Abstract
Aims
Sudden death is a leading cause of mortality in heart failure with reduced ejection fraction (HFrEF). In PARADIGM‐HF, sacubitril/valsartan reduced the incidence of sudden death. The purpose of this post hoc study was to analyse the effect of sacubitril/valsartan, compared to enalapril, on the incidence of ventricular arrhythmias.
Methods and results
Adverse event reports related to ventricular arrhythmias were examined in PARADIGM‐HF. The effect of randomized treatment on two arrhythmia outcomes was analysed: ventricular arrhythmias and the composite of a ventricular arrhythmia, implantable cardioverter defibrillator (ICD) shock or resuscitated cardiac arrest. The risk of death related to a ventricular arrhythmia was examined in time‐updated models. The interaction between heart failure aetiology, or baseline ICD/cardiac resynchronization therapy‐defibrillator (CRT‐D) use, and the effect of sacubitril/valsartan was analysed. Of the 8399 participants, 333 (4.0%) reported a ventricular arrhythmia and 372 (4.4%) the composite arrhythmia outcome. Ventricular arrhythmias were associated with higher mortality. Compared with enalapril, sacubitril/valsartan reduced the risk of a ventricular arrhythmia (hazard ratio [HR] 0.76, 95% confidence interval [CI] 0.62–0.95; p = 0.015) and the composite arrhythmia outcome (HR 0.79, 95% CI 0.65–0.97; p = 0.025). The treatment effect was maintained after adjustment and accounting for the competing risk of death. Baseline ICD/CRT‐D use did not modify the effect of sacubitril/valsartan, but aetiology did: HR in patients with an ischaemic aetiology 0.93 (95% CI 0.71–1.21) versus 0.53 (95% CI 0.37–0.78) in those without an ischaemic aetiology (p for interaction = 0.020).
Conclusions
Sacubitril/valsartan reduced the incidence of investigator‐reported ventricular arrhythmias in patients with HFrEF. This effect may have been greater in patients with a non‐ischaemic aetiology.
Keywords: Neprilysin inhibitor, Heart failure, Ventricular tachyarrhythmia
PARADIGM‐HF trial design and main findings. HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio.
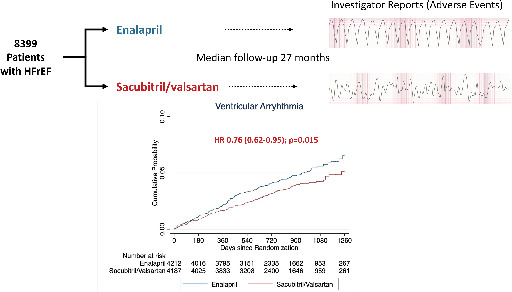
Introduction
In the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure trial (PARADIGM‐HF), 1 sacubitril/valsartan, compared with enalapril, reduced the risk of death and heart failure hospitalization in patients with heart failure and reduced ejection fraction (HFrEF). Further analysis showed a reduction in both death due to worsening heart failure (‘pump failure’) and sudden cardiac death. 2 Importantly, in PARADIGM‐HF, sudden cardiac death was reduced to a similar extent in patients with and without an implanted cardioverter defibrillator (ICD). 3 Although ICDs reduce the risk of sudden death, and rates of sudden death have been declining over time with improving pharmacological therapy, 4 this mode of death remains the principal cause of mortality in ambulatory patients with HFrEF.
The reduction in sudden death with sacubitril/valsartan, compared with enalapril, raises the hypothesis that neprilysin inhibition, added to standard care, including a renin–angiotensin blocker, reduces the risk of ventricular arrhythmias, although there are other causes of sudden death in patients with heart failure. 5 A potential antiarrhythmic action is consistent with the favourable effects of sacubitril/valsartan on left ventricular remodelling, neurohumoral activity, potassium and circulating markers of collagen turnover, potentially reflecting myocardial fibrosis. 6 , 7 , 8 , 9 In pre‐clinical studies, neprilysin inhibition reduces cardiac fibrosis, sympathetic nervous system activity and inducibility of ventricular arrhythmias. 10 , 11 Several observational clinical case‐series have also reported a decrease in frequency of ventricular arrhythmias, after initiation of sacubitril/valsartan. 12 , 13
To investigate the hypothesis that sacubitril/valsartan reduces the incidence of ventricular arrhythmias, we undertook a post hoc analysis of PARADIGM‐HF, examining adverse event reports of ventricular arrhythmias, ICD discharges or resuscitated cardiac arrest, according to randomized treatment assignment.
Methods
Study design and participants
PARADIGM‐HF was a multicentre, double‐blind randomized controlled trial comparing the effect of treatment with the angiotensin receptor–neprilysin inhibitor (ARNI) sacubitril/valsartan against treatment with an angiotensin‐converting enzyme (ACE) inhibitor, enalapril, in patients with HFrEF. 1 History of ventricular arrhythmias did not determine eligibility for the trial. Inclusion criteria included a left ventricular ejection fraction (LVEF) of ≤40% and New York Heart Association (NYHA) functional class II, III or IV. Patients were required to have a plasma B‐type natriuretic peptide (BNP) level of ≥150 pg/ml (or an N‐terminal proBNP [NT‐proBNP] level ≥600 pg/ml). If patients had been hospitalized for heart failure within the previous year, a BNP of ≥100 pg/ml (or NT‐proBNP ≥400 pg/ml) was required. The main exclusion criteria included symptomatic hypotension, a systolic blood pressure (SBP) <100 mmHg at screening or <95 mmHg at randomization, an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2 of body surface area at screening or at randomization or a decrease in the eGFR of >35% between screening and randomization, a serum potassium level of >5.2 mmol/L at screening (or >5.4 mmol/L at randomization), or a history of angioedema or unacceptable intolerance of angiotensin receptor blocker (ARB) or ACE inhibitor treatment. After screening patients entered a run‐in period taking 2 weeks of enalapril before being switched to sacubitril/valsartan for 4 to 6 weeks and then randomized to either treatment in a 1:1 ratio. The trial was conducted in accordance with the Declaration of Helsinki, was approved by an ethics committee at each study centre and all patients provided written informed consent. The design and main findings of PARADIGM‐HF are published. 1 , 14
Pre‐specified trial outcomes
The primary composite outcome in PARADIGM‐HF was time to cardiovascular death or first heart failure hospitalization, whichever occurred first. All‐cause death was a secondary outcome. All occurrences of death and suspected heart failure hospitalization were adjudicated against standardized criteria by a blinded clinical endpoints committee at the Brigham and Women's Hospital, Boston, MA. Where possible, death was classified as cardiovascular or non‐cardiovascular, and cardiovascular deaths were further subclassified into categories which included sudden death and pump failure death (sudden death was defined only as death occurring unexpectedly in an otherwise stable patient). Patients who were resuscitated from cardiac arrest were also identified (meaningful recovery of consciousness following successful cardioversion, defibrillation or cardiopulmonary resuscitation). Patients resuscitated from a cardiac arrest, confirmed by adjudication, were included in the analysis of the composite of time‐to‐first occurrence of a ventricular arrhythmia or ICD discharge or resuscitated cardiac arrest.
Identification of ventricular arrhythmias
All adverse events reported by the investigators during PARADIGM‐HF were examined for any report of a ventricular arrhythmia or an ICD discharge. The adverse events were identified using the MedDRA preferred terms ‘ventricular tachycardia (sustained and non‐sustained)’ (VT), ‘ventricular fibrillation’ (VF), ‘ventricular flutter’, ‘torsades de pointes’, ‘ventricular tachyarrhythmia’ and ‘ventricular arrhythmia’. Adverse events were not reviewed by a blinded committee unless one of the pre‐specified endpoints occurred (e.g. a sudden death or resuscitated cardiac arrest) in which case the events were classed according to the committee's adjudication. A serious adverse event (SAE) was defined as an event which was either fatal or life‐threatening, resulted in persistent significant disability or incapacity, caused or prolonged a hospitalization, constituted a congenital anomaly/birth defect or was medically significant (requiring a medical or surgical intervention to prevent one of the other outcomes listed).
Two time‐to‐first event ventricular arrhythmia outcomes were examined: (1) any ventricular arrhythmia, and (2) the composite of a ventricular arrhythmia, resuscitated cardiac arrest or an ICD discharge. For the purposes of this analysis, ventricular arrhythmias were defined as VT, VF, ventricular flutter, torsades de pointes, ventricular tachyarrhythmia and ventricular arrhythmia (reflecting MedDRA preferred terms used for reporting adverse events). Premature ventricular ectopic events were not included in this analysis. For participants who experienced more than one type of ventricular arrhythmia, only the first event was included in the analysis of the composite endpoint.
Statistical analysis
Baseline characteristics were compared for participants experiencing no ventricular arrhythmia, any ventricular arrhythmia, or a ventricular arrhythmia/ICD discharge/resuscitated cardiac arrest. Categorical variables are reported as whole numbers with percentages. Continuous variables are reported by their mean value with standard deviations or median value with interquartile ranges depending on a respective normal or skewed distribution. The effect of sacubitril/valsartan compared with enalapril on the incidence of each ventricular arrhythmia outcome was examined in a time‐to‐first event analysis using Cox proportional‐hazard regression models. Additionally, we examined the effect of sacubitril/valsartan, compared with enalapril, on the narrower composite of VT, VF, ventricular flutter or torsades de pointes (i.e. excluding the MedDRA preferred terms ‘ventricular tachyarrhythmia’ and ‘ventricular arrhythmia’). In a further sensitivity analysis, we examined each of the ventricular arrhythmia outcomes including only events that were reported as SAEs. The primary models included factors for randomized treatment assignment and the randomization stratification variable of region. Multivariable models were adjusted for factors known to influence prognosis including beta‐blocker use, ACE inhibitor or ARB use, mineralocorticoid receptor antagonist (MRA) use, ischaemic aetiology, LVEF, presence of an ICD or cardiac resynchronization therapy (CRT) device, eGFR, NYHA class, hypertension, diabetes, past hospitalization for heart failure, log‐transformed NT‐proBNP. Event rates per 100 patient years were calculated and are presented with 95% confidence intervals (CIs). The cumulative incidences of outcomes are presented graphically using the Kaplan–Meier method. To account for the fact that death precludes the future occurrence of ventricular arrhythmias, a proportional hazards competing risk regression model was used as a sensitivity analysis. 15 To examine the relative hazard of mortality before or after the occurrence of a ventricular arrhythmia, Cox proportional‐hazard regression models were performed with the occurrence of ventricular arrhythmia or the composite outcome modelled as a time‐varying covariate. 16 The effect of randomized treatment was examined in Cox proportional‐hazard regression models, and the interaction with randomized therapy tested in two important subgroups. The first group was patients with an ischaemic or non‐ischaemic aetiology for heart failure and the second patients with or without an implanted defibrillating device at baseline. The relationship between change in NT‐proBNP from baseline to 8 months and the incidence of ventricular arrhythmias was examined using change in NT‐proBNP modelled as a continuous variable in a restricted cubic spline model adjusted for baseline value. Only arrhythmic events occurring after 8 months were included.
A p‐value <0.05 was considered statistically significant. Statistical analyses were performed using Stata 16.1 (Stata Corp., College Station, TX, USA).
Results
A total of 8399 patients were included in the present analysis, of whom 333 patients (4.0%) had a report of a ventricular arrhythmia. The events accounting for a ventricular arrhythmia included VT in 246 patients (241 as a first event), VF in 64 patients (60 as a first event), ventricular flutter in one patient (1 as a first event), torsades de pointes in two patients (2 as a first event), a ‘ventricular tachyarrhythmia’ in one patient (0 as a first event) and a ‘ventricular arrhythmia’ in 33 patients (29 as a first event). Among the 246 patients experiencing VT, 43 patients had non‐sustained VT (35 as a first event). Figure 1 outlines the occurrence of adjudicated fatal events and resuscitated cardiac arrest in patients who had a ventricular arrhythmia reported. Overall, 200 of 333 (60.1%) first ventricular arrhythmia events were reported as SAEs.
Figure 1.
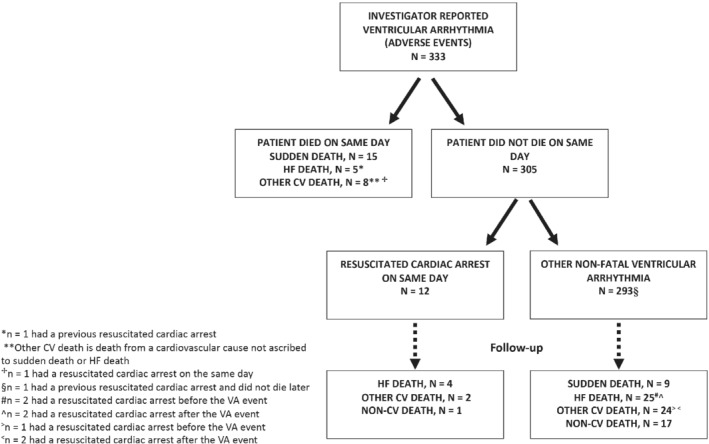
Incidence of adjudicated fatal events and resuscitated cardiac arrest in patients with a reported ventricular arrhythmia (VA). CV, cardiovascular; HF, heart failure.
A total of 372 patients (4.4%) experienced the composite of a ventricular arrhythmia, an ICD shock or resuscitated cardiac arrest. Among these 372 patients, the first event was a ventricular arrhythmia in 311 patients. An ICD shock was reported in 31 participants (23 as a first event) and resuscitated cardiac arrest in 44 patients (38 as a first event). The occurrence of adjudicated fatal events in patients who experienced this composite outcome is outlined in online supplementary Figure S1 .
Baseline characteristics
The baseline characteristics of patients who did and did not experience a ventricular arrhythmia are shown in Table 1 . Compared to those without a report of a ventricular arrhythmia, patients with a report of a ventricular arrhythmia were more likely to be male, White, to have a longer duration of heart failure, and a history of myocardial infarction. Heart rate, SBP, eGFR and LVEF were lower in patients with a report of a ventricular arrhythmia, but body mass index was higher. Age, NYHA class, and the Kansas City Cardiomyopathy Questionnaire clinical summary score did not differ between these two groups. Patients with a report of a ventricular arrhythmia after randomization were more likely to have a history of previous ventricular arrhythmia, to be treated with amiodarone and to have an ICD or received CRT with a defibrillator (CRT‐D). Participants with a report of a ventricular arrhythmia also had a wider QRS duration (but no excess of either right or left bundle branch block) and were less likely to have atrial fibrillation on their baseline electrocardiogram, although the proportion of patients with a history of atrial fibrillation did not differ between groups.
Table 1.
Baseline characteristics of participants who had no ventricular arrhythmia compared with those who had a ventricular arrhythmia and the composite of a ventricular arrhythmia/implantable cardioverter defibrillator shock/resuscitated cardiac arrest
| No ventricular arrhythmia | Ventricular arrhythmia a | p‐value b | Ventricular arrhythmia/ICD shock/resuscitated cardiac arrest c | p‐value b | |
|---|---|---|---|---|---|
| n (%) | 8066 (96.0) | 333 (4.0) | 372 (4.4) | ||
| Age (years) | 64 ± 11 | 64 ± 11 | 0.450 | 64 ± 11 | 0.570 |
| Race, n (%) | <0.001 | <0.001 | |||
| White | 5291 (65.6) | 253 (76.0) | 277 (74.5) | ||
| Black | 406 (5.0) | 22 (6.6) | 27 (7.3) | ||
| Asian | 1477 (18.3) | 32 (9.6) | 37 (9.9) | ||
| Other | 892 (11.1) | 26 (7.8) | 31 (8.3) | ||
| Region, n (%) | <0.001 | <0.001 | |||
| North America | 538 (6.7) | 64 (19.2) | 73 (19.6) | ||
| Latin America | 1388 (17.2) | 45 (13.5) | 52 (14.0) | ||
| Western Europe | 1920 (23.8) | 131 (39.3) | 143 (38.4) | ||
| Central Europe | 2764 (34.3) | 62 (18.6) | 68 (18.3) | ||
| Asia‐Pacific and other | 1456 (18.1) | 31 (9.3) | 36 (9.7) | ||
| Male sex, n (%) | 6282 (77.9) | 285 (85.6) | <0.001 | 320 (86.0) | <0.001 |
| SBP (mmHg) | 122 ± 15 | 118 ± 15 | <0.001 | 117 ± 15 | <0.001 |
| Heart rate (bpm) | |||||
| Sinus rhythm | 72 ± 11 | 69 ± 11 | 0.002 | 69 ± 11 | <0.001 |
| AF/flutter d | 74 ± 13 | 69 ± 12 | <0.001 | 70 ± 12 | 0.002 |
| BMI (kg/m2) | 28 ± 6 | 29 ± 6 | 0.003 | 29 ± 6 | 0.003 |
| eGFR (ml/min/1.73 m2) | 68 ± 20 | 64 ± 22 | <0.001 | 64 ± 21 | <0.001 |
| eGFR <60 ml/min/1.73 m2, n (%) | 2908 (36.1) | 153 (45.9) | <0.001 | 173 (46.5) | <0.001 |
| LVEF (%) | 30 [25–34] | 30 [25–33] | <0.001 | 30 [25–32] | <0.001 |
| LVEF, n (%) | 0.002 | <0.001 | |||
| < median | 3218 (39.9) | 161 (48.3) | 184 (49.5) | ||
| ≥ median | 4847 (60.1) | 172 (51.7) | 188 (50.5) | ||
| NT‐proBNP (pg/ml) ‐ no AF/flutter d | 1447 [814–2955] | 1377 [768–3111] | 0.880 | 1477 [775–3140] | 0.920 |
| NT‐proBNP (pg/ml) ‐ AF/flutter d | 1885 [1095–3646] | 1981 [1053–3954] | 0.850 | 2009 [1138–3976] | 0.590 |
| Troponin (µg/L) e | 0.015 [0.010–0.023] | 0.018 [0.012–0.026] | 0.013 | 0.017 [0.011–0.025] | 0.055 |
| Plasma aldosterone (pmol/L) e | 243 [152–386] | 258 [159–372] | 0.420 | 268 [160–386] | 0.230 |
| Galectin (ng/ml) e | 18.7 ± 6.9 | 18.6 ± 6.7 | 0.800 | 18.8 ± 6.8 | 0.940 |
| Cystatin C (mg/L) e | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.400 | 1.2 ± 0.4 | 0.270 |
| Urinary cyclic‐GMP (nmol/L) e | 1109 [683–1813] | 1417 [827–1956] | 0.015 | 1397 [827–1920] | 0.021 |
| Potassium (mmol/L) | 4.5 ± 0.5 | 4.5 ± 0.5 | 0.680 | 4.5 ± 0.5 | 0.760 |
| Sodium (mmol/L) | 141 ± 3 | 141 ± 3 | 0.300 | 141 ± 3 | 0.097 |
| RBBB, n (%) | 604 (7.5) | 23 (6.9) | 0.690 | 24 (6.5) | 0.450 |
| LBBB, n (%) | 1583 (19.6) | 70 (21.0) | 0.530 | 79 (21.2) | 0.440 |
| QRS duration (ms) | 117 ± 36 | 134 ± 35 | <0.001 | 134 ± 35 | <0.001 |
| NYHA class, n (%) | 0.120 | 0.200 | |||
| I | 379 (4.7) | 10 (3.0) | 12 (3.2) | ||
| II | 5666 (70.4) | 253 (76.0) | 280 (75.3) | ||
| III | 1949 (24.2) | 69 (20.7) | 78 (21.0) | ||
| IV | 59 (0.7) | 1 (0.3) | 2 (0.5) | ||
| KCCQ‐CSS | 80 [63–92] | 80 [67–91] | 0.840 | 80 [67–91] | 0.800 |
| Medical history, n (%) | |||||
| Duration of heart failure | <0.001 | <0.001 | |||
| <1 year | 2455 (30.7) | 45 (13.5) | 52 (14.0) | ||
| 1–5 years | 3085 (38.6) | 118 (35.4) | 131 (35.2) | ||
| >5 years | 2445 (30.6) | 170 (51.1) | 189 (50.8) | ||
| Ischaemic aetiology | 4820 (59.8) | 216 (64.9) | 0.062 | 239 (64.2) | 0.084 |
| Previous ventricular arrhythmia | 185 (2.3) | 47 (14.1) | <0.001 | 50 (13.4) | <0.001 |
| Hypertension | 5716 (70.9) | 224 (67.3) | 0.160 | 256 (68.8) | 0.410 |
| Diabetes | 2768 (34.3) | 128 (38.4) | 0.120 | 139 (37.4) | 0.230 |
| AF history | 2951 (36.6) | 107 (32.1) | 0.098 | 127 (34.1) | 0.350 |
| AF/flutter on baseline ECG | 2036 (25.2) | 54 (16.2) | <0.001 | 64 (17.2) | <0.001 |
| Prior HF hospitalization | 5069 (62.8) | 205 (61.6) | 0.640 | 232 (62.4) | 0.860 |
| MI | 3460 (42.9) | 174 (52.3) | <0.001 | 196 (52.7) | <0.001 |
| PCI | 1702 (21.1) | 99 (29.7) | <0.001 | 112 (30.1) | <0.001 |
| CABG | 1215 (15.1) | 88 (26.4) | <0.001 | 97 (26.1) | <0.001 |
| Stroke | 693 (8.6) | 32 (9.6) | 0.520 | 35 (9.4) | 0.590 |
| COPD | 1035 (12.8) | 45 (13.5) | 0.720 | 52 (14.0) | 0.510 |
| Anaemia f | 1626 (20.2) | 66 (19.8) | 0.880 | 76 (20.4) | 0.890 |
| Medical therapy, n (%) | |||||
| Loop diuretic | 6053 (75.0) | 264 (79.3) | 0.079 | 294 (79.0) | 0.081 |
| Thiazide/thiazide‐related diuretic | 1133 (14.0) | 52 (15.6) | 0.420 | 57 (15.3) | 0.490 |
| Prior ACE inhibitor | 6275 (77.8) | 257 (77.2) | 0.790 | 287 (77.2) | 0.770 |
| Prior ARB | 1814 (22.5) | 78 (23.4) | 0.690 | 89 (23.9) | 0.510 |
| Beta‐blocker | 7500 (93.0) | 311 (93.4) | 0.770 | 350 (94.1) | 0.400 |
| MRA | 4847 (60.1) | 184 (54.8) | 0.078 | 208 (55.9) | 0.110 |
| Digoxin | 2449 (30.4) | 90 (27.0) | 0.190 | 107 (28.8) | 0.530 |
| Amiodarone | 728 (9.0) | 55 (16.5) | <0.001 | 62 (16.7) | <0.001 |
| Sotalol | 37 (0.5) | 4 (1.2) | 0.057 | 4 (1.1) | 0.097 |
| ICD or CRT‐D | 1078 (13.4) | 165 (49.5) | <0.001 | 182 (48.9) | <0.001 |
| CRT‐D | 371 (4.6) | 53 (15.9) | <0.001 | 59 (15.9) | <0.001 |
| CRT‐P | 145 (1.8) | 5 (1.5) | 0.690 | 6 (1.6) | 0.800 |
Values are mean ± standard deviation, or median [interquartile range], unless otherwise indicated.
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy‐defibrillator; CRT‐P, cardiac resynchronization therapy‐pacemaker; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; KCCQ‐CSS, Kansas City Cardiomyopathy Questionnaire clinical summary score; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; RBBB, right bundle branch block; SBP, systolic blood pressure.
Ventricular arrhythmia was defined as any adverse event report using the MedDRA preferred terms ‘ventricular tachycardia’ (VT), ‘ventricular fibrillation’, ‘ventricular flutter’, ‘torsades de pointes’, ‘ventricular tachyarrhythmia’ and ‘ventricular arrhythmia’. Premature ventricular ectopics were excluded.
p‐value compared to no ventricular arrhythmia.
372 patients with a ventricular arrhythmia/ICD shock/resuscitated cardiac arrest compared to 8027 patients with no ventricular arrhythmia.
Based on a history of AF or baseline ECG documenting AF or atrial flutter.
Biomarkers measured in subsets of patients: plasma troponin n = 1947; plasma aldosterone n = 1976; galectin‐3 n = 2043; cystatin C n = 2056; urinary cyclic‐GMP n = 2033.
Anaemia was defined as haemoglobin <130 g/L in males and <120 g/L in females.
The NT‐proBNP level did not differ between patients with and without a ventricular arrhythmia, but troponin and urinary cGMP levels were higher in patients with a ventricular arrhythmia. Sodium, potassium, and other biomarkers, including aldosterone and galectin‐3, did not differ between patients with and without a ventricular arrhythmia. The pattern of differences described was essentially identical when comparing patients with a report of a ventricular arrhythmia, ICD discharge or resuscitated cardiac arrest, to those with no report of a ventricular arrhythmia.
Effect of randomized treatment on incidence of ventricular arrhythmias
Table 2 shows the incidence of the ventricular arrhythmia outcomes, according to randomized treatment. Compared to patients randomly assigned to enalapril, participants assigned to sacubitril/valsartan had lower rate of ventricular arrhythmia (hazard ratio [HR] 0.76, 95% CI 0.62–0.95; p = 0.015) and the composite outcome of a ventricular arrhythmia, ICD shock or resuscitated cardiac arrest (HR 0.79, 95% CI 0.65–0.97; p = 0.025) (Graphical Abstract, Figures 2 and 3 ). The rate of the narrower ventricular arrhythmia composite of VT, VF, ventricular flutter or torsades de pointes events was also lower in patients treated with sacubitril/valsartan compared to enalapril (HR 0.77, 95% CI 0.62–0.97; p = 0.027). The effect of treatment was essentially unchanged in the multivariable adjusted analyses. In a sensitivity analysis including only ventricular arrhythmia events that were reported as SAEs, the favourable effect of a reduction in ventricular arrhythmias when treated with sacubitril/valsartan, compared to enalapril, was consistent with the main analysis findings (online supplementary Table S1 ). Analyses modelling all‐cause mortality as a competing risk also gave similar results (online supplementary Table S2 and Figure S2 ) for the ventricular arrhythmia outcome and the composite ventricular arrhythmia, ICD shock or resuscitated cardiac arrest.
Table 2.
Cox proportional‐hazard models for each ventricular arrhythmia outcome according to randomized treatment assignment
| Outcome | Sacubitril/valsartan | Enalapril | Hazard ratio (95% CI) | |||
|---|---|---|---|---|---|---|
| n/N (%) | Event rate per 100 patient years (95% CI) | n/N (%) | Event rate per 100 patient years (95% CI) | Primary analysis a | Adjusted analysis b | |
| Ventricular arrhythmia | 145/4187 (3.5) | 1.6 (1.4–1.9) | 188/4212 (4.5) | 2.1 (1.8–2.4) | 0.76 (0.62–0.95); p = 0.015 | 0.78 (0.62–0.96); p = 0.021 |
| Ventricular arrhythmia/ICD shock/resuscitated cardiac arrest | 165/4187 (3.9) | 1.8 (1.6–2.1) | 207/4212 (4.9) | 2.3 (2.0–2.6) | 0.79 (0.65–0.97); p = 0.025 | 0.81 (0.66–0.99); p = 0.039 |
| VT/VF/ventricular flutter/torsades de pointes | 133/4175 (3.2) | 1.5 (1.2–1.7) | 171/4195 (4.1) | 1.9 (1.6–2.2) | 0.77 (0.62–0.97); p = 0.027 | 0.79 (0.63–0.99); p = 0.043 |
CI, confidence interval; ICD, implantable cardioverter defibrillator; VF, ventricular fibrillation; VT, ventricular tachycardia.
Primary analysis included randomized treatment and region.
Adjusted analysis included randomized treatment, region, beta‐blocker use, angiotensin‐converting enzyme inhibitor use, angiotensin receptor blocker use, mineralocorticoid receptor antagonist use, ischaemic aetiology, ejection fraction, presence of ICD or cardiac resynchronization therapy, New York Heart Association class, hypertension, diabetes, past hospitalization for heart failure, estimated glomerular filtration rate, log‐transformed N‐terminal pro B‐type natriuretic peptide.
Figure 2.
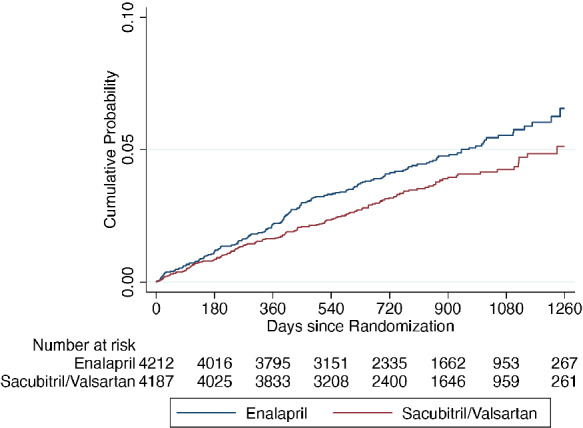
Kaplan–Meier curves for the time‐to‐first ventricular arrhythmia according to treatment assignment.
Figure 3.
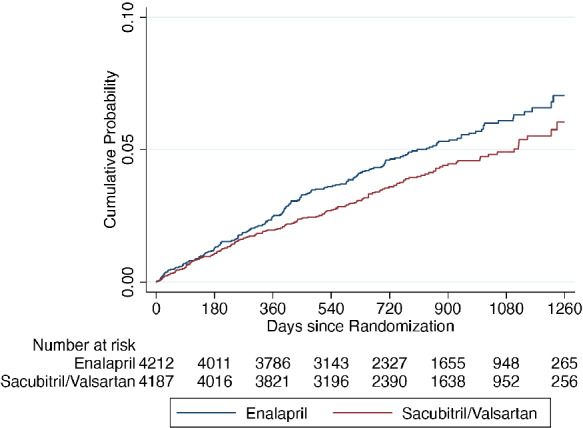
Kaplan–Meier curves for time‐to‐first ventricular arrhythmia/implantable cardioverter defibrillator shock/resuscitated cardiac arrest according to treatment assignment.
Effect of sacubitril/valsartan on ventricular arrhythmias according to heart failure aetiology and baseline implanted defibrillator use
Of the 5036 patients with an ischaemic aetiology, 216 (4.3%) experienced at least one ventricular arrhythmia; the corresponding number for the 3363 patients without an ischaemic aetiology was 117 (3.5%). The HR for the effect of sacubitril/valsartan, compared with enalapril, on ventricular arrhythmias in patients with an ischaemic aetiology was 0.93 (95% CI 0.71–1.21), compared with 0.53 (95% CI 0.37–0.78) in those without an ischaemic aetiology (p for interaction = 0.020) (Table 3 ).
Table 3.
Cox proportional‐hazard models for a ventricular arrhythmia outcome according to randomized treatment assignment in two key patient subgroups
| Outcome | Sacubitril/valsartan | Enalapril | Hazard ratio (95% CI) | Interaction p‐value | |||
|---|---|---|---|---|---|---|---|
| n/N (%) | Event rate per 100 patient years (95% CI) | n/N (%) | Event rate per 100 patient years (95% CI) | Unadjusted analysis a | Adjusted analysis b , c | ||
| Ischaemic aetiology | 0.020 | ||||||
| Yes | 103/2506 (4.1) | 1.9 (1.6–2.3) | 113/2530 (4.5) | 2.1 (1.7–2.5) | 0.93 (0.71–1.21) | 0.92 (0.70–1.20) | |
| No | 42/1681 (2.5) | 1.1 (0.8–1.5) | 75/1682 (4.5) | 2.1 (1.7–2.6) | 0.53 (0.37–0.78) | 0.57 (0.39–0.83) | |
| ICD/CRT‐D at baseline | 0.952 | ||||||
| Yes | 72/623 (11.6) | 5.4 (4.3–6.8) | 93/620 (15.0) | 7.0 (5.7–8.6) | 0.77 (0.57–1.05) | 0.81 (0.59–1.11) | |
| No | 73/3564 (2.0) | 0.9 (0.7–1.2) | 95/3592 (2.6) | 1.2 (1.0–1.5) | 0.76 (0.56–1.04) | 0.76 (0.56–1.04) | |
CI, confidence interval; CRT, cardiac resynchronization therapy; CRT‐D, CRT, cardiac resynchronization therapy‐defibrillator; ICD, implantable cardioverter defibrillator.
Unadjusted analysis included randomized treatment and region.
Adjusted analysis for ischaemic aetiology included randomized treatment, region, beta‐blocker use, angiotensin‐converting enzyme inhibitor use, mineralocorticoid receptor antagonist use, ejection fraction, presence of ICD or CRT, New York Heart Association class, hypertension, diabetes, past hospitalization for heart failure, log‐transformed N‐terminal pro B‐type natriuretic peptide.
Adjusted analysis for ICD/CRT‐D at baseline included randomized treatment, region, beta‐blocker use, angiotensin‐converting enzyme inhibitor use, mineralocorticoid receptor antagonist use, ejection fraction, ischaemic aetiology, New York Heart Association class, hypertension, diabetes, past hospitalization for heart failure, log‐transformed N‐terminal pro B‐type natriuretic peptide.
Of the 1243 patients with a defibrillating device (ICD or CRT‐D) implanted at baseline, 165 (13.3%) experienced at least one ventricular arrhythmia. Among the 7156 participants without a defibrillating device, 168 (2.3%) experienced at least one ventricular arrhythmia. The HR for the effect of sacubitril/valsartan, compared with enalapril, on ventricular arrhythmias in patients with an ICD/CRT‐D was 0.77 (95% CI 0.57–1.05) compared with 0.76 (95% CI 0.56–1.04) in those without such a device (p for interaction = 0.952) (Table 3 ).
Association between any report of a ventricular arrhythmia and subsequent mortality
When occurrence of ventricular arrhythmia was modelled as a time‐varying covariate, there was a strong association with mortality. For a ventricular arrhythmia, the unadjusted HR for all‐cause mortality was 3.89 (95% CI 3.19–4.75; p < 0.001), and for the composite of a ventricular arrhythmia, ICD shock or resuscitated cardiac arrest, the HR for all‐cause mortality was 3.86 (95% CI 3.19–4.67; p < 0.001). The corresponding adjusted HRs were 4.15 (95% CI 3.39–5.09; p < 0.001) and 4.06 (95% CI 3.34–4.93; p < 0.001), respectively. The occurrence of a ventricular arrhythmia was also associated with cardiovascular death and both heart failure death (adjusted HR 4.93, 95% CI 3.38–7.19; p < 0.001) and sudden death (adjusted HR 3.38, 95% CI 2.22–5.15; p < 0.001) (online supplementary Table S3 ).
Association between any report of a ventricular arrhythmia and change in NT‐proBNP
Data were available to calculate change in NT‐proBNP between baseline and 8 months in 1798 patients. When change in NT‐proBNP was modelled as a continuous variable, an increase in NT‐proBNP >3255 pg/ml was associated with a higher incidence of ventricular arrhythmia (online supplementary Figure S3 ).
Discussion
The main findings of this analysis were that sacubitril/valsartan reduced the risk of investigator‐reported ventricular arrhythmias in patients with HFrEF, the occurrence of which was strongly associated with subsequent death.
Ambulatory monitoring and other systematic approaches to arrhythmia detection identify ventricular premature beats and non‐sustained VT in most patients with HFrEF. 17 , 18 , 19 The rate of ventricular arrhythmias detected in the present study was lower because they were identified through spontaneous adverse event reporting by investigators, rather than by systematic monitoring. However, in our recent report from the DAPA‐HF trial using a similar approach to identify arrhythmic events, the rate of ventricular arrhythmias was almost identical to that observed in PARADIGM‐HF. 20 Events reported spontaneously probably reflect the most clinically significant episodes, compared with the more complete burden identified by systematic monitoring. 21 The view that spontaneously reported events are the more clinically significant episodes is also supported by the high subsequent mortality rate in patients with an adverse event report of this type in PARADIGM‐HF. When analysed as a time‐varying covariate, the occurrence of a ventricular arrhythmia was associated with a three to four‐fold increased risk of death. In past studies, there has been an inconsistent association between non‐sustained VT and mortality in patients with HFrEF, especially when other prognostic variables were accounted for. 17 , 21 , 22 However, despite extensive adjustment, including for NT‐proBNP, an adverse event report of a ventricular arrhythmia remained an independent and statistically significant predictor of death in PARADIGM‐HF. The effectiveness of sacubitril/valsartan in reducing sudden death has been clearly demonstrated in the PARADIGM‐HF trial. 2 The present analysis adds mechanistic insight into this benefit, through a reduction in potentially lethal ventricular arrhythmias.
The baseline characteristics of participants with adverse event reports related to a ventricular arrhythmia were also consistent with what would be expected in patients at high risk of such events, including male sex, history of coronary disease, lower LVEF, more frequent treatment with amiodarone and higher rates of prior ventricular arrhythmia and device implantation. 23 , 24 We also examined a composite of clinically more severe events, in which we included ICD shocks and patients experiencing cardiac arrest who were resuscitated, in addition to adverse event reports of VT and VF, whichever occurred first. Neither of the former were common, adding only 23 ICD discharge and 38 resuscitated cardiac arrest first events.
Whether we analysed an adverse event report of a ventricular arrhythmia or the composite of VT, VF, ICD shock or resuscitated cardiac arrest, sacubitril/valsartan reduced these events by approximately 20%, compared with enalapril. Although enalapril was shown not to reduce the frequency or complexity of ventricular arrhythmias in patients with HFrEF in the Studies Of Left Ventricular Dysfunction, 25 both beta‐blockers and MRAs reduce ventricular arrhythmias and sudden death and the rate of use of these other therapies was high in PARADIGM‐HF. 26 , 27 , 28 , 29 The effect of sacubitril/valsartan on ventricular arrhythmias has not been studied in any prior randomized trial, although our findings are consistent with the reduction in sudden cardiac death reported in PARADIGM‐HF and several observational analyses of the effect of sacubitril/valsartan on the burden of ventricular arrhythmias in patients with HFrEF. 12 , 13 , 30 For example, in a single centre study of 167 HFrEF patients with dual‐chamber ICD, Russo and colleagues observed significantly fewer episodes of VF and VT, both sustained and non‐sustained, and appropriate ICD shock events, over a period of up to 12 months after starting sacubitril/valsartan, compared to before treatment. 12 Similar findings have been reported in other smaller studies. 13 , 30
Ventricular arrhythmias were reported more commonly in patients with an implanted defibrillating device. We were unable to tell whether the higher incidence of ventricular arrhythmias in patients with devices reflected the reason why they had the device (i.e. because of a prior arrhythmia or for primary prevention in a patient at perceived high risk) or because of the ability of the device to detect arrhythmias. Our findings support the recent recommendation in the European Society of Cardiology (ESC) guidelines on the management of heart failure that the implantation of a primary prevention ICD is delayed until medical therapy has been optimized for at least 3 months in the hope that the LVEF may increase to above 35%, obviating the need for an ICD. 31 Although this strategy may cause concern about the risk of early sudden death, the absolute rate in a 90‐day period is very small, especially in lower‐risk patients such as those with non‐ischaemic cardiomyopathy. 32 Moreover, most recommended pharmacological therapies, as well as (or maybe because of) improving LVEF, also reduce the risk of sudden death. The data reported in this paper and our recent findings with dapagliflozin 20 extend this evidence to these newer recommended therapies and show that their benefit is additional to that of renin–angiotensin system blockers, beta‐blockers and MRAs. However, we found that sacubitril/valsartan reduced arrhythmias to a similar extent in patients with and without such devices. The decision of whether to implant an ICD and the appropriate timing to do so, particularly in patients with a non‐ischaemic aetiology for heart failure, remains a subject of debate since the results of the DANISH trial were reported. 32 The recent 2021 ESC heart failure guidelines reduced the strength of recommendation for ICD implantation in patients with a non‐ischaemic aetiology from Class I to Class IIa, with the recommendation that medical therapy should be optimized over a minimum of 3 months before implantation of a device. 31 Our data support this recommendation, especially as sacubitril/valsartan has favourable effects on cardiac remodelling and may obviate the need for an ICD should the LVEF increase to more than 35%. 8 Conversely, sacubitril/valsartan seemed to be more effective in reducing ventricular arrhythmias in patients with a non‐ischaemic aetiology, compared to an ischaemic aetiology. Patients with an ischaemic aetiology in PARADIGM‐HF were more likely to have an ICD than non‐ischaemic patients (16.5% vs. 12.2%, p < 0.001) which may have attenuated the potential benefit of sacubitril/valsartan in these patients. Extensive scar after myocardial infarction may also represent an arrhythmia substrate that responds less favourably to sacubitril/valsartan. In this context, the TAROT‐HF study showed more favourable cardiac remodelling with sacubitril/valsartan in non‐ischaemic patients compared to those with an ischaemic aetiology. 33
The mechanisms by which sacubitril/valsartan affects ventricular arrhythmias are unknown. 34 Sacubitril/valsartan did not affect cardiac repolarization in healthy human volunteers 35 although, recently, neprilysin inhibition with sacubitrilat (the active metabolite of sacubitril) was shown to directly decrease potentially pro‐arrhythmogenic diastolic sarcoplasmic reticulum calcium leak in human ventricular cardiomyocytes from patients with end‐stage heart failure. 36 Other potential mechanisms have been suggested by studies in experimental animals, where the combination of a neprilysin inhibitor with a renin–angiotensin system blocker reduces cardiac fibrosis and remodelling, compared to renin–angiotensin system blockade alone. 36 , 37 , 38 , 39 Chamber dilatation and myocardial stretch, reflected in elevation of natriuretic peptide levels, are associated with the occurrence of ventricular arrhythmias. In two randomized trials and one observational study in patients with HFrEF, sacubitril/valsartan reduced cardiac chamber size, and sacubitril/valsartan also reduced NT‐proBNP level, consistent with decreased wall stress. 7 , 8 , 40 These actions would be expected to reduce the propensity to ventricular arrhythmias. Indeed, we found a relationship between increasing NT‐proBNP over time and risk of ventricular arrhythmia, consistent with this hypothesis. This is consistent with the findings of Rohde et al. 3 that the reduction in risk of sudden death with sacubitril/valsartan, compared with enalapril, tended to be greater in patients with a non‐ischaemic aetiology. It is also possible that more favourable cardiac remodelling with sacubitril/valsartan in non‐ischaemic patients, as suggested by the TAROT‐HF study, might explain the greater reduction in ventricular arrhythmias in these participants, compared to patients with an ischaemic aetiology. 33 Lastly, the accuracy of the aetiological classification of heart failure depends on the extent of investigation and this varies globally. Therefore, some patients thought to have a non‐ischaemic aetiology may have had undiagnosed coronary disease.
Finally, while there is a clear link between ventricular arrhythmias and sudden death, it is important to note that not all sudden deaths are due to an arrhythmia or indeed any electrical disturbance, which is why ICDs do not eliminate the risk of sudden death. In this context, it is important to note that sacubitril/valsartan also appeared to reduce the risk of sudden death in patients with an ICD, although this analysis was based on a small number of events. 2 Conversely, ventricular arrhythmias are also predictive of non‐sudden death because they are often a marker of a sicker patient with worse ventricular function or more advanced heart failure as found in the present analyses.
Our study has several limitations. Firstly, this was not a pre‐specified analysis. Secondly, our analysis relied on adverse event reporting which will have resulted in underestimation of the overall prevalence of ventricular arrhythmias. We were unable to ascertain whether ICD discharges were appropriate or inappropriate and did not have information on anti‐tachycardia pacing. There was no electrocardiographic validation of arrhythmias and standardized criteria for reporting of specific ventricular arrhythmias were not provided. However, a similar approach to the one used in the present study identified a benefit of a beta‐blocker on arrhythmias in patients with left ventricular systolic dysfunction, consistent with that found in studies using systematic monitoring. 21 Nevertheless, our findings could be strengthened in future trials by systematic assessment of ventricular arrhythmias using either ambulatory monitoring or by using implanted cardiac devices.
In summary, in this post hoc analysis, sacubitril/valsartan, compared with enalapril, reduced the incidence of investigator‐reported (but not adjudicated) ventricular arrhythmias in patients with HFrEF, most of whom were treated with a beta‐blocker and, in over half of cases, an MRA as well. This possible antiarrhythmic effect is additional to the known benefits of sacubitril/valsartan in HFrEF.
Funding
The PARADIGM‐HF trial was funded by Novartis. J.J.V.M. is supported by a British Heart Foundation Centre of Research Excellence Grant (RE/18/6/34217) and A.M.J. is supported by a British Heart Foundation Clinical Research Training Fellowship (FS/18/14/33330). L.E.R. is partly supported by the National Council for Scientific and Technological Development (CNPq, Brazil, research fellowship 30833/2017‐1) and by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, senior fellowship).
Conflict of interest: P.S.J. has received consulting fees, advisory board fees, and lecture fees from Novartis; advisory board fees from Cytokinetics; and grant support from Boehringer Ingelheim. M.C.P. has received lecture fees from AstraZeneca and Eli Lilly, personal fees from Novo Nordisk, AstraZeneca, NAPP Pharmaceuticals, Takeda Pharmaceutical, Alnylam, Bayer, Resverlogix, and Cardiorentis and grants and personal fees from Boehringer Ingelheim and Novartis. A.S.D. reported receiving personal fees from Abbott, Biofourmis, Boston Scientific, Boehringer Ingelheim, Merck, Regeneron, and Relypsa and grants and personal fees from AstraZeneca, Alnylam, and Novartis, outside the submitted work. L.E.R. has been a consultant or served on advisory board for Amgen, AstraZeneca, Merck and Novartis. M.P.L. is an employee of Novartis. J.L.R. has received grants and consulting fees from Novartis and consulting fees from Abbott, AstraZeneca, MyoKardia, and Sanofi. M.R.Z. has received research funding from Novartis and has been a consultant for Novartis, Abbott, Boston Scientific, CVRx, EBR, Endotronics, Ironwood, Merck, Medtronic, and Myokardia V Wave. S.D.S. has received research grants from Actelion, Alnylam, Amgen, AstraZeneca, Bellerophon, Bayer, BMS, Celladon, Cytokinetics, Eidos, Gilead, GSK, Ionis, Lilly, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Neurotronik, Novartis, NovoNordisk, Respicardia, Sanofi Pasteur, Theracos, and has consulted for Abbott, Action Akros, Alnylam, Amgen, Arena, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardior, Cardurion, Corvia, Cytokinetics, Daiichi Sankyo, Gilead, GSK, Ironwood, Lilly, Merck, Myokardia, Novartis, Roche, Takeda, Theracos, Quantum Genetics, Cardurion, AoBiome, Janssen, Cardiac Dimensions, Tenaya, Sanofi‐Pasteur, Dinaqor, Tremeau, CellProThera, Moderna, American Regent. K.S. reports consulting for Novartis. M.P. has received consulting fees from AbbVie, Akcea, Actavis, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cardiorentis, Daiichi Sankyo, Gilead, Johnson & Johnson, Novo Nordisk, Pfizer, Relypsa, Sanofi, Synthetic Biologics, and Theravance. J.J.V.M. has received payments through Glasgow University from work on clinical trials, consulting and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, BMS, Cardurion, Cytokinetics, DalCor, GSK, KBP Biosciences, Novartis, Pfizer, Theracos; and personal payments from Abbott, Hikma, Ionis, Sun Pharmaceuticals, Servier. All other authors have nothing to disclose.
Supporting information
Figure S1. Incidence of adjudicated fatal and non‐fatal events in patients who experienced a ventricular arrhythmia/ICD shock/resuscitated cardiac arrest.
Figure S2. (A) Cumulative incidence of first ventricular arrhythmia in a competing risk regression according to treatment assignment. (B) Cumulative incidence of first ventricular arrhythmia/ICD shock/resuscitated cardiac arrest in a competing risk regression according to treatment assignment.
Figure S3. Restricted cubic spline of the relationship between change in NT‐proBNP (range −10 000 pg/ml to +10 000 pg/ml) from baseline to 8 months and the incidence of ventricular arrhythmia.
Table S1. Cox proportional‐hazard models for each ventricular arrhythmia outcome (serious adverse events only) according to randomized treatment assignment.
Table S2. Competing risk regression for time‐to‐first ventricular arrhythmia outcomes with all‐cause death as a competing risk (Fine and Gray model).
Table S3. (A) Cox regression of mortality outcomes with the development of a ventricular arrhythmia as a time varying covariate. (B) Cox regression of mortality outcomes with the development of ventricular arrhythmia/ICD shock/resuscitated cardiac arrest as a time varying covariate.
References
- 1. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al.; PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 2. Desai AS, McMurray JJ, Packer M, Swedberg K, Rouleau JL, Chen F, et al. Effect of the angiotensin‐receptor‐neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36:1990–7. [DOI] [PubMed] [Google Scholar]
- 3. Rohde LE, Chatterjee NA, Vaduganathan M, Claggett B, Packer M, Desai AS, et al. Sacubitril/valsartan and sudden cardiac death according to implantable cardioverter‐defibrillator use and heart failure cause: a PARADIGM‐HF analysis. JACC Heart Fail. 2020;8:844–55. [DOI] [PubMed] [Google Scholar]
- 4. Shen L, Jhund PS, Petrie MC, Claggett BL, Barlera S, Cleland JGF, et al. Declining risk of sudden death in heart failure. N Engl J Med. 2017;377:41–51. [DOI] [PubMed] [Google Scholar]
- 5. Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J. 2020;41:1757–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zile MR, O'Meara E, Claggett B, Prescott MF, Solomon SD, Swedberg K, et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. 2019;73:795–806. [DOI] [PubMed] [Google Scholar]
- 7. Desai AS, Solomon SD, Shah AM, Claggett BL, Fang JC, Izzo J, et al.; EVALUATE‐HF Investigators . Effect of sacubitril‐valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322:1077–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, et al.; PROVE‐HF Investigators . Association of change in N‐terminal pro‐B‐type natriuretic peptide following initiation of sacubitril‐valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferreira JP, Mogensen UM, Jhund PS, Desai AS, Rouleau JL, Zile MR, et al. Serum potassium in the PARADIGM‐HF trial. Eur J Heart Fail. 2020;22:2056–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pu Q, Amiri F, Gannon P, Schiffrin EL. Dual angiotensin‐converting enzyme/neutral endopeptidase inhibition on cardiac and renal fibrosis and inflammation in DOCA‐salt hypertensive rats. J Hypertens. 2005;23:401–9. [DOI] [PubMed] [Google Scholar]
- 11. Abdulsalam TM, Hasanin AH, Mohamed RH, El Sayed Badawy A. Angiotensin receptor‐neprilysin inhibitior (thiorphan/irbesartan) decreased ischemia‐reperfusion induced ventricular arrhythmias in rat; in vivo study. Eur J Pharmacol. 2020;882:173295. [DOI] [PubMed] [Google Scholar]
- 12. Russo V, Bottino R, Rago A, Papa AA, Liccardo B, Proietti R, et al. The effect of sacubitril/valsartan on device detected arrhythmias and electrical parameters among dilated cardiomyopathy patients with reduced ejection fraction and implantable cardioverter defibrillator. J Clin Med. 2020;9:1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Diego C, Gonzalez‐Torres L, Nunez JM, Centurion Inda R, Martin‐Langerwerf DA, Sangio AD, et al. Effects of angiotensin‐neprilysin inhibition compared to angiotensin inhibition on ventricular arrhythmias in reduced ejection fraction patients under continuous remote monitoring of implantable defibrillator devices. Heart Rhythm. 2018;15:395–402. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al.; PARADIGM‐HF Committees and Investigators . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail. 2013;15:1062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16. Jann B. Splitting time‐span records with categorical time‐varying covariates. Stata J. 2004;4:221–2. [Google Scholar]
- 17. Boas R, Thune JJ, Pehrson S, Kober L, Nielsen JC, Videbaek L, et al. Prevalence and prognostic association of ventricular arrhythmia in non‐ischaemic heart failure patients: results from the DANISH trial. Europace. 2021;23:587–95. [DOI] [PubMed] [Google Scholar]
- 18. Teerlink JR, Jalaluddin M, Anderson S, Kukin ML, Eichhorn EJ, Francis G, et al. Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. Circulation. 2000;101:40–6. [DOI] [PubMed] [Google Scholar]
- 19. Singh SN, Fisher SG, Carson PE, Fletcher RD. Prevalence and significance of nonsustained ventricular tachycardia in patients with premature ventricular contractions and heart failure treated with vasodilator therapy. Department of Veterans Affairs CHF STAT Investigators. J Am Coll Cardiol. 1998;32:942–7. [DOI] [PubMed] [Google Scholar]
- 20. Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Kober L, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA‐HF. Eur Heart J. 2021;42:3727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMurray J, Kober L, Robertson M, Dargie H, Colucci W, Lopez‐Sendon J, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post‐Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol. 2005;45:525–30. [DOI] [PubMed] [Google Scholar]
- 22. Arnar DO, Mairesse GH, Boriani G, Calkins H, Chin A, Coats A, et al.; ESC Scientific Document Group; EHRA Scientific Documents Committee . Management of asymptomatic arrhythmias: a European Heart Rhythm Association (EHRA) consensus document, endorsed by the Heart Failure Association (HFA), Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), Cardiac Arrhythmia Society of Southern Africa (CASSA), and Latin America Heart Rhythm Society (LAHRS). Europace. 2019. 10.1093/europace/euz046. [DOI] [PubMed] [Google Scholar]
- 23. Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med. 2016;375:111–21. [DOI] [PubMed] [Google Scholar]
- 24. Connolly SJ, Hallstrom AP, Cappato R, Schron EB, Kuck KH, Zipes DP, et al. Meta‐analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J. 2000;21:2071–8. [DOI] [PubMed] [Google Scholar]
- 25. Pratt CM, Gardner M, Pepine C, Kohn R, Young JB, Greenberg B, et al. Lack of long‐term ventricular arrhythmia reduction by enalapril in heart failure. SOLVD Investigators. Am J Cardiol. 1995;75:1244–9. [DOI] [PubMed] [Google Scholar]
- 26. Rossello X, Ariti C, Pocock SJ, Ferreira JP, Girerd N, McMurray JJV, et al. Impact of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with heart failure and left‐ventricular systolic dysfunction: an individual patient‐level meta‐analysis of three randomized‐controlled trials. Clin Res Cardiol. 2019;108:477–86. [DOI] [PubMed] [Google Scholar]
- 27. Al‐Gobari M, Al‐Aqeel S, Gueyffier F, Burnand B. Effectiveness of drug interventions to prevent sudden cardiac death in patients with heart failure and reduced ejection fraction: an overview of systematic reviews. BMJ Open. 2018;8:e021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei J, Ni J, Huang D, Chen M, Yan S, Peng Y. The effect of aldosterone antagonists for ventricular arrhythmia: a meta‐analysis. Clin Cardiol. 2010;33:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldstein S, Kennedy HL, Hall C, Anderson JL, Gheorghiade M, Gottlieb S, et al. Metoprolol CR/XL in patients with heart failure: a pilot study examining the tolerability, safety, and effect on left ventricular ejection fraction. Am Heart J. 1999;138:1158–65. [DOI] [PubMed] [Google Scholar]
- 30. Martens P, Nuyens D, Rivero‐Ayerza M, Van Herendael H, Vercammen J, Ceyssens W, et al. Sacubitril/valsartan reduces ventricular arrhythmias in parallel with left ventricular reverse remodeling in heart failure with reduced ejection fraction. Clin Res Cardiol. 2019;108:1074–82. [DOI] [PubMed] [Google Scholar]
- 31. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 32. Kober L, Thune JJ, Nielsen JC, Haarbo J, Videbaek L, Korup E, et al.; DANISH Investigators . Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–30. [DOI] [PubMed] [Google Scholar]
- 33. Lee YH, Chiou WR, Hsu CY, Lin PL, Liang HW, Chung FP, et al. Different left ventricular remodeling patterns and clinical outcomes between non‐ischemic and ischemic etiologies in heart failure patients receiving sacubitril/valsartan treatment. Eur Heart J Cardiovasc Pharmacother. 2022;8:118–29. [DOI] [PubMed] [Google Scholar]
- 34. Sarrias A, Bayes‐Genis A. Is sacubitril/valsartan (also) an antiarrhythmic drug? Circulation. 2018;138:551–3. [DOI] [PubMed] [Google Scholar]
- 35. Langenickel TH, Jordaan P, Petruck J, Kode K, Pal P, Vaidya S, et al. Single therapeutic and supratherapeutic doses of sacubitril/valsartan (LCZ696) do not affect cardiac repolarization. Eur J Clin Pharmacol. 2016;72:917–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eiringhaus J, Wunsche CM, Tirilomis P, Herting J, Bork N, Nikolaev VO, et al. Sacubitrilat reduces pro‐arrhythmogenic sarcoplasmic reticulum Ca2+ leak in human ventricular cardiomyocytes of patients with end‐stage heart failure. ESC Heart Fail. 2020;7:2992–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sung YL, Lin TT, Syu JY, Hsu HJ, Lin KY, Liu YB, et al. Reverse electromechanical modelling of diastolic dysfunction in spontaneous hypertensive rat after sacubitril/valsartan therapy. ESC Heart Fail. 2020;7:4040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Torrado J, Cain C, Mauro AG, Romeo F, Ockaili R, Chau VQ, et al. Sacubitril/valsartan averts adverse post‐infarction ventricular remodeling and preserves systolic function in rabbits. J Am Coll Cardiol. 2018;72:2342–56. [DOI] [PubMed] [Google Scholar]
- 39. Chang PC, Wo HT, Lee HL, Lin SF, Chu Y, Wen MS, et al. Sacubitril/valsartan therapy ameliorates ventricular tachyarrhythmia inducibility in a rabbit myocardial infarction model. J Card Fail. 2020;26:527–37. [DOI] [PubMed] [Google Scholar]
- 40. Kang DH, Park SJ, Shin SH, Hong GR, Lee S, Kim MS, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139:1354–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Incidence of adjudicated fatal and non‐fatal events in patients who experienced a ventricular arrhythmia/ICD shock/resuscitated cardiac arrest.
Figure S2. (A) Cumulative incidence of first ventricular arrhythmia in a competing risk regression according to treatment assignment. (B) Cumulative incidence of first ventricular arrhythmia/ICD shock/resuscitated cardiac arrest in a competing risk regression according to treatment assignment.
Figure S3. Restricted cubic spline of the relationship between change in NT‐proBNP (range −10 000 pg/ml to +10 000 pg/ml) from baseline to 8 months and the incidence of ventricular arrhythmia.
Table S1. Cox proportional‐hazard models for each ventricular arrhythmia outcome (serious adverse events only) according to randomized treatment assignment.
Table S2. Competing risk regression for time‐to‐first ventricular arrhythmia outcomes with all‐cause death as a competing risk (Fine and Gray model).
Table S3. (A) Cox regression of mortality outcomes with the development of a ventricular arrhythmia as a time varying covariate. (B) Cox regression of mortality outcomes with the development of ventricular arrhythmia/ICD shock/resuscitated cardiac arrest as a time varying covariate.


