To the Editor:
Patients with newly diagnosed acute myeloid leukemia (AML) who are older or have significant comorbidities have a poor prognosis and are often ineligible for intensive induction chemotherapy. Low‐intensity frontline therapies, such as hypomethylating agents (HMAs) or low‐dose cytarabine (LDAC), are available alternatives, but they are associated with poor outcomes in older patients when given alone. 1 Venetoclax, a potent, selective, and orally available small‐molecule inhibitor of B‐cell lymphoma 2 (BCL‐2), has been approved for use in combination with azacitidine, decitabine, or LDAC for the treatment of newly diagnosed AML in adults who are ineligible for intensive induction chemotherapy. 2 , 3 Treatment with venetoclax combination regimens results in high rates of complete remission (CR)/CR with incomplete blood count recovery (CRi) (48%–66%), prolonged overall survival (OS) (median of 7.2–14.7 months), and rapid responses, with median time to first response ranging from 1.2–1.4 months; however, there is a broad distribution of time to first response reported, ranging from 0.6 to 14.9 months. 2 , 3 , 4 Here, we report the timing of response to venetoclax combination regimens and its association with patient outcomes and baseline or post‐baseline characteristics in unfit patients with newly diagnosed AML enrolled in the VIALE‐A and VIALE‐C studies. Study design, patient selection, and study treatment for VIALE‐A (M15‐656; NCT02993523) and VIALE‐C (M16‐043; NCT03069352) have been previously described. 4 , 5
Early responders achieved CR/CRi by the end of Cycle 2, later responders achieved CR/CRi after initiation of Cycle 3, and nonresponders did not achieve CR/CRi before the start of another line of AML therapy or database cutoff; patients who achieved CR/CRi after disease progression or morphologic relapse, or those who achieved morphologic leukemia‐free state (MLFS) on study but did not eventually meet CR/CRi criteria were deemed nonresponders. For patients who achieved CR/CRi after treatment discontinuation but did not start subsequent AML therapy (VIALE‐A, n = 5; VIALE‐C, n = 1), early response was defined as achieving CR/CRi ≤56 days (2 cycles) after the first dose, and later response as achieving CR/CRi ≥57 days after first dose. Baseline characteristics and post‐baseline variables observed up to the end of Cycle 2 as well as safety and efficacy outcomes were evaluated to determine their associations with response timing.
The data cutoff date for VIALE‐A was January 4, 2020 and for VIALE‐C was August 15, 2019. Event‐free survival (EFS), duration of response (DOR), and OS were analyzed using Kaplan–Meier methodology. Patients who achieved a response beyond Cycle 6 were not included in EFS or DOR analyses. A classification and regression tree (CART) model was implemented in rpart package (version 4.1‐15) in R (version 3.6.3) to identify baseline and post‐baseline characteristics associated with CR/CRi occurring in later cycles of therapy in patients who had not achieved CR/CRi in the first 2 cycles. 6 Further details are in the supplemental methods.
Patient baseline characteristics for VIALE‐A and VIALE‐C are shown in Tables S1 and S2. Of 286 patients in the venetoclax + azacitidine arm in VIALE‐A, 190 (66%) achieved CR/CRi, and 96 (34%) were nonresponders. Time to first CR/CRi varied considerably among patients achieving CR/CRi in VIALE‐A (Figure 1A). Of 190 patients achieving CR/CRi, 123 (65%) achieved CR/CRi in ≤1 cycle, 144 (76%) were early responders, and 46 (24%) were later responders. Of 46 later responses, 34 (74%) were achieved during Cycles 3 or 4, 4 (9%) were achieved during Cycles 5 or 6, and 5 (11%) were achieved during or after Cycle 7. There was a small number of responses, only 5 (11%), that were achieved after study treatment discontinuation without any intervening therapy. All later responders who had a CR/CRi beyond Cycle 6 had achieved MLFS before Cycle 6 (Table S3). Of 143 patients enrolled in the venetoclax + LDAC arm in VIALE‐C, 69 (48%) achieved CR/CRi, and 58 (41%), 11 (8%), and 74 (52%) achieved early, late, and no response, respectively (Figure S1).
FIGURE 1.
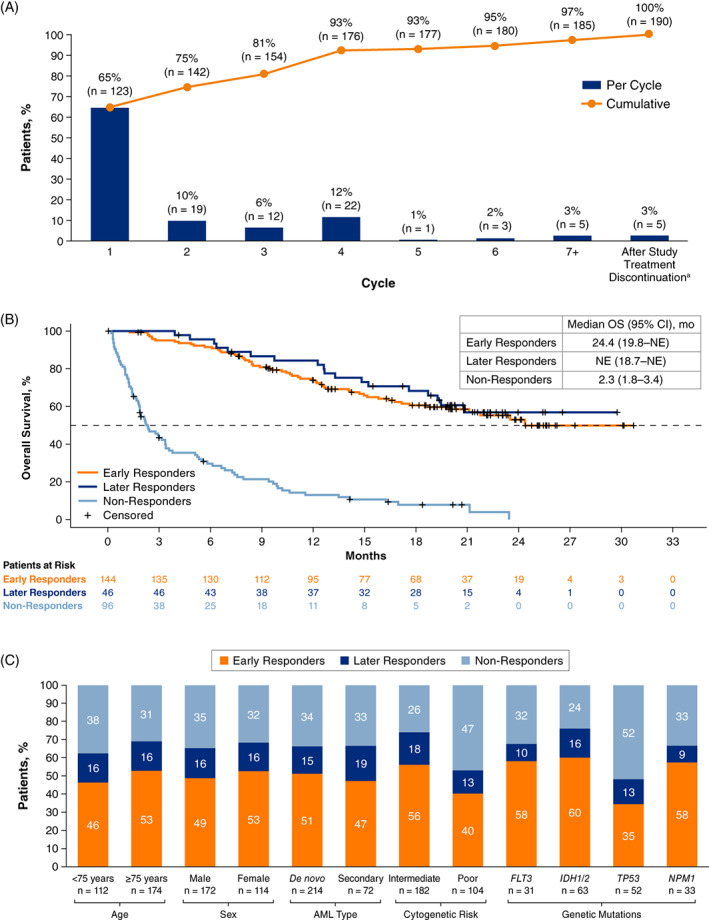
(A) Cumulative time to first response of CR/CRi with venetoclax + azacitidine (VIALE‐A; n = 190); (B) Overall survival by timing of CR/CRi in patients treated with venetoclax + azacytidine (VIALE‐A); (C) Baseline characteristics of patients achieving early or later CR/CRi with venetoclax + azacitidine (VIALE‐A). aOf five patients who achieved CR/CRi after study treatment discontinuation in VIALE‐A, two achieved CR/CRi within 56 days after the first dose of venetoclax and were classified as early responders, and three achieved CR/CRi ≥57 days after the first dose of venetoclax and were classified as later responders. AML, acute myeloid leukemia; CR, complete remission; CRi, complete remission with incomplete blood count recovery
A comparison of efficacy outcomes between early, later, and nonresponders for VIALE‐A is shown in Table S2. In VIALE‐A, 23/46 (50%) later responders had achieved MLFS within the first 2 cycles compared with only 8/96 (8%) nonresponders. EFS was 17.8 months (95% CI, 11.5–19.9) for early responders and 16.2 months (95% CI, 10.2–19.6) for later responders. Later responders had a shorter duration of CR/CRi compared with early responders, with a median duration of CR/CRi of 12.1 months (95% CI, 6.0–not estimable [NE]) versus 18.4 months (95% CI, 15.8–NE), respectively. OS was similar in early and later responders and far exceeded the OS observed in nonresponders (Figure 1B). In early responders, median OS was 24.4 months (95% CI, 19.8–NE), whereas in later responders, median OS was not reached (95% CI, 18.7–NE). Efficacy outcomes by timing of response in VIALE‐C are presented in Table S4. Similar to VIALE‐A, OS was improved in early/later responders compared with nonresponders (Figure S2).
Proportions of patients in VIALE‐A who achieved early CR/CRi, later CR/CRi, or who never achieved CR/CRi are presented by baseline characteristics in Figure 1C. Patients with poor cytogenetic risk or TP53 mutation had the lowest probability of achieving CR/CRi. Patients with intermediate cytogenetic risk or IDH1/2 mutations had the highest probability of achieving CR/CRi. Most responses occurred early, irrespective of patient baseline characteristics. Mean (SD) bone marrow blast count percentages at baseline for early, later, and nonresponders, respectively, were 49.2% (25.1%), 46.5% (24.9%), and 51.6% (23.2%; Table S5). Similar findings were observed for patients in VIALE‐C (Table S5 and Figure S3).
The CART model identified achievement of MLFS within the first 2 cycles of venetoclax + azacitidine as the most important factor in predicting later CR/CRi for VIALE‐A (Figure S4). Patients who achieved MLFS by the end of Cycle 2 had a much higher likelihood of achieving later CR/CRi compared with those who did not (74% vs 21%, respectively). When included in the CART model for CR/CRi response, bone marrow blast counts helped predict later CR/CRi among patients who did not achieve MLFS by the end of Cycle 2. Patients with a ≥50% reduction in bone marrow blasts from baseline to end of Cycle 2 had a higher likelihood of achieving a later CR/CRi compared with those who had a <50% reduction (31% vs.19%, respectively).
The safety of venetoclax‐based regimens was also assessed by CR/CRi responder status. The most common Grade ≥3 hematologic TEAEs occurred at similar or lower rates in later responders compared with early responders in both studies, except for neutropenia in VIALE‐C (Table S6). Most Grade ≥3 hematologic TEAEs had an onset within the first 2 cycles of treatment (or within 56 days after the first dose of venetoclax; Table S7). In VIALE‐A, later and early responders had comparable rates of infections/infestations (83% and 85%, respectively) and sepsis (2% and 3%), with similar results (infections/infestations: 64% vs. 57%; sepsis: 0% vs. 2%) observed in VIALE‐C. The most common TEAEs in both studies occurred at similar or lower rates in later responders compared with early responders, except hypokalemia and fatigue in VIALE‐A and neutropenia and pneumonia in VIALE‐C (Tables S8 and S9). Notably, the incidence of most TEAEs was lower in nonresponders compared with early or later responders, which likely reflects the relatively shorter time that nonresponders were on study treatment (median of 1.2 and 1.6 months in VIALE‐A and VIALE‐C, respectively; Tables S3 and S4).
Data on the timing of response, efficacy outcomes modeling, and safety for patients who achieved CR/CRi with partial hematologic recovery (CRh) can be found in supplementary results (Figures S5–S8 and Tables S10–S14) and is consistent with the CR/CRi data.
In summary, most patients in VIALE‐A and VIALE‐C who achieved CR/CRi responded early after the first 2 cycles of venetoclax combination therapy. Analyses of OS demonstrated similar benefit for both early and later responders compared with, suggesting that responding patients derive a similar survival benefit from venetoclax combination therapy irrespective of whether CR/CRi is achieved early or later. Later responders achieved MLFS within the first 2 cycles of treatment at a higher rate than nonresponders. Accordingly, the CART model identified achievement of MLFS within the first 2 cycles as the most influential factor in predicting whether a patient would achieve a later response.
The safety profiles of VIALE‐A and VIALE‐C were consistent with previous reports. 4 , 5 The analysis demonstrated comparable or lower rates of most Grade ≥3 hematologic TEAEs in later versus early responders within the first two treatment cycles, indicating that a later timing of response does not appear to have a detrimental impact on the safety profile of venetoclax combination therapy.
Overall, these analyses demonstrate that a considerable number of patients achieve CR/CRi after the first 2 cycles of venetoclax combination regimens. Importantly, patients who achieve later responses derive a similar clinical benefit from venetoclax combination regimens as those who achieve early responses. These results indicate that the possibility of attaining a late response should be considered before discontinuation of venetoclax‐based combination therapy in patients who do not achieve a response within the first 2 cycles.
AUTHOR CONTRIBUTIONS
Brian A. Jonas conceptualized the study and designed methodology; Yinghui Duan, Wellington Mendes, Jalaja Potluri, and Björn Tews verified the overall replication/reproducibility of results/experiments and other research outputs; Yinghui Duan applied statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data; Brian A. Jonas, Andrew H. Wei, Christian Recher, Courtney D. DiNardo, Jun‐Ho Jang, Panayiotis Panayiotidis, Pau Montesinos, Su‐Peng Yeh, Vladimir Ivanov, Walter Fiedler, Takahiro Yamauchi, Wellington Mendes, Jalaja Potluri, Björn Tews, and Yishai Ofran conducted the research and investigation process, performed experiments, or collected data/evidence; Brian A. Jonas, Andrew H. Wei, Christian Recher, Courtney D. DiNardo, Jun‐Ho Jang, Panayiotis Panayiotidis, Pau Montesinos, Su‐Peng Yeh, Walter Fiedler, Takahiro Yamauchi, and Yishai Ofran provided study materials, reagents, patients, laboratory samples, animals, instrumentation, computing resources, or other tools; Yinghui Duan, Wellington Mendes, Jalaja Potluri, and Björn Tews managed and coordinated for research activity planning and execution; Brian A. Jonas, Yinghui Duan, Wellington Mendes, Jalaja Potluri, and Björn Tews wrote the original draft; all authors reviewed and edited the manuscript.
FUNDING INFORMATION
Venetoclax is being developed in collaboration between AbbVie and Genentech. AbbVie and Genentech funded the VIALE‐A study, and AbbVie funded the VIALE‐C study. AbbVie and Genentech participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
CONFLICT OF INTEREST
B.A.J. reports consultancy/advisory role for AbbVie, BMS, Genentech, Gilead, GlycoMimetics, Jazz, Pfizer, Servier, Takeda, Tolero, and Treadwell; protocol steering committee for GlycoMimetics; data monitoring committee for Gilead; travel support from AbbVie; research funding to institution from 47, AbbVie, Accelerated Medical Diagnostics, Amgen, AROG, BMS, Celgene, Daiichi Sankyo, F. Hoffmann‐La Roche, Forma, Genentech/Roche, Gilead, GlycoMimetics, Hanmi, Immune‐Onc, Incyte, Jazz, Loxo, LP Therapeutics, Pfizer, Pharmacyclics, Sigma Tau, and Treadwell. A.H.W. reports consultancy/advisory role for Novartis, Janssen, Amgen, Roche, Pfizer, AbbVie, Servier, Gilead, BMS, Macrogenics and Agios; speakers' bureau participation for AbbVie, Novartis, and BMS; royalties from the Walter and Eliza Hall Institute of Medical Research related to venetoclax; research funding to institution from Novartis, AbbVie, Servier, BMS, AstraZeneca, and Amgen. C.R. reports consultancy/advisory role for AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, Celgene, Otsuka, Astellas, Daiichi‐Sankyo, Macrogenics, Roche, Takeda, and Pfizer; research funding from AbbVie, Amgen, Novartis, Celgene, Jazz Pharmaceuticals, Agios, Chugai, MaatPharma, Astellas, Roche, Iqvia, and Daiichi‐Sankyo. C.D.D. reports consultancy/advisory role for AbbVie, Agios, Aprea, Celgene/BMS, ImmuneOnc, Kura, Novartis, Takeda, and Notable Labs; research funding to institution from AbbVie, Agios, Calithera, Cleave, BMS/Celgene, Daiichi‐Sankyo, ImmuneOnc, and Loxo. J‐H.J. declares no conflicts to disclose. K.P. reports consultancy/advisory role for AbbVie, Astellas, Boston BioMedical, Bristol Myers Squibb, Novartis, Servier, Jazz Pharmaceuticals, and Celgene; research funding from AbbVie, Agios, Daiichi Sankyo, and Millennium. P.P. reports honoraria from AbbVie, Amgen, Celgene, Roche, Genesis, Novartis, Gilead, and Janssen; research funding from AbbVie, Genesis, and Roche. P.M. reports consultancy/advisory role, speakers' bureau participation, and research funding from AbbVie. S‐P.Y. reports consultancy/advisory role for AbbVie, Amgen, Janssen, Astellas, Astex, and Takeda. V.I. reports being a clinical investigator in clinical trials for AbbVie, Pfizer, MSD, Takeda, ABScience, Ascentage Pharma Group, Astellas, Novartis, and GSK. W.F. reports consultancy/advisory role for AbbVie, Amgen, ARIAD, Celgene, Clinigen, Jazz Pharmaceuticals, Novartis, Stemline, Morphosys, Servier, and Pfizer; patents, royalties, or other intellectual property from a patent on immunotherapy in AML obtained together with Amgen; travel support from Amgen, Daiichi Sankyo, Jazz Pharmaceuticals, and Servier; research funding from Amgen. T.Y. reports honoraria from Pfizer, AbbVie, Nihon‐Shin‐Yaku, Astellas, and Pfizer; consultancy/advisory role for Janssen, Novartis, and Pfizer; research funding from Chugai, Pfizer, and Solasia. Y.D., W.M., J.P., and B.T. report employment with AbbVie and may hold stock or other options. Y.O. reports honoraria from AbbVie; consultancy/advisory role for AbbVie, Pfizer, Takeda, JnJ, Amgen, Astellas, Novartis, and Roche.
ETHICS STATEMENT
These studies were approved by the institutional review board or independent ethics committee at each institution, and patients provided written informed consent before enrollment. These studies were conducted in accordance with the international conference on harmonization good clinical practice guidelines and the Declaration of Helsinki.
Supporting information
Data S1 Supporting information.
ACKNOWLEDGMENTS
AbbVie and authors thank all the trial investigators and the patients who participated in this clinical trial. Medical writing support was provided by Diana Avery, PhD, and Rohina Rubicz, PhD, of Bio Connections, LLC, and funded by AbbVie.
Funding information AbbVie; Genentech
DATA AVAILABILITY STATEMENT
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
REFERENCES
- 1. Estey EH. Acute myeloid leukemia: 2021 uptake on risk‐stratification and management. Am J Hematol. 2020;95(11):1368‐1398. [DOI] [PubMed] [Google Scholar]
- 2. DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment‐naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei AH, Strickland SA Jr, Hou JZ, et al. Venetoclax combined with low‐dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J Clin Oncol. 2019;37(15):1277‐1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617‐629. [DOI] [PubMed] [Google Scholar]
- 5. Wei AH, Montesinos P, Ivanov V, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo‐controlled trial. Blood. 2020;135(24):2137‐2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lewis R. An introduction to classification and regression tree (CART) analysis. Presented at: Society for Academic Emergency Medicine (SAEM); May 22‐25, 2000; San Francisco, CA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting information.
Data Availability Statement
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial‐level data (analysis data sets), as well as other information (eg, protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal, Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the US and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.


