Abstract
BACKGROUND
Clinical pathways are care plans established to describe essential steps in the care of patients with a specific clinical problem. They translate (inter)national guidelines into local applicable protocols and clinical practice. The purpose of this article is to establish a multidisciplinary integrated care pathway for specialists and allied health care professionals in caring for individuals with von Hippel–Lindau (VHL) disease.
METHODS
Using a modified Delphi consensus‐making process, a multidisciplinary panel from 5 Dutch University Medical Centers produced an integrated care pathway relating to the provision of care for patients with VHL by medical specialists, specialized nurses, and associated health care professionals. Patient representatives cocreated the pathway and contributed quality criteria from the patients' perspective.
RESULTS
The panel agreed on recommendations for the optimal quality of care for individuals with a VHL gene mutation. These items were the starting point for the development of a patient care pathway. With international medical guidelines addressing the different VHL‐related disorders, this article presents a patient care pathway as a flowchart that can be incorporated into VHL expertise clinics or nonacademic treatment clinics.
CONCLUSIONS
Medical specialists (internists, urologists, neurosurgeons, ophthalmologists, geneticists, medical oncologists, neurologists, gastroenterologists, pediatricians, and ear‐nose‐throat specialists) together with specialized nurses play a vital role alongside health care professionals in providing care to people affected by VHL and their families. This article presents a set of consensus recommendations, supported by organ‐specific guidelines, for the roles of these practitioners in order to provide optimal VHL care. This care pathway can form the basis for the development of comprehensive, integrated pathways for multiple neoplasia syndromes.
Keywords: care pathway, hemangioblastoma, pancreatic neuroendocrine tumor, renal cell carcinoma, retinal angioma, von Hippel–Lindau (VHL)
Introduction
Von Hippel–Lindau (VHL) disease is a hereditary disease characterized by various malignant and benign vascular and visceral lesions arising from a heterozygous germline loss of function of the VHL gene. 1 The prototype lesions are hemangioblastomas of the retina and central nervous system, renal cysts and renal clear cell carcinomas, pancreatic cysts and neuroendocrine tumors, pheochromocytomas/paragangliomas, endolymphatic sac tumors (ELST), and epididymal and broad ligament cysts. 2 The heterogeneity and complexity of the disease, associated with the chronic and often deteriorating course, require a multidisciplinary approach with close monitoring and interventions by several medical specialists, nurses, and general practitioners. 3
An incidence of 1 per 36,000 to 91,000 has been reported, and although 80% have an affected parent, 20% of patients, being the first in their family, have a de novo mutation. 2 , 4 , 5 , 6 An estimated 5% of the patients who fulfill the diagnostic criteria for VHL harbor a mosaic variant (a somatic mutation in early embryonic development). 7 The overall penetrance of VHL has been reported to be almost 100% by the age of 75 years. 8
Several organ‐specific clinical VHL guidelines have recently been published to improve care for germline VHL gene mutation carriers. 9 , 10 , 11 , 12 In the Netherlands, complex and highly specialized care for rare diseases such as VHL has been funneled to nationally reviewed and accredited “expertise centers,” which are linked to a European Reference Network (https://ec.europa.eu/health/ern_en). The purpose is to improve and standardize the quality of care and to implement cost‐savings by coordinated care across disciplines. However, despite clear advantages, an integrated care pathway for VHL has never been implemented.
The heterogeneity and complexity of VHL, associated with the chronic and often deteriorating course of the disease, require a multidisciplinary approach with close monitoring and interventions by several medical specialists, nurses, and general practitioners. Care pathways help physicians to organize care around patients with specific clinical problems. Patients with VHL require many hospital visits from a young age and receive care from numerous different specialists throughout their patient journey. Families have to oversee complex screening scheduling, which often involves several affected individuals within the same family, with appointments at multiple clinics; this scenario is the best case for the well patient. When tumors require intervention or if complications occur, the scheduling and oversight can become extremely challenging and even form an obstacle to best care. A multidisciplinary care pathway addresses all the different specialists and diagnostic investigations that patients require according to their individual manifestations. A Cochrane systematic review and meta‐analysis defined clinical care pathways as structured, multidisciplinary care plans that provide professionals with detailed guidance for the care of patients with a specific problem to translate evidence to practice in order to optimize clinical outcomes and maximize clinical efficiency. 13 Clinical pathways may lead to reductions in hospital complications, improved documentation, significant reductions in the length of stay, and decreases in hospital costs 13 and reduce disparities and heterogeneity in treatment. To address this need, a generic care pathway for patients with rare diseases was developed. 14 Clinical pathways can be appended to already existing guidelines. 15
Although national and international guidelines exist, they are not consistently integrated into clinical practice. Integrated care pathways are task‐oriented plans that describe the essential steps in the care of patients with a given clinical problem. They set the patient journey and can also identify why the clinical care described in guidelines sometimes falls short in daily practice. 16 Care pathways set the framework for standardized care and should be cocreated with patients to improve compliance and adherence. Additionally, they decrease variation in practice, increase quality standards, improve patient satisfaction, and address research and development questions. 17 In this article, we describe a VHL care pathway based on the recent international VHL guidelines cocreated with the Dutch VHL patient advocacy group.
Materials and Methods
To establish this VHL‐specific care pathway, we used the VHL‐related international guidelines 9 , 10 , 11 , 12 , 18 as the framework. Furthermore, a consensus working group was established that consisted of experts from 5 Dutch academic hospitals, patients, and the Dutch VHL advocacy group. The consensus process took place from January 2021 until September 2021 and followed a modified Delphi model. 19 In the first round of the process, the items of interest were presented, and the outline of the care pathway was proposed and circulated by email and teleconference. In the second round, the feedback of the participants was incorporated and distributed by email. A third round featured face‐to‐face meetings with participants to reach a consensus regarding the topics still open for discussion. In the final round, the working group was sent the final consensus statement after all group members had consented. Details about this process can be found in the supporting information.
Results and Discussion
The consensus care pathway is shown schematically in Figure 1.
Figure 1.
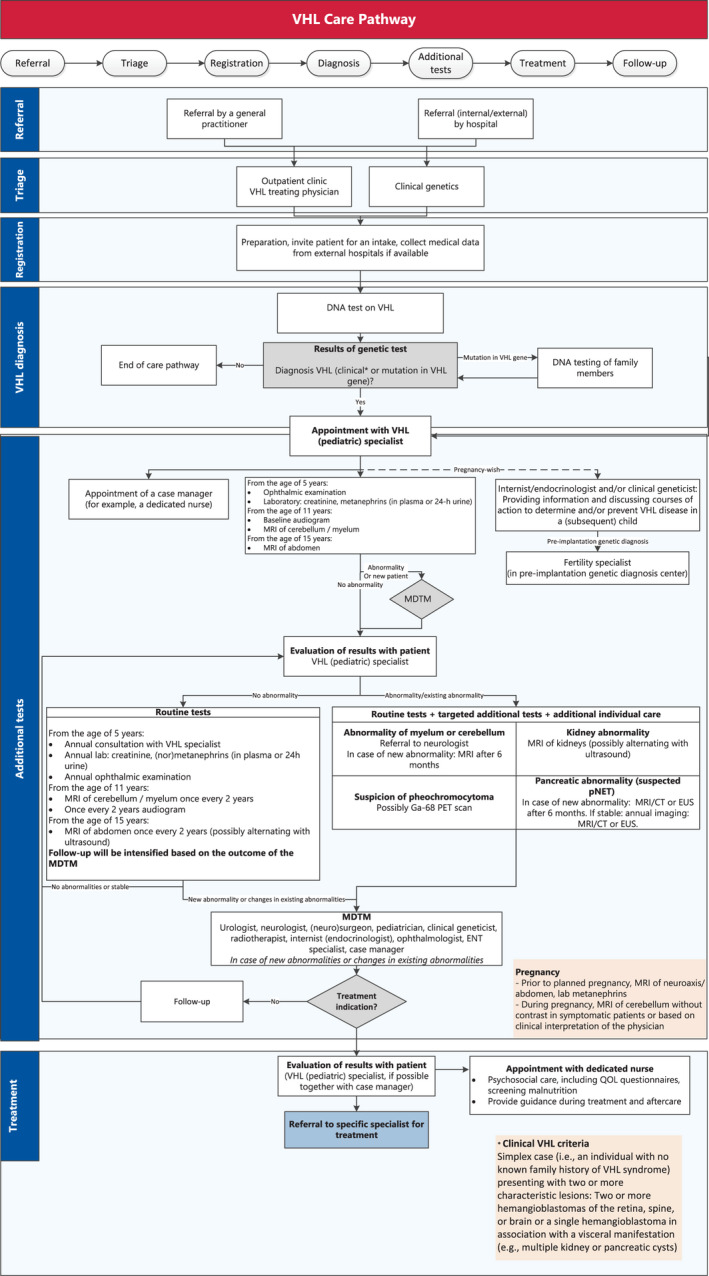
VHL care pathway. CT indicates computed tomography; ENT, ear‐nose‐throat; EUS, endoscopic ultrasound; MDTM, multidisciplinary team meeting; MRI, magnetic resonance imaging; PET, positron emission tomography; pNET, pancreatic neuroendocrine tumor; QOL, quality of life; VHL, von Hippel–Lindau.
The pathway was based on criteria for quality care from the patient perspective, which were generated independently from the national patient advocacy group in the Netherlands through 3 rounds of consensus methodology (Table 1).
TABLE 1.
Criteria of VHL Care From the Patient Perspective
|
|
|
|
|
|
|
|
Abbreviations: GP, general practitioner; VHL, von Hippel–Lindau.
Genetic and Clinical Diagnosis
A VHL diagnosis can be made by genetic confirmation of a pathogenic mutation in the VHL gene or clinically according to international, 20 Danish, 21 or Dutch criteria 22 (Table 2).
TABLE 2.
VHL Clinical Diagnostic Criteria
| VHL Clinical Diagnostic Criteria | Danish Criteria 21 | International Criteria 20 | Dutch Criteria 22 |
|---|---|---|---|
| First‐ or second‐degree family member with VHL and/or 1 or more typical VHL‐associated tumors | 1 VHL‐associated tumor | 1 VHL‐associated tumor | 1 VHL‐associated tumor |
| No known family history of VHL | At least 2 VHL‐related manifestations | 2 HBs (retinal and/or CNS) | At least 2 VHL‐related manifestations |
| 1 HB and a visceral manifestation | |||
| VHL‐related manifestation criteria | Retinal HB | Retinal HB | Retinal HB |
| HB in cerebellum, medulla oblongata, or spinal cord | CNS HB | CNS HB | |
| ELST | RCC | RCC | |
| RCC | Pheo | Pheo/paraganglioma | |
| Pheo, paraganglioma, and/or glomus tumor | pNET | ELST | |
| pNET and/or multiple pancreatic cysts | ELST | (Multiple) kidney cysts | |
| Pancreatic cysts | (Multiple) pancreatic cysts or NET | ||
| Epididymal cystadenomas |
Abbreviations: CNS, central nervous system; ELST, endolymphatic sac tumor; HB, hemangioblastoma; Pheo, pheochromocytoma; pNET, pancreatic neuroendocrine tumor; RCC, renal cell carcinoma; VHL, von Hippel–Lindau (VHL).
Patients are referred from inside or outside the treating hospital. In cases where a VHL diagnosis is suspected, referral to the genetic department for DNA testing should be discussed with the patient. In the case of genetic confirmation, family members should be offered DNA testing accordingly, and guidelines for informing family members should be followed.
A VHL diagnosis should be suspected when a patient has 1 VHL‐related manifestation. DNA analysis of the VHL gene should be considered if a hemangioblastoma occurs under the age of 50 years, if a renal cell carcinoma (RCC) occurs under the age of 40 years, if a pheochromocytoma occurs under the age of 50 years, if multiple retinal angiomas occur at any age, or if an ELST occurs at any age. A patient with a hemangioblastoma under the age of 50 years, multiple retinal angiomas at any age, or an ELST at any age should have a full clinical examination, including imaging of the central nervous system, abdomen, and retina, to exclude other manifestations of VHL. In the case of a pheochromocytoma under the age of 50 years or an RCC under the age of 40 years, imaging to detect other manifestations is not advised. The likelihood of finding another VHL‐related manifestation is not high after a negative VHL germline test.
DNA is primarily extracted from blood and is sequenced via Sanger sequencing or next‐generation sequencing–based techniques for patients who fulfill the clinical criteria or have 1 manifestation as mentioned previously with single‐gene testing. 7 The VHL gene is also added to the targeted panel for pheochromocytoma (diagnosed at any age) and RCC (panel testing is indicated when it is diagnosed under the age of 40 years, there is a family history of RCC, or there is bilateral RCC).
If no germline mutation is found in the VHL gene and a patient fulfills the clinical criteria or has 1 manifestation as mentioned previously, somatic mosaicism has to be examined. In these cases, sequencing of the tumor tissue or testing of a second tissue, such as a skin biopsy, should be considered. If a somatic VHL pathogenic variant is found, the diagnosis of mosaic VHL syndrome can be made.
To patients with a diagnosis of mosaic VHL, full VHL surveillance is offered (Table 3). After the age of 60 years, stopping surveillance can be considered if there are no other VHL manifestations because it is very rare for a new manifestation of VHL to be revealed after this age. DNA testing in the children of patients with a mosaic variant can be considered.
TABLE 3.
Surveillance Protocol for Patients with VHL
| Starting Age | ||||
|---|---|---|---|---|
| 5 y | 11 y | 15 y or Older | 65 y or Older | |
| Consultation with VHL specialist/case manager (PE, including BP) | Annual | Annual | Annual | Annual |
| Lab: Creatinine (nor)metanephrines | Annual | Annual | Annual | If indicated |
| Ophthalmic examination | Annuala | Annual | Annual | Annual |
| MRI of cerebellum/myelum | — | Biannual | Biannual | If indicated |
| Audiogram | — | Biannual | Biannual | If indicated |
| MRI of abdomen (possibly alternating with ultrasound) | — | — | Biannual | If indicated |
Abbreviations: BP, blood pressure; MRI, magnetic resonance imaging; PE, physical examination; VHL, von Hippel–Lindau (VHL).
In the case of an occurrence of a manifestation, the protocol will deviate to the specific tumor protocol.
An ophthalmic examination should be performed at the latest at the age of 5 years. If it is indicated, it should be performed from the age of 1 year.
When DNA analysis does not identify a (likely) pathogenic variant, clinical examination and imaging according to screening protocols should be performed to determine whether the criteria for a clinical diagnosis of VHL are met. The clinical diagnostic criteria are a simplex case (ie, an individual with no known family history of VHL syndrome) presenting with 2 or more characteristic lesions: 2 or more hemangioblastomas of the retina, myelum, or brain or a single hemangioblastoma in association with a visceral manifestation (eg, multiple kidney or pancreatic cysts). Another criterion is that these tumors manifest at a young age. 21 If a genetic analysis does not support a clinical VHL diagnosis, surveillance is warranted for the patient but not for the family members.
VHL Specialist
After the confirmation of a VHL diagnosis, patients meet their VHL specialist. A VHL specialist can be any medical specialist, such as an internist, (pediatric) endocrinologist, nephrologist, neurologist, neurosurgeon, ophthalmologist, or urologist. This specialist should be adequately educated in VHL and capable of keeping an overview of the patients' journey. This specialist is, therefore, responsible not just for screening his or her own organ system but also for ensuring that all organ systems are appropriately monitored. Therefore, the VHL specialist should be well versed in the manifestations outside his or her own medical specialty. For example, the VHL specialist should be able to request central nervous system and abdominal imaging in a timely manner and refer patients to their neurologist and urologist when indicated. A VHL specialist is preferred because of the complexity of the disease, with many specialists being involved. The VHL specialist will refer the patient to the neurologist or neurosurgeon if a hemangioblastoma is observed on regular screens. The urologist should by consulted when an RCC is seen on imaging. The endocrinologist should be consulted if increased (nor)metanephrines are measured or if there is any suspicion of a paraganglioma, pheochromocytoma, or pancreatic neuroendocrine tumor. The ear‐nose‐throat physician should examine a patient with hearing complaints or when an ELST is visible on imaging studies. Accordingly, the VHL specialist should invite colleagues involved in VHL care from all relevant specialties for a periodic multidisciplinary team meeting (MDTM), at which VHL cases at the treating center are centrally reviewed regularly. Preferably, a dedicated nurse with expertise in VHL disease can support the VHL specialist as the navigating case manager or primary point of contact for patients.
A consultation with the VHL specialist and preferably also a dedicated nurse should occur at least once a year or more often if indicated. During the consultation, VHL‐related complaints should be discussed, and a physical examination, including a neurological evaluation and blood pressure measurements, should be performed. Furthermore, the need for psychosocial support should be assessed.
From the ages of 5 to 18 years, children should visit a pediatrician with adequate knowledge of potential VHL symptoms. Additionally, VHL gene mutation carriers, or children who have not received a VHL genetic diagnosis but have a parent with VHL, should receive an ophthalmological examination once a year from the age of 5 years onward.
By regular surveillance, VHL‐related manifestations are usually diagnosed in a timely manner. Patients with abnormalities in the screening will be treated accordingly. This should be described in the (electronic) patient file.
Routine Tests
As part of the surveillance, routine tests based on the international organ‐specific VHL guidelines are shown in the care pathway (Fig. 1). 9 , 10 , 11 , 12 , 18
MDTM
Ideally, all patients with newly diagnosed VHL and patients with new VHL‐related manifestations or comorbidities should be discussed at an MDTM. The MDTM is a requirement to be considered as a VHL expert center by the European Rare Cancer Network (a European Reference Network). An MDTM solely for patients with VHL is most ideal. Additionally, this is also an important aspect of becoming a VHL Clinical Care Center according to the VHL Alliance, which in turn supports guideline implementation. 23 The following specialists should preferably be present at an MDTM: the VHL specialist, an internist (endocrinologist/oncologist), a neurologist/neurosurgeon, an (endocrine) surgeon, a clinical geneticist, a urologist, a pediatrician, an ophthalmologist, a radiotherapist, and a dedicated nurse specialist. A psychologist should also be available for consultation. The MDTM advises on the follow‐up or treatment policy for each individual patient. The transition from a pediatrician to a VHL specialist can also be facilitated through the MDTM and should follow specified transition protocols. The results of the MDTM are reported in the electronic patient file.
If there is an indication for treatment, the patient is referred accordingly. The outcome of the examinations and the MDTM will be discussed with the patient during a follow‐up consultation with the VHL treating physician.
Dedicated Nurse
It is recommended that every patient with VHL have a dedicated specialized nurse. This can be a registered nurse or a nurse practitioner if one is available. The dedicated nurse can perform the role of the case manager. This case manager supports the treating physician in providing psychosocial care, is easily accessible in case of (urgent) matters, and checks whether the patient has all the necessary VHL‐related appointments. Psychosocial care consists of providing information about illness and treatment, emotional support and normalization of complaints, and decision support around treatment options; signaling the disease burden; and referring the patient for additional care (social, psychological, or medical) based on identified problems. The dedicated nurse can also identify factors affecting other family members, such as the need for early psychological support in partners or parents of affected individuals. 24 Specialized nurses are highly appreciated by the patients for their central role in patient management and adherence to the care pathway. In addition, they are appreciated for the psychosocial support that they provide. 25 The authors acknowledge that a dedicated nurse is not available in all health care systems. However, the psychosocial aspects of the disease are significant, 26 so psychosocial screening and support are strongly recommended.
For managing and measuring specific problems in adult patients with VHL, the following tools can be used:
The health‐related 36‐Item Short Form Health Survey questionnaire. 27
The single‐item Visual Analogue Scale (VAS) for cancer‐related fatigue. 28
The Center for Epidemiological Studies Depression Scale (CES‐D) for emotional problems. 29
The Patient‐Specific Functional Scale for specific physical problems. 30
If necessary, the patient can be referred as follows:
To a psychologist or psychotherapist if indicated.
For oncological rehabilitation intake in case of concurrent problems (on at least 2 of the 3 scales: CES‐D > 16 and/or VAS ≥ 4 and/or PSK ≥ 4 on at least 1 item).
For (oncology) rehabilitation care intake for complex problems.
Case Manager
Coordinated care is essential for managing VHL. This coordinated care can be provided by the VHL specialist (and the dedicated nurse if available). Patients/parents should be aware of who their (child's) case manager is and how to contact the person. For pediatric patients, specific attention will be given to the well‐being of patients as well as parents and siblings if a family is affected by VHL.
The case manager should be approachable and easily accessible for patients and caregivers. Patients are introduced to the case manager at the outpatient clinic or during a remote consultation. During the first meeting between the patient and the case manager, expectations and needs are aligned.
Another task of the case manager is to monitor the care process and intervene when it is necessary. This is primarily done by addressing patients' problems. In addition, the case manager monitors the process when patients are referred for treatment or diagnosis to other specialists inside or outside the primary center. Furthermore, the case manager annually reviews the patients in treatment to ensure that no patients have unintentionally been lost to follow‐up. In addition, the case manager notices structural or important problems in the provided care and provides input for improving the quality of care at the MDTM.
Family Planning
Patients who are planning to start a family should be informed about the consequences and options regarding family planning in relation to VHL. This counseling is preferably performed by the clinical geneticist or the VHL specialist. In the Netherlands, approximately 6.5% of VHL pregnancies are assisted by pre‐implantation genetic diagnosis. 31 In a patient with DNA‐proven somatic mosaicism and a pregnancy wish, an invasive prenatal diagnosis or even pre‐implantation genetic testing can be discussed by appropriate, comprehensive, and nondirective genetic counselling. For patients with suspected mosaicism but no pathogenic variant in the VHL gene determined, children are treated as first‐degree relatives, and no prenatal options are available.
Because men affected by VHL can experience reduced fertility due to epididymal cysts, a fertility specialist may be consulted. 32 Although limited data on the effects of pregnancy on female patients with VHL do not indicate significant risk, careful observation is warranted. All scenarios should be discussed, and patients should be supported in their decision with consideration of their cultural/religious backgrounds. It is recommended that any required surveillance be performed before a planned pregnancy to prevent complications during the pregnancy. 20 For each pregnant patient, a plan should be made that is based on current VHL manifestations. There are patient cases in which pregnancy in patients with VHL disease induces cerebellar hemangioblastoma progression. 33 If a patient has VHL manifestations, particularly cerebellar hemangioblastomas before the pregnancy, it is strongly recommended that magnetic resonance imaging without contrast be performed when a patient becomes symptomatic during pregnancy. 10 Furthermore, metanephrines should be measured before pregnancy and in the second trimester. When there are no VHL manifestations present during the pregnancy, there is no indication for extra follow‐up.
The following topics preferably should be discussed in family planning counseling:
A spontaneous pregnancy will result in a 50% risk that the child will be affected.
Invasive prenatal diagnostics and DNA tests can be performed to assess whether the child is affected.
Pre‐implantation genetic diagnosis is an option if the VHL genetic mutation is known.
Egg or sperm donation.
Surrogacy if the mother is in suboptimal health.
Adoption or fostering.
Quality of Care Evaluation
It is essential to assess quality and safety improvements in health care. Clinical pathways are increasingly being used in health care to improve daily clinical practice. When these pathways are used, an evaluation of their implementation is necessary. Assessments should be repeated even when the process of implementation is over. It is, therefore, important to identify specific indicators to measure outcome measures and improve health outcomes. 34 For the care of patients with VHL, we therefore recommend planning an annual self‐assessment via an internal audit. Indicators that can be used for this audit can be based on local hospital criteria for quality improvement, international criteria for VHL specialized treatment centers, 23 or the European Reference Network core indicators for continuous monitoring (https://ec.europa.eu/health/publications/set‐ern‐core‐indicators‐18_en).
Patient and Resource Advocacy
Some hospitals will argue that the adoption of a VHL care pathway such that as outlined here will be costly. However, patients with VHL require coordinated care from multiple specialties throughout their entire postdiagnostic lifetime, and streamlining processes to reduce the surgical burden is imperative both to protect quality of life and from the perspective of health economics. The majority of VHL care consists of wait and see strategies; often, tumor growth is followed longitudinally in more than 1 organ to detect growth and malignancy in order to optimize interventions. Currently, expensive medical imaging is the mainstay of this process, but those costs could be ameliorated once appropriate validated surrogate biomarkers (eg, circulating tumor DNA) are developed. Aggressive screening and the application of VHL guidelines have dramatically increased the mean life expectancy for patients in the last decades from 49 to 67 years, and it has become significantly closer to that of nonaffected siblings and the general population. 35 The consequences of uncoordinated care outside a care pathway could have a profound impact on patient mortality and morbidity, and resources need to be allocated for pathway implementation with payers at the national level. A recent poster presented at the American Society of Clinical Oncology's Genitourinary Cancers Symposium estimated that among 960 VHL carriers with insurance claims for nephrectomy, laser therapy to the retina, surgical excision of cerebellar or spinal hemangioblastomas, or surgical resection of pancreatic neuroendocrine tumors in the United States, in comparison with sporadic RCC patients without germline VHL mutations, significantly more all‐cause hospitalizations, inpatient days, outpatient visits, emergency room visits, and other medical visits per person‐year were found. This translated into 4‐fold higher mean adjusted annualized all‐cause health care costs for VHL in comparison with a control cohort with sporadic RCC. These data set a baseline on which improved and streamlined health services can optimize health resources. 36
In conclusion, the VHL patient care pathway is based on recent guidelines and was developed by health care professionals with vast experience in treating patients with VHL. Patient representatives cocreated the pathway and contributed quality criteria from patients' perspectives.
This Dutch national pathway is the first integrated care pathway for patients with VHL. The flowchart can be easily implemented in hospitals worldwide because of its practical applicability. However, because of local logistical limitations, the pathway might not be fully applicable at all centers treating patients with VHL. A case manager (preferably a specialized nurse) who coordinates and maintains care is an essential asset in complex VHL care. In addition, the VHL specialist, who preferably works closely with the specialized nurse, is responsible for adherence to the care pathway and chairing the MDTM.
Funding Support
Wouter W. de Herder has received speakers fees from Ipsen, Novartis and AAA. He has received research support: from Novartis and AAA.
Conflict of Interest Disclosures
Wouter T. Zandee has received reimbursement for a conference visit from Ipsen. The other authors made no disclosures.
Supporting information
Supplementary Material
Wolters WPG, Dreijerink KMA, Giles RH, van der Horst‐Schrivers ANA, van Nesselrooij B, Zandee WT, Timmers HJLM, Seute T, de Herder WW, Verrijn Stuart AA, Kilic E, Brinkman WM, Zondervan PJ, Vandertop WP, Daniels AB, Wolbers T, Links TP, van Leeuwaarde RS. Multidisciplinary integrated care pathway for von Hippel–Lindau disease. Cancer. 2022. 10.1002/cncr.34265
References
- 1. Friedrich CA. Von Hippel–Lindau syndrome. A pleomorphic condition. Cancer. 1999;86(suppl):2478‐2482. [PubMed] [Google Scholar]
- 2. Maddock IR, Moran A, Maher ER, et al. A genetic register for von Hippel–Lindau disease. J Med Genet. 1996;33:120‐127. doi: 10.1136/jmg.33.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Šimerka P. Council recommendation of 8 June 2009 on an action in the field of rare diseases. EUR‐Lex. Accessed May 10, 2022. https://eur‐lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007:0010:EN:PDF [Google Scholar]
- 4. Maher ER, Iselius L, Yates JR, et al. Von Hippel–Lindau disease: a genetic study. J Med Genet. 1991;28:443‐447. doi: 10.1136/jmg.28.7.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neumann HP, Wiestler OD. Clustering of features of von Hippel–Lindau syndrome: evidence for a complex genetic locus. Lancet. 1991;337:1052‐1054. doi: 10.1016/0140-6736(91)91705-y [DOI] [PubMed] [Google Scholar]
- 6. Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor‐prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A:327‐332. doi: 10.1002/ajmg.a.33139 [DOI] [PubMed] [Google Scholar]
- 7. Coppin L, Plouvier P, Crépin M, et al. Optimization of next‐generation sequencing technologies for von Hippel Lindau (VHL) mosaic mutation detection and development of confirmation methods. J Mol Diagn. 2019;21:462‐470. doi: 10.1016/j.jmoldx.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 8. Kruizinga RC, Sluiter WJ, de Vries EGE, et al. Calculating optimal surveillance for detection of von Hippel–Lindau‐related manifestations. Endocr Relat Cancer. 2014;21:63‐71. doi: 10.1530/erc-13-0308 [DOI] [PubMed] [Google Scholar]
- 9. Chahoud J, McGettigan M, Parikh N, et al. Evaluation, diagnosis and surveillance of renal masses in the setting of VHL disease. World J Urol. 2021;39:2409‐2415. doi: 10.1007/s00345-020-03441-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huntoon K, Shepard MJ, Lukas RV, McCutcheon IE, Daniels AB, Asthagiri AR. Hemangioblastoma diagnosis and surveillance in von Hippel–Lindau disease: a consensus statement. J Neurosurg. Published online October 1, 2021. doi: 10.3171/2021.3.jns204203 [DOI] [PubMed] [Google Scholar]
- 11. Laks S, van Leeuwaarde R, Patel D, et al. Management recommendations for pancreatic manifestations of von Hippel–Lindau disease. Cancer. 2022;128:435‐446. doi: 10.1002/cncr.33978 [DOI] [PubMed] [Google Scholar]
- 12. Mehta GU, Kim HJ, Gidley PW, et al. Endolymphatic sac tumor screening and diagnosis in von Hippel–Lindau disease: a consensus statement. J Neurol Surg B Skull Base. Published online April 8, 2021. doi: 10.1055/s-0041-1725033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rotter T, Kinsman L, Machotta A, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;3:1‐141. doi: 10.1111/j.1744-1609.2011.00223.x [DOI] [PubMed] [Google Scholar]
- 14. Choukair D, Hauck F, Bettendorf M, et al. An integrated clinical pathway for diagnosis, treatment and care of rare diseases: model, operating procedures, and results of the project TRANSLATE‐NAMSE funded by the German Federal Joint Committee. Orphanet J Rare Dis. 2021;16:474. doi: 10.1186/s13023-021-02092-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demirdas S, Van Kessel IN, Korndewal MJ, et al. Clinical pathways for inborn errors of metabolism: warranted and feasible. Orphanet J Rare Dis. 2013;8:2‐4. doi: 10.1186/1750-1172-8-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campbell H, Hotchkiss R, Bradshaw N, Porteous M. Integrated care pathways. BMJ. 1998;316:133‐137. doi: 10.1136/bmj.316.7125.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ryll B. From good to great: what patients can do for your medical research. Nat Med. 2020;26:1508. doi: 10.1038/s41591-020-1097-8 [DOI] [PubMed] [Google Scholar]
- 18. Rednam SP, Erez A, Druker H, et al. Von Hippel–Lindau and hereditary pheochromocytoma/paraganglioma syndromes: clinical features, genetics, and surveillance recommendations in childhood. Clin Cancer Res. 2017;23:e68‐e75. doi: 10.1158/1078-0432.ccr-17-0547 [DOI] [PubMed] [Google Scholar]
- 19. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008‐1015. [PubMed] [Google Scholar]
- 20. VHLA suggested active surveillance guidelines. VHL Alliance. Published 2017. Accessed May 10, 2022. https://www.vhl.org/wp‐content/uploads/2020/10/Active‐Surveillance‐Guidelines‐2020.pdf [Google Scholar]
- 21. Binderup ML, Bisgaard ML, Harbud V, et al. Von Hippel–Lindau disease (vHL). National clinical guideline for diagnosis and surveillance in Denmark. 3rd edition. Dan Med J. 2013;60:B4763. [PubMed] [Google Scholar]
- 22. Hes FJ, van der Luijt RB, Lips CJ. Clinical management of von Hippel–Lindau (VHL) disease. Neth J Med. 2001;59:225‐234. doi: 10.1016/s0300-2977(01)00165-6 [DOI] [PubMed] [Google Scholar]
- 23. Flowers A, Rathmell K, Friedman D, Daniels AB. Universal reflex referral to VHL comprehensive clinical care center of patients presenting to ophthalmologists leads to dramatic improvement in guideline‐concordant screening: results of a pilot study. Invest Ophthalmol Vis Sci. 2019;60:2781.31260519 [Google Scholar]
- 24. Rochette C, Baumstarck K, Canoni‐Zattara H, et al. Psychological impact of von Hippel–Lindau genetic screening in patients with a previous history of hemangioblastoma of the central nervous system. J Psychosoc Oncol. 2018;36:624‐634. doi: 10.1080/07347332.2018.1450320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Viklund P, Wengström Y, Lagergren J. Supportive care for patients with oesophageal and other upper gastrointestinal cancers: the role of a specialist nurse in the team. Eur J Oncol Nurs. 2006;10:353‐363. doi: 10.1016/j.ejon.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 26. Lammens CR, Bleiker EM, Verhoef S, et al. Psychosocial impact of von Hippel–Lindau disease: levels and sources of distress. Clin Genet. 2010;77:483‐491. doi: 10.1111/j.1399-0004.2010.01333.x [DOI] [PubMed] [Google Scholar]
- 27. Brazier JE, Harper R, Jones NM, et al. Validating the SF‐36 Health Survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160‐164. doi: 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Escalante CP, Manzullo EF. Cancer‐related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412‐S416. doi: 10.1007/s11606-009-1056-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saracino RM, Weinberger MI, Roth AJ, Hurria A, Nelson CJ. Assessing depression in a geriatric cancer population. Psychooncology. 2017;26:1484‐1490. doi: 10.1002/pon.4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hefford C, Abbott JH, Arnold R, Baxter GD. The Patient‐Specific Functional Scale: validity, reliability, and responsiveness in patients with upper extremity musculoskeletal problems. J Orthop Sports Phys Ther. 2012;42:56‐65. doi: 10.2519/jospt.2012.3953 [DOI] [PubMed] [Google Scholar]
- 31. Dommering CJ, Henneman L, van der Hout AH, et al. Uptake of prenatal diagnostic testing for retinoblastoma compared to other hereditary cancer syndromes in the Netherlands. Fam Cancer. 2017;16:271‐277. doi: 10.1007/s10689-016-9943-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gomella PT, Shin P, Srinivasan R, Linehan WM, Ball MW. Obstructive azoospermia secondary to bilateral epididymal cystadenomas in a patient with von Hippel–Lindau. Urol Case Rep. 2019;27:100922. doi: 10.1016/j.eucr.2019.100922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frantzen C, Kruizinga RC, Van Asselt SJ, et al. Pregnancy‐related hemangioblastoma progression and complications in von Hippel–Lindau disease. Neurology. 2012;79:793‐796. doi: 10.1212/wnl.0b013e3182661f3c [DOI] [PubMed] [Google Scholar]
- 34. Latina R, Salomone K, D’Angelo D, et al. Towards a new system for the assessment of the quality in care pathways: an overview of systematic reviews. Int J Environ Res Public Health. 2020;17:8634. doi: 10.3390/ijerph17228634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Binderup ML, Jensen AM, Budtz‐Jørgensen E, Bisgaard ML. Survival and causes of death in patients with von Hippel–Lindau disease. J Med Genet. 2017;54:11‐18. doi: 10.1136/jmedgenet-2016-104058 [DOI] [PubMed] [Google Scholar]
- 36. Jonasch E, Song Y, Freimark J, et al. Healthcare resource utilization and costs among patients with von Hippel–Lindau disease–associated renal cell carcinoma: a retrospective administrative claims analysis. J Clin Oncol. 2022;40(suppl):305. doi:10.1200/jco.2022.40.6_suppl.305 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


