Summary
Treatment with high‐dose chemotherapy followed by autologous stem cell transplantation (ASCT) is considered standard of care (SOC) second‐line treatment for relapsed or refractory large B‐cell lymphoma (LBCL). However, outcomes remain suboptimal. A systematic review and meta‐analysis of randomised controlled trials comparing efficacy and safety of SOC versus chimeric antigen receptor T‐cell (CAR‐T) therapy as second‐line for patients with LBCL refractory or relapsing within 12 months. Outcomes included overall survival (OS), event‐free survival (EFS), overall response rate (ORR) and safety. Three trials published in 2021 (involving 865 participants) fulfilled the eligibility criteria. EFS as well as OS were significantly improved with CAR‐T therapy as compared to SOC, hazard ratio (HR) 0.57 (95% confidence interval [CI] 0.49–0.68) and HR 0.77 (95% CI 0.60–0.98) respectively. CAR‐T therapy was associated with significantly better ORR, relative risk (RR) 1.55 (95% CI 1.12–2.13, p = 0.001). The risk of Grade III/IV adverse event was comparable between the two arms, RR 1.03 (95% CI 0.93–1.14). In summary, CAR‐T therapy has superior outcomes as compared to SOC in patients with LBCL refractory or relapsing within 12 months, without excess of toxicity. Longer follow‐up is needed to confirm these results and determine the optimal sequencing of CAR‐T therapy in the management of LBCL.
Keywords: B‐cell, CAR‐T, CD19 chimeric antigen receptor, diffuse large cell, lymphoma, meta‐analysis, relapse: refractory
INTRODUCTION
Despite progress in the up‐front treatment of large B‐cell lymphoma (LBCL), up to a third of patients with LBCL will be refractory to first‐line immuno‐chemotherapy or will relapse after achieving complete response (CR). Of them, at least half are unlikely to be eligible for aggressive approaches due to advanced age and comorbidities. Therefore, only 50% of these patients can be approached with curative intent. 1 , 2 , 3 Based on the results of the Collaborative Trial in Relapsed Aggressive Lymphoma (CORAL) study, patients with previous rituximab exposure have a response rate to salvage therapy of 50%; therefore, at most, only 25% of all relapsing patients today will undergo autologous stem cell transplantation (ASCT). The 3‐year event‐free survival (EFS) of those treated with ASCT in the CORAL study was 50%, so only 10% patients of the relapsed/refractory (R/R) patients are ultimately cured of lymphoma with ASCT. 4 , 5 , 6 Subset of patients, either refractory or relapsing within 12 months after the completion of first‐line immuno‐chemotherapy have even worse outcomes. Response rates to salvage therapy are much inferior; in the CORAL study only 46% of the refractory and early relapse patients achieved CR or partial response (PR) versus 88% of patients relapsing at >12 months after therapy completion (p < 0.001). 4 ASCT outcomes are also inferior, with higher relapse rate during the first 6 months. 7
First autologous anti‐CD19 chimeric antigen receptor T‐cell (CAR‐T) therapy, axicabtagene ciloleucel (Axi‐cel), was approved in 2017 for third‐line R/R LBCL, followed by approval of tisagenlecleucel (Tisa‐cel) in 2018 and lisocabtagene maraleucel (Liso‐cel) last year. 8 , 9 , 10 With an unprecedented overall response rate (ORR) of 50%–80% and durable responses of 30%–40% at 4–5 years, 11 , 12 CAR‐T therapy is now considered standard of care (SOC) for patients with R/R LBCL after two or more lines of therapy.
Recently, several randomised controlled trials (RCTs) evaluating the role of anti‐CD19 CAR‐T therapy as second‐line treatment for R/R LBCL have been published. The aim of the present study was to compile all evidence and compare the efficacy and safety of CAR‐T therapy versus high‐dose chemotherapy followed by ASCT as second‐line treatment for R/R LBCL.
MATERIALS AND METHODS
We searched PubMed until February 2022, the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library, until January 2022, and the following conference proceedings until 2021: Annual Meeting of the American Society of Haematology, Annual Meeting of the American Society of Clinical Oncology, Annual Meeting of the European Haematology Association, International Conference of Malignant Lymphoma, Annual Meeting of the European Society of Blood and Marrow Transplantation and Transplantation and Cellular Therapy Meetings of the American Society for Transplantation and Cellular Therapy (ASTCT) and Center for International Blood and Marrow Transplant Research (CIBMTR).
We cross searched the terms ‘large B cell lymphoma’ or ‘aggressive lymphoma’ and similar terms, ‘CAR‐T’ or ‘CD19 chimeric antigen receptor’ and ‘second line’ and ‘autologous stem cell transplantation’ and similar terms. For PubMed, we added the Cochrane highly sensitive search term for identification of clinical trials. 13 In addition, we scanned references of all included trials and reviews identified for additional studies.
Study selection
We included all RCTs comparing second‐line treatment of high‐dose salvage chemotherapy followed by ASCT versus anti‐CD19 CAR‐T therapy for R/R LBCL.
Data extraction and quality assessment
Two reviewers (L.S., R.G.) independently extracted data regarding case definitions, characteristics of patients, and outcomes from included trials. In the event of disagreement between the two reviewers regarding any of the above, a third reviewer (A.G.) extracted the data. Data extraction was discussed, and decisions were documented.
Two reviewers independently assessed the trials for the following domains: allocation concealment, generation of the allocation sequence, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data reporting, and selective outcome reporting. We made critical assessment separately for each domain and graded it as low‐, unclear‐, or high‐risk of bias according to the criteria specified in the Cochrane Handbook version 6.1.0.7. 13
Outcome measures
Primary outcomes included both EFS and overall survival (OS). Secondary outcomes included ORR, CR rate, progression‐free survival (PFS) and safety. OS was defined as the time from randomisation to death from any cause. EFS in the included trials was defined as: time from randomisation to death from any cause, progressive disease (PD), failure to achieve CR or PR by 9 weeks (TRANSFORM; 14 ClinicalTrials.gov Identifier: NCT03575351) or day 150 (21 weeks) [ZUMA‐7; 15 ClinicalTrials.gov Identifier: NCT03391466) after randomisation, or start of new anti‐neoplastic therapy, whichever occurred first. The BELINDA trial 16 (ClinicalTrials.gov Identifier: NCT03570892) defined EFS as the time from randomisation to stable disease (SD) or PD at or after the week 12 assessment or death at any time. Response assessment was conducted in all trials by independent review committee according to the Lugano criteria. 17 Dates of initial response assessment varied; TRANSFORM – weeks 9 and 18, ZUMA‐7 – day 50 and 100 and BELINDA – weeks 6 and 12.
Data synthesis and statistical analysis
Hazard ratios (HRs) and variances for time‐to‐event outcomes were estimated and pooled in Review Manager (version 5.4 for Windows; The Cochrane Collaboration, Oxford, UK). A HR of <1.0 was in favour of CAR‐T therapy.
Relative risks (RRs) and 95% confidence intervals (CIs) for dichotomous data were estimated and pooled using the Mantel–Haenszel method.
We assessed heterogeneity of trial results by the chi‐square test of heterogeneity, and the I 2 statistic of inconsistency. 12 Statistically significant heterogeneity was defined as p < 0.1 or an I 2 statistic >50%. We conducted the meta‐analysis of dichotomous outcomes using a fixed‐effect model (FEM), and in case of high heterogeneity, we used the random‐effects model (REM). 13
We planned to perform subgroup analyses, according to:
Cell of origin – Germinal centre B‐cell (GCB) and non‐GCB.
High‐grade B‐cell lymphoma (HGBL) defined as HGBL with MYC rearrangement plus rearrangement of BCL2, BCL6, or both in all the studies and also HGBL not otherwise specified in BELINDA trial.
Age >65 years.
RESULTS
Description of trials
The literature search yielded 75 trials, of which 23 were considered as potentially relevant. In all, 20 were excluded for various reasons (Figure 1). Two trials published in peer review journals fulfilled the inclusion criteria, 15 , 16 in addition to one abstract. 14 Overall, only three trials fulfilled the inclusion criteria. Trials were conducted between the years 2018 and 2021, and all were published in 2021. Each trial assessed one of the three United States Food and Drug Administration (FDA) approved anti‐CD19 CAR‐T products: Axi‐cel (ZUMA‐7 15 ), Tisa‐cel, (BELINDA 16 ) and Liso‐cel (TRANSFORM 14 ). Table 1 depicts the characteristics of the included trials.
FIGURE 1.
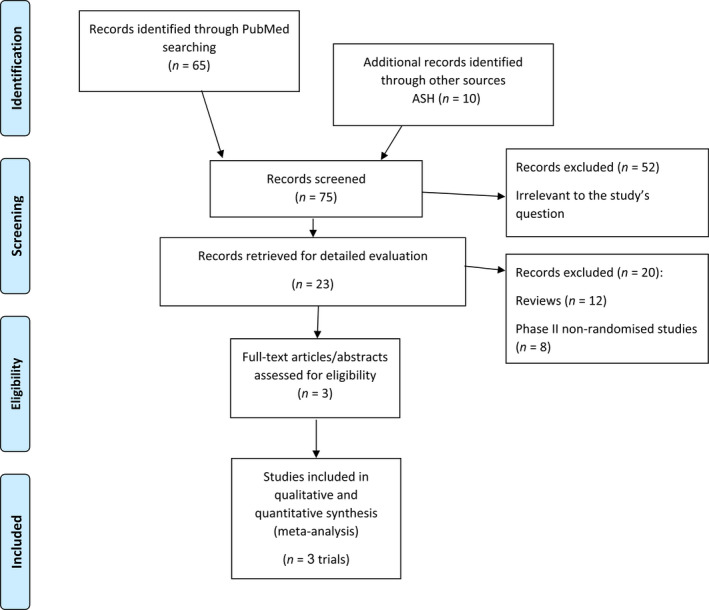
Flow chart of study selection
TABLE 1.
Characteristics of trials
| ZUMA‐7 | BELINDA | TRANSFORM | |
|---|---|---|---|
| Locke et al. 15 2021 | Bishop et al. 16 2021 | Kamdar et al. 14 2021 | |
|
Autologous Anti‐CD19 CAR |
Axicabtagene ciloleucel | Tisagenlecleucel | Lisocabtagene maraleucel |
| Co‐stimulatory domain | CD28 | 4‐1BB | 4‐1BB |
| T‐cell selection | No | No | CD4:CD8 |
| Inclusion criteria | RD and relapse <1 year | RD and relapse<1 year | RD and relapse <1 year |
| LBCL |
LBCL PMBL FL3B Hx of CNS |
LBCL PMBL FL3B Sec. CNS lymphoma |
|
| Randomisation | 1:1 | 1:1 | 1:1 |
| Randomisation stratification |
sAAIPI RD vs. relapsed disease. |
sAAIPI RD and relapse <6 months vs. relapse 6–12 months. US vs. non‐US |
sAAIPI RD and relapse <3 months vs. relapse 3–12 months. |
| Primary outcome | EFS | EFS | EFS |
| Definition of EFS | Time from randomisation to death or PD, or failure to achieve CR or PR by day 150 or start of new anti‐neoplastic therapy. | Time from randomisation to SD or PD at or after the week 12 assessment or death at any time. | Time from randomisation to death or PD or failure to achieve CR or PR by 9 weeks or start of new anti‐neoplastic therapy. |
| Date of initial response assessment | Day 50 and 100 |
Weeks 6 and 12 |
Weeks 9 and 18 |
| CAR‐T therapy arm | |||
| Bridging chemotherapy | No (only steroids) | Allowed (switching allowed) | Allowed |
| Lymphodepletion | Flu 30/Cy500 mg/m2 X3d | Flu 25/Cy250 mg/m2 X3d | Flu 30/Cy300 mg/m2 X3d |
| Cell dose | 2 × 106 cell/kg |
0.6‐6 × 108 cells Median 2.9 × 108 |
1 × 108 cells |
| SOC arm | |||
| Salvage regimen |
ICE DHAP GDP ESHAP |
ICE DHAP GDP GEMOX |
ICE DHAP GDP |
| Cycle number | 2–3 | 2 (Switching allowed) | 3 |
Abbreviations: DHAP, dexamethasone‐cytarabine‐cisplatin; ESHAP, etoposide‐methylprednisolone‐cytarabine‐cisplatin; FL3B, follicular lymphoma grade 3B; GDP, gemcitabine‐dexamethasone‐cisplatin; GEMOX, gemcitabine‐oxaliplatin; ICE, ifosfamide‐carboplatin‐etoposide; PD, progressive disease; PMBL, primary mediastinal B cell lymphoma; RD, refractory disease; sAAIPI, second‐line age‐adjusted International Prognostic Index; US, United States.
Patient characteristics
Our analysis included 865 patients. Patients' age ranged between 19 and 81 years. The median follow‐up ranged between 6 and 25 months. All trials included patients with LBCL, either refractory or relapsed, within 12 months after the completion of first‐line immunochemotherapy including an anti‐CD20 monoclonal antibody and an anthracycline‐containing regimen. In addition to histologically confirmed LBCL, two trials also included patients with primary mediastinal B‐cell lymphoma and follicular lymphoma Grade 3B. 14 , 16 Regarding central nervous system (CNS) involvement, one trial included patients with secondary CNS lymphoma, 14 another study – only if past history,16 while the third one excluded any patient with a history of CNS involvement. 15 Patients had to be eligible for ASCT according to the investigator's assessment and have an Eastern Cooperative Oncology Group (ECOG) Performance Status score of 0 or 1. Table 2 shows the characteristics of the included patients.
TABLE 2.
Characteristics of patients included in the trials
| Study | CAR‐T arm/SOC arm | Patients, n | Age, years, median (range) | Age ≥65 years, n (%) | Sex, male, n (%) | High IPI, n (%) | High‐grade LBCL (IRC), n (%) | Follow‐up, months, median (range) | CAR‐T – bridging chemotherapy, n/N (%) | Cross over to CAR‐T among SOC arm, n/N (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Locke et al. 15 2021 (ZUMA‐7) |
Axi‐cel 2–3 salvage+ASCT |
180 179 |
58 (21–80) 60 (26–81) |
51 (28) 58 (32) |
110 (61) 127 (71) |
sAAIPI 2–3 ‐ 82 (46) sAAIPI 2–3 ‐ 79 (44) |
17 (9) 15 (8) |
24.9 | None | 100/179 (56) |
| Bishop et al. 16 2021 (BELINDA) |
Tisa‐cel 2–3 salvage+ASCT |
162 160 |
59.5 (19–79) 58 (19–77) |
54 (33) 46 (29 |
103 (63.6) 98 (61.2) |
IPI score ≥2106 (65) IPI score ≥2 92 (57) |
39 (24) 27 (17) |
10 (2.9–23.2) | 134/162 (83) | 81/160 (51) |
| Kamdar et al. 14 2021 (TRANSFORM) |
Liso‐cel 3 salvage+ASCT |
92 92 |
60 (20–74) 58 (26–75) |
36 (39) 27 (29) |
44 (48) 61 (66) |
sAAIPI 2–3 ‐ 36 (39) sAAIPI 2–3 ‐ 37 (40) |
22 (24) 21 (23) |
6.2 (0.9–20) | 58/92 (63) | 50/92 (54) |
Abbreviations: ASCT, Autologous stem cell transplantation; CAR‐T, chimeric antigen receptor T‐cell therapy; sAAIPI, second‐line age‐adjusted International Prognostic Index; SOC, standard of care.
SOC and CAR‐T therapy procedure
After screening, patients underwent randomisation in a 1:1 ratio to receive SOC immunochemotherapy or CAR‐T cell therapy. Patients in the SOC arm received two or three cycles of investigator‐selected, platinum‐based immunochemotherapy. Responding patients (either CR or PR) were to proceed to ASCT, mostly with BEAM (carmustine, etoposide, cytarabine, melphalan) conditioning. The BELINDA trial allowed patients with an inadequate response to first protocol to switch to another immunochemotherapy protocol. 16
Lymphocyte collection by leukapheresis was carried out before randomisation in the TRANSFORM and BELINDA trials, 14 , 16 while in the ZUMA‐7 trial, leukapheresis was done after randomisation to the CAR‐T therapy arm only. 15 Bridging immunochemotherapy with SOC regimens was allowed in two trials, 14 , 16 while the third trial allowed only bridging with glucocorticoids. 15 Lympho‐depletion conditioning included cyclophosphamide and fludarabine in all trials in different dosing schedules.
Crossover was included in the protocol of BELINDA and TRANSFORM trials and allowed for SOC patients demonstrating SD or PD to receive CAR‐T therapy. 14 , 16 The ZUMA‐7 trial allowed crossover outside the protocol. 15 All SOC‐arm patients who crossed over to receive CAR‐T therapy continued to be followed for OS/EFS in the SOC arm in all three trials.
Risk of bias of included trials
The ZUMA‐7 and BELINDA trials were judged as low risk of selection bias in terms of randomisation generation and allocation concealment. 15 , 16 In the TRANSFORM trial, which was published as an abstract, methods of allocation concealment and generation were not reported. 14 Blinding of patients and personnel was not done in all trials. All trials were judged as low risk of attrition bias, and at low risk of selective outcome reporting bias as clinically important outcomes including OS were well addressed. 14 , 15 , 16
Primary outcomes
Data from all three trials were available for analysis of OS and EFS. 14 , 15 , 16 OS was significantly improved with CAR‐T therapy as compared to SOC (HR 0.77, 95% CI 0.60–0.98; I 2 = 29%, 865 patients, three trials) (Figure 2). EFS was significantly improved with CAR‐T therapy as compared to SOC (HR 0.57, 95% CI 0.49–0.68; I 2 = 94%, 865 patients, three trials) (Figure 3). 14 , 15 , 16 Due to considerable heterogeneity in this analysis, we conducted a sensitivity analysis excluding the BELINDA trial (HR 0.39, 95% CI 0.31–0.48; I 2 = 0%, two trials).
FIGURE 2.
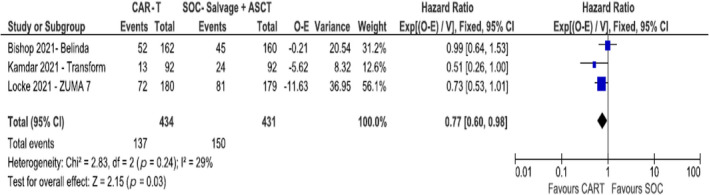
Overall survival
FIGURE 3.
Event‐free survival
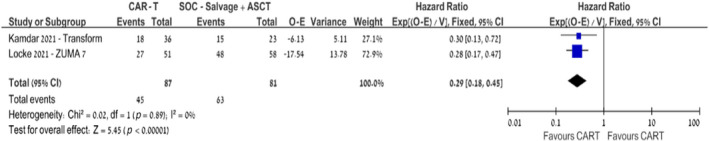
The advantage in terms of EFS with CAR‐T therapy remained consistent across subgroup analyses among patients with HGBL (HR 0.57, 95% CI 0.38–0.87; I 2 = 22%, 179 patients, three trials) and GCB cell of origin (HR 0.47, 95% CI 0.36–0.62; I 2 = 59%, 402 patients, three trials). 14 , 15 , 16 Yet, among non‐GCB patients, the EFS advantage was not statistically significant (HR 0.87, 95% CI 0.58–1.32; I 2 = 80%, 170 patients, three trials) (Figures S1–S2). 14 , 15 , 16 Subgroup analysis of EFS among patients aged >65 years also favoured CAR‐T therapy over SOC (HR 0.29, 95% CI 0.18–0.45; I 2 = 0%, 168 patients, two trials). 14 , 15
Secondary outcomes
The CAR‐T therapy was associated with significantly better ORR (RR 1.55, 95% CI 1.12–2.13; I 2 = 85%, REM, 865 patients, three trials, p = 0.001) and CR rates (RR 1.49, 95% CI 1.09–2.05; I 2 = 72%, REM, 865 patients, three trials, p = 0.03) (Figures S3–S4). 14 , 15 , 16 Data from two trials were available for PFS analysis and showed improved PFS in the CAR‐T therapy group compared with SOC (HR 0.47, 95% CI 0.37–0.60; I 2 = 0%, 543 patients, two trials). 14 , 15
Safety
All trials reported Grade III or IV adverse events (N = 739). The risk of any Grade III or IV adverse event was comparable between the two arms (RR 1.03, 95% CI 0.93–1.14; I 2 = 74%, REM, 865 patients) (Figure 4).
FIGURE 4.
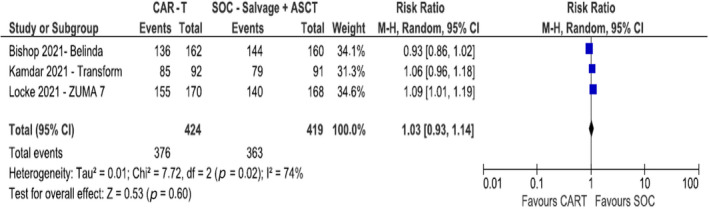
Rate of serious adverse events
Regarding haematological toxicity, there was an increased risk of Grade III–IV neutropenia among CAR‐T therapy patients, 257/424 (61%) in the CAR‐T therapy arm versus 178/419 (42%) in the SOC arm (RR 1.41, 95% CI 1.06–1.90; I 2 = 79%, REM, three trials). However, the risk of febrile neutropenia, was decreased among CAR‐T therapy patients compared to the SOC arm, 40/424 (9%) versus 110/419 (26%) (RR 0.33, 95% CI 0.13–0.88; I 2 = 85%, REM, three trials). Also, the risk of Grade III–IV thrombocytopenia was significantly decreased in the CAR‐T therapy arm versus the SOC arm, 122/424 (29%) versus 229/419 (55%) (RR 0.52, 95% CI 0.28–0.95; I 2 = 92%, REM, three trials). There was no difference in the risk of Grade III‐IV anaemia between the two arms (RR 0.76, 95% CI 0.55–1.04; I 2 = 73%, REM, three trials) (Figures S5–S8). 14 , 15 , 16
DISCUSSION
In this systematic review and meta‐analysis of RCTs, we demonstrate that CAR‐T therapy has statistically significant superior outcomes compared to high‐dose chemotherapy and ASCT in patients with LBCL refractory or relapsing within 12 months. CAR‐T therapy was associated with statistically significant improved ORRs and CR rates and longer EFS and PFS, translating into improved OS, without excess of toxicity.
All three trials are ground‐breaking in their design. They represent the first attempt to challenge the superiority of ASCT in patients with R/R LBCL set in the 1990s following the PARMA trial. 2 Until now, efforts to improve outcomes of patients eligible for transplant focused on incorporation of novel agents to high‐dose chemotherapy, ASCT conditioning regimen or maintenance with anti‐CD20. Yet, results of RCTs were discouraging. 18 , 19 , 20
Now, for the first time since the PARMA trial, investigators assessed a completely new treatment modality, which undermines the concept of employing mega doses of chemotherapy to overcome front‐line chemotherapy resistance in patients with LBCL. The concept of an immunotherapy, chemotherapy free treatment, is increasingly being implemented not only in patients with lymphoma but also among patients with leukaemia, myeloma, and even solid oncology. Recently, the potential role of several novel immunotherapies alone or in combinations; tafasitamab, polatuzumab, lenalidomide and anti‐CD20xCD3 T‐cell engagers, has been evaluated among patients with R/R LBCL. However, none has been compared with high‐dose chemotherapy and ASCT in transplant‐eligible patients. 21 , 22 , 23 , 24
The main strength of this meta‐analysis stems from its large sample size and the pooling of results. While two trials demonstrated only improved EFS and the third one demonstrated no advantage compared to high‐dose chemotherapy, pooled analysis of all three trials showed that CAR‐T therapy significantly improved both EFS and OS compared to the SOC. One can argue that as a third of transplant‐eligible patients can be cured with ASCT, and remaining patients can be ‘salvaged’ with third‐line CAR‐T therapy, ASCT should not be abandoned. This is especially relevant, taking into consideration the substantially higher cost of CAR‐T therapy and the heavy burden on health system budget if applied as second‐line with greater patient potential as assessed by Lin et al. 25 Yet our outcomes contest this claim. Owing to high rate of crossover to CAR‐T therapy in the SOC arms (51%–56% in the different trials), all three trials are essentially comparing second‐line CAR‐T therapy to third‐line CAR‐T therapy (rather than to high‐dose chemotherapy with ASCT). Pooled analysis of all trials allows us to see that early administration of CAR‐T therapy provides a statistically significant survival advantage.
Another strength of the meta‐analysis, is the fact that the results can also be applied to the older population, not fit for transplant. Usually, patients aged >70 years are not considered eligible for high‐dose chemotherapy and ASCT due to substantial morbidity. 26 Yet, patients aged >65 years comprise about a third of all three trials (272/865, 31%). Subgroup analysis of EFS per age group demonstrated similar efficacy among patients aged >65 years. These findings are supported by several encouraging reports on third‐line CAR‐T therapy among elderly patients with lymphoma including pivotal studies and real‐world data that describe comparable outcomes for CAR‐T therapy among older patients. 27 , 28
Interestingly, both the GCB and non‐GCB cohorts had better results with CAR‐T therapy compared to the SOC. However, results were statistically significant only for GCB patients. These findings may be related to the smaller sample size of the non‐GCB subgroup or to the less favoured prognosis of this cohort. Recent retrospective studies reported inferior results for the non‐GCB patients treated with CAR‐T therapy compared to GCB, although not statistically significant. 29 , 30 , 31 Future studies exploring the association between cell of origin and response to CAR‐T therapy are warranted.
Several limitations of our analysis merit consideration. The main limitation lies in the fact that CAR‐T therapy is an innovative treatment, researched in randomised studies only in the last 2 years with preliminary results only recently published. We therefore have a very small number of studies, one of which has only been published as an abstract. The median follow‐up time, ranging between 6 and 24 months, is much too short and impairs our ability to draw strict conclusions.
Another important limitation is the variability in the design of the studies, translating into high heterogeneity between the studies, especially between the BELINDA trial and the other two trials. Even though there are many similarities between the trials, still there are two major differences: first, the definition of EFS in each of the trials. In addition to death and disease progression, all three trials included SD at different time points as events (ZUMA‐7: day 150, BELINDA: week 12, TRANSFORM: week 9). Although initiation of new anti‐lymphoma therapy was considered an event within ZUMA‐7 and TRANSFORM, BELINDA did not include initiation of a second salvage within the first 12 weeks as an event. Second, there is a difference in the product manufacturing time. The ZUMA‐7 trial, with a median manufacturing time of 13 days, did not allow chemotherapy bridging and reported a median (interquartile range [IQR]) time from randomisation to infusion of 29 (27–34) days. Whereas, Tisa‐cel manufacturing time in BELINDA trial was twice as long (median 26 days) and 83% of BELINDA patients received chemotherapy. The median (IQR) time from leukapheresis to infusion day was 52 (43–61) days. The third trial did not report on manufacturing or infusion time. The inability to provide bridging therapy has a considerable selection bias potential. Treating physicians might have feared to enrol patients with high kinetics aggressive lymphoma, thus recruiting more favourable prognosis patients to such a trial.
In conclusion, treatment of patients with LBCL refractory or relapsing early after front‐line therapy has been considered an unmet need for the last several decades. Pooled analysis of three pioneering trials demonstrated that CAR‐T therapy has significantly superior outcomes compared to the SOC. This analysis demonstrates that early administration of CAR‐T therapy provides a statistically significant survival advantage. Nevertheless, results are quite preliminary. Longer follow‐up and future trials designed to compare CAR‐T therapy with and without bridging to ASCT are needed to confirm these results and determine the optimal sequencing of CAR‐T therapy in the management of R/R LBCL.
AUTHOR CONTRIBUTIONS
Liat Shargian, Ronit Gurion and Anat Gafter‐Gvili contributed to conception and design, extracted and analysed the data and wrote the paper. Pia Raanani and Moshe Yeshurun reviewed the paper.
CONFLICT OF INTEREST
Liat Shargian–Advisory board and honoraria payment from Gilead and Novartis. Anat Gafter‐Gvili–Honoraria payment from Novartis. Ronit Gurion–Advisory board and honoraria payment from Gilead and Novartis.
Supporting information
Figures S1‐S8
Shargian L, Raanani P, Yeshurun M, Gafter‐Gvili A, Gurion R. Chimeric antigen receptor T‐cell therapy is superior to standard of care as second‐line therapy for large B‐cell lymphoma: A systematic review and meta‐analysis. Br J Haematol. 2022;198:838–846. 10.1111/bjh.18335
REFERENCES
- 1. Coiffier B, Thieblemont C, Den Van NE, Lepeu G, Plantier I, Castaigne S, et al. Long‐term outcome of patients in the LNH‐98.5 trial, the first randomized study comparing rituximab‐CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Philip T, Guglielmi C, Hagenbeek A, Somers R, van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy‐sensitive non‐Hodgkin's lymphoma. N Engl J Med. 1995;333(23):1540–5. [DOI] [PubMed] [Google Scholar]
- 3. Mounier N, Canals C, Gisselbrecht C, Cornelissen J, Foa R, Conde E, et al. High‐dose therapy and autologous stem cell transplantation in first relapse for diffuse large B cell lymphoma in the rituximab era: an analysis based on data from the European blood and marrow transplantation registry. Biol Blood Marrow Transplant. 2012;18(5):788–93. [DOI] [PubMed] [Google Scholar]
- 4. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B‐cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van Den Neste E, Schmitz N, Mounier N, Gill D, Linch D, Trneny M, et al. Outcome of patients with relapsed diffuse large B‐cell lymphoma who fail second‐line salvage regimens in the international CORAL study. Bone Marrow Transplant. 2016;51:51–7. [DOI] [PubMed] [Google Scholar]
- 6. Friedberg JW. Relapsed/refractory diffuse large B‐cell lymphoma. Hematol Am Soc Hematol Educ Program. 2011;2011:498–505. [DOI] [PubMed] [Google Scholar]
- 7. Hamadani M, Hari PN, Zhang Y, Carreras J, Akpek G, Aljurf MD, et al. Early failure of frontline rituximab‐containing chemo‐immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20(11):1729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. N Engl J Med. 2017;377:2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 10. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B‐cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52. [DOI] [PubMed] [Google Scholar]
- 11. Jacobson CA, Locke FL, Ghobadi A, Miklos, DB , Lekakis LJ, Oluwole OO, et al. Long‐term (4‐ and 5‐year) overall survival in ZUMA‐1, the pivotal study of axicabtagene ciloleucel in patients with refractory large B‐cell lymphoma. Presented at the 63rd American Society of Hematology Annual Meeting and Exposition, December 11–14, 2021:1764. poster.
- 12. Schuster T, Tam C, Borchmann P, Worel N, JP MG, Holte H, et al. Long‐term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B‐cell lymphomas (JULIET): a multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol. 2021;22:1403–15. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JPT, Thomas J, Chandler J. Cochrane handbook for systematic reviews of interventions. Version 6.0 (updated July 2019). Hoboken (NJ): Cochrane; 2019. [Google Scholar]
- 14. Kamdar M, Solomon SR, Arnason JE, Johnston PB, Glass P, Bachanova V, et al. Lisocabtagene Maraleucel (liso‐cel), a CD19‐directed chimeric antigen receptor (CAR) T cell therapy, versus standard of care (SOC) with salvage chemotherapy (CT) followed by autologous stem cell transplantation (ASCT) as second‐line (2L) treatment in patients (pts) with relapsed or refractory (R/R) large B‐cell lymphoma (LBCL): results from the randomized phase 3 transform study. Blood. 2021;138(Supplement 1):91.33881503 [Google Scholar]
- 15. Locke FL, Miklos DB, Jacobson C, Perales M‐A, Kersten M‐A, Oluwole OO, et al. Axicabtagene ciloleucel as second‐line therapy for large B‐cell lymphoma. N Engl J Med. 2021;386:640–54. 10.1056/NEJMoa2116133 [DOI] [PubMed] [Google Scholar]
- 16. Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second‐line tisagenlecleucel or standard care in aggressive B‐cell lymphoma. N Engl J Med. 2021;386:629–39. 10.1056/NEJMoa2116596 [DOI] [PubMed] [Google Scholar]
- 17. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Van Imhoff GW, McMillan A, Matasar MJ, Radford J, Ardeshna KM, Kuliczkowski K, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B‐cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35:544–51. [DOI] [PubMed] [Google Scholar]
- 19. Chahoud J, Sui D, Erwin WD, Gulbis AM, Korbling M, Zhang M, et al. Updated results of rituximab pre‐ and post‐BEAM with or without 90yttrium‐ibritumomab tiuxetan during autologous transplant for diffuse large b‐cell lymphoma. Clin Cancer Res. 2018;24(10):2304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gisselbrecht C, Schmitz N, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Rituximab maintenance therapy after autologous stem‐cell transplantation in patients with relapsed CD20(+) diffuse large B‐cell lymphoma: final analysis of the collaborative trial in relapsed aggressive lymphoma. J Clin Oncol. 2012;30(36):4462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duell J, Maddocks KJ, González‐Barca E, Jurczak W, Liberati AM, de Vos S, et al. Long‐term outcomes from the phase II L‐MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B‐cell lymphoma. Haematologica. 2021;106(9):2417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Magid Diefenbach CS, Abrisqueta P, Gonzalez‐Barca E, Panizo C, Arguinano Perez JM, Miall F, et al. Polatuzumab vedotin (Pola) + rituximab (R) + lenalidomide (Len) in patients (pts) with relapsed/refractory (R/R) diffuse large B‐cell lymphoma (DLBCL): primary analysis of a phase 1b/2 trial. J Clin Oncol. 2021;39:7512. [Google Scholar]
- 23. Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B‐cell non‐Hodgkin lymphoma: an open‐label, phase 1/2 study. Lancet. 2021;398(10306):1157–69. [DOI] [PubMed] [Google Scholar]
- 24. Hutchings M, Sureda A, JoséTerol M, Albareda FB, Corradini P, Larsen TS, et al. Glofitamab (Glofit) in combination with polatuzumab vedotin (Pola): phase Ib/II preliminary data support manageable safety and encouraging efficacy in relapsed/refractory (R/R) diffuse large B‐cell lymphoma (DLBCL). Blood. 2021;138(Supplement 1):525. [Google Scholar]
- 25. Lin JK, Muffly LS, Spinner MA, Barnes JI, Owens DK, Goldhaber‐Fiebert JD. Cost effectiveness of chimeric antigen receptor T‐cell therapy in multiply relapsed or refractory adult large B‐cell lymphoma. J Clin Oncol. 2019;37(24):2105–19. [DOI] [PubMed] [Google Scholar]
- 26. Thieblemont C, Coiffier B. Lymphoma in older patients. J Clin Oncol. 2007;25(14):1916–23. [DOI] [PubMed] [Google Scholar]
- 27. Locke FL, Jacobson C, Ma L, Dong H, Hu Z‐H, Siddiqi T, et al. Real‐world outcomes of axicabtagene ciloleucel (Axi‐cel) for the treatment of large B‐cell lymphoma (LBCL): impact of age and Specic organ dysfunction. Blood. 2021;138(Supplement 1):530.25. [Google Scholar]
- 28. Ram R, Grisariu S, Shargian‐Alon L, Amit O, Bar‐On Y, Stepensky P, et al. Toxicity and efficacy of chimeric antigen receptor T‐cell in patients with diffuse large B cell lymphoma above the age of 70 years compare to younger patients ‐ a matched control multi‐center cohort study. Haematologica. 2021;107:1111–8. 10.3324/haematol.2021.278288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lacoboni G, Villacampa G, Martinez‐Cibrian N, Bailén R, Lopez Corral L, Sanchez JM, et al. Real‐world evidence of tisagenlecleucel for the treatment of relapsed or refractory large B‐cell lymphoma. Cancer Med. 2021;10(10):3214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Basher F, Coughlin C, Kwon D, Lekakis L, Schatz J. AACR abstract PO‐55: single‐center experience of chimeric antigen receptor T‐cell (CAR‐T) immunotherapy in relapsed/refractory large B‐cell lymphoma identifies association of acute toxicities with inferior disease outcomes. Blood Cancer Discov. 2020;1(3_Supplement):PO‐55. [Google Scholar]
- 31. Rutherford SC, Leonard JP. DLBCL cell of origin: what role should it play in care today? Oncology (Williston Park). 2018;32(9):445–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1‐S8


