with diabetes, but also in those with insulin resistance, dyslipidaemia or obesity, either independently, or when there is an overlap of conditions. While it is well known that diabetes is associated with worse outcomes in patients with HFpEF, especially if treated with insulin, much less is known about the prevalence of pre‐diabetes and risk associated with pre‐diabetes. 18 Indeed, the few prior analyses of pre‐diabetes in HF either only described patients with HFrEF or did not provide outcome data by LVEF phenotype. 19 , 20 , 21
In PARAGON‐HF, a high proportion (36%) of participants without diabetes had pre‐diabetes. Many of the phenotypic characteristics of these patients were intermediate between those of participants with normal HbA1c and those with diabetes. This is consistent with the view that pre‐diabetes represents an earlier stage of a dysglycaemic continuum. However, there are already metabolic cardiac consequences at this stage. For example, myocardial glucose uptake is depressed in HF patients with pre‐diabetes, compared to HF patients with a normal oral glucose tolerance test. 22 It was striking that patient‐reported wellbeing, as reflected in the KCCQ‐CSS, was as impaired in patients with pre‐diabetes as in those with diabetes in PARAGON‐HF. There was also an elevated incidence of both first and repeat HF hospitalizations in patients with pre‐diabetes (compared to those with normal HbA1c), which persisted despite adjustment for other predictive variables, including NT‐proBNP. However, the excess risk of hospitalization in pre‐diabetes was not as great as seen in patients with diabetes (compared to those with normal HbA1c). By contrast, mortality did not appear to be elevated in people with pre‐diabetes (compared to those with normal HbA1c), whereas mortality was clearly higher in participants with diabetes. It is possible that the association between elevated glucose and mortality may be related to duration, as well as degree, of hyperglycaemia. However, diagnosis of diabetes also leads to commencement of glucose‐lowering therapy and the safety of many types of antihyperglycaemic treatment, including insulin, is unknown in patients with HF. 23 The risk of the worsening renal function endpoint was elevated only in patients with diabetes, and not in pre‐diabetes. Although here there is some uncertainty about this difference, because of the very small number of events, it too may reflect the duration of hyperglycaemia.
We also report novel findings in relation to established diabetes. Its prevalence varied considerably across geographic regions, being most common in North America and Asia. The high prevalence of diabetes in Asia (particularly East Asia) is of interest given the lower average BMI in individuals from this region, with a ‘lean diabetes’ phenotype being common there. 6 , 24 Greater deposition of adipose tissue in the visceral space and more beta‐cell dysfunction are possible explanations for this phenomenon. 25 Others have noted that Asian patients with ‘lean diabetes’ were more likely to have HFpEF than HFrEF. 24 Although some have reported patients with ‘lean diabetes’ to be at higher risk of death than patients with diabetes with a higher BMI, we did not confirm this. Moreover, we observed that there were fewer HF hospitalizations in patients with ‘lean diabetes’, compared to diabetes patients with a BMI ≥25 kg/m2. By contrast, we were able to confirm the well‐known association between insulin and poor outcomes in HF, even after comprehensive adjustment for other prognostic variables. 12 , 13 , 26 Indeed, patients with diabetes treated with insulin were 1.3‐times more likely to experience the primary composite outcome in the trial.
We made other new observations in relation to diabetes; in addition to the expected comorbidities (e.g. a greater prevalence of obesity, hypertension, coronary heart disease), anaemia and sleep apnoea were also more common than in participants with normal HbA1c. Both comorbidities were even more common among those taking insulin, and each is clearly relevant to the symptom profile of patients with HFpEF. 27 Although sleep apnoea is likely to be associated with obesity, the higher prevalence of anaemia is harder to explain, especially as mean eGFR was similar across patient subgroups. However, an increased risk of anaemia has been described in patients with type 2 diabetes without chronic kidney disease previously and may be related to iron deficiency, among other factors. 28 A further explanation might be that plasma volume is greater in patients with diabetes, who had more signs of congestion and worse symptoms of HF than in patients without. 29
The documentation of symptoms and signs at baseline, along with an accompanying natriuretic peptide measurement in PARAGON‐HF, suggests a discrepancy between greater congestion in patients with diabetes, compared to those without, despite similar NT‐proBNP levels. This raises the possibility of a relative natriuretic peptide deficiency in patients with diabetes, which contributes to their greater sodium and water overload. If correct, the deficiency might be related to obesity. 30 , 31 , 32 It is therefore of interest that patients with ‘lean diabetes’ had higher concentrations of NT‐proBNP and less evidence of congestion, compared to non‐lean diabetics. Another possibility is that the worse symptoms in patients with diabetes (and in the group treated with insulin versus those not) might be related to a greater degree of obesity or larger waist circumference. Unfavourable haemodynamic findings, including increased left ventricular filling pressure, have been described in women with HFpEF with greater visceral adiposity. 33
Another important finding in the present study was the relatively high proportion of patients with HFpEF with undiagnosed diabetes (7% of all participants, 12% of participants without an existing diagnosis of diabetes), raising the question of awareness among cardiologists about the possibility of comorbid diabetes in their patients and, perhaps, the difficulty in diagnosing diabetes in individuals who may already be fatigued and experience urinary frequency and thirst because of diuretic therapy.
This leaves individuals with normal HbA1c, who represented just under one third of the overall trial cohort. These patients are at high risk developing dysglycaemia and diabetes. Therefore, prevention, as well as treatment, of diabetes in these patients may be a therapeutic goal, along with reduction in weight. Although there have been important developments in some of these therapeutic areas, including a benefit of SGLT2 inhibitors in HFpEF, detailed discussion is beyond the scope of this paper. 34 , 35 , 36
Finally, the effect of sacubitril‐valsartan on the primary outcome in PARAGON‐HF, which was of borderline statistical significance, did not differ according to baseline HbA1c level.
Limitations
As with any study of this type there are limitations. This was not a pre‐planned analysis. The protocol had specific inclusion and exclusion criteria and patients self‐selected by agreeing to participate. Consequently, they only partially reflect the entire spectrum of patients with HFpEF, although they do share many of the typical characteristics described in epidemiologic cohorts and registries. Of relevance to this study is the BMI threshold used for exclusion of patients in PARAGON‐HF (BMI >40 kg/m2), which may impact the prevalence of diabetes and pre‐diabetes in this particular cohort. The definitions of pre‐diabetes and undiagnosed diabetes were based on single HbA1c measurements and not on fasting plasma glucose measurements or a 2‐h plasma glucose value during a 75‐g oral glucose tolerance test; the latter approaches are known to give a different prevalence of pre‐diabetes. Duration of diabetes and data on diabetic complications, such as retinopathy and neuropathy, were not available. We did not report longitudinal changes in HbA1c. The PARAGON‐HF trial enrolled patients before the use of SGLT2 inhibitors had become common.
Conclusion
In summary, diabetes and pre‐diabetes together affect around two thirds of HFpEF patients, highlighting the highly dysglycaemic character of this HF phenotype, globally. Diabetes, and to a lesser extent pre‐diabetes, was associated with worse clinical status and higher risk of adverse cardiovascular outcomes. Although a focus on developing safe and effective glucose‐lowering therapies for patients with HF is clearly important, attempts to prevent the development of, and even to reverse, diabetes (and, perhaps, even pre‐diabetes) should not be neglected.
Funding
PARAGON‐HF was funded by Novartis Pharmaceuticals. J.J.V.McM is supported by British Heart Foundation Centre of Research Excellence Grant RE/18/6/34217. A.M.J. is supported by a British Heart Foundation Clinical Research Training Fellowship (FS/18/14/33330).
Conflict of interest: I.S.A. has received consulting fees from Amgen, ARCA Biopharma, AstraZeneca, Boston Scientific Corporation, Boehringer Ingelheim, LivaNova, Novartis, and Zensun. B.L.C. has been a consultant for Amgen, AO Biome, Biogen, Boehringer Ingelheim, Corvia, Gilead, Myokardia, and Novartis. J.G.F.C. has received research grants from Bayer, Bristol‐Myers Squibb, Pharmacosmos and Vifor and has received honoraria for advisory boards, lectures and or trial committees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, HeartFelt Technologies, Idorsia, Johnson & Johnson, Medtronic, Myokardia, Novartis, Pharmacosmos, Respicardia, Servier, Torrent, Vifor and Viscardia. A.S.D. reported receiving personal fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, Merck, Regeneron, and Relypsa and grants and personal fees from AstraZeneca, Alnylam, and Novartis, outside the submitted work. C.S.P.L. has received grant support and fees for serving on an advisory board from Boston Scientific and Roche Diagnostics; grant support, fees for serving on an advisory board, and fees for serving on steering committees from Bayer; grant support from Medtronic; grant support and fees for serving on a steering committee from Vifor pharma; fees for serving on an advisory board and for serving on steering committees from AstraZeneca and Novartis; fees for serving on an advisory board from Amgen, Boehringer Ingelheim, and Abbott Diagnostics; consulting fees from Merck and Stealth BioTherapeutics; fees for serving on a steering committee from Janssen Research and Development; lecture fees and consulting fees from Menarini; and fees for serving on a scientific committee from Corvia Medical. J.G., M.P.L. and A.R.R. are employees of Novartis and A.R.R. owns Novartis stock. A.P.M. reports receiving fees for serving on a study committee from Bayer and Fresenius. F.M. has received personal fees from Novartis. M.P. has received consulting fees from AbbVie, Akcea, Actavis, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Gilead, Johnson & Johnson, Novo Nordisk, Pfizer, Relypsa, Sanofi, Synthetic Biologics, and Theravance. M.A.P. has received grants paid to his institution for serving on the Steering Committee of PARAGON‐HF, and for serving as Study Chair of Prospective ARNI vs ACE Inhibitor Trial to DetermIne Superiority in Reducing Heart Failure Events After MI (PARADISE‐MI) from Novartis and personal fees for consulting from AstraZeneca, CinCor, Corvidia, DalCor, GlaxoSmithKline, Novartis, NovoNordisk, Pharmascience and Sanofi, and also owns Stock Options of DalCor. B.P. reports receiving fees for serving on a steering committee, fees for serving on an advisory board, and lecture fees from Bayer HealthCare Pharmaceuticals and MSD; lecture fees from AstraZeneca; fees for serving on an advisory board and lecture fees from Bristol‐Myers Squibb; fees for serving on an advisory board from Daiichi Sankyo; and lecture fees and honoraria from Medscape. M.M.R. reports being an unpaid consultant for Novartis. J.L.R. has received grants and consulting fees from Novartis and consulting fees from Abbott, AstraZeneca, MyoKardia, and Sanofi. P.M.S. has received honoraria for lectures from Medtronic, Abbott, Servier, AstraZeneca, Respicardia; consultancy agreement and honoraria for lecturer from Boehringer Ingelheim, Novartis; and consultancy agreement from Vifor Pharma. J.T. has received personal fees from Roche Diagnostics, Olink Proteomics, and Us2.ai, outside the submitted work, and has a patent 16/216929 licensed. D.J.vV. reports receiving fees for serving on a steering committee and travel support from ARCA biopharma and Corvia Medical. M.B.Y. reports grants from Amgen, Inc., Novartis, Bayer and Dalcor Pharmaceuticals. F.Z. has received personal fees from Novartis, Janssen, Bayer, Boston Scientific, Amgen, CVRx, Boehringer, Cardiorenal, AstraZeneca, Vifor Fresenius, Cardior, Cereno Pharmaceutical, Applied Therapeutics, Merck and CardioVascular Clinical Trialists (CVCT). M.R.Z. has received research funding from Novartis and has been a consultant for Novartis, Abbott, Boston Scientific, CVRx, EBR, Endotronics, Ironwood, Merck, Medtronic, and Myokardia V Wave. L.K. has received honoraria from Novartis, Boehringer, Novo, and AstraZeneca. M.C.P. has received lecture fees from AstraZeneca and Eli Lilly, personal fees from NovoNordisk, AstraZeneca, NAPP Pharmaceuticals, Takeda Pharmaceutical, Alnylam, Bayer, Resverlogix, and Cardiorentis and grants and personal fees from Boehringer Ingelheim and Novartis. P.S.J. has received consulting fees, advisory board fees, and lecture fees from Novartis; advisory board fees from Cytokinetics; and grant support from Boehringer Ingelheim. S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and has consulted for Abbott, Action Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi‐Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent. J.J.V.McM. has received grants and his employer paid by AstraZeneca, Theracos, and GlaxoSmithKline during the conduct of the study and grants and his employer being paid by Novartis, Amgen, Bristol‐Myers Squibb, Bayer, Abbvie, Dal‐Cor, Kidney Research UK, and Cardurion and grants from British Heart Foundation. All other authors have nothing to disclose.
Supporting information
Table S1. Change in NYHA class and KCCQ clinical summary score from baseline to 8‐month follow‐up, according to diabetes status.
Table S2. Treatment effects of sacubitril‐valsartan, compared with valsartan, according to diabetes status.
|Figure S1. Histogram showing the distribution of glycated haemoglobin.
Figure S2. Primary composite outcome of cardiovascular death and total heart failure hospitalizations (both first and recurrent) across the range of glycated haemoglobin 5% to 9%.
Figure S3. Primary composite outcome of cardiovascular death and total heart failure hospitalizations (both first and recurrent) and total heart failure hospitalizations (both first and recurrent) in patients with diabetes, according to whether they had a ‘lean diabetes’ phenotype or not.
Figure S4. Composite outcome of cardiovascular death and heart failure hospitalization, the components of the composite and death from any cause (all analysed as time‐to‐first event) in patients with diabetes, according to whether they had a ‘lean diabetes’ phenotype or not.
Figure S5. Primary composite outcome of cardiovascular death and total heart failure hospitalizations (both first and recurrent) and total heart failure hospitalizations (both first and recurrent) in patients with diabetes, according to whether they were treated with insulin or not.
Figure S6. Composite outcome of cardiovascular death and heart failure hospitalization, the components of the composite and death from any cause (all analysed as time‐to‐first event) in patients with diabetes, according to whether they were treated with insulin or not.
References
- 1. Khan SS, Butler J, Gheorghiade M. Management of comorbid diabetes mellitus and worsening heart failure. JAMA. 2014;311:2379–80. [DOI] [PubMed] [Google Scholar]
- 2. Haffner S, Taegtmeyer H. Epidemic obesity and the metabolic syndrome. Circulation. 2003;108:1541–5. [DOI] [PubMed] [Google Scholar]
- 3. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. [DOI] [PubMed] [Google Scholar]
- 4. MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al.; CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–85. [DOI] [PubMed] [Google Scholar]
- 5. Huxley RR, Barzi F, Woo J, Giles G, Lam TH, Rahimi K, et al.; Asia Pacific Cohort Studies Collaboration. A comparison of risk factors for mortality from heart failure in Asian and non‐Asian populations: an overview of individual participant data from 32 prospective cohorts from the Asia‐Pacific region. BMC Cardiovasc Disord. 2014;14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chandramouli C, Teng TK, Tay WT, Yap J, MacDonald MR, Tromp J, et al.; ASIAN‐HF Investigators. Impact of diabetes and sex in heart failure with reduced ejection fraction patients from the ASIAN‐HF registry. Eur J Heart Fail. 2019;21:297–307. [DOI] [PubMed] [Google Scholar]
- 7. Arnold SV, Yap J, Lam CSP, Tang F, Tay WT, Teng THK, et al. Management of patients with diabetes and heart failure with reduced ejection fraction: an international comparison. Diabetes Obes Metab. 2019;21:261–6. [DOI] [PubMed] [Google Scholar]
- 8. Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, et al.; PARADIGM‐HF Investigators and Committees. Risk related to pre‐diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial. Circ Heart Fail. 2016;9:e002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solomon SD, Rizkala AR, Gong J, Wang W, Anand IS, Ge J, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON‐HF trial. JACC Heart Fail. 2017;5:471–82. [DOI] [PubMed] [Google Scholar]
- 10. Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, et al. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON‐HF trial. Circ Heart Fail. 2018;11:e004962. [DOI] [PubMed] [Google Scholar]
- 11. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al.; PARAGON‐HF Investigators and Committees. Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–20. [DOI] [PubMed] [Google Scholar]
- 12. Shen L, Rørth R, Cosmi D, Kristensen SL, Petrie MC, Cosmi F, et al. Insulin treatment and clinical outcomes in patients with diabetes and heart failure with preserved ejection fraction. Eur J Heart Fail. 2019;21:974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cosmi F, Shen L, Magnoli M, Abraham WT, Anand IS, Cleland JG, et al. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail. 2018;20:888–95. [DOI] [PubMed] [Google Scholar]
- 14. George AM, Jacob AG, Fogelfeld L. Lean diabetes mellitus: an emerging entity in the era of obesity. World J Diabetes. 2015;6:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. International Diabetes Federation . IDF diabetes atlas, 9th ed. Brussels: International Diabetes Federation; 2019. Available at: . https://www.diabetesatlas.org. [Google Scholar]
- 16. Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Stat Soc Series B Stat Methodol. 2000;62:711–30. [Google Scholar]
- 17. Seferović PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two‐faced disease with restrictive and dilated phenotypes. Eur Heart J. 2015;36:1718–27. [DOI] [PubMed] [Google Scholar]
- 18. Kristensen SL, Jhund PS, Lee MMY, Køber L, Solomon SD, Granger CB, et al.; CHARM Investigators and Committees. Prevalence of prediabetes and undiagnosed diabetes in patients with HFpEF and HFrEF and associated clinical outcomes. Cardiovasc Drugs Ther. 2017;31:545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dauriz M, Targher G, Temporelli PL, Lucci D, Gonzini L, Nicolosi GL, et al.; GISSI‐HF Investigators. Prognostic impact of diabetes and prediabetes on survival outcomes in patients with chronic heart failure: a post‐hoc analysis of the GISSI‐HF (Gruppo Italiano per lo studio della Sopravvivenza nella Insufficienza Cardiaca‐Heart Failure) trial. J Am Heart Assoc. 2017;6:e005156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mai L, Wen W, Qiu M, Liu X, Sun L, Zheng H, et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes Metab. 2021;23:2476–83. [DOI] [PubMed] [Google Scholar]
- 21. Matsue Y, Suzuki M, Nakamura R, Abe M, Ono M, Yoshida S, et al. Prevalence and prognostic implications of pre‐diabetic state in patients with heart failure. Circ J. 2011;75:2833–9. [DOI] [PubMed] [Google Scholar]
- 22. Nielsen R, Jorsal A, Iversen P, Tolbod L, Bouchelouche K, Sørensen J, et al. Heart failure patients with prediabetes and newly diagnosed diabetes display abnormalities in myocardial metabolism. J Nucl Cardiol. 2018;25:169–76. [DOI] [PubMed] [Google Scholar]
- 23. Seferović PM, Coats AJS, Ponikowski P, Filippatos G, Huelsmann M, Jhund PS, et al. European Society of Cardiology/Heart Failure Association position paper on the role and safety of new glucose‐lowering drugs in patients with heart failure. Eur J Heart Fail. 2020;22:196–213. [DOI] [PubMed] [Google Scholar]
- 24. Tromp J, Tay WT, Ouwerkerk W, Teng TK, Yap J, MacDonald MR, et al.; ASIAN‐HF Authors. Multimorbidity in patients with heart failure from 11 Asian regions: a prospective cohort study using the ASIAN‐HF registry. PLoS Med. 2018;15:e1002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. [DOI] [PubMed] [Google Scholar]
- 26. Huynh T, Harty BJ, Claggett B, Fleg JL, McKinlay SM, Anand IS, et al. Comparison of outcomes in patients with diabetes mellitus treated with versus without insulin + heart failure with preserved left ventricular ejection fraction (from the TOPCAT study). Am J Cardiol. 2019;123:611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qie R, Zhang D, Liu L, Ren Y, Zhao Y, Liu D, et al. Obstructive sleep apnea and risk of type 2 diabetes mellitus: a systematic review and dose‐response meta‐analysis of cohort studies. J Diabetes. 2020;12:455–64. [DOI] [PubMed] [Google Scholar]
- 28. Sahay M, Kalra S, Badani R, Bantwal G, Bhoraskar A, Das AK, et al. Diabetes and anemia: International Diabetes Federation (IDF) ‐ Southeast Asian Region (SEAR) position statement. Diabetes Metab Syndr. 2017;11Suppl 2:S685–95. [DOI] [PubMed] [Google Scholar]
- 29. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pivovarova O, Gögebakan Ö, Klöting N, Sparwasser A, Weickert MO, Haddad I, et al. Insulin up‐regulates natriuretic peptide clearance receptor expression in the subcutaneous fat depot in obese subjects: a missing link between CVD risk and obesity? J Clin Endocrinol Metab. 2012;97:E731–9. [DOI] [PubMed] [Google Scholar]
- 31. Coué M, Badin PM, Vila IK, Laurens C, Louche K, Marquès MA, et al. Defective natriuretic peptide receptor signaling in skeletal muscle links obesity to type 2 diabetes. Diabetes. 2015;64:4033–45. [DOI] [PubMed] [Google Scholar]
- 32. Kovacova Z, Tharp WG, Liu D, Wei W, Xie H, Collins S, et al. Adipose tissue natriuretic peptide receptor expression is related to insulin sensitivity in obesity and diabetes. Obesity (Silver Spring). 2016;24:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sorimachi H, Obokata M, Takahashi N, Reddy YNV, Jain CC, Verbrugge FH, et al. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J. 2021;42:1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, et al. Sodium‐glucose co‐ transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1495–503. [DOI] [PubMed] [Google Scholar]
- 35. Inzucchi SE, Docherty KF, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, et al.; DAPA‐HF Investigators and Committees. Dapagliflozin and the incidence of type 2 diabetes in patients with heart failure and reduced ejection fraction: an exploratory analysis from DAPA‐HF. Diabetes Care. 2021;44:586–94. [DOI] [PubMed] [Google Scholar]
- 36. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al.; EMPEROR‐Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Change in NYHA class and KCCQ clinical summary score from baseline to 8‐month follow‐up, according to diabetes status.
Table S2. Treatment effects of sacubitril‐valsartan, compared with valsartan, according to diabetes status.
|Figure S1. Histogram showing the distribution of glycated haemoglobin.
Figure S2. Primary composite outcome of cardiovascular death and total heart failure hospitalizations (both first and recurrent) across the range of glycated haemoglobin 5% to 9%.
Figure S3. Primary composite outcome of cardiovascular death and total heart failure hospitalizations (both first and recurrent) and total heart failure hospitalizations (both first and recurrent) in patients with diabetes, according to whether they had a ‘lean diabetes’ phenotype or not.
Figure S4. Composite outcome of cardiovascular death and heart failure hospitalization, the components of the composite and death from any cause (all analysed as time‐to‐first event) in patients with diabetes, according to whether they had a ‘lean diabetes’ phenotype or not.
Figure S5. Primary composite outcome of cardiovascular death and total heart failure hospitalizations (both first and recurrent) and total heart failure hospitalizations (both first and recurrent) in patients with diabetes, according to whether they were treated with insulin or not.
Figure S6. Composite outcome of cardiovascular death and heart failure hospitalization, the components of the composite and death from any cause (all analysed as time‐to‐first event) in patients with diabetes, according to whether they were treated with insulin or not.


