Abstract
The spore coat protein CotA of Bacillus subtilis displays similarities with multicopper oxidases, including manganese oxidases and laccases. B. subtilis is able to oxidize manganese, but neither CotA nor other sporulation proteins are involved. We demonstrate that CotA is a laccase. Syringaldazine, a specific substrate of laccases, reacted with wild-type spores but not with ΔcotA spores. CotA may participate in the biosynthesis of the brown spore pigment, which appears to be a melanin-like product and to protect against UV light.
The spore-forming bacterium Bacillus subtilis synthesizes and deposits a protein coat around the developing endospore during differentiation (8). The spore coat consists of at least 25 different polypeptides of 5 to 65 kDa, and some of them are highly cross-linked. These proteins are assembled into a lamella-like inner coat and an electron-dense outer coat, which protects the spore from a diverse range of stresses (8).
The cotA gene codes for a 65-kDa protein belonging to the outer spore coat of B. subtilis. It corresponds to the former pig locus (7, 21) and was one of the first cot genes to be cloned (7). The cotA gene is expressed under the control of sigma K, with GerE acting as a transcriptional repressor instead of its more usual function of transcriptional activator (24). The absence of CotA has no apparent effect on spore resistance, but it results in the loss of the usual brownish pigmentation of the colonies. Moreover, wild-type spore-forming colonies are darker at higher manganese concentrations (13). This is similar to marine Bacillus sp. strain SG1, which has spores that express manganese oxidase (30).
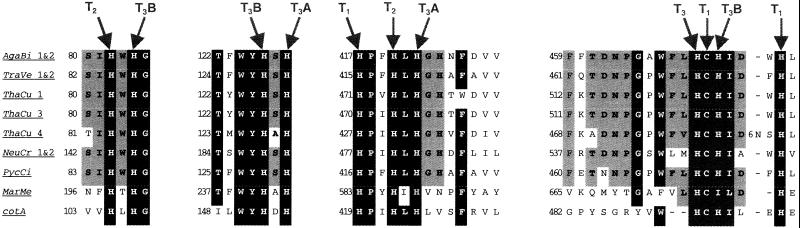
The CotA protein displays similarities with multicopper oxidases (for a review, see reference 28). In particular, it contains the four copper-binding sites, the type I blue copper center (T1) and the T2/T3 trinuclear cluster, which differ in their spectroscopic features (Fig. 1). The multicopper oxidase family includes ascorbate oxidase (EC 1.10.3.3), ceruloplasmin (EC 1.16.3.1), laccase (EC 1.10.3.2), various manganese oxidases, and other enzymes. Among the proteins most closely related to CotA are the manganese oxidase of Leptothrix discophora (4) and the CumA protein of Pseudomonas putida (1), which is essential for manganese oxidation. CotA also shares similarities with a polyphenoloxidase of Acremonium murorum and, with much lower similarity scores, laccases including those of Neurospora crassa (9), Agaricus bisporus (18), and Marinomonas mediterranea—formerly an Alteromonas species (MMB1) (23). Laccase was first found in the sap of the Japanese lacquer tree (hence its name). Laccases are widespread in plants, where they are involved in lignin biosynthesis, and in fungi, where they are involved in ligninolysis, development-associated pigmentation, detoxification, and pathogenesis (28, 29). Laccases are polyphenoloxidases which oxidize a wide range of polyphenols, methoxy-substituted phenols, and diamines. Substrate specificity differs from one laccase to another, but most laccases do not oxidize tyrosine, in contrast to the classic tyrosinase (EC 1.14.18.1) (for a review, see reference 29). Tyrosinase is also a polyphenoloxidase, but it contains only one coupled binuclear copper center (type III; T3).
FIG. 1.
The four copper-binding sites in CotA and in some fungal laccases. Abbreviations: AgaBi, A. bisporus; TraVe, Trametes versicolor; ThaCu, Thanatephorus cucumeris; NeuCr, N. crassa; PycCi, Pycnoporus cinnabarinus; MarMe, M. mediterranea. There are two laccases (laccases 1 and 2) which have identical copper-binding sites in A. bisporus, T. versicolor, and N. crassa. Conserved amino acids are shaded in black (90% conservation or more) or in grey (70 to 90% conservation). T1, T2, T3A, and T3B are the type 1, 2, and 3 copper centers, which differ by their spectroscopic features (T3 center contains two copper atoms called A and B). For each copper center, arrows point to the copper-binding residues, which upon folding of the protein come into close proximity and coordinate copper.
Despite similarities with the multicopper oxidases, it is not known if CotA has any oxidase activity in B. subtilis. We tested whether CotA is a manganese oxidase or a laccase with or without tyrosinase activity. We report that CotA is a classical laccase.
To identify the function of CotA, a cotA::aphA3 mutant was constructed. A DNA fragment including the cotA gene was amplified by PCR using chromosomal DNA as the template. Oligonucleotides allowing the creation of a HindIII site 294 bp upstream from the start codon of cotA and an EcoRI site 114 bp downstream from the stop codon were used. The resulting fragment was ligated between the EcoRI and HindIII sites of pUC18. A kanamycin cassette was then inserted between a PstI site (95 bp upstream of the translation start) and an XbaI site (54 bp upstream from the stop codon), deleting the whole coding sequence except for the last 18 amino acids. The resulting plasmid was linearized and used to transform B. subtilis strain 168 to give the cotA::aphA3 mutant (ΔcotA).
All experiments were performed in accordance with European regulation requirements concerning the contained use of genetically modified organisms from group 1 (regulation no. 2735).
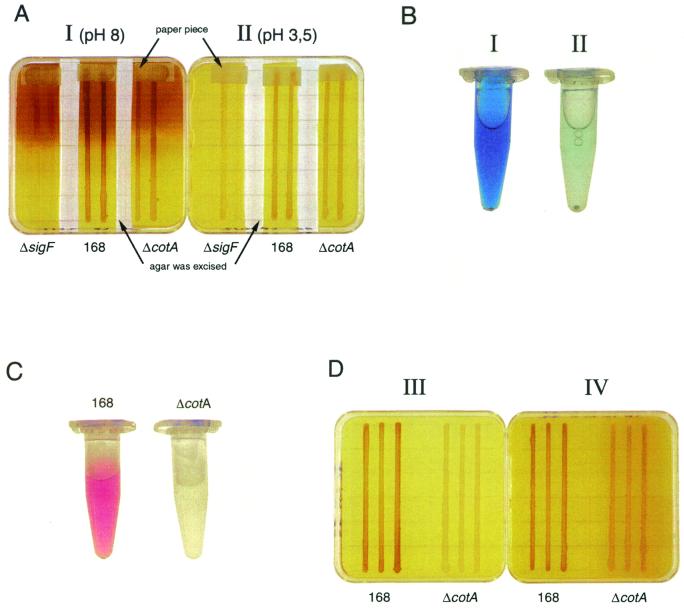
To define the relationship between spore pigmentation and manganese, the formation of the brownish color was studied in several culture conditions and in various mutants. Precultures were diluted to an optical density at 600 nm of 0.1. Using a home-made replicator (for details, see http://locus.jouy.inra.fr/genmic/madbase/mutant/home.gif), the bacteria were streaked on SP agar medium (pH 7.0) (26) in square Falcon plates (Fig. 2A). The bacteria were grown for 48 h at 37°C. To modify the pH, a Whatman paper (8 by 8 cm) was then laid in the plate lid and soaked with 1.5 ml of either distilled water (Fig. 2A, plate I) or 3 M sodium acetate, pH 4.8 (Fig. 2A, plate II). After 24 h, the pH was checked and found to be 8 in plate I and 3.5 in plate II. Small pieces of Whatman paper containing 10 μl of 5 M MnCl2 were then placed at one end of the streaks. After 48 h, a brown pigmentation appeared near the site of MnCl2 application in plate I, but not in plate II (Fig. 2A). There was no pigmentation on plates similarly treated with manganese in the absence of bacteria (data not shown). Thus, the appearance of brown pigmentation in the agar around the colonies depends on the presence of B. subtilis and manganese and also on the pH of the plates. Similar results were obtained with strain 168, a ΔsigF mutant which is blocked early in the sporulation process, and the ΔcotA mutant (Fig. 2A). This pigmentation found at high pH is therefore independent of sporulation and particularly of CotA.
FIG. 2.
B. subtilis pigmentation in several culture conditions. (A) Manganese oxidation on plates by wild-type, sigF, and ΔcotA strains. Ten microliters of 5 M MnCl2 in Whatman paper was deposited at the top of the plates. An agar band was excised to separate each strain from its neighbors on the plate. (B) Leucoberbelin blue test. For each strain, a piece of 0.15 g of browned agar was excised from plates I and II shown in panel A near the paper band containing MnCl2. After being melted in 800 μl of water acidified by the addition of 400 μl of 3 M sodium acetate (pH 4.8), 40 μl of leucoberbelin blue solution was added. Tubes I and II correspond to plates I and II of panel A, respectively. All three strains gave the same result on plate I, on the one hand, and on plate II, on the other hand. (C) The syringaldazine test was performed using a spore suspension obtained by the water-washing method (20). (D) Production of spore pigment on SP plates supplemented with 10 μM CuSO4 and with (plate IV) or without (plate III) 1 mM MnCl2.
To determine whether the observed brown color was due to manganese oxide, leucoberbelin blue, which specifically reacts with MnIII to MnVII, was tested with the browned agar (14). For each strain, dark blue (tube I, corresponding to plate I) and pale blue (tube II, corresponding to plate II) staining revealed the presence of manganese oxide on plate I but not on plate II (Fig. 2B). Therefore, B. subtilis is able to oxidize manganese, but neither CotA nor sporulation is involved. Colonies tranferred to nylon filters did not react with the leucoberbelin blue (data not shown). This indicates that the bacterial spore pigment is not manganese oxide, which agrees with the report of van Waasbergen and coworkers (31). Manganese oxide in the agar was also detected when the plates were autoclaved before the addition of manganese (data not shown) and is therefore not produced by enzymatic activity. It is probably formed by the alkalinization of the medium, resulting from bacterial growth (5).
So, the CotA-dependent pigmentation remains to be explained. Four copper-binding sites are present in CotA and in laccases (Fig. 1). In spite of the overall low similarities, we then tested whether CotA could be a laccase. A substrate specific for laccase, syringaldazine [N,N′-bis(3,5-dimethoxyhydroxybenzylidenehydrazine)], was used (10). Spore suspensions were incubated with 50 μM syringaldazine and 10 μM CuSO4 in 100 mM phosphate buffer. A purple color developed with 168 spores, but not with ΔcotA spores, indicating that CotA may be a laccase (Fig. 2C). The optimal pH and temperature for this reaction were pH 7 and 45°C (data not shown). To confirm the laccase activity, the CotA protein was overproduced in Escherichia coli. The cotA gene (nucleotides −1 to +1656 from the start codon) was cloned under the control of the strong inducible T7 promoter in the pET20b+ vector (Novagen). The pET20b+-cotA plasmid was transformed into E. coli strain BL21(DE3) (Novagen). Cells were grown at 37°C in Luria-Bertani medium until they reached an optical density at 600 nm of 3. IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM) was then added to induce cotA expression. Cells were harvested 2 h later, resuspended in 100 mM phosphate buffer (pH 7), and treated with toluene. After the addition of 50 μM syringaldazine and 10 μM CuSO4, the purple color appeared at 45°C. No color appeared with pET20b+ alone. This demonstrates that CotA is a laccase.
Since fungal laccase synthesis is often induced by the copper cofactor or by their substrates, the expression of a transcriptional cotA-lacZ fusion (19) was tested on Luria-Bertani plates enriched with various concentrations of copper or with putative substrates. Neither copper, p-anisidine, veratric acid, resorcin, p-toluidine, p-coumaric acid, lignosulfonic acid, orcinol, ferulic acid, p-xylidine, nor lignosulfonic acid elicited any induction. In addition, no ligninolytic activity was found with lignosulfonic acid (data not shown).
As some Bacillus strains produce pigment on agar media supplemented with tyrosine and copper salts (27), the CotA protein could be a laccase with tyrosinase activity, like the multipotent polyphenoloxidase of M. mediterranea (22). The addition of 1 mM tyrosine to SP medium did not modify the color of the colonies. In contrast, addition of 10 μM CuSO4 to SP medium enhanced the difference in the spore pigmentation between strain 168 and the ΔcotA mutant (Fig. 2D, plate III). This substantiates the role of copper in the enzymatic activity of CotA. Further addition of 1 mM tyrosine did not modify this result, suggesting that CotA has no tyrosinase activity (data not shown). The color of the CotA-dependent pigment and that of manganese oxide are quite similar. The effect of Cu and Mn on the pigmentation of 168 and ΔcotA strains was then tested. MnCl2 (1 mM) completely masked the difference between both strains on SP plates and reduced it on SP plates with 10 μM CuSO4 (Fig. 2D, plate IV). This may explain why the spore pigmentation has been thought to be manganese dependent (13).
Laccase activity has been detected in Bacillus sphaericus. It correlates closely with spore formation and the appearance of dipicolinic acid (3). It has also been found in Streptomyces galbus (15), in Azospirillum lipoferum (6), and in M. mediterranea (22), all of which produce a melanic pigment. This suggests a possible link between laccase activity and melanin production, at least in S. galbus and A. lipoferum, as tyrosinase is responsible for pigmentation in M. mediterranea (23). This is reminiscent of some fungal laccases involved in melanization (2). CotA participates in the biosynthesis of the brown spore pigment, which is also thought to be a melanin-like product (25). A few bacilli, among them a strain of B. subtilis, produce a black melanic pigment (11, 12, 17, 25).
To test whether the pigment produced by B. subtilis has some properties of melanin, spores of strains 168 and ΔcotA were purified following Riesenman and Nicholson (20) after growth on SP plates with and without 10 μM CuSO4. The effect of 15% H2O2 was tested on the brownish spores of strain 168. The spore pigment was immediately bleached by this treatment, as would be expected for melanin. The spore coat has been previously shown to protect against hydrogen peroxide (20). The role of CotA in the resistance of spores to H2O2 was therefore tested. The 168 and the ΔcotA strains were treated 1 h in 5% H2O2 as described by Riesenman and Nicholson (20). A total of 2.2 × 10−3 cells survived in the 168 strain versus 10−8 cells in the ΔcotA strain. Weakly pigmented 168 spores obtained on SP plates were as resistant as brownish 168 spores obtained on SP plates supplemented with 10 μM CuSO4. This shows that the CotA protein and/or the pigment is involved in protection against hydrogen peroxide.
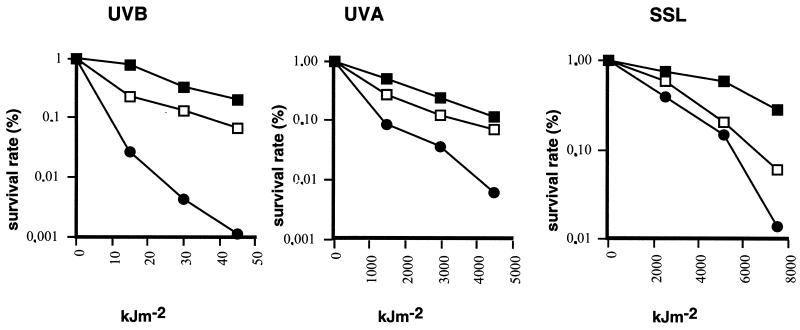
Melanin shields against radiation (16), and the spore coat confers resistance to UV light (20). The effects of UVB, UVA, and simulated solar light were therefore tested on spore survival following Riesenman and Nicholson (20). At the highest level of irradiation energy applied, the brownish spores of strain 168 were 177-, 18-, and 19-fold more resistant than the ΔcotA spores to UVB, UVA, and simulated solar light, respectively (Fig. 3). The weakly pigmented spores of strain 168 obtained in the absence of copper display an intermediate protection (Fig. 3). This suggests a possible correlation between the protective effect and the amount of pigment.
FIG. 3.
UV light resistance of wild-type and ΔcotA strains. The UVB source was a Fluolink TFL-35 M (Vilber Lourmat, Torcy, France). The UVA source was a Supersun 5000 lamp (Mutzhas) emitting only UVA2 radiation (λ > 340 nm). Intensity was 1,130 J m−2 as measured by a VLX 3W radiometer equipped with interferential filters (Vilber Lourmat). For modeling the environmental solar radiation, a Kratos solar simulator equipped with a 2,500-W xenon compact arc lamp (Conrad-Hanovia, Inc., Newark, N.J.) was used in conjunction with a Schott WG 320 cutoff filter (3 mm) to eliminate UVC and shorter UVB radiation. The fluence rate was 1,700 J m−2 s−1, measured with a YSI Kettering 65a thermopile (Yellow Spring Instruments). Each experiment was done with two different batches of spores. ▪, strain 168 grown on SP plus 10 μM CuSO4; □, strain 168 grown on SP without CuSO4 supplementation; ●, ΔcotA grown on SP plus 10 μM CuSO4.
To our knowledge, this is the first time that an enzymatic function is attributed to a spore coat protein. CotA seems to be responsible for most of the protection afforded by the spore coat against UV light and hydrogen peroxide. This effect is probably at least partly mediated by the spore pigment. However, identification of this pigment as a melanin would require further study.
Acknowledgments
We are deeply indebted to Evelyne Sage for helping with the UV work. We are grateful to W. Krumbein for kindly providing leucoberbelin blue, to G. Rapoport for helpful discussion, to O. Soutourina for translation of reference 27, to Cécilia Fabry for her bibliographic work, and to P. Ollivon, mechanic at the Pasteur Institute, for the construction of the replicator.
REFERENCES
- 1.Brouwers G-J, de Vrind J P M, Corstjens P L A M, Cornelis P, Baysse C, de Vrind-de Jong E W. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1. Appl Environ Microbiol. 1999;65:1762–1768. doi: 10.1128/aem.65.4.1762-1768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler M J, Day A W. Fungal melanins: a review. Can J Microbiol. 1998;44:1115–1136. [Google Scholar]
- 3.Claus H, Filip Z. The evidence of a laccase-like enzyme activity in a Bacillus sphaericus strain. Microbiol Res. 1997;152:209–216. [Google Scholar]
- 4.Corstjens P L A M, de Vrind J P M, Goosen T, Vrind-de Jong E W de. Identification and molecular analysis of the Leptothrix discophora SS-1 mofA gene, a gene putatively encoding a manganese-oxidizing protein with copper domains. Geomicrobiol J. 1997;14:91–108. [Google Scholar]
- 5.Deutscher M P, Kornberg A. Biochemical studies of bacterial sporulation and germination. VIII. Patterns of enzyme development during growth and sporulation of Bacillus subtilis. J Biol Chem. 1968;243:4653–4660. [PubMed] [Google Scholar]
- 6.Diamantidis G, Effosse A, Potier P, Bally R. Purification and characterization of the first bacterial laccase in the rhizospheric bacterium Azospirillum lipoferum. Soil Biol Biochem. 2000;32:919–927. [Google Scholar]
- 7.Donovan W, Zheng L B, Sandman K, Losick R. Genes encoding spore coat polypeptides from Bacillus subtilis. J Mol Biol. 1987;196:1–10. doi: 10.1016/0022-2836(87)90506-7. [DOI] [PubMed] [Google Scholar]
- 8.Driks A. Bacillus subtilis spore coat. Microbiol Mol Biol Rev. 1999;63:1–20. doi: 10.1128/mmbr.63.1.1-20.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Germann U A, Müller G, Hunziker P E, Lerch K. Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J Biol Chem. 1988;263:885–896. [PubMed] [Google Scholar]
- 10.Harkin J M, Obst J R. Syringaldazine, an effective reagent for detecting laccase and peroxidase in fungi. Experientia (Basel) 1973;29:381–387. [Google Scholar]
- 11.Hinojosa-Rebollar E, Rangel-Mandujano A, Ortigoza-Ferado J, Mesta-Howard A M, Hernandez-Rodriguez C. Synthesis and partial characterization of a melanin-like pigment of Bacillus subtilis. Rev Latinoam Microbiol. 1993;35:399–406. [Google Scholar]
- 12.Hoti S L, Balaraman K. Formation of melanin pigment by a mutant of Bacillus thuringiensis H-14. J Gen Microbiol. 1993;139:2365–2369. doi: 10.1099/00221287-139-10-2365. [DOI] [PubMed] [Google Scholar]
- 13.Iichinska E. Some physiological features of asporogenous mutants of bacilli. Microbiology (New York) 1960;29:147–150. [Google Scholar]
- 14.Krumbein W E, Altmann H J. A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgol Wiss Meeresunters. 1973;25:347–356. [Google Scholar]
- 15.Kuznetsov V D, Filippova S N, Rybakova A M. Nature of the brown pigment and the composition of the phenol oxidases of Streptomyces galbus. Microbiology (New York) 1984;53:193–197. [PubMed] [Google Scholar]
- 16.Margalith P Z. Pigment microbiology. London, United Kingdom: Chapman & Hall; 1992. pp. 5–31. [Google Scholar]
- 17.Patel K R, Wyman J A, Patel K A, Burden B J. A mutant of Bacillus thuringiensis producing a dark-brown pigment with increased UV resistance and insecticidal activity. J Invertebr Pathol. 1996;67:120–124. [Google Scholar]
- 18.Perry C R, Smith M, Britnell C H, Wood D A, Thurston C F. Identification of two laccase genes in the cultivated mushroom Agaricus bisporus. J Gen Microbiol. 1993;139:1209–1218. doi: 10.1099/00221287-139-6-1209. [DOI] [PubMed] [Google Scholar]
- 19.Popham D L, Stragier P. Cloning, characterization, and expression of the spoVB gene of Bacillus subtilis. J Bacteriol. 1991;173:7942–7949. doi: 10.1128/jb.173.24.7942-7949.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riesenman P J, Nicholson W L. Role of the spore coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl Environ Microbiol. 2000;66:620–626. doi: 10.1128/aem.66.2.620-626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogolsky M. Genetic mapping of a locus which regulates the production of pigment associated with spores of Bacillus subtilis. J Bacteriol. 1968;95:2426–2427. doi: 10.1128/jb.95.6.2426-2427.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Amat A, Solano F. A pluripotent polyphenol oxidase from the melanogenic marine Alteromonas sp. shares catalytic capabilities of tyrosinases and laccases. Biochem Biophys Res Commun. 1997;240:787–792. doi: 10.1006/bbrc.1997.7748. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Amat A, Lucas-Elio P, Fernandez E, Garcia-Borron J C, Solano F. Molecular cloning and functional characterization of a unique multipotent polyphenol oxidase from Marinomonas mediterranea. Biochim Biophys Acta. 2001;1547:104–116. doi: 10.1016/s0167-4838(01)00174-1. [DOI] [PubMed] [Google Scholar]
- 24.Sandman K, Kroos L, Cutting S, Youngman P, Losick R. Identification of the promoter for a spore coat protein gene in Bacillus subtilis and studies on the regulation of its induction at a late stage of sporulation. J Mol Biol. 1988;200:461–473. doi: 10.1016/0022-2836(88)90536-0. [DOI] [PubMed] [Google Scholar]
- 25.Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969;33:48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaeffer P, Millet J, Aubert J-P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shliakhov E N, Burdenko T A, Simonova L L, Buracheva S A. The effect of copper compounds on pigment formation in Bacillus subtilis and Bacillus megaterium. Mikrobiol Zh (Kiev) 1985;47:33–36. . (In Russian.) [PubMed] [Google Scholar]
- 28.Solomon E I, Sundaram U M, Machonkin T E. Multicopper oxidases and oxygenases. Chem Rev. 1996;96:2563–2605. doi: 10.1021/cr950046o. [DOI] [PubMed] [Google Scholar]
- 29.Thurston C F. The structure and function of fungal laccases. Microbiology (Reading) 1994;140:19–26. [Google Scholar]
- 30.van Waasbergen L G, Hildebrand M, Tebo B M. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J Bacteriol. 1996;178:3517–3530. doi: 10.1128/jb.178.12.3517-3530.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Waasbergen L G, Hoch J A, Tebo B M. Genetic analysis of the marine manganese-oxidizing Bacillus sp. strain SG-1: protoplast transformation, Tn917 mutagenesis, and identification of chromosomal loci involved in manganese oxidation. J Bacteriol. 1993;175:7594–7603. doi: 10.1128/jb.175.23.7594-7603.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]