Abstract
The recent increase in life expectancy has resulted in an increase in the number of older adults with diabetes mellitus. In addition to type 2 diabetes, in which aging is a well‐known risk factor, individuals with type 1 and other types of diabetes are also increasing owing to longevity in the general population and improved prognosis of the disease and comorbidities. Insulin‐dependent state in type 1 diabetes and other types of diabetes, such as diabetes after pancreatectomy, inevitably requires insulin treatment for survival; however, daily injection of insulin is often hampered in older adults due to impaired cognitive function or limited activities of daily living. In this review, we aimed to discuss the current situation of insulin‐dependent diabetes mellitus in older adults and highlight future prospects. Geriatr Gerontol Int 2022; 22: 549–553.
Keywords: immune checkpoint inhibitors, insulin‐dependent diabetes, older adults, pancreatectomy, type 1 diabetes
Insulin‐dependent state in type 1 diabetes and other types of diabetes, such as diabetes after pancreatectomy, inevitably requires insulin treatment for survival; however, daily injection of insulin is often hampered in older adults due to impaired cognitive function or limited activities of daily living. In this review, we aimed to discuss the current situation of insulin‐dependent diabetes mellitus in older adults and highlight future prospects.
Introduction
The recent increase in life expectancy has resulted in an increase in the number of older adults with diabetes mellitus. In addition to type 2 diabetes, in which aging is a well‐known risk factor, patients with type 1 and other types of diabetes are also increasing owing to longevity in the general population and improved prognosis of the disease and other associated conditions. 1 , 2 The insulin‐dependent state in type 1 diabetes and other types of diabetes, such as diabetes after total pancreatectomy, requires insulin treatment for survival. The recent progress in insulin preparations, devices for insulin treatment, and glucose monitoring systems has contributed to a better prognosis of patients with insulin‐dependent diabetes mellitus. However, older adults with diabetes cannot fully benefit from such new technologies, and even daily injection of insulin is often hampered by age‐dependent or disease‐associated decline in cognitive and physical functions. Moreover, these important issues have not been well discussed, particularly in Japan, where insulin‐dependent diabetes mellitus is relatively rare compared with European countries. In this review, we aimed to discuss the current situation of insulin‐dependent diabetes mellitus in older adults and highlight future prospects.
Insulin‐dependent state in diabetes mellitus
The term “insulin‐dependent” is used when patients require insulin treatment to sustain their lives. Without insulin treatment, patients with insulin‐dependent diabetes develop ketoacidosis and coma, which can lead to death. Insulin treatment may be necessary in patients in a non‐insulin‐dependent state to achieve better glycemic control, but such cases are not “insulin‐dependent,” but termed “insulin‐requiring” diabetes.
Insulin‐dependent diabetes develops when insulin‐producing beta cells in the pancreas are markedly decreased due to destruction or removal. The most common type of diabetes is type 1, in which immune‐mediated destruction of beta cells leads to insulin‐dependent diabetes. Patients with type 1 diabetes are insulin‐dependent, with the exception of the early stage of slowly progressive type 1 diabetes, which is initially non‐insulin dependent but eventually becomes insulin‐dependent over several months or years. 3
Another example of insulin‐dependent diabetes is diabetes after total pancreatectomy, in which the removal of the whole pancreas leads to the absence of beta cells and an insulin‐dependent state. 4 In addition to beta cells, the whole endocrine cells of the islet, including glucagon‐producing alpha cells, are also removed, leading to the lack of hormones controlling hyperglycemia, hypoglycemia, insulin and glucagon. Furthermore, the exocrine pancreas is also removed, leading to nutritional problems due to the lack of digestive enzymes for major nutrients, amylase for carbohydrates, trypsin and chymotrypsin for proteins, and lipase for lipids. The lack of endocrine and exocrine functions of the pancreas leads to serious problems in energy metabolism and nutrition unless appropriately controlled. 4 As total pancreatectomy is often performed in patients with pancreatic cancer, the frequency of older adults is high, resulting in an increase in the number of older patients with insulin‐dependent diabetes.
Heterogeneity in insulin dependency
Among insulin‐dependent type 1 diabetes, heterogeneity exists in residual beta‐cell function, ranging from the complete lack of endogenous insulin to some residual beta‐cell function. 5 The complete lack of endogenous insulin makes glycemic control unstable, leading to considerable fluctuation in glucose levels, known as brittle diabetes, even with intensive insulin therapy. 6 , 7 , 8 Stable glycemic control by adjusting the time‐to‐time fluctuation of insulin demand with exogenous insulin is particularly difficult in such patients. Fine‐tuning of the insulin dose is often required by using continuous subcutaneous insulin infusion (CSII) with a pre‐programming function of time‐to‐time infusion dose in younger cases (Fig. 1). 9
Figure 1.
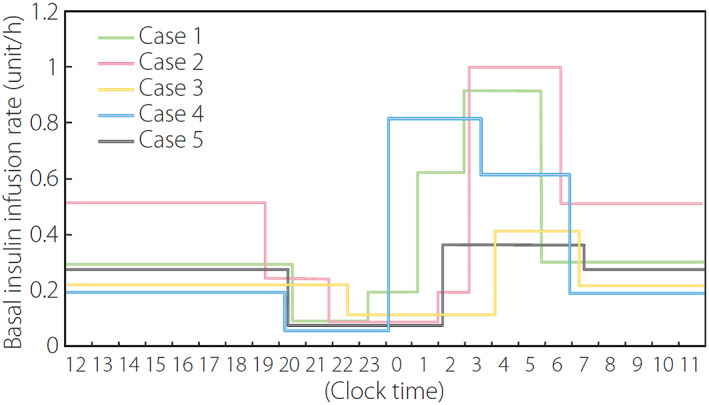
Basal insulin infusion rate in patients with type 1 diabetes and complete lack of endogenous insulin. Note the very dynamic changes in basal insulin infusion rate to achieve stable glycemic control with near‐normal glycemia at bedtime and before breakfast with no nocturnal hypoglycemia. Adapted from Ikegami H et al. J Diabetes Investig 2011; 2: 415–420 with permission. 9
The frequency of type 1 diabetes with a complete lack of endogenous insulin is reported to be higher in Japanese than in populations of European descent. 5 Most Japanese patients with acute‐onset type 1 diabetes are reported to lose endogenous insulin secretion within 5 years from onset, 10 , 11 while many patients with type 1 diabetes in the European population have retained residual beta‐cell function for a long time after the onset of type 1 diabetes. 12 , 13
In addition to typical acute‐onset type 1 diabetes, patients with fulminant type 1 diabetes are relatively common in the Japanese and East Asian populations, whereas they are almost absent in European populations. 14 Fulminant type 1 diabetes is the most severe form of type 1 diabetes, characterized by a markedly abrupt onset and complete destruction of insulin‐producing beta cells of the pancreas at onset. In contrast to typical acute‐onset type 1 diabetes, fulminant type 1 diabetes is rare in children, and most patients, including older adults, develop the disease in adulthood. 14 The complete lack of beta cells in fulminant type 1 diabetes makes stable glycemic control very difficult, particularly in older adults who have difficulty using new technologies, such as CSII and continuous glucose monitoring (CGM).
In addition to the spontaneous development of type 1 diabetes, the incidence of type 1 diabetes induced by immune checkpoint inhibitors is also increasing. 15 Immune checkpoint inhibitors, such as anti‐programmed cell death 1, anti‐programmed cell death ligand 1 and anti‐cytotoxic T‐lymphocyte associated protein 4 antibodies, are increasingly being used for cancer immunotherapy, and remarkable effects mediated by accelerating immune reactions against cancer cells have been reported. However, immune checkpoint inhibitors strengthen immune reactions to not only cancer cells but also self‐antigens to autoimmune reactions, leading to the development of immune‐related adverse events, including type 1 diabetes. 15 Type 1 diabetes associated with immune checkpoint inhibitors is characterized by a high frequency of fulminant type 1 diabetes and an insulin‐dependent state. 16
Insulin‐dependent diabetes in older adults
Although the development of type 1 diabetes is common in childhood and around puberty, type 1 diabetes develops at all ages, including older adults. 17 In addition to new‐onset diabetes in older adults, the improved prognosis of type 1 diabetes results in an increase in the number of older adults with a young‐onset of type 1 diabetes with a long duration. 18 Figure 2 shows the distribution of age of patients with type 1 diabetes in an insulin‐dependent state in the Department of Endocrinology, Metabolism and Diabetes, Kindai University Faculty of Medicine (Osaka, Japan). Insulin dependency was defined as fasting C‐peptide of <0.6 ng/mL. 19 Among a total of 205 patients with type 1 diabetes in an insulin‐dependent state, 29.3% (60 of 205) were older adults (≥65 years). Even in patients with a complete lack of endogenous insulin (fasting C‐peptide less than detection limit), 28.9% (24 of 83) are older adults (Fig. 2), indicating that a substantial fraction of older adults with type 1 diabetes is insulin‐dependent, including those with a complete lack of endogenous insulin.
Figure 2.
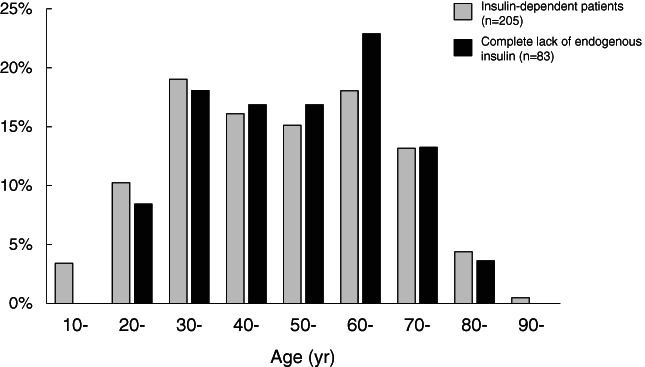
Distribution of patients with type 1 diabetes according to age. Gray bar: insulin‐dependent patients (fasting plasma C‐peptide <0.6 ng/mL, n = 205). Black bar: insulin‐dependent patients with complete lack of endogenous insulin (fasting plasma C‐peptide less than detection limit, n = 83).
In addition to the insulin‐dependent state in typical type 1 diabetes, insulin‐dependent diabetes induced by immune checkpoint inhibitors and after total pancreatectomy often develops in older adults because these treatments are applied to cancers that are more common in older adults. The number of such patients is also increasing due to an increase in the number of patients treated with immune checkpoint inhibitors and improved prognosis after pancreatectomy. A complete lack of endogenous insulin is frequently observed in type 1 diabetes induced by immune checkpoint inhibitors 15 , 16 and in all patients after pancreatectomy, contributing to an increase in the number of older adults with insulin‐dependent diabetes with a complete lack of endogenous insulin.
Treatment of insulin‐dependent diabetes in older adults
Patients with insulin‐dependent diabetes inevitably require insulin treatment for survival. Treatment regimens and glycemic targets must be individualized in older adults because of the large inter‐individual variations. Several factors should be considered when determining treatment regimens. In the case of older adults with insulin‐dependent diabetes, two major factors to be considered are the disease status and the ability of patients to self‐manage. These two factors should be balanced when determining the treatment regimen in older adults with insulin‐dependent diabetes (Fig. 3).
Figure 3.
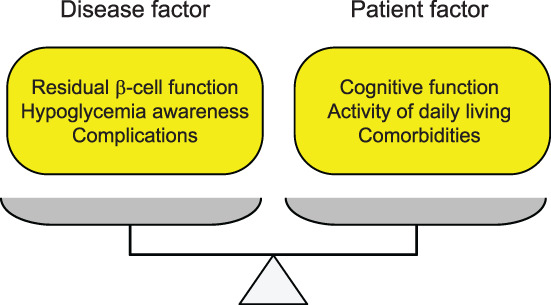
Factors to be considered in determining treatment regimen. The severity of the disease and ability of the patient should be balanced to determine the treatment regimen and glycemic targets.
Factors to be considered in the disease status include residual beta‐cell function, hypoglycemic awareness and chronic complications (Fig. 3). Regarding residual beta‐cell function, patients with a complete lack of endogenous insulin are particularly difficult to treat 6 , 7 , 8 and may need to use new technologies, such as CSII and sensor‐augmented pumps, in which the insulin pump is connected to a glucose sensor and can automatically suspend insulin to prevent hypoglycemia. Patients with impaired awareness of hypoglycemia are prone to severe hypoglycemia, a life‐threatening situation 20 ; therefore, special attention should be paid to avoid hypoglycemia. These patients may also need to use new technologies, such as CGM with built‐in alarms and alerts for hypoglycemia and a sensor‐augmented pump. 21 , 22 Among the chronic complications, impaired vision due to retinopathy potentially becomes an obstacle to insulin treatment.
The ability of patients to self‐manage is also an important factor in determining the treatment regimen because the success of each treatment option depends on the ability of each patient to carry out the prescribed treatment. Impaired cognitive function, limited activities of daily living (ADL) and comorbidities are major obstacles in the application of appropriate treatment regimens in older adults (Fig. 3). New technologies, such as CGM, CSII and the sensor‐augmented pump described above provide better glycemic control with a lower risk for hypoglycemia, 21 , 22 indicating the beneficial effect of these technologies in older adults with insulin‐dependent diabetes. 23 , 24 However, older adults cannot fully benefit from these new technologies because their limited ability to manage these instruments due to cognitive and functional impairments makes it difficult to use these technologies. 21 The hybrid closed loop system, which has recently been approved in the Japanese market, can automatically modulate insulin delivery based on sensor glucose levels to control both hyper‐ and hypoglycemia. 25 Therefore, it is expected to be an ideal treatment for older adults to achieve better glycemic control with minimal risk for hypoglycemia. Even in such devices, several obstacles exist in applying to older adults because the refilling of insulin, changing of infusion sets and insertion of cannula are required every 2–3 days, and appropriate management of devices in daily life, such as before and after bathing or showering, requires sufficient self‐management ability. In older adults with impaired cognitive or physical functions, self‐management is difficult even with multiple injections of insulin, making patients at risk of hypoglycemia due to overdose, and ketoacidosis due to missed doses.
Treatment targets in older adults with insulin‐dependent diabetes
Treatment targets should be set based on the balance between the disease status and the self‐management ability of the patient (Fig. 3). The Joint Committee of the Japan Diabetes Society and the Japan Geriatric Society on Improving Care for Elderly Patients with Diabetes has been working on these issues and published a committee report in 2016 with glycemic targets for older adults based on functional categories of cognitive function, ADL and comorbidities (Fig. 4). 26 To classify patients into appropriate categories, several screening tools, such as DASC‐8 (Dementia Assessment Sheet for Community‐based Integrated Care System 8‐items), were developed. 27 DASC‐8 is a short version of DASC‐21, which consists of 21 items, 28 and was confirmed strongly correlated with the original DASC‐21 in classifying older adults with diabetes into categories for determining glycemic targets. 27
Figure 4.
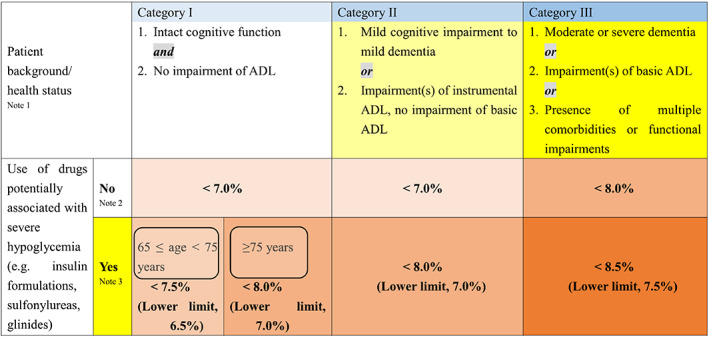
Glycemic targets for older adults with diabetes. ADL, activities of daily living. Adapted from JDS/JGS Joint Committee on Improving Care for Elderly Patients with Diabetes: Geriatr Gerontol Int 2016; 16: 1243–1245 with permission. 26
These targets were subdivided according to the presence or absence of medications conferring a risk of hypoglycemia. In the case of insulin‐dependent diabetes, all patients are treated with insulin; therefore, the glycemic targets described in the lower panel of the table were applied to avoid potentially associated severe hypoglycemia. These targets are recommended as individualized for each patient, which is particularly important in older adults with insulin‐dependent diabetes because of the unique aspects of the disease, as described above. DASC‐8 may be used for older adults with both insulin‐dependent and non‐insulin‐dependent diabetes; however, it is noteworthy that DASC‐8 for category classification was validated in 410 individuals, including 70 subjects with diabetes, 27 of which no or a very small number of patients with insulin‐dependent diabetes are suspected to be included. Preventing hypoglycemia is important in both non‐insulin‐dependent and insulin‐dependent diabetes. In addition, preventing hyperglycemic crisis and ketoacidosis due to insulin skip is important in insulin‐dependent diabetes because administration of sufficient insulin is indispensable to sustain life in these patients.
Among the factors affecting the disease side (Fig. 3), a complete lack of endogenous insulin and hypoglycemic unawareness are factors to be considered in insulin‐dependent diabetes because these factors make patients highly susceptible to severe hypoglycemia. 20 , 29 The lack of endogenous insulin makes older adult patients prone to hypoglycemia, hyperglycemia and ketoacidosis unless basal insulin is appropriately administered. This is not always possible in older adults, particularly in those with impaired cognitive function and limited ADL, and those without nearby relatives or caregivers.
Among patient factors, impaired cognitive function limits the treatment options and glycemic target of diabetes, as described above. On the other hand, diabetes and poor glycemic control are risk factors for cognitive decline and dementia, 30 , 31 indicating that the relationship between cognitive function and diabetic control is bi‐directional. Better glycemic control is desirable to prevent cognitive decline, but is hampered by impaired cognitive function.
Future prospects
With the increase in the number of older adults with insulin‐dependent diabetes, we are facing serious challenges in the treatment of these patients in daily clinical practice. Daily insulin therapy must be continued to sustain life; however, self‐management is not always possible in older adults. As both missed doses and overdoses lead to life‐threatening events, ketoacidosis and hypoglycemia, an appropriate treatment regimen must be constructed in each patient to sustain insulin treatment. In addition to efforts by caregivers and support by social systems, progress in insulin preparations and new technologies are awaited to assist older adults with insulin‐dependent diabetes. New basal insulin analogs designed for once‐weekly injections are now being developed. 32 , 33 , 34 Such insulin may provide a stable supply of basal insulin with the injection in clinics or by visiting doctors once a week, thereby contributing to the prevention of insulin‐dependent diabetes from ketosis and ketoacidosis. The risk of hypoglycemia may be avoided by titrating the insulin dose to the minimal dose necessary to prevent marked hyperglycemia and ketosis.
Beta‐cell replacement therapy may be another solution in the future. 35 , 36 In a previous study, encapsulated neonatal porcine islets were transplanted into the peritoneal cavity via laparoscopy in patients with unstable type 1 diabetes without immunosuppressive drugs. 35 Improved glycemic control (glycated hemoglobin <7.0%) was reported to be maintained for more than 600 days, with significant reduction in unaware hypoglycemic events. Long‐lasting basal insulin secretion without immunosuppressive drugs is promising for older adults with insulin‐dependent diabetes. Transplanting macro‐encapsulated islet cells derived from human stem cells may be another solution in the future. 36 In a recent first‐in‐human phase 1/2 clinical trial in patients with type 1 diabetes, subcutaneous implantation of a device containing stem cell‐derived pancreatic endodermal cells secreted insulin in a physiologically regulated fashion. 37 , 38 Although the main purpose of beta‐cell replacement therapy is to cure insulin‐dependent diabetes, the newly developed methods described above are potentially applicable to older adults with insulin‐dependent diabetes who have difficulty in self‐managing insulin injections.
Conclusions
Insulin‐dependent diabetes inevitably requires insulin treatment for survival, but a substantial number of older adults cannot self‐manage insulin injections due to cognitive and physical impairments. In addition to assistance from caregivers and healthcare systems, new insulin preparations and technologies, such as once‐weekly insulin and implantation of encapsulated islet cells, may potentially contribute to stable insulin supplementation for survival in older adults with insulin‐dependent diabetes.
Author contributions
YH contributed to the data acquisition. HI wrote the manuscript. All authors were involved in the interpretation of the data, writing, and reviewing drafts of the manuscript, and approved the final version for submission.
Disclosure statement
The authors declare that they have no competing interests.
Acknowledgements
This research has been supported by Grants‐in Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science: 21 K08589 and 21 K08567 awarded to HI and SN, respectively.
Ikegami H, Hiromine Y, Noso S. Insulin‐dependent diabetes mellitus in older adults: Current status and future prospects. Geriatr. Gerontol. Int. 2022;22:549–553. 10.1111/ggi.14414
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Livingstone SJ, Levin D, Looker HC et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008‐2010. JAMA 2015; 313: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh epidemiology of diabetes complications study cohort. Diabetes 2012; 61: 2987–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanaka S, Ohmori M, Awata T et al. Diagnostic criteria for slowly progressive insulin‐dependent (type 1) diabetes mellitus (SPIDDM) (2012): report by the committee on slowly progressive insulin‐dependent (type 1) diabetes mellitus of the Japan diabetes society. Diabetol Int 2015; 6: 1–7. [Google Scholar]
- 4. Niwano F, Hiromine Y, Noso S et al. Insulin deficiency with and without glucagon: a comparative study between total pancreatectomy and type 1 diabetes. J Diabetes Investig 2018; 9: 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ikegami H, Babaya N, Noso S. Beta‐cell failure in diabetes: common susceptibility and mechanisms shared between type 1 and type 2 diabetes. J Diabetes Investig 2021; 12: 1526–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukuda M, Tanaka A, Tahara Y et al. Correlation between minimal secretory capacity of pancreatic beta‐cells and stability of diabetic control. Diabetes 1988; 37: 81–88. [DOI] [PubMed] [Google Scholar]
- 7. Shibasaki S, Imagawa A, Terasaki J, Hanafusa T. Endogenous insulin secretion even at a very low level contributes to the stability of blood glucose control in fulminant type 1 diabetes. J Diabetes Investig 2010; 1: 283–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Babaya N, Noso S, Hiromine Y et al. Relationship of continuous glucose monitoring‐related metrics with HbA1c and residual β‐cell function in Japanese patients with type 1 diabetes. Sci Rep 2021; 11: 4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikegami H, Noso S, Babaya N, Kawabata Y. Genetics and pathogenesis of type 1 diabetes: prospects for prevention and intervention. J Diabetes Investig 2011; 2: 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uno S, Imagawa A, Kozawa J, Fukui K, Iwahashi H, Shimomura I. Complete loss of insulin secretion capacity in type 1A diabetes patients during long‐term follow up. J Diabetes Investig 2018; 9: 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sugihara S, Kikuchi T, Urakami T et al. Residual endogenous insulin secretion in Japanese children with type 1A diabetes. Clin Pediatr Endocrinol 2021; 30: 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keenan HA, Sun JK, Levine J et al. Residual insulin production and pancreatic ß‐cell turnover after 50 years of diabetes: Joslin medalist study. Diabetes 2010; 59: 2846–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oram RA, Jones AG, Besser RE et al. The majority of patients with long‐duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014; 57: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanafusa T, Imagawa A. Fulminant type 1 diabetes: a novel clinical entity requiring special attention by all medical practitioners. Nat Clin Pract Endocrinol Metab 2007; 3: 36–45. [DOI] [PubMed] [Google Scholar]
- 15. Ikegami H, Kawabata Y, Noso S. Immune checkpoint therapy and type 1 diabetes. Diabetol Int 2016; 7: 221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Baden MY, Imagawa A, Abiru N et al. Characteristics and clinical course of type 1 diabetes mellitus related to anti‐programmed cell death‐1 therapy. Diabetol Int 2018; 10: 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Green A, Hede SM, Patterson CC et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia 2021; 64: 2741–2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Otani T, Kasahara T, Miura J, Uchigata Y, Babazono T. Clinical background of Japanese patients with type 1 diabetes mellitus who have received insulin therapy for 50 years or longer. Diabetol Int 2019; 10: 288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kawasaki E, Maruyama T, Imagawa A et al. Diagnostic criteria for acute‐onset type 1 diabetes mellitus (2012): report of the Committee of Japan Diabetes Society on the research of fulminant and acute‐onset type 1 diabetes mellitus. J Diabetes Investig 2014; 5: 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weinstock RS, DuBose SN, Bergenstal RM et al. Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care 2016; 39: 603–610. [DOI] [PubMed] [Google Scholar]
- 21. Toschi E, Munshi MN. Benefits and challenges of diabetes technology use in older adults. Endocrinol Metab Clin N Am 2020; 49: 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pratley RE, Kanapka LG, Rickels MR et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA 2020; 323: 2397–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matejko B, Cyganek K, Katra B et al. Insulin pump therapy is equally effective and safe in elderly and young type 1 diabetes patients. Rev Diabet Stud 2011; 8: 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Briganti EM, Summers JC, Fitzgerald ZA, Lambers LNJ, Cohen ND. Continuous subcutaneous insulin infusion can be used effectively and safely in older patients with type 1 diabetes: long‐term follow‐up. Diabetes Technol Ther 2018; 20: 783–786. [DOI] [PubMed] [Google Scholar]
- 25. Boughton CK, Hovorka R. New closed‐loop insulin systems. Diabetologia 2021; 64: 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Japan Diabetes Society/Japan Geriatrics Society Joint Committee on Improving Care for Elderly Patients with Diabetes . Glycemic targets for elderly patients with diabetes. Geriatr Gerontol Int 2016; 16: 1243–1245. [DOI] [PubMed] [Google Scholar]
- 27. Toyoshima K, Araki A, Tamura Y et al. Development of the dementia assessment sheet for community‐based integrated care system 8‐items, a short version of the dementia assessment sheet for community‐based integrated care system 21‐items, for the assessment of cognitive and daily functions. Geriatr Gerontol Int 2018; 18: 1458–1462. [DOI] [PubMed] [Google Scholar]
- 28. Awata S, Sugiyama M, Ito K et al. Development of the dementia assessment sheet for community‐based integrated care system. Geriatr Gerontol Int 2016; 16 (Suppl 1): 123–131. [DOI] [PubMed] [Google Scholar]
- 29. Namba M, Iwakura T, Nishimura R et al. The current status of treatment‐related severe hypoglycemia in Japanese patients with diabetes mellitus: a report from the committee on a survey of severe hypoglycemia in the Japan diabetes society. J Diabetes Investig 2018; 9: 642–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia 2005; 48: 2460–2469. [DOI] [PubMed] [Google Scholar]
- 31. Rawlings AM, Sharrett AR, Schneider AL et al. Diabetes in midlife and cognitive change over 20 years: a cohort study. Ann Intern Med 2014; 161: 785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenstock J, Del Prato S. Basal weekly insulins: the way of the future! Metabolism 2022; 126: 154924. [DOI] [PubMed] [Google Scholar]
- 33. Nishimura E, Pridal L, Glendorf T et al. Molecular and pharmacological characterization of insulin icodec: a new basal insulin analog designed for once‐weekly dosing. BMJ Open Diabetes Res Care 2021; 9: e002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heise T, Chien J, Beals J et al. Basal insulin fc (BIF), a novel insulin suited for once weekly dosing for the treatment of patients with diabetes mellitus. J Endocr Soc 2021; 5: A329. [Google Scholar]
- 35. Matsumoto S, Abalovich A, Wechsler C, Wynyard S, Elliott RB. Clinical benefit of islet xenotransplantation for the treatment of type 1 diabetes. EBioMedicine 2016; 12: 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Russ HA, Shilleh AH, Sussel L. From the dish to humans: a stem cell recipe for success. Cell Metab 2022; 34: 193–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramzy A, Thompson DM, Ward‐Hartstonge KA et al. Implanted pluripotent stem‐cell‐derived pancreatic endoderm cells secrete glucose‐responsive C‐peptide in patients with type 1 diabetes. Cell Stem Cell 2021; 28: 2047–2061. [DOI] [PubMed] [Google Scholar]
- 38. Shapiro AMJ, Thompson D, Donner TW et al. Insulin expression and C‐peptide in type 1 diabetes subjects implanted with stem cell‐derived pancreatic endoderm cells in an encapsulation device. Cell Rep Med 2021; 2: 100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


