Background:
Probiotics and rifaximin are treatments for gut microbiota dysbiosis in patients with traveler’s diarrhea (TD), and they both proved beneficial for the prevention of TD. However, comparative effectiveness research between them has not been performed. A systematic review and network meta-analysis are to be performed to clarify which of them is more effective in the prevention of TD.
Methods:
Literature concerning the effectiveness of probiotics or rifaximin in the prevention of TD was searched in Medline, Embase, the Cochrane Central Register of Controlled Trials, and clinical registries for randomized controlled trials (RCTs) from inception of these databases to November 30, 2021 without any language restrictions. The primary efficacy outcome was the incidence of TD, and the safety outcome was the incidence of adverse events. The effect size of probiotics was measured by using relative ratio (RR), and the network meta-analysis was performed by using a frequentist approach and a random-effect model.
Results:
Totally 17 RCTs after screening 1119 retrieved records were included in analysis and 9 RCTs were with low risk of bias. Compared with placebo, both probiotics and rifaximin were associated with lower incidence of TD (probiotics, RR 0.85, 95% CI 0.76–0.95; rifaximin, RR 0.47, 95% CI 0.35–0.63), and rifaximin was more effective than probiotics (RR 0.56, 95% CI 0.4–0.78). Further analysis showed that sodium butyrate, rifaximin and L. acidophilus + L. bulgaricus + Bifido.bifidum + Strept. Thermophilus were the three most effective treatments for TD.
Conclusions:
Both rifaximin and probiotics are superior over placebo, and rifaximin has better treatment effect than probiotics in reducing the incidence of TD. Different types of probiotics have heterogeneous treatment effects.
Keywords: differential probiotics, network meta-analysis, traveler’s diarrhea
1. Introduction
Traveler’s diarrhea (TD), as one of the most common medical conditions, has affected at least 60% of travelers.[1,2] Although TD is commonly non-fatal and self-healing, it can cause severe conditions like fever, vomiting, abdominal pain or cramp, and dehydration, which cause disruptions to travel plans or hospitalization.[3,4]
The pathological mechanism of TD is acknowledged to be bacterial infection, and the most commonly reported pathogens are Escherichia coli, Campylobacter jejuni, Salmonella species and Shigella species.[1,2,5] Therefore, antibiotics are usually recommended for the treatment of TD. In the clinical practice guideline released by the American College of Gastroenterology (ACG) for acute diarrheal infections, treatment with antibiotics is recommended for patients with moderate-to-severe watery stools that were travel-related.[5] Another guideline for the management of TD also recommends that moderate-to-severe TD should be treated with antibiotics, specifically azithromycin or fluoroquinolones.
Despite solid evidence for the use of antibiotics in the treatment of TD, evidence for their effectiveness in the prevention of TD is insufficient. Two previous meta-analyses confirmed the efficacy of using rifaximin in the preventive treatment of TD,[1,6] and the meta-analysis conducted recently concluded that rifaximin has favorable tolerability and safety profile and was especially effective against diarrheagenic Escherichia coli.[1] Although rifaximin seems promising for the prevention of TD, its profile local antimicrobial resistance/susceptibility data and comparative effectiveness against other options were rarely investigated.
Given that the pathological mechanism of TD is associated with intestinal flora, probiotics are also proposed for the prevention of TD. The advantages of using probiotics for TD prophylaxis include easy accessibility, no antibiotic resistance, and mild adverse events. A recent meta-analysis published in 2018 reached the conclusion that probiotics have exhibited statistically significant efficacy in TD prophylaxis.[7] However, the meta-analysis did not address the question of which probiotic strains or probiotic combinations are most effective for TD prophylaxis, which has actually long been concerned by both clinicians and whose interested in travel.
Therefore a systematic review and network meta-analysis are performed to examine the comparative effectiveness of probiotics and rifaximin in the prevention of TD, and to find out the most effective probiotic strains.
2. Methods
A systematic review and network meta-analysis were carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension statement for reporting systematic reviews incorporating network meta-analyses of health care interventions.[8]
2.1. Data source
Relevant literature was searched and retrieved in MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, and clinical registries from the inception of these databases up to November 30, 2021 without any language restrictions. It should be noted that studies that were completed but have not been reported yet were also searched in clinical registries (clinicaltrials.gov and chictr.org.cn). Previously published reviews were searched and their reference lists were also scrutinized to avoid missing out on any important literature.
2.2. Study selection
Randomized controlled trials (RCTs) examining the effect of probiotics on the prevention of TD were eligible; RCTs with parallel design were preferentially selected, and RCTs with crossover design would be included when the results of the first phase (before crossover) were separately reported. The study population was constrained to healthy adults (aged over 18, without comorbidities that might affect the results, and those excluded according to the exclusion criteria) who planned to travel and took preventive treatments. RCTs that assessed the efficacy of probiotics (including synbiotics and prebiotics) or rifaximin for the prevention of TD were qualified; the controls included no treatment, placebo treatment, or active controls recommended by guidelines or suggested effective by previously published systematic reviews. RCTs that investigated the incidence and outcome of TD or the adverse events of TD interventions were eligible. Two reviewers independently screened the retrieved articles in accordance with the predefined criteria, first screening the article title and abstract of retrieved literature and then the full text. Discrepancies in literature selection were solved by panel discussion and arbitrated by a third reviewer.
2.3. Study outcomes
The primary efficacy outcome was the incidence of TD. With reference to the ACG clinical guideline[5] and the approach adopted by a previous systematic review,[6] TD was defined as the passage of at least three unformed stools within 24 hours accompanied with at least one of the following conditions: abdominal pain or cramps, nausea, vomiting, fever (≥37.8°C), fecal urgency, passage of gross blood or mucus in stool, tenesmus, or moderate to severe intestinal gas. The safety outcome was treatment-related adverse events.
2.4. Data extraction
Two reviewers independently extracted data from the included RCTs using standardized extraction forms. The extracted data included characteristics of the included trials, baseline parameters of the participants, details of the interventions and controls, and outcome measures. The characteristics of the included trials contained the name of the first author, year of publication, study design and settings, sample size, and length of follow-up. Baseline parameters of the participants included the mean age, proportion of females, and mean body mass index of the participants. The details of interventions and controls comprised the types of interventions or controls, dosage and frequency of treatment drug, and total duration of treatment. The outcome parameters included the types of interventions or controls, the number of participants in the intervention group, the number of participants with TD, and the assessment time points. Missing data were acquired by email communications with the authors.
2.5. Risk of bias assessment and evidence grading
The risk of bias (RoB) of the included trials were assessed by using the revised Cochrane “Risk of bias” tool for randomized trials (RoB 2).[9] Each trial was evaluated in five domains, including randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result, Each domain was rated into low RoB, high RoB, or some concerns according to the response to signaling questions in each domain. RCTs with all the five low-ROB domains was judged as overall low RoB.
2.6. Statistical analysis
The comparative effectiveness of differential probiotics in reducing the incidence of TD was calculated by adopting a frequentist approach for network meta-analysis. Placebo was treated as a common comparator, and the effect size of each treatment was determined by the comparison with placebo. The results of the included RCTs were pooled through random-effects model, and relative ratios (RRs) and the corresponding 95% confidence interval (95% CIs) were estimated for each treatment in comparison with placebo. The comparative effectiveness of the treatments were ranked by using P-score, an index of the mean probability that a treatment is the most efficacious treatment in all of the included treatments.[10]
The consistency of the network meta-analysis was checked by comparing the estimates from indirect evidence with those from direct evidence, and the significance of the consistency was texted by Z test. The heterogeneity of the network meta-analysis was examined by using Cochran’s Q test and the I2 statistics. In Cochran’s Q test, both within-trial heterogeneity and between-trial heterogeneity were evaluated. According the Cochrane Handbook, an I2 value <40% indicates unimportant heterogeneity.[11]
The network meta-analyses was performed in two steps. Firstly, all probiotics were combined into one category to study the effect size of probiotics when compared with that of placebo and rifaximin. Secondly, the effect sizes of differential probiotics were analyzed separately in comparison with that of placebo or rifaximin.
3. Results
3.1. Characteristics of the included RCTs
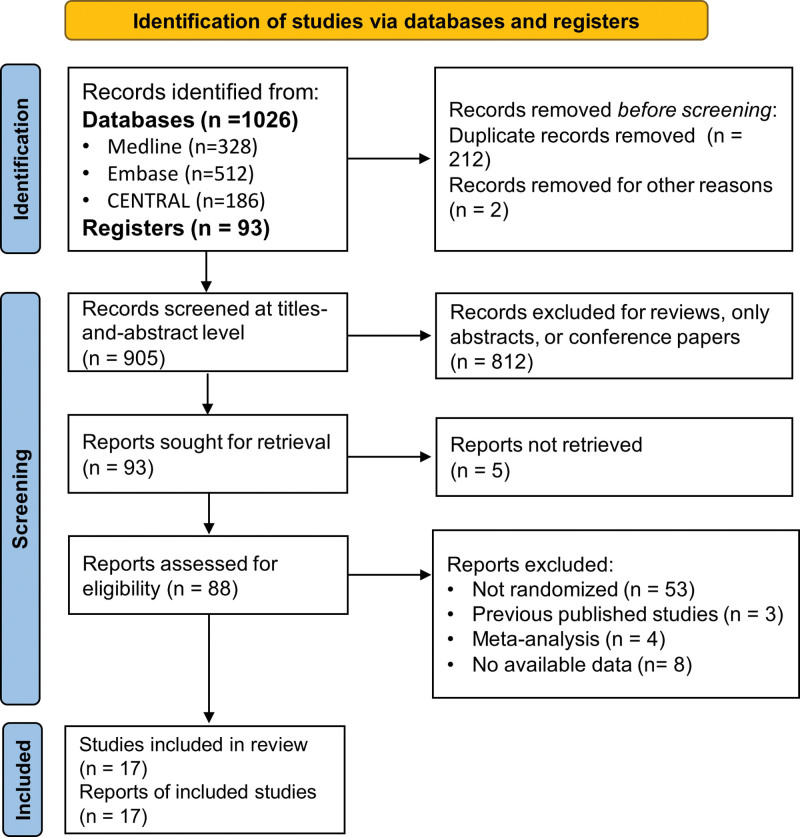
Totally 1119 records were obtained after literature search, among which 905 records were screened through the review of article titles and abstracts and 93 records were screened through full-text review. A total of 17 RCTs were finally included into analysis[12–28] with 6012 participants involved. Details of the literature screening process were provided in Figure 1. The participants included were mostly from European countries and the US, and most of the participants had received 14 to 21 days of treatments. Characteristics of the included RCTs are shown in Table 1.
Figure 1.
Study flowchart.
Table 1.
Trial characteristics.
| Study ID | Study population | Travel destination | Mean age (range) | Treatment (no.) | Control (no.) | Mean treatment duration, d | Overall RoB |
|---|---|---|---|---|---|---|---|
| Armstrong 2010[15] | US | Turkey | 36 | Rifaximin (48) | Placebo (47) | 14 | Low risk of bias |
| Black 1989[27] | Danish | Egypt | 51 | Probiotics (47) | Placebo (47) | 14 | Some concerns |
| Briand 2006[17] | French | West Africa or Asia | 38 (36–40) | Probiotics (79) | Placebo (72) | 14 | Low risk of bias |
| de dios Pozo-Olano 1978[19] | US | Mexico | -- | Probiotics (26) | Placebo (24) | 8 | Some concerns |
| Drakoularakou 2010[12] | British | Varied destinations | 38 | Probiotics (81) | Placebo (78) | >7 | Low risk of bias |
| Dupont 2005[16] | Mexico | Mexico | -- | Rifaximin (54) | Placebo (54) | 14 | Low risk of bias |
| Flores 2011[14] | US | Mexico | 25 (18–67) | Rifaximin (50) | Placebo (48) | 14 | Low risk of bias |
| Hasle 2017[18] | Norwegian | Varied destinations with high risks of TD | 43 | Probiotics (167) | Placebo (167) | 14 | Low risk of bias |
| Hilton 1997[21] | US | Varied destinations | 50 (17–80) | Probiotics (126) | Placebo (119) | 21 | Some concerns |
| Katelaris 1995[22] | British | Belize | -- | Probiotics (181) | Placebo (101) | 21 | Some concerns |
| Kollaritsch 1989[24] | Austrian | Varied destinations, hot climates | -- | Probiotics (1148) | Placebo (712) | 23 | Some concerns |
| Kollaritsch 1993[23] | Austrian | Varied destinations, hot climates | -- | Probiotics (655) | Placebo (361) | 21 | Low risk of bias |
| Krokowicz 2014[28] | Polish | Varied destinations | -- | Probiotics (22) | Placebo (20) | >3 | Low risk of bias |
| Martinez-Sandoval 2010[25] | Mexico | Mexico | 24 (18–75) | Rifaximin (99) | Placebo (102) | 14 | Low risk of bias |
| Oksanen 1990[26] | Finish | Turkey | 43.8 (10–80) | Probiotics (373) | Placebo (383) | 14 | Some concerns |
| Virk 2013[13] | US | High TD risk areas | 48.7 | Probiotics (94) | Placebo (102) | 21 | Low risk of bias |
| Zanger 2013[20] | German | Southeast Asia | 29 (24–37) | Rifaximin (122) | Placebo (117) | 28 | Low risk of bias |
RoB = risk of bias, TD = travelers’ diarrhea.
Among the included RCTs, 9 of them were evaluated with low RoB, and 8 RCTs were rated with some concerns. The most frequent concerns included deviations from the intended interventions (5 studies) and missing outcome data (5 studies). Detailed assessment results of RoB were shown in supplementary Figure 1, http://links.lww.com/MD/H449.
3.2. Incidence of TD
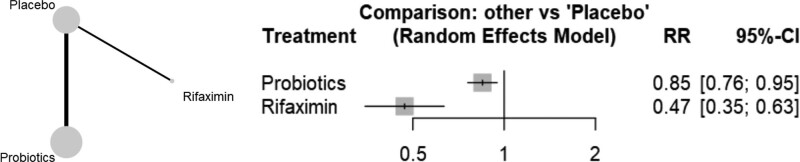
In network meta-analysis performed with aggregate-level data, it was found that both probiotics (RR 0.85, 95% CI 0.76–0.95) and rifaximin (RR 0.47, 95% CI 0.35–0.63) were associated with significantly lower incidence of TD when compared with placebo (Fig. 2). Rifaximin was superior over probiotics (RR 0.56, 95% CI 0.4–0.78) in reducing the incidence of TD (Table 2). There is no evidence of inconsistency between direct and indirect estimates. The test of heterogeneity showed unimportant heterogeneity (I2 = 39.6%, tau2 = 0.016, Cochran’s Q = 24.85, P = .052).
Figure 2.
The effectiveness of probiotics and rifaximin. CI = confidence interval, RR = relative ratio.
Table 2.
Pairwise comparison between probiotics and rifaximin.
| Rifaximin | – | 0.47 (0.35–0.64) |
| 0.56 (0.40–0.78) | Probiotics | 0.84 (0.74–0.95) |
| 0.47 (0.35–0.64) | 0.84 (0.74–0.95) | Placebo |
The top half showed the estimates of direct comparisons between two treatments, and the bottom half showed the estimates of network meta-analysis. Comparisons between treatments should be read from left to right, and the comparison estimate is in the cell between the column-defining treatment and the row-defining treatment. RRs > 1 favors row-defining treatment.
RR = relative ratio.
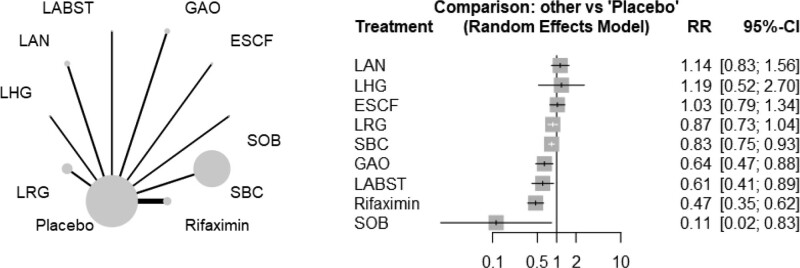
It was found from the network meta-analysis of differential probiotics and rifaximin that sodium butyrate (SOB), rifaximin, L. acidophilus + L. bulgaricus + Bifido.bifidum + Strept. Thermophilus (LABST), galacto-oligosaccharide (GAO), S. boulardii CNCM I-745 (SBC) were associated with significantly lower incidence of TD when compared with placebo (Fig. 3). Among these treatments superior over placebo, rifaximin was more effective than SBC (RR 0.56, 95% CI 0.42–0.76). Pairwise comparisons between these treatments were shown in Table 3. No inconsistency was found between direct and indirect estimates. The test of heterogeneity showed unimportant heterogeneity (I2 = 4.3%, tau2 = 0.0013, Cochran’s Q = 8.36, P = .399).
Figure 3.
The comparative effectiveness of differential probiotics and rifaximin. CI = confidence interval, ESCF = Entero. faecium SF68 + S. cerevisiae CNCM I-4444 + fructo-oliogosaccharide, GAO = galacto-oligosaccharide, LABST = L. acidophilus + L. bulgaricus + Bifido.bifidum + Strept. Thermophilus, LAN = L. acidophilus nr, LHG = L. helveticus ATCC33409 + L. gasseri ATCC4962, LRG = L. rhamnosus GG, RR = relative ratio, SBC = S. boulardii CNCM I-745, SOB = sodium butyrate.
Table 3.
Pairwise comparisons of differential probiotics and rifaximin.
| SOB | – | – | – | – | – | 0.11 (0.02–0.83) | – | – | – |
| 0.24 (0.03–1.81) | Rifaximin | – | – | – | – | 0.47 (0.35–0.62) | – | – | – |
| 0.19 (0.02–1.42) | 0.77 (0.48–1.25) | LABST | – | – | – | 0.61 (0.41–0.89) | – | – | – |
| 0.18 (0.02–1.32) | 0.73 (0.48–1.11) | 0.94 (0.57–1.55) | GAO | – | – | 0.64 (0.47–0.88) | – | – | – |
| 0.14 (0.02–1.00) | 0.56 (0.42–0.76) | 0.73 (0.49–1.09) | 0.77 (0.56–1.07) | SBC | – | 0.83 (0.75–0.93) | – | – | – |
| 0.13 (0.02–0.96) | 0.54 (0.39–0.75) | 0.70 (0.46–1.07) | 0.74 (0.52–1.05) | 0.96 (0.78–1.18) | LRG | 0.87 (0.73–1.04) | – | – | – |
| 0.11 (0.02–0.83) | 0.47 (0.35–0.62) | 0.61 (0.41–0.89) | 0.64 (0.47–0.88) | 0.83 (0.75–0.93) | 0.87 (0.73–1.04) | Placebo | 0.97 (0.75–1.27) | 0.84 (0.37–1.91) | 0.88 (0.64–1.21) |
| 0.11 (0.01–0.82) | 0.46 (0.31–0.67) | 0.59 (0.37–0.94) | 0.63 (0.42–0.94) | 0.81 (0.61–1.08) | 0.85 (0.62–1.17) | 0.97 (0.75–1.27) | ESCF | – | – |
| 0.10 (0.01–0.82) | 0.40 (0.17–0.94) | 0.51 (0.21–1.27) | 0.54 (0.23–1.30) | 0.70 (0.31–1.61) | 0.73 (0.32–1.70) | 0.84 (0.37–1.91) | 0.86 (0.37–2.05) | LHG | – |
| 0.10 (0.01–0.75) | 0.41 (0.27–0.63) | 0.53 (0.32–0.88) | 0.57 (0.36–0.88) | 0.73 (0.53–1.02) | 0.77 (0.53–1.10) | 0.88 (0.64–1.21) | 0.90 (0.60–1.36) | 1.04 (0.43–2.51) | LAN |
The top half showed the estimates of direct comparisons between two treatments, and the bottom half showed the estimates of network meta-analysis. Comparisons between treatments should be read from left to right, and the comparison estimate is in the cell between the column-defining treatment and the row-defining treatment. RRs > 1 favors row-defining treatment. The sequence of the treatments was arranged according to the P-scores, and the treatment with the highest P-score was arranged at left.
ESCF = Entero. faecium SF68 + S. cerevisiae CNCM I-4444 + fructo-oliogosaccharide, GAO = galacto-oligosaccharide, LABST = L. acidophilus + L. bulgaricus + Bifido.bifidum + Strept. Thermophilus, LAN = L. acidophilus nr, LHG = L. helveticus ATCC33409 + L. gasseri ATCC4962, LRG = L. rhamnosus GG, RR = relative ratio, SBC = S. boulardii CNCM I-745, SOB = sodium butyrate.
3.3. Adverse events
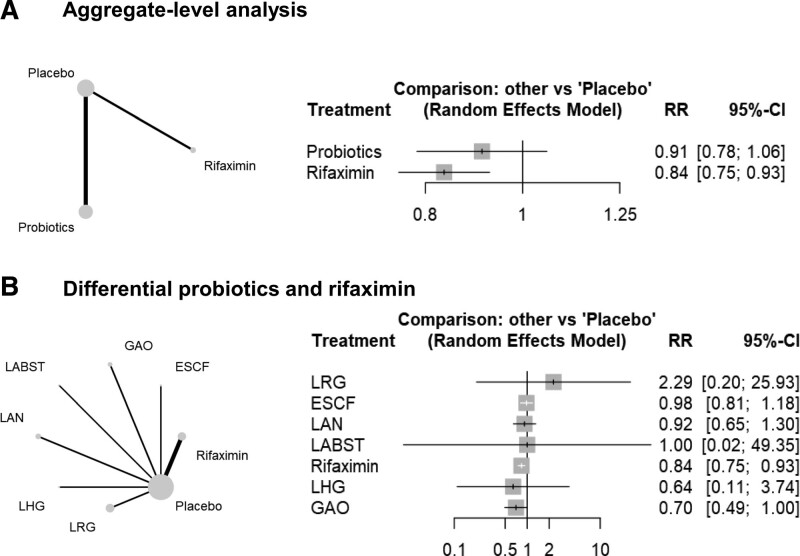
The aggregate-level analysis suggested that rifaximin had a relatively lower rate of adverse events than placebo, while probiotics had a similar adverse event rate as rifaximin (I2 = 0%, tau2 = 0, Cochran’s Q = 4.48, P = .973) (Fig. 4A). Further analysis indicated that LRG might have a higher rate of adverse events compared with placebo, while the other remaining treatments did not show higher adverse event rates than placebo (I2 = 0%, tau2 = 0, Cochran’s Q = 1.11, P = .993) (Fig. 4B).
Figure 4.
The adverse events. CI = confidence interval, ESBL-PE = Extended-spectrum beta-lactamase-producing Enterobacteriaceae, ESCF = Entero. faecium SF68 + S. cerevisiae CNCM I-4444 + fructo-oliogosaccharide, GAO = galacto-oligosaccharide, LABST = L. acidophilus + L. bulgaricus + Bifido.bifidum + Strept. Thermophilus, LAN = L. acidophilus nr, LHG = L. helveticus ATCC33409 + L. gasseri ATCC4962, LRG = L. rhamnosus GG, RR = relative ratio.
4. Discussion
As founded in this systematic review and network meta-analysis, both rifaximin and probiotics are superior over placebo, and rifaximin has better treatment effect than probiotics in reducing the incidence of TD. Different types of probiotics have heterogeneous treatment effects. SOB, rifaximin, LABST, GAO and SBC were associated with significantly lower incidence of TD when compared with placebo, rather than L. rhamnosus GG (LRG), Entero. faecium SF68 + S. cerevisiae CNCM I-4444 + fructo-oliogosaccharide (ESCF), L. helveticus ATCC33409 + L. gasseri ATCC4962 (LHG), and L. acidophilus nr. (LAN). Heterogeneity in the network meta-analysis of different probiotics decreased significantly, which supports the differential effects of different probiotics. In general, both probiotics and rifaximin are safe for the prevention of TD, since no severe adverse events of the two treatments have been reported, and they have the adverse event rate similar to or lower than placebo.
Travelers are susceptible to pathogens that cause TD—Escherichia Coli or other pathogens, especially when protective intestinal microbiome is disrupted. Due to significant changes identified in the intestinal microbiome between the pre-travel and post-travel samples,[29] treatments for regulating intestinal microbiome have been proposed to prevent TD. Two recently conducted systematic reviews confirmed the efficacy of probiotics and rifaximin, which is in agreement with our findings. However, it should be noted that since these two systematic reviews separately assessed the efficacy of probiotics and rifaximin in preventive treatment of TD, comparative effectiveness of probiotics and rifaximin remains unclear.
Our study enriches the knowledge that rifaximin has better preventive effect on TD than probiotics. Although ample studies assessing probiotic/prebiotic effectiveness in the prevention of TD infection among adults have been conducted,[12,13,23,24,30] these investigations suffer from several methodological shortcomings, such as variability in experimental settings, causes of acute diarrhea, probiotic strains, lack of adequate follow up time and person-time analysis. In addition, other limitations include large variations in the dosage of probiotics, and different frequency of administration and formulations used. In summary, in considering the totality of evidence, the panel reached a consensus that the data supporting the use of probiotics and prebiotics for either prevention or treatment of TD was not adequate enough to make a graded recommendation.[2] Meanwhile, in two RCTs rifaximin has been proved to be a more effective agent, as compared to placebo, in treating noninvasive pathogen-associated TD, and in another two RCTs, it was found to be non-inferior to ciprofloxacin.[31] So far there was only limited data on resistance against rifaximin,[31] but there is no indication of MICs increase among recovered enteric pathogen isolates in contrast to fluoroquinolones and azithromycin.[32] The safety profile is excellent with this poorly absorbed agent. So far there is a lack of documentation as to whether rifaximin therapy is associated with ESBL-PE acquisition, but it has been demonstrated to alter the microbiome, possibly in a beneficial way.[33,34]
In addition, our study reveals that the effect sizes of differential probiotics were not consistent in that only four probiotic formulas were found significantly superior over placebo, while the other four had similar effects to placebo. This finding indicates that before recommending the use of probiotics in the prevention of TD, it is important to first clarify which probiotic strains or combinations are relatively more effective than the others. This also applies to rifaximin, the optimal dose should be further determined, which was not clarified in previous systematic reviews[1] and our study because of the lack of original studies.
Several factors might bias the conclusions of our study. The first is the possible effect of diets on the incidence of TD. Whether diet changes have had causal effects on TD pathogenesis was inconclusive. Analysis of the correlation between diets and intestinal microbiome may suggest the role of diet changes in the incidence of TD. However, diet changes were not clearly documented in the included studies, so the impact of diets could not be assessed. Second, the study population might focus on the travel itself, rather than complying to the health advice provided by doctors, and abnormal daily life during travel might trigger TD, all of which were not documented in the included studies either. Third, owing to the short follow-up period, the possibility that the study population might have delayed onset was impossible to be clarified. Fourth, the national income of the travel destination as one of the factors affecting the incidence of TD should have been considered. Although the included studies specified low-income countries, the association of the national income with the incidence of TD was ambiguous. All effect of the above mentioned important factors on the incidence of TD should be investigated in future studies.
Our meta-analysis has several limitations. Firstly, despite the comprehensive literature search in major databases, the limited sample size may affect the generalizability of conclusions reached in this paper. Secondly, many of the studies included in this paper were conducted before 2000, and the participants in these studies might have strikingly different living styles which might affect the objectivity of our study results. However, the heterogeneity was insignificant and unimportant in our network meta-analysis, indicating that living style variations might not significantly affect our conclusions.
In summary, our meta-analysis showed that both probiotics and rifaximin are efficacious in the prevention of TD; rifaximin is well-tolerated and superior over probiotics, which necessitates further investigations into its optimal dose for the prevention of TD.
Author contributions
All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. HF and HZ designed the study. LG and ZY acquired the study data. HF analyzed and interpreted the data. HF wrote the first draft of the manuscript. HZ, SY and QP contributed to revision. All authors revised the manuscript and approved it for publication.
Supplementary Material
Abbreviations:
- ACG =
- American College of Gastroenterology
- CI =
- confidence interval
- ESBL-PE =
- Extended-spectrum beta-lactamase-producing Enterobacteriaceae
- ESCF =
- Entero. faecium SF68 + S. cerevisiae CNCM I-4444 + fructo-oliogosaccharide
- GAO =
- galacto-oligosaccharide
- LABST =
- L. acidophilus + L. bulgaricus + Bifido.bifidum + Strept. Thermophilus
- LAN =
- L. acidophilus nr
- LHG =
- L. helveticus ATCC33409 + L. gasseri ATCC4962
- LRG =
- L. rhamnosus GG
- RCT =
- randomized controlled trial
- RoB =
- risk of bias
- RR =
- relative ratio
- SBC =
- S. boulardii CNCM I-745
- SOB =
- sodium butyrate
- TD =
- traveler’s diarrhea
Scientific and Technological Research Program of Chongqing Municipal Education Commission (grant no. KJQN202001603). General project of Sichuan Tourism Development Research Center (Grant No. LY21-43). Regional tourism industry development research collaborative innovation center (Grant No. 2021XJPT07).
This meta-analysis study was exempt from ethics approval because it only involved in published data.
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Fan H, Gao L, Yin Z, Ye S, Zhao H, Peng Q. Probiotics and rifaximin for the prevention of travelers’ diarrhea: A systematic review and network meta-analysis. Medicine 2022;101:40(e30921).
Contributor Information
Hao Fan, Email: fanhao@cque.edu.cn.
Lei Gao, Email: 1149693277@qq.com.
Hua Zhao, Email: zhaohua76132@163.com.
References
- [1].Ng QX, Ho CYX, Shin D, et al. A meta-analysis of the use of rifaximin to prevent travellers’ diarrhoea. J Travel Med. 2017;24:10.1093/jtm/tax025. [DOI] [PubMed] [Google Scholar]
- [2].Riddle MS, Connor BA, Beeching NJ, et al. Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med. 2017;24(suppl_1):S57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Giddings SL, Stevens AM, Leung DT. Traveler’s diarrhea. Med Clin North Am. 2016;100:317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Steffen R, Hill DR, DuPont HL. Traveler’s diarrhea: a clinical review. JAMA. 2015;313:71–80. [DOI] [PubMed] [Google Scholar]
- [5].Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol. 2016;111:602–22. [DOI] [PubMed] [Google Scholar]
- [6].Hu Y, Ren J, Zhan M, et al. Efficacy of rifaximin in prevention of travelers’ diarrhea: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Travel Med. 2012;19:352–6. [DOI] [PubMed] [Google Scholar]
- [7].Bae JM. Prophylactic efficacy of probiotics on travelers’ diarrhea: an adaptive meta-analysis of randomized controlled trials. Epidemiol Health. 2018;40:e2018043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [9].Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- [10].Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane; 2022. Available at: www.training.cochrane.org/handbook. [Google Scholar]
- [12].Drakoularakou A, Tzortzis G, Rastall RA, et al. A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers’ diarrhoea. Eur J Clin Nutr. 2010;64:146–52. [DOI] [PubMed] [Google Scholar]
- [13].Virk A, Mandrekar J, Berbari EF, et al. A randomized, double blind, placebo-controlled trial of an oral synbiotic (AKSB) for prevention of travelers’ diarrhea. J Travel Med. 2013;20:88–94. [DOI] [PubMed] [Google Scholar]
- [14].Flores J, Dupont HL, Jiang ZD, et al. A randomized, double-blind, pilot study of rifaximin 550 mg versus placebo in the prevention of travelers’ diarrhea in Mexico during the dry season. J Travel Med. 2011;18:333–6. [DOI] [PubMed] [Google Scholar]
- [15].Armstrong AW, Ulukan S, Weiner M, et al. A randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of rifaximin for the prevention of travelers’ diarrhea in US military personnel deployed to Incirlik Air Base, Incirlik, Turkey. J Travel Med. 2010;17:392–4. [DOI] [PubMed] [Google Scholar]
- [16].DuPont HL, Jiang ZD, Okhuysen PC, et al. A randomized, double-blind, placebo-controlled trial of rifaximin to prevent travelers’ diarrhea. Ann Intern Med. 2005;142:805–12. [DOI] [PubMed] [Google Scholar]
- [17].Briand V, Buffet P, Genty S, et al. Absence of efficacy of nonviable Lactobacillus acidophilus for the prevention of traveler’s diarrhea: a randomized, double-blind, controlled study. Clin Infect Dis. 2006;43:1170–5. [DOI] [PubMed] [Google Scholar]
- [18].Hasle G, Raastad R, Bjune G, et al. Can a galacto-oligosaccharide reduce the risk of traveller’s diarrhoea? A placebo-controlled, randomized, double-blind study. J Travel Med. 2017;24:10.1093/jtm/tax057. [DOI] [PubMed] [Google Scholar]
- [19].de dios Pozo-Olano J, Warram JH, Gómez RG, et al. Effect of a lactobacilli preparation on traveler’s diarrhea. A randomized, double blind clinical trial. Gastroenterology. 1978;74:829–30. [PubMed] [Google Scholar]
- [20].Zanger P, Nurjadi D, Gabor J, et al. Effectiveness of rifaximin in prevention of diarrhoea in individuals travelling to south and southeast Asia: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2013;13:946–54. [DOI] [PubMed] [Google Scholar]
- [21].Hilton E, Kolakowski P, Singer C, et al. Efficacy of lactobacillus GG as a diarrheal preventive in travelers. J Travel Med. 1997;4:41–3. [DOI] [PubMed] [Google Scholar]
- [22].Katelaris PH, Salam I, Farthing MJG. Lactobacilli to prevent traveler’s diarrhea? N Engl J Med. 1995;333:1360–1. [DOI] [PubMed] [Google Scholar]
- [23].Kollaritsch H, Holst H, Grobara P, et al. [Prevention of traveler’s diarrhea with Saccharomyces boulardii. Results of a placebo controlled double-blind study]. Fortschr Med. 1993;111:152–6. [PubMed] [Google Scholar]
- [24].Kollaritsch HH, Wiedermann G. Prevention of traveler’s diarrhea: a double-blind randomized trial with Saccharomyces cerevisiae Hansen CBS 5926. Steffen R, Lobel H, Haworth J, Bradley DJ. (eds). In: Travel Medicine: Proceedings of the First Conference on International Travel Medicine, 5–8 April 1988. Zürich, Switzerland: Springer, 1989:328–32. [Google Scholar]
- [25].Martinez-Sandoval F, Ericsson CD, Jiang ZD, et al. Prevention of travelers’ diarrhea with rifaximin in US travelers to Mexico. J Travel Med. 2010;17:111–7. [DOI] [PubMed] [Google Scholar]
- [26].Oksanen PJ, Salminen S, Saxelin M, et al. Prevention of travellers’ diarrhoea by Lactobacillus GG. Ann Med. 1990;22:53–6. [DOI] [PubMed] [Google Scholar]
- [27].Black FT, Andersen PL, Ørskov J, et al. Prophylactic efficacy of Lactobacilli on traveler’s diarrhea. Steffen R, Lobel H, Haworth J, Bradley DJ. (eds). In: Travel Medicine: Proceedings of the First Conference on International Travel Medicine, 5–8 April 1988. Zürich, Switzerland: Springer, 1989:333–5. [Google Scholar]
- [28].Krokowicz L, Kaczmarek BF, Krokowicz P, et al. Sodium butyrate and short chain fatty acids in prevention of travellers’ diarrhoea: a randomized prospective study. Travel Med Infect Dis. 2014;12:183–8. [DOI] [PubMed] [Google Scholar]
- [29].Riddle MS, Connor BA. The traveling microbiome. Curr Infect Dis Rep. 2016;18:29. [DOI] [PubMed] [Google Scholar]
- [30].McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97–105. [DOI] [PubMed] [Google Scholar]
- [31].Charlebois TSWPBP; approved by CATMAT. statement for travellers and yellow fever: an Advisory Committee Statement (ACS) Committee to Advise on Tropical Medicine and Travel (CATMAT). Can Commun Dis Rep. 2013;39:1– 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mendez Arancibia E, Pitart C, Ruiz J, et al. Evolution of antimicrobial resistance in enteroaggregative Escherichia coli and enterotoxigenic Escherichia coli causing traveller’s diarrhea. J Antimicrob Chemother. 2009;64:343–7. [DOI] [PubMed] [Google Scholar]
- [33].Acosta A, Camilleri M, Shin A, et al. Effects of rifaximin on transit, permeability, fecal microbiome, and organic acid excretion in irritable bowel syndrome. Clin Transl Gastroenterol. 2016;7:e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ponziani FR, Scaldaferri F, Petito V, et al. The role of antibiotics in gut microbiota modulation: the eubiotic effects of rifaximin. Dig Dis. 2016;34:269–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.