Abstract
Previous studies have reported that serum klotho and vitamin B12 levels are valuable aging-related markers. However, studies supporting the association between serum klotho and vitamin B12 levels are lacking. We investigated the association between serum klotho and vitamin B12 concentrations in adults in the United States. The analytic study sample was 2065 aged 40 to 79 who participated in the 2011 to 2014 National Health and Nutrition Examination Survey (NHANES). Serum klotho and vitamin B12 collected from adults who consented to the use of their samples in the future. The participants were divided into 2 groups based on estimated glomerular filtration rate (eGFR) levels (high: ≥90 mL/min/1.73 m2 or low: <90 mL/min/1.73 m2). Of the 2065 participants, the log-transformed klotho concentration was significantly correlated with log-transformed vitamin B12 in the high eGFR group, but not in the low eGFR group. After adjusting for all potential covariates, there was a significant association between klotho and vitamin B12 concentrations in the high eGFR groups (beta = 0.100, SE = 0.040). In contrast, there was no significant relationship between klotho and vitamin B12 concentrations in the low eGFR group (beta = 0.012, SE = 0.019). Serum klotho concentration was significantly associated with vitamin B12 increases in US adults with high kidney function. Vitamin B12 concentration may be an important marker of klotho concentration in older adults.
Keywords: elderly, GH/IGF-1, kidney function, nutrients
1. Introduction
Klotho is an anti-aging gene that was discovered in 1997. It encodes a single-pass transmembrane protein and is expressed in limited tissues, including the distal convoluted tubules in the kidney and the choroid plexus in the brain. As a transmembrane protein, klotho can be cleaved, shed, and act as a circulating hormone.[1,2] Decreased expression of mouse klotho protein is associated with premature aging phenotypes and a shortened lifespan, whereas mice overexpressing klotho have an increased lifespan.[3,4] In humans, serum klotho concentrations decline with increasing age.[5,6] Serum klotho levels are higher in children and decrease with aging.[5] Among elderly subjects, low klotho concentrations in elderly individuals have been associated with increased mortality,[7] cardiovascular disease,[8] and disability in activities of daily living.[9] The functional role of klotho protein is controversial, but it is known to bind fibroblast growth factor-23 as a cofactor, which regulates phosphate homeostasis and vitamin D metabolism.[10–12] Several lines of evidence suggest a relationship between klotho and growth hormone (GH) secretion.[3] Klotho is also known to inhibit the activity of insulin and the signaling of insulin-like growth factor-1 (IGF-1), a negative regulator of GH secretion, presumably by interfering with receptor-ligand interactions.[11] Therefore, reduced klotho levels increase IGF-1 signaling, resulting in reduced GH secretion.[6] GH and IGF-1 are crucial hormones. Diminished GH and IGF-1 levels are associated with lifespan extension and health benefits in animals and humans.[13] The circulating levels of GH and IGF-1 are affected by multiple factors such as hormonal and genetic levels and especially nutrients (i.e., carbohydrates, amino acids, vitamins, and calcium).[1,14]
Vitamin B12 is an essential micronutrient required for metabolic function.[15] It is a water-soluble vitamin that regulates diverse cellular processes in vertebrates.[16] Humans are unable to synthesize vitamin B12, which is typically found in animal products. Vitamin B12 is crucial in maintaining the normal metabolism and function of all cells in the body. Vitamin B12 deficiency causes significant adverse effects in organ systems, with high cell turnover and metabolism.[17] Unfortunately, vitamin B12 deficiency is globally prevalent due to inadequate intake as a result of high animal product costs, cultural or religious beliefs, and age-related malabsorption. This particularly influences biological aging.[18] Vitamin B12 deficiencies can result in clinical consequences such as dementia,[19,20] decreased cognitive functioning, numbness, muscle weakness,[21,22] and low bone mineral density.[23] Although the physiological role of vitamin B12 is multifactorial, some studies have suggested that vitamin B12 carrier proteins positively regulate GH.[24–26] GH regulates taurine synthesis; taurine, a semi-essential amino acid important for growth and metabolism, is an upstream regulator of IGF-1 synthesis in the liver, and it is involved in osteoblast action.[25]
Based on circumstantial evidence, research has suggested that klotho has a strong interaction with GH, IGF-1 secretion gradually decreases with age, and the GH/IGF-1 axis is influenced by the nutritional state.[27] Circulating klotho is an anti-aging factor associated with the GH/IGF-1 axis, and vitamin B12 is an important micronutrient that controls aging-related metabolic markers and can influence GH and IGF-1 secretion.[15] Therefore, we focused on the possibility of a plausible association between klotho and vitamin B12 levels. A reliable method for measuring klotho concentrations in the blood has been developed, and the National Health and Nutrition Examination Survey (NHANES) provides valuable data to investigate the association between serum klotho and vitamin B12 concentrations in a nationally representative sample of the US population. In the present study, we aimed to investigate the association between anti-aging klotho and vitamin B12 levels in adults.
2. Materials and Methods
2.1. Study population
The NHANES, a cross-sectional study conducted by the Centers for Disease Control and Prevention, is a nationally representative survey of the non-institutionalized civilian population in the United States. This study used publicly available data on serum klotho and vitamin B12 concentrations collected during the 2011 to 2012 and 2013 to 2014 waves. The National Centers for Health Statistics Institutional Review Board approved the study protocols of the NHANES.[28]
Serum klotho and vitamin B12 samples were collected from participants aged 40 to 79 years and >20 years, respectively. This study needed data on serum klotho and vitamin B12 levels to investigate this association. Therefore, of the 19,931 participants, we selected 5225 who were aged 40 to 79 years old and had provided pristine serum samples as well as consent for their samples to be used in future research. Among these individuals, we excluded 2770 participants who had no serum vitamin B12 data, as well as 390 participants who had missing data on other variables (i.e., education, household income, smoking status, alcohol consumption, body mass index [BMI], and disease history information). The final sample size was 2065 participants from the 2011 to 2014 NHANES.
2.2. Measurement of serum klotho
Available pristine serum samples were collected from 40 to 79 years old participants who had consented to the use of their samples for future research. Klotho concentration measurements were performed on frozen samples from 2011 to 2012 and 2013 to 2014 waves that were received and tested during 2019 to 2020. All samples were stored at −80℃ until daily predefined batches of samples were provided to the technicians for the analyses. The analyses were performed by a commercially available ELISA kit (IBL International, Japan). The assay sensitivity was calculated to be 4.33 pg/mL. Two samples, including very high and high klotho concentrations were used to evaluate the assay linearity at different dilutions. Plots on the expected (r2 = 0.998) and obtained values (r2 = 0.997) demonstrated an excellent linearity in the assay measurement range. The intra-assay precision was obtained for 2 recombinant klotho, and 2 human samples exhibited a coefficient of variation of 3.2% and 3.9% for the recombinant and 2.3% and 3.3% for the human samples, respectively. Analyses were performed on all samples (excluding four fresh-frozen [pristine] samples received from the Centers for Disease Control and Prevention) by the Northwest Lipid Metabolism and Diabetes Research Laboratories, Division of Metabolism, Endocrinology, and Nutrition, University of Washington. The serum samples were analyzed in duplicate, and the average of the 2 values was used as the final value. Each sample plate also contained 2 quality control samples with low and high concentrations of klotho analyzed in duplicate. Results of analyses were automatically transmitted from the instrument to the laboratory Oracle Management System for evaluation. The average of 2 duplicate analyses was used for quality assurance, and samples with duplicate results exceeding 10% were reanalyzed. If the value of a quality control sample was not within 2 standard deviations of the known value, the entire plate was repeated. The lower detection limit was 6 pg/mL. However, there are no specific cutoff points for klotho concentrations to be used as indicators of biological age.
2.3. Measurement of serum vitamin B12
Blood samples were collected from adults aged more than 20 years old and were processed, stored, and shipped to the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, for analysis. The sample vials were stored under the appropriate conditions (−30°C) until they were shipped to the National Center for Environmental Health. Serum vitamin B12 concentrations were measured using fully automated Roche electrochemiluminescence immunoassay on a Modular Analytics E170 system (Roche Diagnostics, IN). Serum specimens were collected in regular red-top Vacutainers or tubes containing separating gel, and plasma specimens were collected using Na-heparin or K3-EDTA as an anticoagulant. The NHANES quality control showed significant shifts associated with reagent lot-to-lot variations and adjusted the serum B12 measurements using mean quality control pool data using Deming regression. The quality control limits for all pools were established by analyzing duplicates of each pool for at least 20 consecutive runs. The lower limit of detection for vitamin B12 was 30 pg/mL (i.e., 22.14 pmol/L).
2.4. Other variables
Questionnaire information included age (40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, or 75–79 years old), sex (male or female), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other), education (less than high school, high school, or College or more), and annual household income (<$20,000 or ≥$20,000). Health behavior variables included smoking status (current, former, or never), alcohol consumption (drinker or nondrinker), moderate recreational activities (yes or no), and BMI. BMI was calculated using the standard formula, which divides the individual’s weight (kg) by his or her height squared (m2), and categorized into the following 4 groups: underweight (<18.5 kg/m2), normal weight (18.5–22.9 kg/m2), overweight (23–24.9 kg/m2), and obese (≥25 kg/m2). Disease history information such as a physical diagnosis of diabetes (yes or no) or hypertension (yes or no) was also considered in this study. We calculated the estimated glomerular filtration rate (eGFR) using the equation (141 × min(serum creatinine/κ, 1)α × max(serum creatinine/κ, 1)- 1.209 × 0.993Age × 1.018 [if female] × 1.159 [if Black]) developed by the Chronic Kidney Disease Epidemiology Collaboration[29] and grouped the participants into 2 eGFR levels (≥90 mL/min/1.73 m2 or <90 mL/min/1.73m2).
2.5. Statistical analyses
The study was performed for weighted estimates of population parameters based on the NHANES analytic and reporting guidelines. All statistical analyses were applied to the PROC SURVEY procedures in SAS 9.4 (SAS Institute, Cary, NC), and the statistical significance was set at α = 0.05.
For each variable, the chi-square test and analysis of variance were performed to test for significance in each group. The participants were divided into 2 groups based on their eGFR levels (high: ≥90 mL/min/1.73 m2 or low: <90 mL/min/1.73 m2) to determine the statistical differences between the mean klotho concentration in each group. Pearson correlation coefficients between the klotho and vitamin B12 concentrations were calculated. Linear regression analysis was used to evaluate the association, and to provide beta coefficients and standard errors (SE).
Additionally, regression models were adjusted for age, sex, race/ethnicity, education, annual household income, smoking status, drinking consumption, moderate recreational activities, BMI, the presence of diabetes and/or hypertension, and eGFR.
3. Results
Table 1 shows the mean (standard deviation) klotho concentrations according to the characteristics of the study population. As age increased, the klotho concentrations tended to increase; the subjects aged 40 to 45 years (mean = 942.86) had higher klotho concentrations than those 70 years and older (mean = 820.31). The Black participants (mean = 919.98) had the highest klotho concentrations, whereas White participants had the lowest concentrations (mean = 858.82). Participants who had a college or high education (mean = 906.44) and a higher household income (mean = 891.40) had higher klotho concentrations than those who had lower concentrations of education and household income. Health behavior variables such as smoking status (current smoke vs never smoker, 852.72 vs 915.23), alcohol drinking (drinker vs nondrinker, 874.85 vs 909.92), and moderate recreational activities (yes vs no, 900.12 vs. 873.28) resulted in differences in klotho concentrations in each group. Participants who did not have diabetes and/or hypertension were more likely to have higher klotho concentrations than those who did.
Table 1.
Characteristics of study populations for klotho concentration (n = 2065).
| N | (%) | Mean | SD | P value | ||
|---|---|---|---|---|---|---|
| Age (yr) | ||||||
| 40–44 | 270 | (13.1) | 942.86 | (333.14) | <.0001 | |
| 45–49 | 270 | (13.1) | 875.96 | (297.98) | ||
| 50–54 | 307 | (14.8) | 937.84 | (365.07) | ||
| 55–59 | 266 | (12.9) | 840.63 | (273.56) | ||
| 60–64 | 376 | (18.2) | 889.35 | (283.37) | ||
| 65–69 | 235 | (11.4) | 890.78 | (388.20) | ||
| 70–74 | 202 | (9.8) | 820.31 | (268.36) | ||
| 75–79 | 139 | (6.7) | 820.61 | (261.55) | ||
| Sex | ||||||
| Male | 1014 | (49.1) | 863.13 | (302.68) | .4155 | |
| Female | 1051 | (50.9) | 904.75 | (329.05) | ||
| Race/ethnicity | ||||||
| White | 824 | (39.9) | 858.82 | (295.63) | .0056 | |
| Black | 547 | (26.5) | 919.98 | (380.12) | ||
| Hispanic | 419 | (20.3) | 881.33 | (275.62) | ||
| Others | 275 | (13.3) | 894.31 | (293.17) | ||
| Education | ||||||
| Less than high school | 502 | (24.3) | 857.62 | (305.64) | .0026 | |
| High school | 446 | (21.6) | 858.93 | (295.99) | ||
| College or more | 1117 | (54.1) | 906.44 | (328.47) | ||
| Household income, $ | ||||||
| <20,000 | 514 | (24.9) | 862.93 | (315.76) | <.0001 | |
| ≥20,000 | 1551 | (75.1) | 891.40 | (317.17) | ||
| Smoking status | ||||||
| Current smoker | 408 | (19.8) | 852.72 | (299.84) | <.0001 | |
| Former smoker | 587 | (28.4) | 849.92 | (309.52) | ||
| Never smoker | 1070 | (51.8) | 915.23 | (324.45) | ||
| Alcohol drinking | ||||||
| Drinker | 1508 | (73.0) | 874.85 | (318.07) | <.0001 | |
| Nondrinker | 557 | (27.0) | 909.92 | (312.87) | ||
| Moderate recreational activities for at least 10 min | ||||||
| Yes | 849 | (41.1) | 900.12 | (311.52) | <.0001 | |
| No | 1216 | (58.9) | 873.28 | (320.41) | ||
| Body mass index (kg/m2) | ||||||
| Underweight (<18.5) | 15 | (0.7) | 781.77 | (342.73) | .2688 | |
| Normal weight (18.5–24.9) | 493 | (23.9) | 904.14 | (338.90) | ||
| Overweight (25-29.9) | 712 | (34.5) | 878.80 | (312.55) | ||
| Obese (≥30) | 845 | (40.9) | 879.21 | (306.69) | ||
| Diabetes | ||||||
| Yes | 386 | (18.7) | 854.59 | (308.36) | <.0001 | |
| No | 1679 | (81.3) | 891.14 | (318.63) | ||
| Hypertension | ||||||
| Yes | 979 | (47.4) | 862.45 | (319.61) | .0185 | |
| No | 1086 | (52.6) | 904.02 | (313.44) | ||
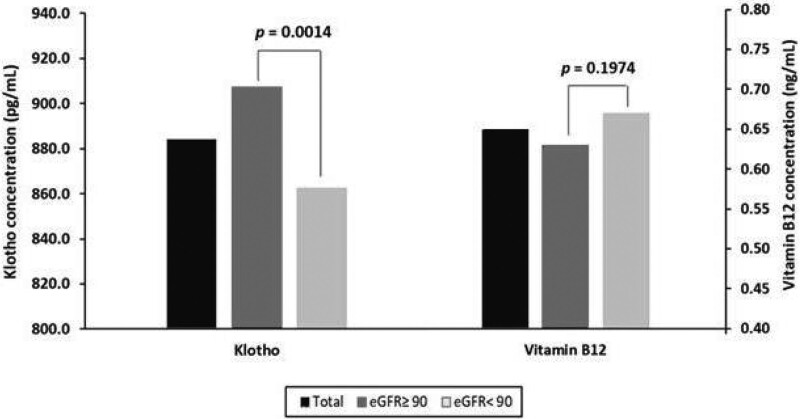
Figure 1 presents the structures of klotho and vitamin B12 concentrations according to the 2 eGFR groups. Of the 2065 participants, the mean klotho and vitamin B12 concentrations were 884.31 and 0.65 ng/mL, respectively. The mean klotho concentration was a significantly higher in the high eGFR group than in the low eGFR group (907.32 vs 862.67 pg/mL; P value = .0014). In contrast to the mean klotho concentration, the mean vitamin B12 concentration was lower in the high eGFR group than in the low eGFR group. However, the differences in the mean vitamin B12 concentrations between the 2 groups are not significant (low eGFR group vs high eGFR group, 0.63 vs 0.67 ng/mL; P value = .1974).
Figure 1.
Concentration of klotho and vitamin B12 according to the 2 eGFR groups. eGFR = estimated glomerular filtration rate.
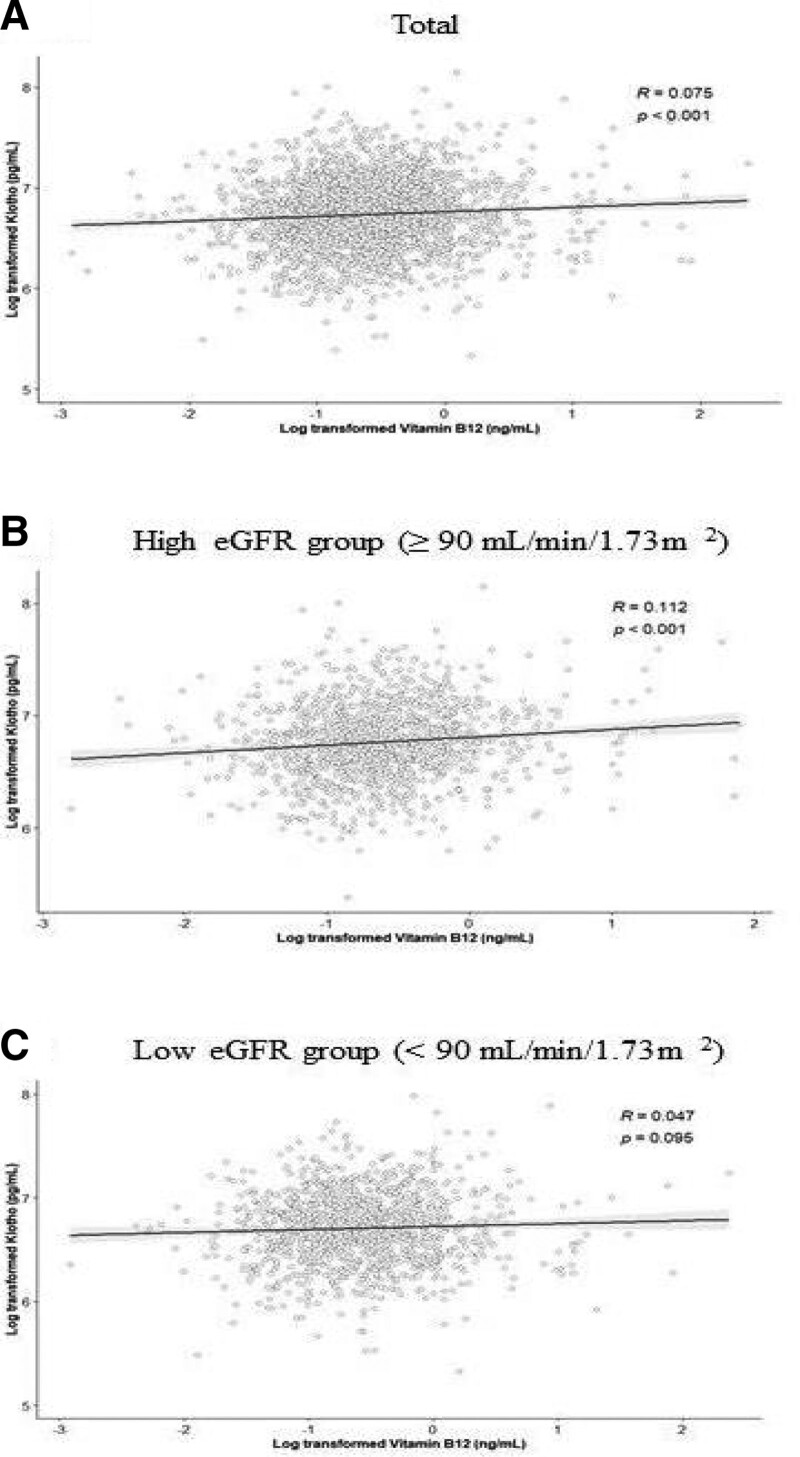
Figure 2 shows the Pearson correlation distributions between the klotho and vitamin B12 concentrations in the 2 eGFR groups. The log transformed klotho concentration was significantly correlated with the log transformed vitamin B12 concentrations (R = 0.075). Statistically significant positive correlation coefficients were observed between the log transformed klotho and vitamin B12 concentrations in the high eGFR group (R = 0.112). Compared to the correlation coefficients in the high eGFR group, the correlation coefficients between the log transformed klotho and vitamin B12 concentrations was not significant in the low eGFR group (R = 0.047).
Figure 2.
Pearson correlation coefficients between the log transformed klotho and vitamin B12 concentrations in total study population (A), high eGFR group (≥90 mL/min/1.73 m2) (B), and low eGFR group (<90 mL/min/1.73 m2) (C). eGFR = estimated glomerular filtration rate.
Table 2 indicates the estimated beta coefficients (SE) of the log transformed klotho and vitamin B12 concentrations in the 2 eGFR groups. In the unadjusted model, there was a significantly positive association with the vitamin B12 concentration as the klotho concentration increased by 1 unit (beta = 0.046, SE = 0.011; P value = .001). In the high eGFR group, the beta coefficient of the klotho concentration for the increasing vitamin B12 concentration was found to be significant at 0.107 (SE = 0.030; P value = .002). However, no significant relationship between the klotho and vitamin B12 concentrations was observed in the low eGFR group. In the adjusted model for all potential covariates, a significant association with an increase in 1 unit of vitamin B12 was observed with the klotho concentration (beta = 0.043, SE = 0.011; P value = .002). The adjusted beta coefficient was also significant in the high eGFR group for the klotho and vitamin B12 concentrations (beta = 0.100, SE = 0.040; P value = .022). In contrast, there was no significant relationship between the klotho and vitamin B12 concentrations in the low eGFR group (beta = 0.012, SE = 0.019; P value = .548).
Table 2.
Beta coefficients (SE) of the log transformed klotho and vitamin B12 concentrations in the 2 eGFR groups.
| Unadjusted model | Adjusted model | |||
|---|---|---|---|---|
| Beta (SE) | P value | Beta (SE) | P value | |
| 1 unit increase Vitamin B12 (ng/mL) | 0.046 (0.011) | .001 | 0.043 (0.011) | .002* |
| Stratified by eGFR | ||||
| High (≥90 mL/min/1.73 m2) | 0.107 (0.030) | .002 | 0.100 (0.040) | .022† |
| Low (<90 mL/min/1.73 m2) | 0.018 (0.022) | .433 | 0.012 (0.019) | .548† |
BMI = body mass index, eGFR = estimated glomerular filtration rate, SE = standard error.
This result was adjusted for age, sex, ethnicity, education, income, smoking status, alcohol drinking, physical activities, BMI, the presence of diabetes, hypertension, and eGFR.
This result was adjusted for age, sex, ethnicity, education, income, smoking status, alcohol drinking, physical activities, BMI, the presence of diabetes, and hypertension.
4. Discussion
The main objective of this study was to investigate the association between anti-aging protein klotho and vitamin B12 concentrations in the general population of the United States during 2011 to 2014. We found that increases in serum klotho concentrations were significantly associated with higher vitamin B12 concentrations in adults aged 40 to 79 years old. Klotho is highly expressed in the kidney, and klotho concentrations are closely correlated with kidney function[30]; we stratified the study population into 2 groups based on the eGFR levels. A significant association between klotho and vitamin B12 concentrations was observed among participants with high eGFR levels, but not among the low eGFR level group. Although we do not yet understand the clinical relevance of circulating anti-aging protein klotho concentrations, increases in vitamin B12 concentrations appeared to affect the increase in klotho concentrations, especially in participants with high eGFR (≥90 mL/min/1.73 m2).
There is no evidence of a significant association between klotho and vitamin B12 concentrations; however, there may be a plausible link among klotho concentrations, the GH/IGF-1 axis, and vitamin B12 concentrations. Population aging poses great challenges for longevity and diseases, and addressing aging-related factors has gained much attention in scientific research. One of the factors is klotho, and research is currently underway to elucidate its anti-aging effects in humans.[7,9]
In a cross-sectional study by Crasto et al,[9] 802 participants (≥65 years old) with low klotho concentrations had disabilities in their activities of daily living (odds ratio = 0.57, 95% CI: 0.35–0.93). Semba et al[7] explained the association between klotho concentrations and mortality in a longitudinal study of 804 subjects aged ≥ 65 years. During the 6 years follow-up period, participants with lower klotho concentrations showed an increased risk of death compared to those with higher klotho concentrations (hazard ratio = 1.78, 95% CI: 1.20–2.63). The results of these studies highlights the positive effect of high klotho concentrations on aging.
In the context of the aging process, the GH/IGF-1 axis is one of the most discussed topics. GH is known to be a regulator of the aging process, and the secretion of GH and IGF-1 tends to progressively decrease with increasing age.[13] Age is a determinant of GH response, with younger individuals showing a more robust response by decreasing in older subjects.[31] The age-related decline in GH and IGF-1 secretion contributes to functional deficits and disorders such as dementia[32] and cardiovascular disease.[33] Recent studies have demonstrated that klotho concentrations are positively correlated with GH and IGF-1 levels.[34–36] Gkentzi et al[34] investigated the relationship between klotho and IGF-1 levels. Although the 159 participants in the study were healthy children, unlike our study participants, the finding of a significant positive correlation between klotho and IGF-1 levels in their study could support our hypothesis on the plausible link between IGF-1 and klotho concentrations (P < .0001). In a prospective controlled study by Neidert et al,[35] klotho concentrations were found to be higher in the active acromegaly group than in the control group with non-GH-producing pituitary adenomas. In a study of patients with excessively high soluble klotho concentrations, similar results were found, which suggest that serum klotho expression or secretion is regulated by GH or IGF-1 levels.[35]
Nutrition is an important regulator of IGF-1 synthesis and secretion. Adequate nutrition is required for GH production, which stimulates IGF-1 production in the liver.[36] Nutrients, through IGF-1, can modulate the pathways that regulate aging and age-related disorders. An imbalance in the levels of macronutrients (e.g., carbohydrates, fibers, proteins, and lipids) or micronutrients (e.g., vitamin D, vitamin A, vitamin B complex, and calcium) can affect GH and IGF-1 secretion and action.[37,38] The vitamin B complex is an essential water soluble vitamin that regulates a multitude of vertebrate cellular processes, including growth, development, and oxidative processes. Of these nutrients, vitamin B12 plays a role in the up-regulation of GH and IGF-1. In a study involving a mouse model, Roman-Garcia et al[25] found that vitamin B12 deficiency caused severe growth retardation and osteoporosis in mice. Vitamin B12 deficiency decreases the hepatic production of taurine, an essential amino acid needed for growth and metabolism; and a decline in taurine production influences GH and IGF-1 synthesis in the liver resulting in growth defects and osteoporosis. We found a significant association between serum klotho and vitamin B12 concentrations, but this was very limited and could not explain their biological link. Taken together, these studies indicate that klotho expression was regulated by GH and IGF-1 levels, despite evidence of a bi-directional interaction between them. Vitamin B12 is related to GH and IGF- 1 synthesis; therefore, high vitamin B12 concentrations may promote klotho concentrations. However, this possibility needs to be verified by further research.
To our knowledge, this is the first study to demonstrate an association between klotho and vitamin B12 concentrations. We analyzed data obtained from the NHANES study, a large-scale, nationally representative study of the US population that provide valuable data. It included potential covariates that enabled us to establish the independence of the relationships between them. However, this study had several limitations. First, as this was a cross-sectional study, these associations could not be examined for causality. Therefore, it is difficult to generalize the causal relationship between klotho and vitamin B12 levels. This should be tested in larger cohorts to determine causality. Second, the study was not free from bias due to the self-reported data. As this study was based on an observational investigation that included several variables, recall bias remained in the characteristics of the participants. Moreover, we could not consider all possible confounding factors, and there may have been unmeasured confounders that affected the relationship between klotho and vitamin B12 concentrations.
5. Conclusions
As described above, our study found that anti-aging protein klotho concentrations increased as vitamin B12 concentrations increased, especially in adults with high kidney function (eGFR ≥ 90 mL/min/1.73 m2). However, there was no significant relationship between klotho and vitamin B12 levels in the low eGFR group. Although this finding suggests that vitamin B12 concentrations may be a valuable marker of klotho concentration, their direct association with human biology is questionable. Further research is needed to investigate the validity of this finding and establish the biological mechanisms.
Author contributions
Conceptualization: Kyoung-Bok Min.
Formal analysis: Ju-Young Choi, Kyoung-Bok Min.
Methodology: Jin-Young Min.
Supervision: Kyoung-Bok Min.
Validation: Jin-Young Min.
Visualization: Ju-Young Choi.
Writing – original draft: Ju-Young Choi.
Writing – review & editing: Jin-Young Min, Kyoung-Bok Min.
Abbreviations:
- BMI =
- body mass index
- eGFR =
- estimated glomerular filtration rate
- GH =
- growth hormone
- IGF-1 =
- insulin-like growth factor-1
- NHANES =
- National Health and Nutrition Examination Survey
- SE =
- standard errors
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (grant numbers 2022R1A2C2010463, 2019R1A2C1004966 and 2020R1A2C1102097). This work was supported by the Education and Research Encouragement Fund of the Seoul National University Hospital. The funding body did not have any role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
The 2011–2014 NHANES was approved by the US National Center for Healthcare Statistics (NCHS) Research Ethics Review Board (ERB) (protocol numbers 2011-17 and continuation of protocol #2011-17). This study was exempt from a formal ethics review as a secondary analysis of existing NHANES public data under the US Health and Human Services regulations at 45 CFR 46.101(b). Participants provided written informed consent before the home interview and health examinations (https://www.cdc.gov/nchs/nhanes/hlthprofess.htm). This study was conducted in accordance with the Declaration of Helsinki guidelines.
The authors have no conflicts of interest to disclose.
Research data supporting this publication are available from the “NHANES” database at located at https://www.cdc.gov/nchs/nhanes/index.htm.
How to cite this article: Choi J-Y, Min J-Y, Min K-B. Anti-aging protein klotho was associated with vitamin B12 concentration in adults. Medicine 2022;101:40(e30710).
Contributor Information
Ju-Young Choi, Email: cjuyoung@snu.ac.kr.
Jin-Young Min, Email: minkb@snu.ac.kr.
References
- [1].Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. [DOI] [PubMed] [Google Scholar]
- [2].Masuda H. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev. 2005;126:1274–83. [DOI] [PubMed] [Google Scholar]
- [3].Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rubinek T, Wolf I, Modan-Moses D. The longevity hormone klotho is a new player in the interacion of the growth hormone/insulin-like growth factor 1 axis. Pediatr Endocrinol Rev. 2016;14:9–18. [DOI] [PubMed] [Google Scholar]
- [5].Yamazaki Y. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: age-dependent change of soluble alpha-klotho levels in healthy subjects. Biochem Biophys Res Commun. 2010;398:513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wolf I. Association between decreased klotho blood levels and organic growth hormone deficiency in children with growth impairment. PLoS One. 2014;9:e107174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Semba RD, Cappola AR, Sun K, et al. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. 2011;66:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Semba RD. Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc. 2011;59:1596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Crasto CL. Relationship of low-circulating “anti-aging” klotho hormone with disability in activities of daily living among older community-dwelling adults. Rejuvenation Res. 2012;15:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Urakawa I. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4. [DOI] [PubMed] [Google Scholar]
- [11].Kurosu H. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kurosu H, Kuro-o M. The Klotho gene family and the endocrine fibroblast growth factors. Curr Opin Nephrol Hypertens. 2008;17:368–72. [DOI] [PubMed] [Google Scholar]
- [13].Bartke A, Darcy J. GH and ageing: pitfalls and new insights. Best Pract Res Clin Endocrinol Metab. 2017;31:113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Caputo M. Regulation of GH and GH signaling by nutrients. Cells. 2021;10:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].de Benoist B. Conclusions of a WHO technical consultation on folate and vitamin B12 deficiencies. Food Nutr Bull. 2008;29(2 Suppl):S238–44. [DOI] [PubMed] [Google Scholar]
- [16].Nielsen MJ. Vitamin B12 transport from food to the body’s cells – a sophisticated, multistep pathway. Nat Rev Gastroenterol Hepatol. 2012;9:345–54. [DOI] [PubMed] [Google Scholar]
- [17].Wong CW. Vitamin B12 deficiency in the elderly: is it worth screening? Hong Kong Med J. 2015;21:155–64. [DOI] [PubMed] [Google Scholar]
- [18].Shin C, Baik I. Leukocyte telomere length is associated with serum vitamin B12 and homocysteine levels in older adults with the presence of systemic inflammation. Clin Nutr Res. 2016;5:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Agnew-Blais JC. Folate, vitamin B-6, and vitamin B-12 intake and mild cognitive impairment and probable dementia in the Women’s health initiative memory study. J Acad Nutr Diet. 2015;115:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Morris MS, Selhub J, Jacques PF. Vitamin B-12 and folate status in relation to decline in scores on the mini-mental state examination in the framingham heart study. J Am Geriatr Soc. 2012;60:1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gröber U, Kisters K, Schmidt J. Neuroenhancement with vitamin B12-underestimated neurological significance. Nutrients. 2013;5:5031–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pavlov CS. Neurological disorders in vitamin B12 deficiency. Ter Arkh. 2019;91:122–9. [DOI] [PubMed] [Google Scholar]
- [23].Morris MS, Jacques PF, Selhub J. Relation between homocysteine and B-vitamin status indicators and bone mineral density in older Americans. Bone. 2005;37:234–42. [DOI] [PubMed] [Google Scholar]
- [24].Lobie PE, García-Aragón J, Waters MJ. Growth hormone (GH) regulation of gastric structure and function in the GH-deficient rat: up-regulation of intrinsic factor. Endocrinology. 1992;130:3015–24. [DOI] [PubMed] [Google Scholar]
- [25].Roman-Garcia P, Quiros-Gonzalez I, Mottram L, et al. Vitamin B12–dependent taurine synthesis regulates growth and bone mass. J Clin Invest. 2014;124:2988–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Guevara-Aguirre J, Guevara A, Palacios I, et al. GH and GHR signaling in human disease. Growth Horm IGF Res. 2018;38:34–8. [DOI] [PubMed] [Google Scholar]
- [27].Rubinek T. Klotho response to treatment with growth hormone and the role of IGF-I as a mediator. Metabolism. 2016;65:1597–604. [DOI] [PubMed] [Google Scholar]
- [28].Center for Disease Control and Prevention. National Health and Nutrition Examination Survey guidelines. Available at: https://www.cdc.gov/nchs/nhanes/hlthprofess.htm.
- [29].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sanchez-Niño MD, Fernandez-Fernandez B, Ortiz A. Klotho, the elusive kidney-derived anti-ageing factor. Clin Kidney J. 2020;13:125–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tanaka K. Age-related decrease in plasma growth hormone: response to growth hormone-releasing hormone, arginine, and L-dopa in obesity. Metabolism. 1991;40:1257–62. [DOI] [PubMed] [Google Scholar]
- [32].Westwood AJ. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology. 2014;82:1613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Laughlin GA. The prospective association of serum insulin-like growth factor I (IGF-I) and IGF-binding protein-1 levels with all cause and cardiovascular disease mortality in older adults: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2004;89:114–20. [DOI] [PubMed] [Google Scholar]
- [34].Gkentzi D. Fibroblast growth factor 23 and Klotho serum levels in healthy children. Bone. 2014;66:8–14. [DOI] [PubMed] [Google Scholar]
- [35].Neidert MC. Soluble α-klotho: a novel serum biomarker for the activity of GH-producing pituitary adenomas. Eur J Endocrinol. 2013;168:575–83. [DOI] [PubMed] [Google Scholar]
- [36].Sze L. Excessively high soluble Klotho in patients with acromegaly. J Intern Med. 2012;272:93–7. [DOI] [PubMed] [Google Scholar]
- [37].Johnson SC. Nutrient sensing, signaling and ageing: the role of IGF-1 and mTOR in ageing and age-related disease. Subcell Biochem. 2018;90:49–97. [DOI] [PubMed] [Google Scholar]
- [38].Brown-Borg HM. The somatotropic axis and longevity in mice. Am J Physiol Endocrinol Metab. 2015;309:E503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]