Abstract
The evolution of risk identification and ultimately the public and private responses that have become known collectively as the “opioid crisis” is an important case study in risk management due to the reach and magnitude of its impacts. This article examines a number of “signals” related to opioid risks using the social amplification of risk framework (SARF) to investigate a limited set of public‐sector activities and policy responses. We evaluate whether the SARF presents an effective lens to examine the serious shortcomings of risk management of opioid use, which has a history of risk attenuation and, more recently, evidence of risk amplification. Our goal in this article is limited to addressing “goodness of fit” of the SARF as a descriptive tool. We consider whether the SARF effectively reveals important gaps in public risk management responses for the opioid example and other similarly situated societal risk problems. Applying SARF supports that its suggested relationship between risk signals and inappropriate attenuated public response does generate useful insights into regulatory efficacy for examples of public risk management. Similar such conclusions about inappropriate public responses stemming from the amplification factors are less supported because, in this case, the risk is, and continues to be, large. Overall, we find that the SARF's particular focus on the signaling function of risk information performs best as an organizational aid to study historical information rather than as a predictive tool for determining inappropriate risk management responses.
Keywords: Opioid crisis, risk management, social amplification of risk
1. INTRODUCTION
The evolution of risk identification and ultimately the public and private responses that have become known collectively as the “opioid crisis” is an important case study in risk management due to the reach and magnitude of its impacts. Understanding the full scope of the opioid crisis and the many factors that have contributed to its consequences is beyond the scope of any one paper. This article pursues a more modest goal of examining a number of “signals” related to opioid risks using the social amplification of risk framework (SARF) to address a limited set of public‐sector activities and policy responses. The SARF is a comprehensive conceptual framework based on social science and communication foundations to at least organize, if not understand, the various dynamic social processes underlying risk perception and response. The SARF uses a metaphor from communications that focuses on signals, receiving stations, rippling effects, and impacts to understand the social fate of risk signals (Kasperson et al., 1988).
Kasperson (2021) summarizes that “[i]n particular, those processes by which certain hazards and events that experts assess as relatively low in risk can become a particular focus of concern and sociopolitical activity within a society (risk amplification), whereas other hazards that experts judge to be more serious receive comparatively less attention from society (risk attenuation).” The SARF has been applied to risk attenuation and amplification across various risk management problems (Kasperson & Kasperson, 1996). The opioid crisis, however, is an example of evolving risk communication and response in which important risk signals were first attenuated and, later, the same or additional risk signals arguably have been amplified within public institutions. Our goal is to highlight risk signals and risk events that resulted in more or less attenuation and amplification with important consequences for delays and accelerations in the evolving public response to this crisis and similar widespread public health epidemics.
The article reviews particular SARF factors such as attributes of the hazard, risk management organization, and risk signals that are relevant to the attenuation and amplification of the seriousness, manageability, and consequences of opioid risks. As a risk management problem, the opioid crisis also exhibits large secondary and tertiary consequences that spread beyond the initial impact (e.g., addiction) to affect other institutions (e.g., response through litigation). We evaluate whether the SARF and certain theoretical enhancements added by practitioners since the original 1988 publication present an effective lens to explain the serious shortcomings of risk management of opioid use. We also consider whether the SARF incrementally advances our understanding for the opioid example and other similarly situated societal risk problems such as use of vaping products and exposures to toxic chemicals.
The next section provides background on the opioid crisis in the United States and an overview of the regulation and operations of pharmaceutical markets. Sections 3 and 4 contain analysis of certain risk response examples from the pre‐ and post‐2010 phases of the opioid crisis and the SARF conditions consistent with risk attenuation and amplification, respectively. Section 5 contains our conclusions about how well the SARF explains and predicts attenuation and amplification of the opioid risks considered.
2. CHARACTERIZATION OF RISKS AND THE OPIOID CRISIS
2.1. Background on the Opioid Crisis in the United States
Information about the opioid crisis in the United States demonstrates that it is first and foremost an example of a risk management problem that is local and national, involving private industry and public agency oversight, and large by any measure of mortality, morbidity, and cost. Public risk management of modern‐day opioids begins with the 1970 Controlled Substances Act (CSA) which formalized a scheduling system to sort drugs into five categories according to their therapeutic value and abuse potential (DEA, 2020a). The Food and Drug Administration (FDA), then‐current government enforcement organizations, and later the Drug Enforcement Administration (DEA) were responsible for adherence to this system. Heroin was, and is, included with other outlawed drugs under Schedule I. Prescription opioids are legal products that can be prescribed by healthcare practitioners to treat pain. Several opioid molecules are currently sold as prescription opioids, some of the most common molecules being oxycodone, hydrocodone, fentanyl, and morphine. Most prescription opioids were classified as Schedule II drugs, but hydrocodone combination products were listed as Schedule III until they were rescheduled to Schedule II in October 2014 (DEA, 2014). The CSA strengthened the closed system of distribution for opioid products by requiring scheduled drugs to be controlled only by lawful registrants until delivered to the intended medical users (DEA, 2006).
In the late 1980s and early 1990s, medical and healthcare professionals began calling for change surrounding the treatment of pain (Dayer et al., 2019).1 State medical board laws governing opioid prescriptions were liberalized in the late 1990s for the treatment of noncancer pain, and new pain management standards were implemented by the nonprofit governing body, the Joint Commission on Accreditation of Healthcare Organizations, in 2000 (Manchikanti, Fellows, Ailinani, & Pampati, 2010). Baker (2017) explains that reported opioid prescriptions followed a clear annual upward trend and increased from 76 million in 1991 to 219 million in 2011, considered to be the “turning point” of the crisis.
Other relevant signals of a growing crisis were also readily available to risk managers. For example, comparisons of oxycodone consumption in the United States and globally indicates that by 2012, the U.S. per capita level was approximately 20 times that of the global level with an expanding divergence between the two measures after 1995 (ECC, 2018). Lyapustina and Alexander (2015), citing statistics on narcotic drugs from the International Narcotics Control Board, report that the United States is by far the largest consumer of prescription opioids. In 2009, the U.S. share of global prescriptions was 99% for hydrocodone, 60% for hydromorphone, and 81% for oxycodone.
Risks from illicit opioids also became increasingly apparent after 2010. The U.S. Centers for Disease Control and Prevention (CDC) describes this history as reflecting three waves in opioid overdose deaths (CDC, 2020a). The first wave from 1999 is driven by commonly prescribed opioid pharmaceuticals. The second wave begins in 2010 and reflects a rise in heroin overdose deaths. The third and steepest wave begins in 2013 and reflects a rise in overdose deaths caused by other synthetic opioids including illicit fentanyl.
From 2000 forward, the United States experienced increasing consequences related to prescription and illicit opioid use. “Over 700,000 people died in the United States from drug overdoses between 1999 and 2017, with 70,237 deaths in 2017 alone. Of these 70,237 deaths, 67.8% involved an opioid” (CDC, 2019, p. 7). Fig. 1 shows that from 1999 to 2010, there were, what should have been received as alarming, increases in such critical risk signals as opioid sales volume, abuse, and deaths, among many other consequences that have been identified (Brill & Ganz, 2018). Degenhardt et al. (2019) present comparative data showing that U.S. consumption of opioids substantially exceeded that of other developed countries by a wide margin. Regulation of opioids clearly failed in the United States even with substantial public risk management and oversight.
Fig 1.
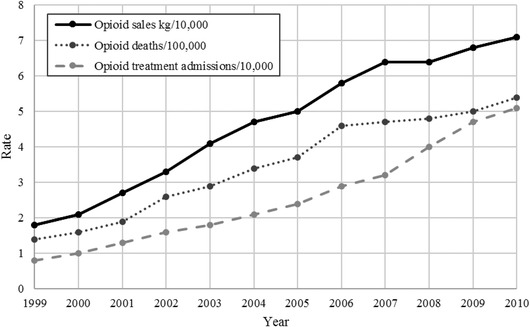
Rates of opioid sales, opioid‐related unintentional overdose deaths, and opioid addiction treatment admissions, 1999–2010 (adapted from Kolodny et al., 2015, p. 560).
2.2. Pharmaceutical Markets and Risk Stations
Opioid prescriptions and related activities in the United States operate in a complex social system with experienced, well‐funded, and well‐trained participants as summarized by Fig. 2. On the supply side, pharmaceutical manufacturers produce approved prescription drugs and generally sell the product to wholesalers (also known as a distributor). Then, wholesalers distribute and sell the product downstream to pharmacies. Pharmacies dispense the product to patients, who receive prescriptions from healthcare practitioners. Pharmacies also interact with the pharmacy benefit managers (PBM) of patients’ insurance plans (MedPac, 2016; Sood, Shih, Van Nuys, & Goldman, 2017).
Fig 2.
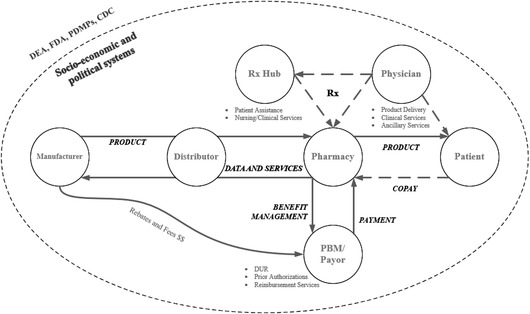
Pharmaceutical system.
There are many public‐sector regulatory and oversight organizations (or “stations” in the SARF) relevant to the risk management of opioid risks. These public agencies have broad authority to track, monitor, and analyze private information to identify drivers with potentially risk‐increasing effects or to monitor the signals of abuse and impact directly. Overlaying the transactions of the many participants in opioid markets are regulation and enforcement by DEA, FDA, and state pharmacy and licensing boards, as well as administration of benefits by the Centers for Medicare & Medicaid Services (CMS) (CMS, 2020, FDA, 2020). The CDC and other public health organizations also are responsible for tracking and understanding risks of addiction and injury (CDC, 2020b). Regarding abuse, opioids are regulated products subject to regular and continuous review by DEA, among other state oversight organizations, such as prescription drug monitoring programs (PDMPs) (DEA, 2020b; DEA ARCOS, 2020). Importantly, the volume of opioids is tracked by DEA, and DEA sets production quotas for Schedule I and II product manufacturers each year (DEA, 2020c, 2020d).
Despite the breadth and depth of public‐sector stations within this highly sophisticated and regulated system, an opioid crisis emerged. Risk signals were apparently attenuated to the point that the intended operation of the system led to a national crisis of significant and widespread unintended consequences. The substantial impacts indicate preliminarily that the risk assessment and communication related to licit and illicit opioids in the United States demonstrated serious attenuation of public health signals and mitigation responses at least up to 2010, when public response became more focused. As such, the period of the opioid crisis up to 2010 presents an ideal candidate to be investigated using the SARF for risk attenuation.
3. RISK ATTENUATION
A large and growing body of studies, such as ECC (2018), supports that numerous “signals” related to the opioid crisis failed to stimulate the social mobilization that the problem required. The SARF factors, associated with inappropriate risk attenuation, emphasize features of the risk signal and characteristics of the social stations. Practitioners identify five types of hazards particularly susceptible to attenuation (Kasperson & Kasperson, 1991; Kasperson, Kasperson, Pidgeon, & Slovic, 2003):
Global elusive hazards that display diffuse, latent, or inarticulate consequences;
Ideological hazards that invoke values and assumptions that minimize consequences, elevate associated benefits, or idealize certain beliefs;
Marginal hazards that affect people who occupy the margins of human populations, cultures, societies, and economies;
Amplification‐driven hazards that have effects inconsistent with conventional risk assessment methods but are easily magnified by other stations, causing the risk controversies to be dismissed or discounted by risk assessors until the consequences are visible to society generally; and
Value‐threatening hazards that alter human institutions, lifestyles, and basic values and overwhelm society's ability to adjust and respond effectively.
Renn (2011) applied the SARF to investigate the attenuation of climate change risks. He added two theoretical concepts to increase the power of the SARF: functional resonance and common pool resources. Renn used these theoretical concepts to complement the SARF and improve its ability to help understand the mechanisms of amplification and attenuation. Functional resonance addresses the relationship between four systems of society (order, meaning, production and distribution, and social relationship) and the corresponding resonance medium (power, values and beliefs, money, and solidarity).
In our investigation, we examine whether certain risk signals failed to resonate with the social station and were therefore attenuated. Common pool resource refers to the resource problem where no or limited access restrictions for the individual user lead the user to overconsume. Here, the “tragedy of the commons” problem potentially manifested as risk attenuation of a common public bad. With vague delineation of the agencies’ respective ownership of the public health consequences of opioid use, each public agency appears to have discounted the increasing public health signals as “somebody else's problem” or beyond their administrative scope.
We investigate the effectiveness of the SARF with the above considerations of resonance and accountability to explain risk attenuation that likely affected public and private responses by way of several selected examples. Ultimately, we want to understand in what ways the attenuation of risk signals delayed putting the opioid crisis at the forefront of the public agenda and muted the societal response to address the related consequences.
3.1. Risk Signals that should have Heightened Control Responses
In the regulation of opioids, DEA, FDA, CDC, state agencies, state medical boards, and professional organizations, such as the American Pain Society (APS), not only receive risk signals but also are primary in the interpretation and communication of signals to industry, healthcare providers, payors, and patients. We use the SARF to examine selected examples in which public station attenuation likely affected prescription volumes and subsequently, changes in abuse or diversion risks.
3.1.1. The Elusive Regulation Hazard
3.1.1.1. Hydrocodone
An example in which the risk signal appears to have been attenuated by regulation is the case of hydrocodone (one of the most highly diverted prescription opioids). The CSA classified hydrocodone, along with most narcotics and opiates, as a Schedule II drug “unless specifically excepted or unless listed in another schedule” (Public Law 91–513, 1970). However, the act included an exception that classified some combinations of narcotics with nonnarcotics (e.g., hydrocodone‐acetaminophen) as Schedule III. This regulatory exception, based on lower risk of abuse rationale (DEA, 2014), was subsequently followed by a market emphasis on combination hydrocodone products and a greater potential for increasing volumes. As Schedule III drugs, the combination products provided refills for patients without the need to obtain additional prescriptions and were less susceptible to state regulatory oversight through PDMPs (Brandeis University, 2018).
Despite requests to increase control hydrocodone combination products as far back as 1999, it was not until October 2014 that DEA rescheduled them as Schedule II after receiving a reversal in the Health and Human Services (HHS) scheduling recommendations. In its analysis, DEA acknowledged multiple risk signals showing that hydrocodone combination products have a high incidence of abuse and are often diverted from approved sales channels (DEA, 2014). DEA's report notes that the addition of a nonnarcotic analgesic may have the intended abuse deterrent properties for a regular user but likely would not deter someone who was already an abuser. Following the reclassification, the 2015 volume of hydrocodone prescribed fell approximately 20% (Steckler, Mosher, Desloover‐Koch, & Lund, 2019; Varisco, Ogunsanya, Barner, & Fleming, 2017).
Applying the SARF, there is support that the classification of hydrocodone combination products and their related risks fell into the category of an ideological hazard, since the regulatory exception was premised on lower risks of abuse based on recommendations from HHS. The DEA report notes that the perception of lower risks of abuse originally supported by HHS was based on the physical condition that combination products required less hydrocodone to achieve the desired effects. In its 2014 analysis, DEA noted that there were no scientific studies to support that perception and that it failed to account for the easier access afforded by the Schedule III classification. The market response to combination products and, relatedly, hidden abuse risks may have failed to resonate adequately with DEA and HHS, since the original classification of the combination products was recognized explicitly by the control framework and designed to encourage access to a relatively safer alternative.
3.1.1.2. Abuse Deterrent Formulations (ADFs) and the Gateway Theory
Other attenuated risks of substance abuse are evident in the opioid crisis. Based on a review of the regulatory history of OxyContin, NAS (2017) investigated the ADF requested by the FDA to produce a product initially thought to have a lower risk of misuse because it could not be crushed, snorted, or injected by an abuser. NAS (2017) cites studies that indicated high percentages of OxyContin misusers reacted to the reformulation by using other drugs, including heroin. This has become known as the gateway theory. Alpert, Powell, and Pacula (2018) find a strong association between the decrease in OxyContin misuse and heroin mortality. Based on their findings, they suggested that 80% of the increase in heroin deaths could be due to the ADF.
NAS (2017) reports data from the National Survey on Drug Use and Health (NSDUH) showing that restrictions on licit opioids resulted in a surge in heroin users after 2010 and is consistent with the gateway theory. However, the theory that reformulation led to increasing abuse and eventually increasing heroin deaths has been challenged by other studies. Jones, Logan, Gladden, and Bohm (2015) indicates that a greater supply of cheap heroin entered the U.S. market coincident with the reformulation, so an association exists, but when examined at the epidemiological level, it might be spurious. Jalal et al. (2018) present evidence that the U.S. drug overdose crisis has displayed an exponential growth curve since at least 1979, but do not reject the gateway theory.
Nonetheless, the ADF information indicates two factors that apparently did not resonate with FDA regulatory oversight: a long‐term trend in opioid drug overdoses and the need for a novel approach to regulatory approval of opioid products. After its extensive review of the opioid crisis in 2017, NAS (2017, pp. 360–361) finds that “the FDA generally has reviewed opioids through the same lens used for other drugs. The committee believes that the preceding chapters of this report establish a scientific and epidemiological basis for special treatment of opioids by the FDA that would involve greater integration of public health considerations at the time of preapproval testing, during regulatory review and approval, and during routine post‐approval oversight.”
3.1.2. The Elusive Diversion Hazard
DEA's enforcement focus has its roots in control of illicit opioids (e.g., heroin) (DEA, 2020e). As the opioid crisis worsened, this focus shifted to diversion of licit opioids through illegal prescriptions and suspicious sales. The attention to suspicious orders, however, did not manifest in penalties until 2008 (DEA, 2020f; Eyre, 2020; OIG, 2019). In addition, a related signal that appeared to have been attenuated by DEA is the diversion occurring through patients.
Reported at least by 2010, there was evidence that nearly 56% of nonmedical users obtained opioid pharmaceuticals illicitly for free from friends/relatives (Manchikanti et al., 2010). Only approximately 4% of the illicit opioid pharmaceuticals were obtained from a drug dealer or stranger. This information also indicated that the patients (i.e., friends and relatives) were primarily obtaining prescriptions from one doctor. The risk of diversion from patients to nonmedical abuse is a signal that would not have resonated easily with DEA given its historical data collection and enforcement functions (Horwitz & Higham, 2017). Indirect diversion through patient demand reflected the characteristics of an elusive hazard in the SARF, because this population was not monitored directly by the agency.
3.1.3. Value Threatening: Attitudes Regarding Pain and Disrupting the Doctor–Patient Relationship
Did the public system of control rely inappropriately on prescribing doctors to monitor and respond to signals of abuse and diversion by their patients? DEA was charged with setting annual quotas for Schedule I and II controlled substances to ensure patients receive the medications they need while limiting the possibility of diversion and abuse. An unwillingness to interfere with patient care, as reflected in written prescriptions by medical providers who were monitored by DEA, would have attenuated the risks associated with the signal of a substantially increasing volume of consumption as shown previously in Fig. 1. Despite the growing crisis, opioid production quotas set by DEA doubled from 2000 to 2013 (DEA, 2010, 2019a).
It also is now suspected that individual doctors, especially those not specialized in pain treatment, had a difficult time recognizing the signals regarding abuse and diversion. Benedetti, Dickerson, and Nichols (2001), Mezei and Murinson (2011), and Tauben and Loeser (2013) criticize U.S. medical schools for the lack of pain treatment education, particularly with respect to opioid abuse and addiction. However, in the case of medical specialists, Rollman et al. (2019) present survey data indicating that some physician groups and patients were well informed about the risks.
Medicare data report the types of prescriptions written by certain medical specialties (CMS, 2016). Not surprisingly, opioid prescriptions in 2016 account for approximately 57% of all prescriptions written by pain management specialists and approximately 4% of the written prescriptions by family and internal medicine providers. Although individual family and internal medicine providers write opioid prescriptions infrequently, they account for approximately 50% of the opioid prescriptions written. Nurses and physician assistants account for approximately another 17%.
Opioid abuse and diversion risk signals likely did not resonate effectively with nonpain specialists in the provider station. IOM (2011), Gaskin and Richard (2012), Garthwaite (2012), and Butikofer and Skira (2018) address differing perspectives about pain and its proper treatment as an ongoing debate in the medical community.
Descriptions of the origins of the opioid crisis identify a series of published challenges to the pre‐1980 practice of using opioids only for relief of acute pain (Porter & Jick, 1980). In 1980, one letter to the editors of the New England Journal of Medicine was subsequently cited hundreds of times (Rummans, Burton, & Dawson, 2018). Leung, Macdonald, Stanbrook, Dhalla, and Juurlink (2017) find that until 2013, the majority of annual articles citing the paper were affirmational. Over 80% of those articles failed to indicate that the patients were hospitalized at the time they received opioid medication, thereby reinforcing a misleading narrative of low risk for prescribers. Other influential articles with limited scientific reliability continued to be published in the 1980s, presenting low rates of addictive habits among the small groups of cancer and noncancer patients and questioning conventional wisdom that cautioned against the use of opioids to treat chronic pain (Meldrum, 2016; Rummans et al., 2018).
In 1987, Dr. Ronald Melzack told members of the IASP that there was a “‘growing body of evidence’ that opioids could be used safely to treat chronic nonmalignant pain and provided reassurance that ‘most patients in pain exposed to narcotics do not become drug abusers’” (Dayer et al., 2019, p. 332). This evidence included a malignant pain program implemented by the Wisconsin State Controlled Substances Board in 1989. The practices of the program were adopted in 20 other states based on a perceived low risk of addiction. Although the data related to these perceptions were collected from patients with malignant pain, the risk assessments affected the adoption of similar models for nonmalignant pain patients.
In 1996, the American Academy of Pain Medicine and the APS issued a statement supporting the use of opioids for treating chronic noncancer pain (Rummans et al., 2018). The APS coined the phrase “pain as the 5th vital sign” and brought visibility to patients’ pain as a measure similar to the four routinely monitored vital signs: body temperature, pulse rate, respiration rate, and blood pressure (National Pharmaceutical Council, Inc., 2001, p. 21). Subsequently, “pain as the 5th vital sign” was referenced by organizations such as the Department of Veterans Affairs in 1999 and the Joint Commission in 2001 (Department of Veterans Affairs, 2000; Mularski et al., 2006). In addition, Gilson and Joranson (2011) report that many states passed Intractable Pain Acts removing sanctions for physicians who prescribed long‐term opioid therapy.
Family physicians were interacting with patients and their requests for chronic pain treatment directly when attitudes about pain and the stigma of opioid treatments were shifting considerably in medical communities. At the same time, certain metrics related to reimbursement tracked patient satisfaction with healthcare providers. Rummans et al. (2018) identify patient satisfaction as an influence on provider willingness to treat chronic pain with opioid therapy. Drug control, however, was guided largely by law enforcement to prevent abuse and diversion. It would take many years for the DEA law enforcement emphasis to give way to a public health perspective focused on treating addiction. The CDC did not add opioid overdose prevention to its top five public health challenges until 2014 although it recognized the growing signals of opioid abuse and overdoses as early as 2006 (Kolodny et al., 2015). Lacking the proper foundation or guidance to be effective gatekeepers of opioids for pain treatment, the SARF would indicate that such providers might have faced an amplification‐driven hazard. Reponses to the risk signals and understanding of the abuse and diversion threats by nonspecialist providers were apparently insufficient to overcome the risk attenuation in the medical community until there was growing public alarm and large unintended consequences.
States might have identified the prescribing influences on the opioid crisis sooner if information about the trends had been available. Today, nearly all U.S. states and Washington, DC, have PDMPs in place to collect and analyze related data, and provide reporting on prescribing, dispensing, and use of prescription drugs within each state, but this capacity was slow to develop (Brandeis University, 2018). The development of state PDMPs has been varied across the United States, and until 1990, only approximately 10 states had programs in place with the first of these requiring electronic transmission of prescription information.
The slow development of PDMPs suggests that state authorities faced an amplification‐driven hazard (i.e., state authorities became responsive to the opioid crisis only once the signals were apparent to society at large). PDMP information collection coincided with the peak and eventual downturn of the opioid crisis. Had the development of this station preceded the development of prescription opioid use in the United States, the prescribing behavior risk might have been recognized and addressed earlier.
3.2. Attenuated Risk Signals or Basic Economics?
An alternative view on the opioid crisis explains the sales, abuse, and diversion outcomes in the United States as predictable consequences of basic economics. Supply and demand are important determinants of outcomes in many markets, including the opioid drug market, where quantity demanded increases as prices decline (CEA, 2019; Grabowski, Long, Mortimer, & Boyo, 2016). Two economic changes in particular have been associated with dramatic declines in opioid prices: increasing access through Medicare coverage for prescription drugs and increasing generic opioid product supply.
In 2006, Medicare, which provides health insurance to Americans age 65 and older, provided coverage for prescription drugs for the first time (CEA, 2019). Information from representative medical expenditures indicates that Medicare coverage substantially expanded consumers’ access to opioid prescriptions. The proportion of Medicare payments for opioid prescriptions after 2000 increased from less than 2% to approximately 45% by 2016 (AHRQ, 2000, 2016). Samuels, Ross, and Dhruva (2017) conduct an analysis of Medicare drug coverage in 2006 and 2011 and show that more than two‐thirds of Medicare prescription plans had no opioid prescribing restrictions.
Regarding pricing, CEA (2019) estimates that between 2001 and 2010, the price of prescription opioids to the patient declined by 81% due to the increasing share of generic opioids and Medicare and Medicaid coverage. CEA finds that the declining prices and the greater access increased the per capita sales, abuse, and diversion substantially contributing to as much as 83% of the prescription opioid overdoses.
For CEA, the severe societal outcomes of the opioid crisis are largely a result of drug economics, not attenuated risk signals. Although CEA's analysis is compelling, it should be noted that FDA (2018) also examines pricing and economic factors and appears to reject their causal influence on the opioid crisis. In addition, the simple economics perspective fails to explain differences in abuse and diversion between the United States and other developed countries. Case and Deaton (2017), however, rescue the economics perspective by documenting that the opioid crisis developed concurrently with a cultural shift in the United States leading to a rise in “deaths of despair,” a term referring to deaths caused by suicide, drug overdose, and alcohol. Case and Deaton (2017) hypothesize that declines in the labor force and decays in traditional social structures formed a cumulative disadvantage for certain demographics, resulting in higher incidences of deaths. The explanations for deaths of despair rely directly on socioeconomic theory and empirical analysis. Compared to a more traditional economics view, the SARF lens adds little explanatory value or advantage to organizing the information to understand the price, access, and sales history. Economic incentives and social opportunities are more compelling explanations for responses to the signals of interest, rather than the hazard conditions associated with risk attenuation as advanced by the SARF.
4. RISK AMPLIFICATION
As discussed above and shown in Fig. 3, the monitored distribution of opioids in the United States responded to the increased regulatory oversight with a substantial decline after peaking in 2011. Turning to the SARF focus on risk amplification, the critical question shifts to one recognizing that now opioid abuse is at the forefront of the public agenda, is the societal response appropriate?
Fig 3.
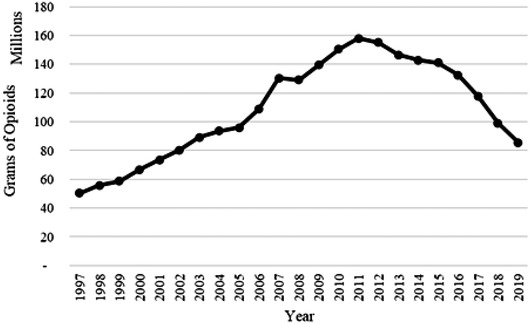
Grams of opioids distributed in the United States, 1997–2019. Includes the following opioid molecules: codeine, dihydrocodeine, oxycodone, hydromorphone, hydrocodone, levorphanol, meperidine, morphine, opium, oxymorphone, tapentadol, and fentanyl. Grams are interpolated for the year 2000 (adapted from DEA, 1997–2019).
4.1. The SARF Factors Supporting Risk Amplification
Kasperson et al. (1988) identify certain attributes of information that can increase the amplification of risk signals. These attributes include volume, the degree to which information is disputed, the extent of dramatization, and the symbolic connotations of the information. As is shown below, beginning in about 2010, the volume of information about opioid risks increased considerably. The analysis above indicates that information about pain treatment and opioid risks has been and continues to be highly disputed inside and outside the medical community. Information is dramatized through graphic personal accounts reported in news stories on the frequent overdoses and signals of drug abuse. The symbolic connotations of terms such as opioids, abuse, and addiction and the potential stigma attached to such terms have existed in the United States for some time (NAS, 2017).
Building on these concepts through an analysis of nearly 170 hazard events, Renn, Burns, Kasperson, Kasperson, and Slovic (1992) summarize various factors associated with an amplification of risk signals and the consequent potentially inappropriate social responses. Their paper provides a concise set of variables to be applied to a risk event that indicate increasing propensity to amplify risk signals. Some of these factors are particularly relevant to the opioid crisis after 2010 and are considered below to investigate the power of the SARF not only in explaining the most recent set of social responses to the opioid crisis, but also to suggest where social responses might be heading.
4.1.1. Physical Consequences
HHS (1999) reinforces that addiction affects not only the individual who is addicted, but also family and community members in numerous measurable and immeasurable ways: loss of productivity, expenses for treatment, reduced quality of life, and abuse and neglect of children, to name a few. Consequently, the opioid crisis, along with alcoholism and other addictions, reflects hazards to a broad population. Based on an opinion poll, APA (2018) found that nearly a third of Americans attest to “know[ing] someone who is or has been addicted to opioids or prescription painkillers.” According to the SARF, this broad distribution of contact with the general population will exacerbate risk perceptions beyond the number of casualties directly related to the opioid crisis.
4.1.2. Media Attention
Media attention to the opioid crisis shows a pattern consistent with risk amplification under the SARF. An in‐depth analysis of the risk signals in the media content is beyond the scope of this article; however, attention as measured by the frequency of articles can be observed. Fig. 4 shows the average daily publications that mention opioid(s) and/or at least one of five major opioid molecules (hydrocodone, oxycodone, fentanyl, morphine, and methadone) in major U.S. publications from 1997 to April 2020.2 The solid‐line series displays news related to opioids, and the dashed‐line series indicates news related to opioids or heroin. After some early and modestly growing attention between 1997 and 2012, the media attention to the opioid crisis increases substantially in 2013 to a peak in 2018, coinciding with the elevation of response to the risk signal by the public organizational stations (e.g., FDA and CDC). Media attention reflects a substantial decline in 2019 to partial year 2020 with the emergence of the global coronavirus pandemic. This information suggests that the media attention to opioids is following—not necessarily leading—the actions of other risk stations.
Fig 4.
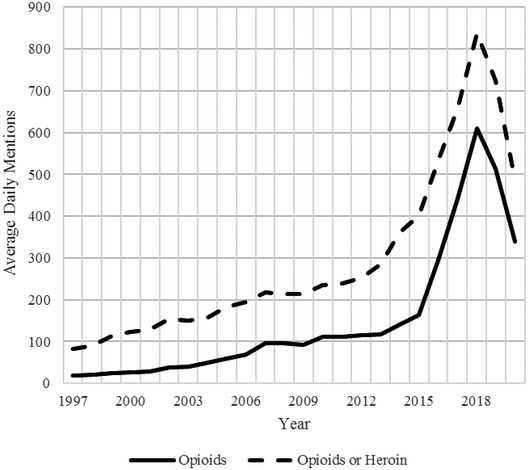
Average daily publications mentioning opioids or opioids and heroin (January 1997 through April 2020) (Factiva, 2020).
4.1.3. Risk Perception
The SARF identifies a number of risk perception factors that exacerbate risk amplification. The opioid crisis reflects certain features indicating a high potential for large future risks, dread, and demands for accountability.
4.1.3.1. Future risks and dread
The opioid crisis in the United States is far from resolved, and both private and public entities continue to perceive opioid abuse and misuse as a public health risk. Although evidence exists that the decreased volume of prescription opioids has contributed to a decline in prescription opioid mortality rates, the United States is still suffering from an increase in overdose deaths involving heroin and synthetic opioids. Chen et al. (2019) conclude that, while overdose deaths due to prescription opioids are expected to level out over the next five years, overdose deaths due to illicit opioids are projected to continue to grow until 2025.
DEA (2017) reports in the National Drug Threat Assessment that fentanyl is often not a drug product demanded by users but is actually a threat reflecting a contamination of the illicit drug supply. Fentanyl first entered the U.S. drug supply through heroin, and heroin users often do not know that fentanyl has been used as an adulterant and mixed with their drug supply. Fentanyl cannot be detected in powder heroin and is incredibly lethal. Increased deaths from potential fentanyl contamination magnify the dread associated with the opioid crisis. In addition, DEA (2019b) reports that drug traffickers are known to be experimenting with fentanyl compounds (such as carfentanil), which often creates even more lethal products. Future risks from opioid abuse projected by academics, media, and both public and private entities are consistent with a high potential for dread and would be expected to exacerbate risk amplification.
4.1.3.2. Search for accountability
By 2019, the failure of the risk responses by public and private management throughout the pharmaceutical system was becoming more apparent. In 2017, the bipartisan House Energy and Commerce Committee investigated the role of the DEA in the monitoring and enforcement of opioid distribution in West Virginia (ECC, 2018). The investigation “identified weaknesses in the DEA's enforcement posture in West Virginia as well as policy approaches that appear to have limited the agency's ability to take enforcement action against registrants suspected of diversion” (ECC, 2018, p. 45). This investigation also addressed the DEA's role in preventing opioid‐related harms across the country. The findings derived from the Committee's report question “the efforts of the DEA, to prevent diversion in other areas of the country that have been impacted by the opioid epidemic” (ECC, 2018, p. 44).
Importantly, the DEA has a means for recording and tracking controlled substances transactions, known as the Automated Reports and Consolidated Ordering System (ARCOS). ARCOS “allows the agency to track controlled substances from the time they are manufactured until they are dispensed to consumers through pharmacies, doctors or other means” (ECC, 2018, p. 53). The investigation finds substantial deficiencies regarding how the DEA used these data to combat diversion. Subsequent news media analysis has also relied on ARCOS to develop an extensive narrative on the scale and geography of opioid prescriptions throughout the United States (Achenbach, Bernstein, O'Harrow, & Boburg, 2019). The public reporting of these data often focused on the culpability of the pharmaceutical industry, the medical community, and the government for opioid addiction, abuse, and overdoses.
Other narratives on accountability have been advanced that do not align easily with any single source of risk management but instead point to socioeconomic changes (Venkataramani, Bair, O'Brien, & Tsai, 2020). Such perspectives place the accountability at both a more general societal level and the level of individual responses to difficult personal circumstances.
Regarding public perceptions of accountability, Blendon and Benson (2018) examine data from national polls conducted in 2016 and 2017 and report that respondents tended to blame doctors for improper prescribing and illicit drug trafficking. In addition, respondents also preferred addiction treatment to incarceration for illegal misuse of opioids. Importantly, although the proportion of respondents viewing prescription drug abuse as a serious public health problem was growing over time, the majority indicated that it was not an emergency.
Rather than uncover a particular social station as the source of managerial incompetence and a clear target for blame, the recent history of the opioid crisis has demonstrated the complexities of the accountability issue for the public. The SARF suggests that a high degree of blame exacerbates risk amplification, but given the diversity in accountability perspectives, the blame effect on public risk perceptions might be low.
4.1.4. Societal Impact
4.1.4.1. Political
Before President Obama released his first National Drug Control Strategy in 2010, there was relatively little investigation at the national or state levels into the opioid crisis (Wilson & Morgan, 2015). The Obama strategy coincided with the peak of the opioid crisis, with a focus on opioid use disorders and overdoses. Since then, there has been a proliferation of actions taken by political entities (on both sides of the political spectrum) to combat the opioid crisis (Hodge et al., 2019). As prescription opioid abuse and misuse entered front stage in the United States, the opioid crisis became a key item on the agendas of the White House, Congress, Senate, and other governmental entities. Table I lists just a few of the public response actions by political entities between 2010 and 2018. Public investigations into the opioid crisis and media attention to these investigations have grown in more recent years, which is directionally consistent with an increasing social amplification of risk.
Table I.
Political or Regulatory Response to the Opioid Crisis (2010–2018)
| Date | Political or Regulatory Response | Source |
|---|---|---|
| May 2010 | President Obama releases his first National Drug Control Strategy | https://obamawhitehouse.archives.gov/sites/default/files/ondcp/newsletters/ondcp_update_may_2010.pdf |
| April 2011 | White House Office of National Drug Control Policy (ONDCP) Report ‐ Epidemic: Responding to America's Prescription Drug Abuse Crisis | https://obamawhitehouse.archives.gov/sites/default/files/ondcp/issues‐content/prescription‐drugs/rx_abuse_plan.pdf |
| 2011/2012 | FDA approves and launches Risk Evaluation and Mitigation Strategy (REMS) system for Transmucosal Immediate Release Fentanyl (TIRF) products | https://www.fda.gov/drugs/information‐drug‐class/questions‐and‐answers‐fda‐approves‐class‐risk‐evaluation‐and‐mitigation‐strategy‐rems‐transmucosal |
| 2012/2013 | FDA approves and launches REMS system for extended‐release and long‐acting opioid products | https://www.fda.gov/drugs/information‐drug‐class/questions‐and‐answers‐fda‐approves‐risk‐evaluation‐and‐mitigation‐strategy‐rems‐extended‐release‐and |
| September 2015 | President Obama launches the Prescription Drug Overdose: Prevention for States Program through the CDC | https://www.ncsl.org/research/health/obama‐administration‐initiatives‐to‐address‐prescription‐drug‐abuse‐and‐heroin‐use.aspx; https://www.cdc.gov/drugoverdose/states/state_prevention.html |
| July 2016 | Senate and House pass the Comprehensive Addiction and Recovery Act (CARA) | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4959617/ |
| May 2017 | House Energy and Commerce Committee began bipartisan investigation into distribution of prescription opioids, specifically within West Virginia | https://republicans‐energycommerce.house.gov/opioids‐pilldumping/ |
| October 2017 | President Trump and HHS declare the opioid crisis a public health emergency | https://trumpwhitehouse.archives.gov/briefings‐statements/president‐donald‐j‐trump‐taking‐action‐drug‐addiction‐opioid‐crisis/ |
| March 2018 | President Trump launches Initiative to Stop Opioid Abuse | https://trumpwhitehouse.archives.gov/briefings‐statements/president‐donald‐j‐trumps‐initiative‐stop‐opioid‐abuse‐reduce‐drug‐supply‐demand/ |
| April to June 2018 | Senate approves Opioid Crisis Response Act (OCRA) and the Helping to End Addiction and Lessen (HEAL) Substance Use Disorders Act | https://bipartisanpolicy.org/blog/congressional‐actions‐to‐address‐the‐opioid‐crisis/ |
| October 2018 | Substance Use‐Disorder Prevention that Promotes Opioid Recovery Treatment (SUPPORT) for Patients and Communities Act amends the CSA | https://www.govinfo.gov/content/pkg/FR‐2020‐11‐02/pdf/2020‐23813.pdf |
4.1.4.2. Litigation
There is now a consolidation of more than 3,000 lawsuits filed against the pharmaceutical supply chain and individual physicians by states, counties, cities, Native American tribes, and individuals throughout the United States. The lawsuits are seeking compensation for the cost of public services needed to address the consequences of addicted communities, ranging from emergency response capabilities to rehabilitation services (Strange, 2019). In addition, plaintiffs seek opioid suppliers’ compliance with heightened independently monitored safeguards and injunctions regarding their alleged false and misleading public statements and omissions.
The sheer volume and reach of the lawsuits are consistent with a strong ripple effect caused by a social amplification of risk by the public‐interest social stations in the legal and government realms (e.g., state attorneys general, the Department of Justice). CUSP (2021) provides a status report which indicates that virtually every level of government is involved in the litigation, some major firms have been forced into bankruptcy, and settlements to date paid by manufacturers such as Purdue Pharma have exceeded $8 billion and by drug distributors have been proposed at $21 billion. When the dust finally settles, litigation related to the opioid crisis will be one of the most salient examples of a ripple effect and course correction for the public stations charged with regulating and managing risk.
4.2. Inappropriate Prescriber Responses?
In 2016, CDC published new guidelines with 12 recommendations to practitioners for opioid prescribing (CDC, 2016). The CDC advises clinicians to prescribe the lowest effective dosage and cites two thresholds of morphine milligram equivalents (MME): 50 MME/day and 90 MME/day. If increasing dosage to 50 MME/day or more, clinicians should carefully reassess evidence of benefits and risks. These new guidelines differ substantially from those published in 2009 by the APS and the American Academy of Pain Medicine, which discussed safe management of high dosage therapy defined as more than 200 mg of oral morphine (or equivalent) (Chou et al., 2009), and other articles published prior to 2016, which suggested increased risks at dosages exceeding 100 MME per day. For example, Ballantyne (2012) surveys literature on the chronic use of opioid analgesics and finds that concerns about unsafe and ineffective treatments reflect these high dose levels. The debate over the dosing level that should trigger increasing restrictions to manage risks is important because too low a level is also problematic.
Over the past five years, the supply of licit prescription opioids has indeed tightened. This tightening of supply has produced additional public health concerns. The recent restrictions on opioid prescribing have raised counterbalancing concerns that such limitations negatively affected patients with true needs. Some examples of the concerns include:
Reports of disruption to treatment for chronic pain patients, which can cause anxiety, depression, self‐harm, suicidal ideation, diminished quality of life, etc. (HHS, 2019; Rubin, 2019; Nicholson, Hoffman, & Kollas, 2018);
A March 6, 2019, letter to the CDC from 300 healthcare professionals urged CDC to clarify its 2016 opioid prescribing guidelines, arguing that they were being “misapplied by doctors and insurer to the detriment of pain patients—even driving some chronic pain patients to suicide” (Finnegan, 2019);
An increase in use of illicit opioids as a substitute, which puts users at risk of HIV/AIDS, hepatitis, and overdose from particularly potent illicit fentanyl (Huecker & Shoff, 2014; Rothstein, 2017);
Increased costs for patients with legitimate pain management needs, due to loss of access to affordable generic opioids (e.g., the imposition of a New York state tax on prescription opioids) (Gliadkovskaya, 2019); and
Physicians are tapering their patients off opioids altogether, out of fear of losing their licenses or criminal charges; pain management specialists are leaving their practices: “It doesn't matter if I practice legally anymore. The DEA will look at my prescribing patterns, and tell me I MUST have known that the ONLY reason any patient would get that much medication is if they are selling it on the street” (Pain News Network, 2018).
If social amplification of risk is now causing inappropriate social responses, the recent constraints on legitimate opioid prescriptions are probably the clearest consequence. Left unchecked, inappropriate reductions in patient access or medical treatments come at a high cost to pain management in the United States.
5. CONCLUSIONS
In the case of the opioid crisis, applying the SARF, with some notable theoretical enhancements, supports that attenuation is at least logically consistent with the overall framework. However, amplification does not seem to fit as easily because, in the case of the opioid crisis, the risk is large, which warrants a large public response to restrict abuse and diversion. There are several conclusions, however, regarding the usefulness of the SARF to understand the progression of risk responses by the public sector to the opioid crisis.
First, the SARF's focus on the treatment of risk signals is instructive to understand the FDA, DEA, and prescribing protocol examples from the pre‐2011 period of managing opioid risks and offers some guidance for early detection of attenuation in pharmaceutical regulation. The regulation and control examples present compelling support that important risk signals were attenuated by sophisticated federal agencies. Augmented by considerations of resonance and traditional agency responsibilities, the SARF appears useful to investigate inappropriate risk responses by public risk managers including inadequate attention to addiction potential, abuse deterrence, and diversion prevention. The hazards of prescription volumes failed to resonate with the lead agencies for reasons consistent with the SARF. The attenuation of these risk signals did not appear to be driven by high uncertainty or ambiguity of the consequences. The information and precise tracking of volume, abuse, diversion, and overdose data were collected routinely and available to these public stations. In addition, states tracked data on prescribing behaviors though PDMPs. Nonetheless, muted public responses to the increasing levels of the risk signals before 2011 allowed the opioid crisis to expand and create high levels of public health consequences.
Second, there are gaps in the SARF metaphor relating hazard conditions to inappropriate public responses to risk signals. Economic incentives and opportunities are not well emphasized in the SARF attenuation factors and analysis of the role of such economic signals is better addressed directly by socioeconomic theory and empirical study. Regarding public policy, however, the economic influences on the opioid crisis continue to be insufficiently evaluated in the context of risk management to avoid another undesirable situation driven by, among other factors, pricing and access.
Third, in the amplification case, the SARF highlights information regarding certain risk perception triggers, media attention, and a growing public search for accountability. Application of the SARF indicates that these factors can lead to certain unintended consequences in this second phase of the public policy problem especially regarding prescribing restrictions. Investigating the levels of the SARF factors using the recent history of the opioid crisis supports a strong risk‐amplification potential. Despite the indications of a strong risk amplification potential, however, the SARF is less useful to test the appropriateness of societal responses that have largely jumped immediately to determining culpability and had an extraordinary litigation impact. There is a lack of guidance in the SARF literature to determine when a response is inappropriate. In this context, it is not clear whether the framework has any capacity to be predictive or if the ripple effects are simply greatly accelerated by the immediate and high levels of the public health outcomes. Even with debate about the critical causes of the opioid crisis and reliability of current risk signals, it is unclear what the appropriate level of media coverage and societal response should be, again given the magnitude of the public health outcomes. The distinction between appropriate and inappropriate public response is a subject warranting further development for the SARF. At this time, these limitations suggest that the SARF performs best as an organizational aid rather than a predictive tool for investigating public risk management problems.
ACKNOWLEDGMENTS
The material in this article was developed partially from project work related to state and federal litigation related to prescription opioid use in the United States. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the opinions, position, or policy of Berkeley Research Group, LLC or its other employees and affiliates. The authors wish to thank Bonnie Ram for continuing the effort to produce the special issue on SARF after the sad and untimely death of her beloved husband, Roger Kasperson. We thank Michael Siegrist and two anonymous reviewers for their helpful review and feedback. We also want to thank Charlotte Brown for assistance with formatting and editing.
Footnotes
In the current litigation, plaintiffs assert, among other claims, that the change in pain treatment practices was manipulated by manufacturers’ marketing related to opioid products, most notably Purdue's Oxycontin. As this subject is now under vigorous public analysis, we leave it for others to examine the merits of these allegations.
Factiva search was limited to major publications. Opioids series reflects the following search terms: “opioid,” “opioids,” “hydrocodone,” “oxycodone,” “methadone,” “morphine,” and “fentanyl.” The Opioids or Heroin series reflects the same search terms, as well as “heroin.” Publications are counted if at least one of the search terms appears in the text of the publication.
REFERENCES
- Achenbach, J. , Bernstein, L. , O'Harrow, R. , Jr., & Boburg, S. (2019). An onslaught of pills, hundreds of thousands of deaths: Who is accountable? The Washington Post. [Google Scholar]
- Agency for Healthcare Research and Quality (AHRQ) . (2000, 2006, and 2016). Medical Expenditure Panel Survey: Prescribed Medicines File. Retrieved from https://meps.ahrq.gov/mepsweb/data_stats/download_data_files_results.jsp?cboDataYear=All&cboDataTypeY=2%2CHousehold+Event+File&buttonYearandDataType=Search&cboPufNumber=All&SearchTitle=Prescribed+Medicines
- Alpert, A. , Powell, D. , & Pacula, R. L. (2018). Supply‐side drug policy in the presence of substitutes: Evidence from the introduction of abuse‐deterrent opioids. American Economic Journal: Economic Policy, 10(4), 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatry Association . (2018). Nearly one in three people know someone addicted to opioids; More than half of millennials believe it is easy to get illegal opioids. Retrieved from https://www.psychiatry.org/newsroom/news‐releases/nearly‐one‐in‐three‐people‐know‐someone‐addicted‐to‐opioids‐more‐than‐half‐of‐millennials‐believe‐it‐is‐easy‐to‐get‐illegal‐opioids
- Baker, D. (2017). The joint commission's pain standards: Origins and evolution. Oakbrook Terrace, IL: The Joint Commission. [Google Scholar]
- Ballantyne, J. C. (2012). “Safe and effective when used as directed”: The case of chronic use of opioid analgesics. Journal of Medical Toxicology, 8(4), 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti, C. , Dickerson, E. , & Nichols, L. (2001). Medical education: A barrier to pain therapy and palliative care. Journal of Pain and Symptom Management, 21(5), 360–362. [DOI] [PubMed] [Google Scholar]
- Blendon, R. J. , & Benson, J. M. (2018). The public and the opioid‐abuse epidemic. New England Journal of Medicine, 378(5), 407–411. [DOI] [PubMed] [Google Scholar]
- Brandeis University . (2018). History of prescription drug monitoring programs. Prescription Drug Monitoring Program Training and Technical Assistance Center. Waltham, MA: Brandeis University. [Google Scholar]
- Brill, A. , & Ganz, S. (2018). The geographic variation in the cost of the opioid crisis. AEI Paper & Studies. [Google Scholar]
- Butikofer, A. , & Skira, M. M. (2018). Missing work is a pain: The effect of Cox‐2 inhibitors on sickness absence and disability pension receipt. Journal of Human Resources, 53(1), 71–122. [Google Scholar]
- Case, A. , & Deaton, A. (2017). Mortality and morbidity in the 21st century. Brookings Papers on Economic Activity, Spring, 397–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (US Centers for Disease Control and Prevention) . (2016). Guidelines for prescribing opioids for chronic pain. Washington, DC: US Department of Health and Human Services. [Google Scholar]
- CDC . (2019). Annual surveillance report of drug‐related risks and outcomes (7pp.). Washington, DC: U.S. Department of Health and Human Services. Retrieved from https://www.cdc.gov/drugoverdose/pdf/pubs/2019‐cdc‐drug‐surveillance‐report.pdf [Google Scholar]
- CDC . (2020a). Opioid overdose, opioid basics: Understanding the epidemic. Washington, DC: U.S. Department of Health and Human Services. Retrieved from https://www.cdc.gov/drugoverdose/epidemic/ [Google Scholar]
- CDC . (2020b). CDC's response to the opioid overdose epidemic, a public health crisis. Washington, DC: U.S. Department of Health and Human Services. Retrieved from https://www.cdc.gov/opioids/strategy.html [Google Scholar]
- CEA (Council of Economic Advisers) . (2019). The role of opioid prices in the evolving opioid crisis. Executive Office of the President, the White House. [Google Scholar]
- Chen, Q. , Larochelle, M. R. , Weaver, D. T. , Lietz, A. P. , Mueller, P. P. , Mercaldo, S. , … Chhatwal, J. (2019). Prevention of prescription opioid misuse and projected overdose deaths in the United States. JAMA Network Open, 2(2), e187621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, R., , Fanciullo, G. J. , Fine, P. G. , Adler, J. A. , Ballantyne, J. C. , Davies, P. , … Miaskowski, C. (2009). Opioid treatment guidelines, clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. Journal of Pain, 10(2), 113–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CMS (Centers for Medicare and Medicaid Services) . (2016). Medicare Provider Utilization and Payment Data: Medicare Part D Prescriber. Retrieved from https://www.cms.gov/Research‐Statistics‐Data‐and‐Systems/Statistics‐Trends‐and‐Reports/Medicare‐Provider‐Charge‐Data/Part‐D‐Prescriber
- CMS . (2020). History. Washington, DC: U.S. Department of Health and Human Services. Retrieved from https://www.cms.gov/About‐CMS/Agency‐Information/History/index.html [Google Scholar]
- CUSP (Center for U.S. Policy) . (2021). Opioid Litigation Status Update. May 3. Retrieved from https://centerforuspolicy.org/opioid‐litigation‐tracker/
- Dayer, L. E. , Painter, J. T. , McCain, K. , King, J. , Cullen, J. , & Foster, H. R. (2019). A recent history of opioid use in the US: Three decades of change. Substance Use & Misuse, 54(2), 331–339. [DOI] [PubMed] [Google Scholar]
- Degenhardt, L. , Grebely, J. , Stone, J. , Hickman, M. , Vickerman, P. , Marshall, B. D. L. , … Larney, S. (2019). Global patterns of opioid use and dependence: Harms to populations, interventions, and future action. Lancet, 394, 1560–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEA (Drug Enforcement Agency) . (1997–2019). ARCOS retail drug summary reports. Washington, DC: U.S. Department of Justice. [Google Scholar]
- DEA . (2006). Practitioners manual. Vol. 19. Washington, DC: U.S. Department of Justice. [Google Scholar]
- DEA . (2010). Aggregate production quota history for selected substances. Washington, DC: U.S. Department of Justice. [Google Scholar]
- DEA . (2014). Schedules of controlled substances: Placement of hydrocodone combination products into schedule ii background, data, and analysis: Eight factors determinative of control and findings pursuant to 21 U.S.C. 812(b). Washington, DC: U.S. Department of Justice. [Google Scholar]
- DEA . (2017). 2017 National drug threat assessment (DEA‐DCT‐DIR‐040‐17). DEA Strategic Intelligence Section. Washington, DC: U.S. Department of Justice. [Google Scholar]
- DEA . (2019a). Aggregate production quota history for selected substances. Washington, DC: U.S. Department of Justice. [Google Scholar]
- DEA . (2019b). 2019 National drug threat assessment (DEA‐DCT‐DIR‐007‐20). DEA Strategic Intelligence Section. Washington, DC: U.S. Department of Justice. [Google Scholar]
- DEA . (2020a). Diversion control division. Controlled substances schedules. Washington, DC: U.S. Department of Justice. Retrieved from www.deadiversion.usdoj.gov/schedules/ [Google Scholar]
- DEA . (2020b). Mission. Washington, DC: U.S. Department of Justice. Retrieved from https://www.dea.gov/mission [Google Scholar]
- DEA . (2020c). ARCOS retail drug summary reports. Washington, DC: U.S. Department of Justice. Retrieved from https://www.deadiversion.usdoj.gov/arcos/retail_drug_summary/ [Google Scholar]
- DEA . (2020d). Quota applications. Washington, DC: U.S. Department of Justice. Retrieved from https://www.deadiversion.usdoj.gov/quotas/quota_apps.htm [Google Scholar]
- DEA . (2020e). DEA history—Early years. Washington, DC: U.S. Department of Justice. Retrieved from https://www.dea.gov/sites/default/files/2018‐07/Early%20Years%20p%2012‐29%20%281%29.pdf [Google Scholar]
- DEA . (2020f). History: 2003–2008. Washington, DC: U.S. Department of Justice. Retrieved from https://www.dea.gov/sites/default/files/2018‐07/2003‐2008%20p%20118‐153.pdf [Google Scholar]
- DEA ARCOS (Automation of Reports and Consolidated Orders System) . (2020). Washington, DC: U.S. Department of Justice. Retrieved from https://www.deadiversion.usdoj.gov/arcos/index.html
- Department of Veterans Affairs. (2000). Pain as the 5th Vital Sign Toolkit. Revised Edition. Washington, DC: Veterans Health Administration.
- ECC (Energy and Commerce Committee) . (2018). Red Flags and warning signs ignored: Opioid distribution and enforcement concerns in West Virginia (22pp). Washington, DC: House Committee on Energy & Commerce. [Google Scholar]
- Eyre, E. (2020). Death in Mud Lick: A coal country fight against the drug companies that delivered the opioid epidemic. New York, NY: Scribner. [Google Scholar]
- Factiva . (2020). Number of Publications by Year. Retrieved from Factiva subscription.
- FDA . (2018). FDA analysis of long‐term trends in prescription opioid analgesic products: Quantity, sales, and price trends. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- FDA . (2020). Timeline of selected FDA activities and significant events addressing opioid misuse and abuse. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- Finnegan, J. (2019). CDC clarifies opioid prescribing guidelines, says doctors should use the “clinical judgment”. FierceHealthcare. [Google Scholar]
- Garthwaite, C. (2012). The economic benefits of pharmaceutical innovations: The case of cox‐2 inhibitors. American Economic Journal: Applied Economics, 4(3), 116–137. [Google Scholar]
- Gaskin, D. J. , & Richard, P. (2012). The economic cost of pain in the United States. Journal of Pain, 13(8), 715–724. [DOI] [PubMed] [Google Scholar]
- Gilson, A. M. , & Joranson, D. E. (2011). Controlled substances and pain management: Changes in knowledge and attitude of state medical regulators. Journal of Pain and Sympton Management, 21(3), 227–237. [DOI] [PubMed] [Google Scholar]
- Gliadkovskaya, A. (2019). New York State drew up a tax to punish opioid makers—But found some unanticipated side effects. Fortune. Retrieved from https://fortune.com/2019/09/16/new‐york‐opioid‐tax‐drugmakers‐side‐effects/ [Google Scholar]
- Grabowski, H. , Long, G. , Mortimer, R. , & Boyo, A. (2016). Updated trends in US brand‐name and generic drug competition. Journal of Medical Economics, 19(9), 836–844. [DOI] [PubMed] [Google Scholar]
- HHS (Health and Human Services) . (1999). Impact of substance abuse on the individual, family, and community. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- HHS. (2019). HHS guide for clinicians on the appropriate dosage reduction or discontinuation of long‐term opioid analgesics. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- Hodge, J. G., Jr., et al. (2019). Exploring legal and policy responses to opioids: America's worst public health emergency. South Carolina Law Review, 70, 481–516. [Google Scholar]
- Horwitz, S. , & Higham, S. (2017). Could the DEA have halted epidemic by cutting off supply? The Washington Post. [Google Scholar]
- Huecker, M. R. , & Shoff, H. W. (2014). The law of unintended consequences: Illicit for licit narcotic substitution. Western Journal of Emergency Medicine, 15(4), 561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) . (2011). Relieving pain in America: A blueprint for transforming prevention, care, education, and research. National Academy of Sciences. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Jalal, H., et al. (2018). Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science, 361, eaau1184. 10.1126/science.aau1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C. M. , Logan, J. , Gladden, R. M. , & Bohm, M. K. (2015). Vital signs: Demographic and substance use trends among heroin users – United States, 2002–2013. Morbidity and Mortality Weekly Report, 64(26), 719–725. [PMC free article] [PubMed] [Google Scholar]
- Kasperson, J. X. , Kasperson, R. E. , Pidgeon, N. , & Slovic, P. (2003). The social amplification of risk: Assessing fifteen years of research and theory. In Pidgeon N., Kasperson R. E., & Slovic P. (Eds.), The social amplification of risk (pp. 13–46). Cambridge, MA: University of Cambridge Press. [Google Scholar]
- Kasperson, R. E. (2021). The Social Amplification and Attenuation of Risk, New Perspectives Since 1988. [Paper for this special issue].
- Kasperson, R. E. , & Kasperson, J. X. (1991). Hidden hazards. In Mayo D. C. & Hollander R. (Eds.), Acceptable evidence: Science and values in hazard management (pp. 9–28). Oxford, UK: Oxford University Press. [Google Scholar]
- Kasperson, R. E. , & Kasperson, J. X. (1996). The social amplification and attenuation of risk. Annals of the American Academy of Political and Social Science, 545, 95–105. [Google Scholar]
- Kasperson, R. E. , Renn, O. , Slovic, P. , Brown, H. S. , Emel, J. , Goble, R. , … Ratick, S. (1988). The social amplification of risk: A conceptual framework. Risk Analysis, 8(2), 177–187. [Google Scholar]
- Kolodny, A. , Courtwright, D. T. , Hwang, C. S. , Kreiner, P. , Eadie, J. L. , Clark, T. W. , & Alexander, G. C. (2015). The prescription opioid and heroin crisis: A public health approach to an epidemic of addiction. Annual Review of Public Health, 36, 559–574. [DOI] [PubMed] [Google Scholar]
- Leung, P. T. M. , Macdonald, E. M. , Stanbrook, M. B. , Dhalla, I. A. , & Juurlink, D. N. (2017). A 1980 letter on the risk of opioid addiction. New England Journal of Medicine, 376(22), 2194–2195. [DOI] [PubMed] [Google Scholar]
- Lyapustina, T. , & Alexander, G. C. (2015). The prescription opioid addiction and abuse epidemic: How it happened and what we can do about it. Pharmaceutical Journal, 294(7866), 1–9. [Google Scholar]
- Manchikanti, L. , Fellows, B. , Ailinani, H. , & Pampati, V. (2010). Therapeutic use, abuse, and nonmedical use of opioids: A ten‐year perspective. American Society of Interventional Pain Physicians, 13(5), 401–435. [PubMed] [Google Scholar]
- MedPac (Medicare Payment Advisory Commission) . (2016). Overview: The drug development and supply chain (pp. 12–16). Washington, DC: Department of Health and Human Services. Retrieved from http://www.medpac.gov/docs/default‐source/fact‐sheets/overview‐of‐the‐drug‐development‐and‐supply‐chain.pdf?sfvrsn=0 [Google Scholar]
- Meldrum, M. (2016). The ongoing opioid prescription epidemic: Historical context. AJPH Perspectives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezei, L. , Murinson, B. B., & Johns Hopkins Pain Curriculum Development Team . (2011). Pain education in North American medical schools. Journal of Pain, 12(12), 1199–1208. [DOI] [PubMed] [Google Scholar]
- Mularski, R. A. , White‐Chu, F. , Overbay, D. , Miller, L. , Asch, S. M. , & Ganzini, L. (2006). Measuring pain as the 5th vital sign does not improve quality of pain management. Journal of General Internal Medicine, 21(6), 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAS (National Academies of Sciences, Engineering, and Medicine) . (2017). Pain management and the opioid epidemic: Balancing societal and individual benefits and risks of prescription opioid use (pp. 360–361). Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- National Pharmaceutical Council, Inc . (2001). Pain: Current understanding of assessment, management, and treatments (p. 21). Washington, DC: NPC.
- Nicholson, K. M. , Hoffman, D. E. , & Kollas, C. D. (2018). Overzealous use of the CDC's opioid prescribing guideline is harming pain patients. STAT. Retrieved from https://www.statnews.com/2018/12/06/overzealous‐use‐cdc‐opioid‐prescribing‐guideline [Google Scholar]
- OIG (Office of the Inspector General) . (2019). Review of the drug enforcement administration's regulatory and enforcement efforts to control the diversion of opioids. Washington, DC: U.S. Department of Justice. [Google Scholar]
- Pain News Network . (2018). Why I Am Closing My Pain Practice. Retrieved from https://www.painnewsnetwork.org/stories/2018/1/17/why‐i‐am‐closing‐my‐pain‐practice
- Porter, J. , & Jick, H. (1980). Addiction rare in patients treated with narcotics. New England Journal of Medicine, 302(2), 123. [DOI] [PubMed] [Google Scholar]
- Public Law 91–513 . (1970). Comprehensive Drug Abuse Prevention and Control Act of 1970 (pp. 1250–1252). U.S. Government Printing Office. Retrieved from https://www.govinfo.gov/content/pkg/STATUTE‐84/pdf/STATUTE‐84‐Pg1236.pdf
- Renn, O. (2011). The social amplification/attenuation of risk framework: Application to climate change. WIREs Clim Change, 2, 154–169. [Google Scholar]
- Renn, O. , Burns, W. J. , Kasperson, J. X. , Kasperson, R. E. , & Slovic, P. (1992). The social amplification of risk: Theoretical foundations and empirical applications. Journal of Social Issues, 48(4), 137–160. [Google Scholar]
- Rollman, J. E., et al. (2019). Assessment of the FDA risk evaluation and mitigation strategy for transmucosal immediate‐release fentanyl products. Journal of American Medical Association, 321(7), 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein, M. A. (2017). The opioid crisis and the need for compassion in pain management. American Journal of Public Health, 107(8), 1253–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, R. (2019). Limits on opioid prescribing leave patients with chronic pain vulnerable. Journal of the American Medical Association, 321(21), 2059–2062. [DOI] [PubMed] [Google Scholar]
- Rummans, T. A. , Burton, M. C. , & Dawson, N. L. (2018). How good intentions contributed to bad outcomes: The opioid crisis. Mayo Foundation for Medical Education and Research, 93(3), 344–350. [DOI] [PubMed] [Google Scholar]
- Samuels, E. , Ross, J. , & Dhruva, S. (2017). Medicare formulary coverage restrictions for prescription opioids, 2006 to 2015. Annals of Internal Medicine, 167(12), 895–896. [DOI] [PubMed] [Google Scholar]
- Sood, N. , Shih, T. , Van Nuys, K. , & Goldman, D. (2017). The flow of money through the pharmaceutical distribution system (2pp.). Leonard D. Schaeffer Center for Health Policy & Economics, University of Southern California. [Google Scholar]
- Steckler, T. J. , Mosher, H. J. , Desloover‐Koch, Y. , & Lund, B. C. (2019). Impact of hydrocodone reclassification on analgesic prescribing in the Veterans Health Administration. American Society of Health‐System Pharmacists, 76(suppl 2), S61–S67. [DOI] [PubMed] [Google Scholar]
- Strange, L. J., III. (2019). A prescription for disaster: How local governments’ abuse of public nuisance claims wrongly elevates courts and litigants into a policy‐making role and subverts the equitable administration of justice. South Carolina Law Review, 70, 517–563. [Google Scholar]
- Tauben, D. J. , & Loeser, J. D. (2013). Pain education at the University of Washington School of Medicine. Journal of Pain, 14(5), 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varisco, T. , Ogunsanya, M. , Barner, J. , & Fleming, M. (2017). Pharmacists’ perceptions regarding the impact of hydrocodone rescheduling on prescription volume, workflow management, and patient outcomes. Journal of the American Pharmacists Association, 57, S51–S62. [DOI] [PubMed] [Google Scholar]
- Venkataramani, A. S. , Bair, E. F. , O'Brien, R. L. , & Tsai, A. C. (2020). Association between automotive assembly plant closures and opioid overdose mortality in the United States: A difference‐in‐differences analysis. JAMA Internal Medicine, 180, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, J. J. , & Morgan, R. B. (2015). Obama administration initiatives to address prescription drug abuse and heroin use. National Conference of State Legislatures. Retrieved from https://www.ncsl.org/research/health/obama‐administration‐initiatives‐to‐address‐prescription‐drug‐abuse‐and‐heroin‐use.aspx


