Abstract
Beards are controversial in the operating room setting because of the possible retention and shedding of pathogens. Surgical site infection poses a significant burden on healthcare systems. All male healthcare workers who entered the operating room were approached to participate in the study. Four facial swab samples were anonymously collected and a hygiene practice questionnaire was administered. Sample A was taken from the upper and lower lips, sample B from cheeks, and samples C and D were collected by 20 and 40 cm shedding below the face. Colony-forming units (CFUs) and minimum inhibitory concentrations (MICs) of meropenem resistance were determined for samples A and B. Random samples from A, B, C, and D, in addition to meropenem-resistant isolates were cultured with chlorohexidine. Sixty-one bearded and 19 nonbearded healthcare workers participated in the study. 98% were positive for bacterial growth with CFU ranging between 30 × 104 and 200 × 106 CFU/mL. Bacterial growth was significantly higher in bearded participants (P < .05). Eighteen (27.1%) isolates were resistant to meropenem; of these which 14 (77.8%) were from bearded participants, this was not statistically significant. Chlorohexidine was effective in inhibiting the growth of all strains including the meropenem-resistant isolates. Bearded men in the operating room had a significantly higher facial bacterial load. Larger-scale resistance studies are needed to address facial bacterial resistance among healthcare workers in the operating room.
This study aimed to estimate the facial microbial load and identify strains and antimicrobial resistance profiles in bearded versus nonbearded male healthcare workers in the operating room of a tertiary hospital in the Middle East.
Keywords: antibiotic resistance, beard, facial flora, microbial flora, nosocomial infection
1. Introduction
Hospital-acquired infections are a major cause of death and increased morbidity among hospitalized patients with significant financial repercussions.[1] The incidence of surgical site infection (SSI) may reach up to 20%, depending on the procedure type and wound classification. SSI increases the morbidity, mortality, hospital stay, and readmission rate,[2,3] which places a greater burden on the healthcare system and its resources. The prevention of potential causes of SSI is essential in patient care, improvement of public health, and preservation of its resources.
Growing Beards has become popular in men across different workplace environments, ranging from full-grown beards to scruffy looks. Beards may be potential reservoirs for microorganisms implicated in hospital-acquired infections.[4] If facial hair harbors more abundant and or more virulent pathogens than facial skin, then healthcare workers’ beards may act as a bacterial source that may contribute to nosocomial SSIs.[4]
Multiple studies tackled bacterial shedding from beards in the operating room setting and attempted to explore the hypothesis that facial hair may be a potential source of bacterial contamination in the healthcare setting. McLure et al conducted a study in 1999 on 30 surgical healthcare workers (10 bearded, 10 clean-shaven, and 10 females) and cultured their facial flora. The study reported that bearded men shed a significantly greater number of bacteria than clean-shaven men and women.[5] This study did not address shaving habits or facial hygiene routines. On the other hand, another study by Wakeam et al suggested that bearded men do not harbor more virulent bacteria in their beards and that clean-shaven men were more likely to be colonized with potential nosocomial pathogens.[6] The authors examined several factors that McLure et al did not consider, such as the use of soap during face washing, but did not take into consideration the shaving habits in clean-shaven men.[5,6] The human skin microbiome differs significantly between individuals from different geographic locations.[4] Both of the studies discussed above were conducted in the western world; the microbiome in the west may differ from that in the Middle East. To our knowledge, there is no available data from the Middle East regarding the facial microbiological flora of bearded versus clean-shaven medical professionals.
This study aimed to estimate the facial bacterial floral load and identify strains and antimicrobial resistance profiles in bearded versus nonbearded men in the operating room in one of the largest tertiary medical centers in the Middle East. Multiple confounding factors were considered, including the use of soap for daily face washing, shaving habits, working hours, smoking status, and comorbidities.
2. Methods
2.1. Study setting
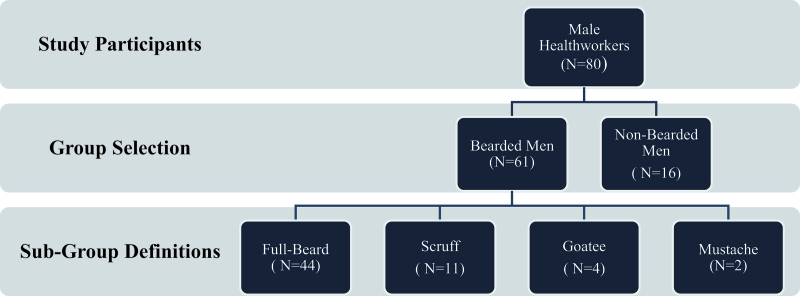
This cross-sectional experimental study was conducted at the American University of Beirut Medical Center, involving healthcare workers in the operating room between April and May 2019. All subjects voluntarily participated and entered the study anonymously. The study’s exclusion criteria included women, presence of any skin condition on the day of sampling, and refusal to contribute to the study. The study was approved by the American University of Beirut Institutional Review Board (IRB) [IRB ID: BIO-2018-0511], which provided core protection for human research participants. All male surgeons, including attendings, residents, and interns, as well as anesthetists (attendings and residents), anesthesia technicians, scrubs, and circulating nurses whose job descriptions require direct contact with patients in the operating room were invited to participate in the study. Beard was defined as facial hair measuring > 2 mm in length. Subdefinitions for subgroup analysis were reported as follows: Scruff: facial hair between 1 and 2 mm without any shaved areas; full beard: facial hair longer than 2 mm without any shaved areas; goatee: facial hair around the mouth and on the chin, but not on the cheeks; mustache: facial hair over the upper lip (Fig. 1).
Figure 1.
Participant flowchart.
2.2. Study population
Power analysis with 2 groups was conducted to determine sample size using a margin of error of 5%, a power of 0.80, and detect a difference of 25% in a load of bacterial flora. Based on the aforementioned assumptions, the desired sample size was 98, with 49 participants per group.
2.3. Sample collection
After obtaining verbal informed consent, participants responded to a questionnaire regarding their grooming habits. Four samples were collected from each participant. Sample A from the skin of the upper and lower lips, sample B from the skin of the cheeks using dry sterile swabs. Samples C and D were used as a test for bacterial shedding, whereby beards were rubbed using a sterile inoculation loop while a blood Petri plate was held at a distance of 20 and 40 cm below the face in resemblance to the actual physician-patient physical distance in the operating room.
2.4. Microbiology testing
From each participant, samples A and B (which were taken using swabs) were first placed in 1 mL of lysogeny broth (LB) separately and then incubated at 37°C for 24 hours. Samples C and D, taken on blood agar plates, were placed in an incubator at 37°C for 24 hours. The next day, bacterial isolates were suspended in Brucella broth medium and stored at −80°C until further use.
The CFU experiment was performed by preparing a 0.5 McFarland bacterial suspension from each sample followed by serial dilutions at 3 different dilution factors: 100, 1000, and 10,000. Then, 30 μL of each dilution factor was inoculated on an LB agar plate and placed in the incubator at 37°C for 24 hours. The following day, the results were documented using the following formula: CFU/mL = (no. of colonies × dilution factor)/volume of the culture plate.
Samples A and B from all the participants were inoculated on blood agar plates supplemented with 3 different concentrations of meropenem separately, which are: 0.5, 1, and 2 µg/mL. A broth microdilution assay was then performed on the 65 isolates that grew on blood agar plates supplemented with 2 µg/mL meropenem. The 18 isolates that had an MIC of meropenem > 4 µg/mL were identified using Gram staining, followed by coagulase, catalase, and mannitol salt tests. The MIC of the isolates was determined by the broth microdilution method, according to the Clinical and Laboratory Standard Institute recommendations.
Finally, to test the efficiency of chlorhexidine, random samples from groups A, B, C, and D were inoculated on LB agar plates supplemented with 4% chlorhexidine.
2.5. Statistical studies
All variables were entered into the Statistical Package of Social Science SPSS 24.0 software (SPSS Inc., Chicago, IL) for analysis. The dependent variables were the bacterial flora load, bacterial strains, and antimicrobial resistance profiles. Continuous variables were expressed as mean ± standard error of the mean (SEM) and were subjected to statistical analysis using one-way analysis of variance (ANOVA) followed by Tukey post hoc test for multiple group comparisons for categorical variables, and independent Student t-test for continuous variables. Statistical significance was set at P < .05. Missing data were not present. Data on both outcome and experimental variables were precisely collected promptly.
3. Results
3.1. Demographics
Eighty male healthcare workers in the operating room were enrolled in this study, including 61 bearded and 19 nonbearded participants (Table 1). The mean age was 34.3 ± 10.8 and 36.1 ± 9.0 years for the bearded and nonbearded groups, respectively. Forty-four (72.1%) of the bearded participants washed their faces daily with soap, while 9 (40.9%) of the men in the nonbearded group washed their faces with soap. Both bearded 32 (52.4%) and nonbearded 10 (52.6%) men mainly used razors or blades for shaving. The majority of the participants spent >8 hours in the hospital. The surgery department hosted the highest number of bearded participants when compared to the anesthesia and nursing departments, with 38 (62.3%) bearded participants from the department of surgery. There was no significant difference between soap utility in daily washing, working hours, smoking status, method of shaving, and hospital division between the bearded and nonbearded participants.
Table 1.
Study demographics.
| Bearded, n = 61 | Nonbearded, n = 19 | P value | |
|---|---|---|---|
| Age (mean ± SD), y | 34.3 ± 10.8 | 36.1 ± 9.0 | .52 |
| Daily wash | 60 (98.4%) | 18 (94.7%) | .42 |
| Daily soap wash | 44 (72.1%) | 9 (47.4%) | .05 |
| Clipper | 29 (47.5%) | 9 (47.4%) | 1.00 |
| Smoker | 23 (37.7%) | 9 (47.4%) | .59 |
| Working hours | |||
| <8 h | 6 (9.8%) | 1 (5.3%) | 1.00 |
| >8 h | 55 (90.2%) | 18 (94.7%) | |
| Hospital division | |||
| Surgery | 38 (62.3%) | 12 (63.2%) | .97 |
| Anesthesia | 11 (18%) | 3 (15.8%) | |
| Nursing | 12 (19.7%) | 4 (21.1%) |
Three dilution factor concentrations were considered to maintain the highest bacterial growth percentage which was achieved at dilution factors. The bacterial growth range was chosen as 30 to 300 CFUs. Any value below this range was considered as low growth, and any value greater was marked as heavy bacterial growth. Eighty samples were included in the analysis. Almost all “A” and “B” samples were positive for bacterial growth with CFU ranging between 30 × 10 4 and 200 × 10 6 CFU/mL.
Bacterial growth was significantly higher in nonbearded participants for both samples A and B with respective P values of .03 and .04 (Table 2). In sample A, 31 (50.8%) isolates from bearded men were associated with heavy bacterial growth as compared to 15 (78.9%) isolates in the nonbearded group; this was statistically significant with a P value of .03. Similarly, in sample B, 40 (65.6%) isolates from bearded men were associated with heavy growth, whereas 17 (89.5%) isolates from nonbearded men were associated with heavy bacterial growth (P = .04).
Table 2.
Bacterial growth at 104 dilution factor in bearded and nonbearded men for samples A, B, C, and D.
| Colony-forming units | Bearded | Nonbearded | P value | |
|---|---|---|---|---|
| A | Low growth | 30 (49.2%) | 4 (21.1%) | .03* |
| Heavy growth | 31 (50.8%) | 15 (78.9%) | ||
| B | Low growth | 21 (34.4%) | 2 (10.5%) | .04* |
| Heavy growth | 40 (65.6%) | 17 (89.5%) | ||
| C | Positive growth | 47 (77%) | 10 (52.6%) | .04* |
| Negative growth | 14 (23%) | 9 (47.4%) | ||
| D | Positive growth | 17 (27.9%) | 4 (21.1%) | .55 |
| Negative growth | 44 (72.1%) | 15 (78.9%) |
A: Sterile swab taken from the skin of the upper and lower lips. B: Sterile swab taken from the skin of the cheeks. C: Beards scrubbed using a sterile inoculation loop while a blood agar petri plate was held at 20 cm distance as a test for bacterial shedding. D: Beards scrubbed using a sterile inoculation loop while a blood agar petri plate was held at 40 cm as a test for bacterial shedding.
In sample C (shedding test at 20 cm), 47 (77.0%) isolates from bearded men were associated with positive bacterial growth as compared to 10 (56%) isolates in the nonbearded group; this was statistically significant (P = .04). Similarly, in sample D (shedding test at 40 cm), 17 (27.9%) of bearded men were associated with positive bacterial growth, whereas 4 (21.1%) of the nonbearded men were associated with positive bacterial growth (P = .5).
3.2. Susceptibility results
Of the 65 tested isolates, 18 (27.6%) meropenem-resistant isolates were detected. All these isolates were identified as Staphylococcus aureus by gram staining, which showed that the isolates were gram-positive staphylococci, followed by positive catalase and coagulase tests, and finally yellow color with the mannitol salt test. Forty-seven (72.3%) of the 65 isolates that grew on blood agar plates supplemented with 2 µg/mL of meropenem were proven to be susceptible to this antibiotic by broth microdilution. The remaining 18 meropenem-resistant isolates, with minimum inhibitory concentrations (MICs) >4 µg/mL, were distributed as 14 (77.8%) from bearded (sample A: 7, sample B: 7) and 4 (22.2%) from nonbearded (sample A: 2, sample B: 2) isolates (Table 3). MIC against meropenem resistance was studied in both bearded and nonbearded participants, and no significant difference was observed between the 2 groups (P = .96 and .84, respectively) (Table 3). Table 4 reporting the MIC values separated by beard category and Figure 2 reporting the MIC values per sample number are added as supplemental files to this manuscript.
Table 3.
MIC against meropenem-resistant (>4 µg/mL) of bacterial isolates among samples A and B.
| Samples | Participant group | Meropenem-resistant isolates (n = 18) | P value |
|---|---|---|---|
| A | Bearded men | 7 (38.9%) | .96 |
| Nonbearded men | 2 (11.1%) | ||
| B | Bearded men | 7 (38.9%) | .84 |
| Nonbearded men | 2 (11.1%) |
Table 4.
MIC samples separated by beard category.
| Sample number | Bearded vs not bearded | ||
|---|---|---|---|
| 16A | No | 68A | Yes |
| 2A | Yes | 68B | Yes |
| 28B | Yes | 69A | Yes |
| 28A | Yes | 69B | No |
| 29A | Yes | 69A | No |
| 32A | Yes | 70A | Yes |
| 32B | Yes | 70B | Yes |
| 33B | Yes | 72A | No |
| 33A | Yes | 73B | Yes |
| 34A | Yes | 74A (2) | Yes |
| 34B | Yes | 74B | Yes |
| 36A | Yes | 74A (1) | Yes |
| 39B | Yes | 75A | Yes |
| 41A | Yes | 76A (1) | No |
| 41B | Yes | 76A (2) | No |
| 41A | Yes | 78B | Yes |
| 41B | Yes | 78A | Yes |
| 43B | No | 79A | Yes |
| 43A | No | 79B | Yes |
| 45A | Yes | 8B | No |
| 45B | Yes | 80B (2) | Yes |
| 48B | No | 80A | Yes |
| 48A | No | 80B (1) | Yes |
| 49A | Yes | ||
| 49B | Yes | ||
| 51A | Yes | ||
| 51B | Yes | ||
| 53A | Yes | ||
| 54A | Yes | ||
| 54B | Yes | ||
| 57B | Yes | ||
| 59A | Yes | ||
| 59B | Yes | ||
| 60B | No | ||
| 62A | No | ||
| 62B | No | ||
| 63B | Yes | ||
| 65A | Yes | ||
| 65B | Yes | ||
| 65A | Yes | ||
| 67A | Yes | ||
| 67B | Yes |
Figure 2.
MIC values per sample number. MIC = minimum inhibitory concentration.
No growth was detected when 160 randomly selected isolates from groups A, B, C, and D were inoculated on LB agar plates supplemented with 4% chlorhexidine for 48 hours. This indicates that chlorohexidine was highly efficient in inhibiting the growth of all strains, even the meropenem-resistant strains.
In sample A, 6 (40%) of the nonbearded participants who used the clipper as a shaving mechanism were associated with heavy bacterial growth, while 9 (60%) of those who used razor/blade were associated with heavy growth; this was not statistically significant (P = .213). In sample B, 8 (47.1%) of men using clippers were associated with heavy bacterial growth as compared to 9 (52.9%) of men reporting to use razor/blade as a shaving mechanism. As for sample C, 6 (60%) of nonbearded participants using clippers reported positive growth on nutrient agar as compared to 4 (40%) of the participants using razor/blade. Finally, only 1 (25%) participant using clipper reported positive bacterial growth in contrast to 3 (75%) participants using razor/blade (Table 5).
Table 5.
Bacterial growth at 104 dilution factor in nonbearded men using clipper as a shaving mechanism versus blades and razors.
| Clipper (n = 9) | Blades and razor (n = 10) | P value | ||
|---|---|---|---|---|
| A | Low growth | 3 (75%) | 1 (25%) | .213 |
| Heavy growth | 6 (40%) | 9 (60%) | ||
| B | Low growth | 1 (50%) | 1 (50%) | .937 |
| Heavy growth | 8 (47.1%) | 9 (52.9%) | ||
| C | Positive growth | 6 (60%) | 4 (40%) | .245 |
| Negative growth | 3 (33.3%) | 6 (66.7%) | ||
| D | Positive growth | 1 (25%) | 3 (75%) | .313 |
| Negative growth | 8 (53.3%) | 7 (46.7%) |
4. Discussion
Nosocomial infections are preventable and may significantly impact the patients and the public health sector by increasing hospital stay and cost, as well as increasing morbidity and mortality.[1,6–9] SSIs are defined as an infection of the surgical incision, organ, or space that occurs after surgery.[10] Surgical volume worldwide has increased over the years, with a 38% increase in surgical volume from 2004 to 2012, according to a study by the World Health Organization.[11] With this significant increase in surgical volume, SSIs will become more detrimental and have an increased negative impact on patients’ health and healthcare systems in general.
The skin is a barrier to microorganisms that may be virulently leading to SSIs. The skin is colonized by many microorganisms.[3] The most commonly isolated organisms in SSI are S. aureus, coagulase-negative Staphylococci, Enterococcus spp., and Escherichia coli.[6] The Center for Disease Control and Prevention guidelines for the prevention of SSI highlight the importance of preoperative patient preparation, aseptic technique, and attention to surgical techniques along with antibiotic prophylaxis.[7,12]
Breaches in the aseptic technique due to possible contamination from the surgical team are a potential cause of SSI. The risk of SSI after microbial contamination of the surgical site depends on the inoculation dose, the virulence of the pathogen, and the patient’s immunity.[6] The risk of SSI increases when the level of contamination exceeds 105 organisms per gram of tissue.[13] Contamination of the surgical field may occur by unintentional breaches in the aseptic technique and may originate from exogenous sources such as the surgical team, operating room environment, and surgical instruments. Contamination can occur when the surgical wound is exposed to the surgical team’s microbial skin flora, possibly through bacterial shedding from various locations such as facial skin or facial hair of the surgical team. Facial hair has become more common across all occupations; therefore, if beards harbor more abundant or virulent pathogens than facial skin, healthcare workers’ facial hair may act as a reservoir for bacteria that may lead to nosocomial infections.[4]
The skin of healthcare workers carries greater quantities of bacteria than the general public. Sumner et al reported that 50% of medical staff carried potentially pathogenic bacteria, such as S. aureus, E. coli, Streptococcus viridans, and Proteus vulgaris in their hair.[14] A study conducted on microbiology personnel where aerosolized bacteria were sprayed on bearded and clean-shaven men showed that bearded individuals retained the bacteria on their faces longer than their clean-shaven counterparts.[15]
The role of surgical facemasks (SFMs) in the operating room and the prevention of SSI remains controversial.[16–18] Tunevall et al conducted a randomized study in which 1537 operations were performed with masks with 4.7% SSI and 1551 operations were performed without a facemask with 3.5% SSI, with no statistical significance between the groups.[19] A National Surgical Quality Improvement Program review of 6517 patients in 2 teaching hospitals that were visited by the Department of Health that imposed more stringent regulations on operating room attire, including coverage of facial hair and full coverage of ears, did not show any significant decrease in SSI before and after the site visits.[16]
Studies investigating areas directly under the operator have shown that SFM almost completely prevents bacterial contamination of agar plates placed 30 cm below the lips.[17,18,20] The theory of bacterial shedding is based on the concept of dermabrasion, where wiggling or facial shedding causes the shedding of bacterial skin microflora onto the sterile surgical field.[4,5,20,21] McLure et al showed that even if the subjects remained still, there was significantly more shedding below the SFM of bearded males than females and clean-shaven males.[5] With clean-shaven men considered to have recently removed a superficial layer of skin containing bacteria while shaving, leading to less shedding as compared to bearded individuals. In contrast, Parry et al performed a similar study but did not show any significant difference in the shedding of bacteria below SFM alone or SFM with a surgical hood of bearded as compared to clean-shaven men.[21] Parry et al showed significantly higher bacterial shedding from unmasked subjects 6.5 CFUs) than masked subjects 1.4 CFUs; P = .02).[21]
A study similar to ours showed that workers with facial hair were less likely to be colonized with S. aureus (41.2% bearded vs 52.6% clean-shaven, P = .02) and methicillin-resistant coagulase-negative staphylococci (2.0% vs 7.0%, P < .01).[4] Wakeam et al concluded that bearded male hospital workers did not harbor more pathogenic bacteria than clean-shaven hospital workers. In contrast, their study showed that clean-shaven individuals were more likely to be colonized with more virulent bacteria. This finding was explained by microtrauma-causing abrasions by shaving, leading to an increase in bacterial proliferation.[4] Our study showed that bearded hospital workers were more likely to shed bacteria than their clean-shaven counterparts.
The principal findings of our study are that samples taken from nonbearded individuals had significantly more bacterial growth than bearded individuals. The test for bacterial shedding has shown a statistically significant difference between the 2 groups when shedding was tested at a 20 cm distance. Significant bacterial growth was not reported when shedding was done at a distance higher than 20 cm; specifically, 40 cm in our case. Meropenem-resistant bacterial growth was detected at a higher frequency in bearded individuals; however, this did not reach statistical significance. In addition, we showed that 4% chlorohexidine was highly efficient in inhibiting the growth of all strains, even the meropenem-resistant strains. Chlorhexidine-based solutions are widely used for prepping surgical sites before procedures to reduce the risk of SSI. Since 2012, our institution has adopted the use of chlorhexidine-alcohol-based solutions for preoperative prepping of surgical sites. Darouiche et al demonstrated the superiority of chlorhexidine-alcohol-based solutions over povidone-iodine-based solutions in the prevention of SSIs (9.5% vs 16.1%, P = .004) in clean-contaminated surgeries, reducing the risk of SSI by 41%.[22]
The activity of operating room personnel is the principal source of airborne bacteria that originate mainly from the skin of people present in the room.[23] The number of airborne bacteria depends on the number of people present, their level of activity, and compliance with infection control practices.[24] Grooming habits and daily soap washing may affect bacterial growth. Individuals from the surgery department were found to harbor the heaviest bacterial growth in samples A and B. Regarding the hair shedding samples, this study showed that the farther the patient-physician distance, the lower the bacterial growth.
Crucial factors to prevent microbial infection expansion by operating room personnel are personal integrity and work ethics. All surgical teams and staff personnel should adhere to the standard guidelines as an essential part of the prevention process.
Despite being aware of the limitations of our study, we initially planned to enroll 49 participants in each group; however, because the majority of the male healthcare workers grew their beards at the time of the study – most of the participants were bearded – we were not able to recruit > 80 participants. Our study limitations entail an unequal sample size. The adopted statistical test specifically the chi-square is a nonparametric test that is a distribution-free test concerning the distribution of the data. Yet, the study results may be potentially skewed.
5. Conclusion
Our study shows that nonbearded healthcare workers in the operating room had a significantly higher bacterial load in their facial flora than their bearded counterparts. The difference in shaving methods and facial washing with or without soap did not translate into a significant difference in bacterial growth between the 2 groups. Even though the bacterial resistance profile was not significantly different between MIC the2 groups, bearded individuals had relatively more resistant strains.
Author contributions
J.H. and M.E.E. are both the first authors and have contributed equally to this manuscript. M.E.E. and A.Z. proposed the study. J.H. and N.M. performed the data collection. J.H., N.M., and A.S. performed the laboratory work. J.H. and R.J. analyzed the data. R.J. is a statistician. A.Z., M.E.E., and J.H. reviewed the literature and wrote the first draft. All authors contributed to the design and interpretation of the study and drafted the manuscript.
Abbreviations:
- ANOVA =
- analysis of variance
- CFU =
- colony-forming units
- CLSI =
- Clinical and Laboratory Standard Institute
- IRB =
- institutional review board
- LB =
- lysogeny broth
- MIC =
- minimum inhibitory concentration
- SEM =
- standard error of the mean
- SFMs =
- surgical facemasks
- SPSS =
- Statistical Package of Social Science
- SSI =
- surgical site infection.
How to cite this article: El Edelbi M, Hassanieh J, Malaeb N, Abou Fayad A, Jaafar RF, Sleiman A, Abedelrahim A, Kanafani Z, Matar GM, Zaghal A. Facial microbial flora in bearded versus nonbearded men in the operating room setting: A single-center cross-sectional STROBE-compliant observational study. Medicine 2022;101:40(e29565).
The authors have no conflicts of interest to disclose.
This study was funded by a SEED Grant from the American University of Beirut.
The relevant datasets and supporting documents generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Contributor Information
Mostapha El Edelbi, Email: me139@aub.edu.lb.
Joelle Hassanieh, Email: jh91@aub.edu.lb.
Nancy Malaeb, Email: nm140@aub.edu.lb.
Antoine Abou Fayad, Email: aa328@aub.edu.lb.
Rola F. Jaafar, Email: rj29@aub.edu.lb.
Ahmad Sleiman, Email: ahmad.zaghal@aub.edu.lb.
Abdelkader Abedelrahim, Email: aia23@mail.aub.edu.
Zeina Kanafani, Email: zk10@aub.edu.lb.
Ghassan M. Matar, Email: gmatar@aub.edu.lb.
References
- [1].Ginawi I, Saleem M, Sigh M, et al. Hospital-acquired infections among patients admitted in the medical and surgical wards of a non-teaching secondary care hospital in northern India. J Clin Diagn Res. 2014;8:81–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. centers for disease control and prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control. 1999;27:97–132; quiz 133. [PubMed] [Google Scholar]
- [3].Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wakeam E, Hernandez RA, Morales DR, et al. Bacterial ecology of hospital workers’ facial hair: a cross-sectional study. J Hosp Infect. 2014;87:63–7. [DOI] [PubMed] [Google Scholar]
- [5].McLure HA, Mannam M, Talboys CA, Azadian BS, Yentis SM. The effect of facial hair and sex on the dispersal of bacteria below a masked subject. Anesthesia. 2000;55:173–6. [DOI] [PubMed] [Google Scholar]
- [6].Owens CD, Stoessel K. Surgical site infections: epidemiology, microbiology, and prevention. J Hosp Infect. 2008;70(Suppl 2):3–10. [DOI] [PubMed] [Google Scholar]
- [7].Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152:784–91. [DOI] [PubMed] [Google Scholar]
- [8].İlhami Çelik NI, Denk A, Sevim E, Yaşar D, Yaşar MA. Prevalence of hospital acquired infections in anesthesiology intensive care unit. Firat Tip Dergisi. 2005;10:132–5. [Google Scholar]
- [9].Shepard J, Ward W, Milstone A, et al. Financial impact of surgical site infections on hospitals: the hospital management perspective. JAMA Surg. 2013;148:907–14. [DOI] [PubMed] [Google Scholar]
- [10].National Healthcare Safety Network, C.f.D.C.a.P. Surgical site infection (SSI) event. 2017. Available at: http://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
- [11].Weiser TG, Haynes AB, Molina G, et al. Size and distribution of the global volume of surgery in 2012. Bull World Health Organ. 2016;94:201–209F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ban KA, Minei JP, Laronga C, et al. American college of surgeons and surgical infection society: surgical site infection guidelines, 2016 update. J Am Coll Surg. 2017;224:59–74. [DOI] [PubMed] [Google Scholar]
- [13].Krizek TJ, Robson MC. Evolution of quantitative bacteriology in wound management. Am J Surg. 1975;130:579–84. [DOI] [PubMed] [Google Scholar]
- [14].Summers MM, Lynch PF, Black T. Hair as a reservoir of staphylococci. J Clin Pathol. 1965;18:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Barbeito MS, Mathews CT, Taylor LA. Microbiological laboratory hazard of bearded men. Appl Microbiol. 1967;15:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Farach SM, Kelly KN, Farkas RL, et al. Have recent modifications of operating room attire policies decreased surgical site infections? An American College of Surgeons NSQIP review of 6,517 patients. J Am Coll Surg. 2018;226:804–13. [DOI] [PubMed] [Google Scholar]
- [17].Romney MG. Surgical face masks in the operating theatre: re-examining the evidence. J Hosp Infect. 2001;47:251–6. [DOI] [PubMed] [Google Scholar]
- [18].Datta R. Use of surgical facemasks in the operation theatre: effective or habit? Med J Armed Forces India. 2010;66:163–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tunevall TG. Postoperative wound infections, and surgical face masks: a controlled study. World J Surg. 1991;15:383–7; discussion 387. [DOI] [PubMed] [Google Scholar]
- [20].McLure HA, Talboys CA, Yentis SM, Azadian BS. Surgical face masks and downward dispersal of bacteria. Anaesthesia. 1998;53:624–6. [DOI] [PubMed] [Google Scholar]
- [21].Parry JA, Karau MJ, Aho JM, Taunton M, Patel R. To beard or not to beard? bacterial shedding among surgeons. Orthopedics. 2016;39:e290–4. [DOI] [PubMed] [Google Scholar]
- [22].Darouiche RO, Wall M, Jr, Itani KM, et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med. 2010;362:18–26. [DOI] [PubMed] [Google Scholar]
- [23].Lidwell OM. Clean air at operation and subsequent sepsis in the joint. Clin Orthop Relat Res. 1986:91–102. [PubMed] [Google Scholar]
- [24].Hambraeus A. Aerobiology in the operating room--a review. J Hosp Infect. 1988;11(Suppl A):68–76. [DOI] [PubMed] [Google Scholar]