Abstract
Objective:
Most radiotherapy patients with prostate cancer are treated with volumetric modulated arc therapy (VMAT). Advantages of VMAT may be limited by daily treatment uncertainties such as setup errors, internal organ motion, and deformation. The position and shape of prostate target as well as normal organ, i.e., rectum volume around the target, may change during the course of treatment. The aim of the present work is to estimate rectal toxicity estimation using a novel two-level biological knowledge-based fuzzy logic method. Both prostate and rectal internal motions as well as setup uncertainties are considered without compromising target dose distribution in the present study.
Materials and Methods:
The Mamdani-type fuzzy logic framework was considered in two levels. The prostate target volume changes from minimum to maximum during the course of treatment. In the first level, the fuzzy logic was applied for determining biological acceptable target margin using tumor control probability and normal tissue complication probability (NTCP) parameters based on prostate target motion limits, and then, fuzzy margin was derived. The output margin of first-level fuzzy logic was compared to currently used margins. In second-level fuzzy, rectal volume variation with weekly analysis of cone-beam computed tomography (CBCT) was considered. The biological parameter (NTCP) was calculated corresponding to rectal subvolume variation with weekly CBCT image analysis. Using irradiated volume versus organ risk relationship from treatment planning, the overlapped risk volumes were estimated. Fuzzy rules and membership function were used based on setup errors, asymmetrical nature of organ motion, and limitations of normal tissue toxicity in Mamdani-type Fuzzy Inference System.
Results:
For total displacement, standard errors of prostate ranging from 0 to 5 mm range were considered in the present study. In the first level, fuzzy planning target volume (PTV) margin was found to be similar or up to 0.5 mm bigger than the conventional margin, but taking the modeling uncertainty into account resulted in a good match between the calculated fuzzy PTV margin and conventional margin formulations under error 0–5 mm standard deviation (SD) range. With application of fuzzy margin obtained from first-level fuzzy, overlapped rectal volumes and corresponding NTCP values were fuzzified in second-level fuzzy using rectal volume variations. The final risk factor (RF) of rectum was qualitatively assessed and found clinically acceptable for each fractional volume of irradiated to total volume and relevant NTCP values. The reason may be at 5 mm SD displacement error range, NTCP values would be within acceptable limit without compromising the tumor dose distribution though the confounding factors such as organ motion, deformation of rectum, and in-house image matching protocols exist.
Conclusion:
A new approach of two-level fuzzy logic may be suitable to estimate possible organ-at-risk (OAR) toxicity biologically without compromising tumor volume that includes both prostate target and OAR rectum deformation even at displacement standard errors of prostate ranging from 0 to 5 mm range which was considered in the present study. Using proposed simple and fast method, there is an interplay between volume-risk relationship and NTCP of OARs to judge real-time normal organ risk level or alter the treatment margins, particularly concern to individual factors such as comorbidities, genetic predisposition, and other lifestyle choices even at high displacement errors >5 mm SD range.
Keywords: Asymmetric margins, fuzzy logic, normal tissue complication probability, prostate cancer, risk factor, volumetric modulated arc therapy
INTRODUCTION
Radiation therapy (RT) is one of the treatment methods used in the treatment of cancer. In typical radiotherapy dose planning, the objective is the irradiation of a diseased volume of tissue with a lethal dose while at the same time causing minimum damage to the surrounding normal tissue. As a consequence, the treatment is always limited by normal tissue tolerance. The goal of radiotherapy depends on the precise definition of target or diseased volumes of interest and nearby healthy normal organs in a treatment course. The induction of adverse side effects of critical organs around target and their severity may depend on proper definition of treatment margins. Thus, the selection of best possible treatment margins is dependent on benefit and risk trade off to achieve favorable treatment outcomes. The currently used margin recipes were formulated to maintain the geometrical accuracy of target volumes using probabilistic dose distributions.[1] In most treatment strategies today, the target margins are of symmetric or linear nature by considering a linear relationship between radiotherapy errors and planning target volume (PTV) margins. However, the effect of organ motion and its associated subvolume of surrounding organ at risks (OARs) are the main limitations of these margin recipes, particularly for moving organs.[2] In case of moving organ radiotherapy like prostate cancer (PC), it is difficult to define target boundaries precisely to derive the exact PTV margin. Since the position of prostate target volume between treatment fractions is likely to change, it was difficult to define the target volume due to this movement-related fuzziness region of target. Therefore in treating the dynamic tumor volumes such as prostate radiotherapy, the currently used conventional margin recipes may have limitations due to their rigidity to account for the displacements caused due to the biological nature of the organ. Besides variations in the prostate gland, in response to rectum and bladder fillings, the total inter-fraction displacement can often range from 0 to 20 mm.[3,4] Therefore in the fractionated RT phase, the actual planned dose may differ from delivered dose due to the presence of organ motion-related deformity, its effect on nearby organs along with other radiotherapy setup errors. Yartsev S et al.[5] studied the effect of organ motion on PTV margins using various approaches that illustrated the fuzziness in defining prostate target volume. Further, this fuzziness of boundaries may affect nearby normal overlapped OARs like rectum in RT planning. This may impact on subvolume-related radiation-induced toxicities.[6,7] The rectal volume receiving 60 Gy or higher is associated with an increased risk of Grade 2 late rectal toxicity or rectal bleeding and can be a limiting factor for dose escalation.
In general, radiation-induced toxicity of healthy organs of interest around target volume depends upon the dose received by them. The dose-volume evaluation is truly based on geometrical position and shape of normal organs to consider actual dosimetric impact in radiotherapy treatment[8] particularly for moving organs. Therefore the effect of organ deformation is significant to estimate possible toxicity of healthy organs. The risk or toxicity of healthy organ of interest depends on acceptance limits of normal tissue complication probability (NTCP), which is calculated based on type of organ, its total volume (VTVO), and subvolume of interest (VVOI) of OAR of particular type. During irradiation, if the total volume of OAR is large, then its expected damage or risk factor (RF) is low and vice versa. The RF and degree of OAR damage was studied by Ansari et al.[9] In their study, the risk estimation was assessed numerically based physical dose distribution and conformity index of the target volume. RF is possibly directly proportional to the fragment of the volume or subvolume and inversely proportional to the total volume of OAR which gets irradiated by its tolerance dose (TD). In moving organ radiotherapy like prostate that varies between patients, the RF estimation of OARs is very difficult to generalize until target as well as critical organs are assessed by an adaptive approach. Further, the currently used margin recipes[2] are based on physical or geometrical dose–volume distributions only. The radiobiological effects of tumor and adjacent critical organs are not accounted for at the planning level. The reason is due to the difficulties in establishing a simple mathematical correlation between radiobiological parameters such as tumor control probability (TCP), NTCP, their margin-dependent real-time effect, and radiotherapy uncertainties at a time. Although if the model is established, it may have a large degree of variability. However, radiobiological effects should be considered at the planning level[10] in view of tumor motion and adjacent critical organs deformation. Therefore, PTV margins should not be of rigid type, and margins should be asymmetrical in proportion to organ displacement and as well as to account for radiobiological effects.
In organ motion management, the image-guided radiotherapy (IGRT) with cone-beam computed tomography (CBCT) is considered as part of standard external beam radiotherapy due to precision and accuracy in radiotherapy of PC. IGRT with daily or weekly (CBCT) can provide information on variation in the shape and size of the prostate target and OAR like rectum. The differences between planned and actual anatomy enable the remedial actions to achieve the aim of radiation therapy.
The basic matching procedures are automatic and or manual in order to access the correctness of the treatment delivery. Based on the lack of sufficient quality of CBCT images and subsequent long acquisition time of images,[11,12,13] automatic matching may be inadequate for most clinical situations. In manual matching IGRT, the procedure is time consuming and for each patient, it needs approximately 6 hrs or more.[14,15] However, CBCT can provide information on the day-to-day anatomical variation in the shape and size of prostate volume, i.e., target, and rectum i.e., critical organ. But in the present practice of radiotherapy, it is not possible to assess real-time damage of OAR using its subvolumes and appropriate NTCP values in the treatment planning system (TPS). This may be possible until there would be intermediate approach to evaluate possible risk of OARs which takes some extra time during treatment. Introducing a simple fuzzy logic in radiotherapy planning has the advantage over other mathematical models because fuzzy logic allows easier and faster linkage of radiobiological and geometrical parameters through knowledge-based fuzzy rules and appropriate membership functions (MFs). Also correlating of these parameters is cumbersome to quantify using a simple mathematical approach without have a large degree of variability.
The present work is related to asymmetric margins of moving organs and limitations of margins using fuzzy logic in PC based on earlier work by Patnaikuni et al.[16] The aim of the present work is to study the qualitative risk estimation (RF) of OAR rectum using a novel biological knowledge-based fuzzy logic method. In the present study, both internal organ motions of prostate and rectum were considered in addition to setup errors in two different fuzzy levels. In the first level, fuzzy PTV margins were applied for prostate target motion from fuzzy PTV margin output which was derived using TCP, NTCP parameters, and fuzzy rules. Furthermore, the derived fuzzy output margin was also compared to currently used margins. In the second-level fuzzy approach, weekly CBCT versus rectal volume variation was considered. To deduce overlapped risk volume estimation in each plan, irradiated volume-risk relationship-based fuzzy rules were considered. The selection of treatment margins should be toxicity specific for optimization and dose escalation in view of true results of radiotherapy while maintaining target dose distribution. In routine radiotherapy practice, the application of rigid treatment margins is under still consideration for the treatment of moving organs in most of treatment centers. In such cases, the current study may be expected to provide the qualitative estimation of RF of OARs for possible outcomes of radiotherapy in order to have a personalized treatment.
MATERIALS AND METHODS
The Fuzzy inference system (FIS) of Mamdani type was used in the present study to consider radiation effects of organ motion and toxicities suited to expect human thinking, because it was accepted widely for capture expert knowledge within reasonable time than other types. The basic workflow of the current study is shown in Figure 1. The FIS was applied in two levels here. In the first level, PTV margin derivation procedure was considered using target motion consideration. In the second level, normal tissue bladder toxicity estimation was studied based on rectum volume variations.
Figure 1.
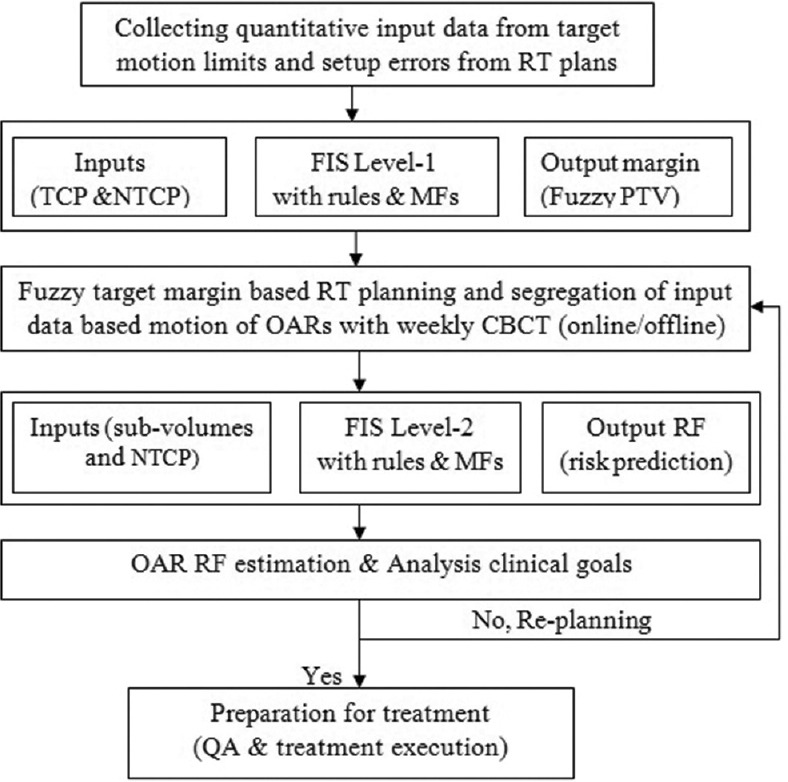
The workflow of study on normal tissue risk estimation using two-level biological fuzzy logic
Sampling and treatment planning
In the present study, eight numbers of localized prostate patients were selected for volumetric modulated arc therapy (VMAT). The dose prescription was 73.5 Gy. Before CT simulation, bowel and bladder preparation was considered in reproducibility of their position during treatment. With co-registration of magnetic resonance imaging, PTV was delineated by expanding each clinical target volume (CTV) as per guidelines.[16,17] CTV to PTV margins were generated asymmetrically using statistical estimation of tracking methods.[16] RT oncology group (RTOG)0621 guidelines were followed in all contouring for a male pelvis. The same investigator completed all contouring per patient to reduce interuser variability. Sample characteristics, plan objectives, and dose–volume constraints were considered for treatment planning, as mentioned in Table 1. TPS, Eclipse 15.6.Varian Medical Systems, was used for all treatment planning with parameters setting.[18] The Matlab R2018a-based simulation tool was used to calculate radiobiological parameters using the equivalent uniform dose (EUD) modeling.[19] For all plans, it was ensured that 95% or more of PTV and entire CTV were covered by 100% of the prescription dose for all plans. For OARs, constraints were followed according to RTOG 0815 guidelines.
Table 1.
Sample characteristics and Planning parameters values used for fuzzy modelling (Patnaikuni et al., 2020, Mzenda B et al., 2010 and AAPM task group 166, AAPM)
| Sample characteristics | Parameter value |
|---|---|
| 1. Clinical details | |
| Number of patients | 8 |
| Age (years) | 45-65 |
| Tumor staging | T1 - T2/N0/M0 |
| Dose prescription/number of fractions | 73.5 (Gy/33) |
| 2. Objectives for target/OARs | |
| PTV prostate | 73.5 Gy (uniform dose), V95% >95% |
| TCP parameters | |
| EDU/γ50/a/D50 | Target EUD=69.3 Gy, a=−10 |
| OAR rectum constraints | V50 Gy <65%, V65 Gy <50%, V70 Gy <35% |
| NTCP parameters | |
| EDU/γ50/a/TD50 | EUD=58 Gy, a=8 |
| OAR bladder constraints | V50 Gy <60%, V65 Gy <35%, V70 Gy <25% |
| NTCP parameters | |
| EDU/γ50/a/TD50 | EUD=59Gy, a=8 |
OAR: Organ at risk, PTV: Planning target volume, NTCP: Normal tissue complication probability, TCP: Tumor control probability, EUD: Equivalent uniform dose, AAPM: American association of physicists in medicine, EDU: Equivalent uniform dose
First-level fuzzy approach based on prostate target motion
In the first-level approach, all treatment plans were performed using adopted asymmetrical PTV margins to CTV from the minimum acceptable margin of PTV to maximum. The margins were studied in all axial views from minimum to maximum margin of PTV. These margins were adopted asymmetrically as Superior-Inferior (SI): 0 to 14 mm; Anterior-Posterior (AP): 0 to 14 mm, Posterior-Anterior (PA): 0 to 12 mm, Left-Right (LR): 0 to 12 mm using tracking methods.[16] Here 1 mm stepped-size was added to subsequent PTV margin up to the maximum limit. For each plan, TCP and NTCP parameters were calculated using EUD tool.
The TCP and NTCP can be calculated as follows:


Here, D50 is absorbed dose to produce 50% of tumor control rate; TD50 is dose producing a 50% complication rate as TD under uniform radiation. γ50 is a unit less model parameter to describe the slope of tumor dose–response curve.
The base values of TCP and NTCP corresponded to plan with minimal PTV margin. New TCP and NTCP values were recalculated for each margin of PTV added by 1 mm stepped increment. Subsequent loss in TCP (i.e., ΔTCP) and increase in NTCP (i.e., ΔNTCP) were also calculated when compared to base values. For combined errors, margin order up to 10 with 1 mm magnitude was used in our study. The first-level FIS consisted of 02 inputs, i.e.; ΔTCP and ΔNTCP and 01 output, i.e., PTV margin. Fuzzy rules were framed mainly on knowledge-based limitations of TCP and NTCP using permutations of MFs for ΔTCP, ΔNTCP, and PTV margin. The increase in NTCP is compensated for by reducing PTV margin. The loss in TCP is compensated for by increasing PTV margin size. In this manner, an optimum number of fuzzy rules[16] were selected using clinical goals imposed on margin limits. The resultant output as 3D surface[16] represents uneven changes in PTV margin with required TCP/NTCP relation of two inputs and one output values, as shown in Figure 2.
Figure 2.
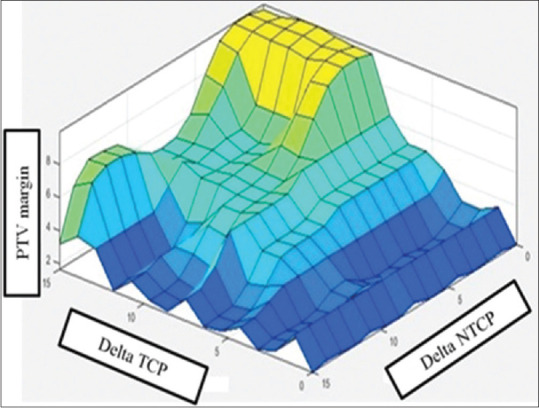
Fuzzy level one output as three-dimensional surfaces for deciding planning target volume margin corresponding to optimal tumor control probability and normal tissue complication probability on the basis of target motion-based deformation (Patnaikuni et al. 2020)
Second-level fuzzy framework on organ-at-risk rectal volume variation and weekly cone-beam computed tomography approach
In the second-level FIS framework, the same first-level PTV output margin-based VMAT plan was considered to assess in the estimation of risk due to volume variation of OAR rectum. In case of OAR rectum, the volume may vary significantly on daily basis. Therefore positional or geometrical reproducibility of rectum is difficult. This rectal volume variation leads to different overlapped rectum volumes, and hence, the actual rectal dose may be different from the planned dose. Therefore, rectal subvolume (%) involvement and its corresponding NTCP values were considered to assess risk of OARs in second-level fuzzy. To estimate rectal volume variations, weekly CBCT images (slice thickness 2 mm) were acquired in standard pelvis mode. For the initial assessment of CBCT images, an automatic match algorithm was used, and further verification was completed using manual matching. The CTV and OAR rectum were re-contoured according to the patient's anatomy of the day. Revised volumes were compared on weekly CBCTs to planning CT (CTp) which was the reference. Five CBCT scans were performed for all patients in 5-day interval. The rectum volume was manually outlined on the CTp versus CBCTs. In this way, for each patient, six rectum contours were outlined and all were co-registered with CTp. Re-optimization and dose calculation was done using the same parameter setting for each CBCT1-5 versus CTp co-registration. Superimposed rectal contours from the CTp and weekly CBCTs of a typical patient on axial (left) and sagittal (right) views are shown in Figure 3. The average, minimum, and maximum rectal volumes for each patient as compared to planned CT rectal volume are listed in Table 2.
Figure 3.
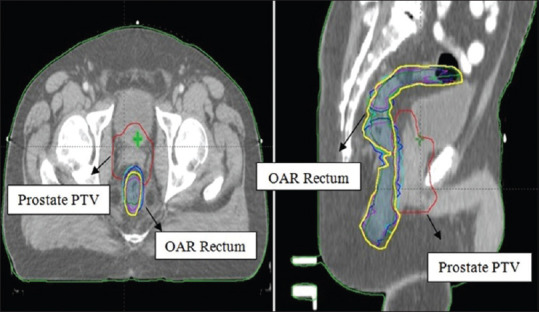
Superimposed rectal contours of a typical patient on axial (left) and sagittal (right) views as ----- Original rectum volume (yellow contour) in planning CT; ----- Rectum volume in CBCT1; ----- Rectum volume in CBCT2; ----- Rectum volume in CBCT3; ----- Rectum volume in CBCT4; ----- Rectum volume in CBCT5
Table 2.
Organ-at-risk rectum volume variations for 8 number of observations: Planning computed tomography volume (cc) versus weekly cone-beam computed tomography volume (cc)
| Number of sample | CTp volume (cc) of OAR rectum | Weekly CBCT volume (cc) range of OAR | Subvolume (%) range of OAR rectum | |
|---|---|---|---|---|
|
| ||||
| CBCT minimum- CBCT maximum | CBCT mean | |||
| 1 | 65.63 | 55.34-70.08 | 63.62 | 8.77-20.79 |
| 2 | 82.55 | 70.21-110.05 | 87.23 | 8.58-18.76 |
| 3 | 90.67 | 81.22-107.61 | 96.97 | 7.02-18.72 |
| 4 | 70.04 | 55.03-113.09 | 89.31 | 5.40-25.53 |
| 5 | 75.11 | 65.10-117.85 | 93.35 | 7.83-28.44 |
| 6 | 95.07 | 78.50-121.91 | 100.55 | 7.54-23.31 |
| 7 | 105.45 | 82.81-164.86 | 132.98 | 6.12-25.35 |
| 8 | 159.37 | 95.50-168.30 | 123.56 | 10.60-28.27 |
OAR: Organ at risk, CBCT: Cone-beam computed tomography, CTp: Planning CT
The volume effect of any healthy organ around the target volume may be significant in risk or toxicity estimation during irradiation by its TD.[9] The expected risk of OAR is dependent on the entire volume (VTVO) type of OAR, and involvement of its fragment or subvolumes. The damage or RF is lesser if the total volume of OAR is larger and vice versa. In addition, TD decides the degree of damage of a normal organ on account of irradiation. For serial organs, TD is the maximum dose, and for parallel organs, TD is the mean dose. If TD is more, then there is less chance of damage during irradiation and vice versa. The RF of an OAR is also inversely proportional to its TD DTD. The RF of OARs was qualitatively assessed for fractional volume in each CBCT to total volume, relevant NTCP values, subvolume of volume of interest (VVOI), and its associated NTCP, as mentioned in equation 2.
RF ∝ VVOI/VTVO × DTD
Therefore, RF = DP × VVOI/VTVO × DTD (3)
where DP is a constant quantity which stands for prescribed dose to the PTV. In the above equation, the ratio of DP and DTD for OAR rectum is nearly insignificant depending on maximum TD. But still, it is considered in clinical experience-based fuzzy rules. In our study, the real-time possible risk of OAR is related to the subvolume of OAR involved and corresponding NTCP values.
For assessing the risk or damage rationally in second-level fuzzy approach, the ratio of VVOI to VTVO was considered as input 1; rectum NTCP parameter was considered as input 2, while normal tissue induced RF was considered as output. MFs were distributed for low risk to high-risk regions as low, medium, high, and very high. For input 1, MFs are as 0%–25%, 25%–50%, 50%–75%, and 75%–100% intervals, and for input 2 as 0%–2%, 2%–4%, 4%–6%, and above 6%, respectively. MFs were selected for output RF using RF scale[9] as low, medium, and high corresponding to 0–0.3, 0.3–0.6, and above 0.6, respectively. When RF is near to zero means, OAR is fully conserved. For rectum-like moving organs, this was likely impractical. Low RF: If the numerical value of RF is 0.3 or less, then the expected damage to OAR may be allowed depending upon tumor conformity as well as acceptable NTCP limits of rectum. Medium RF: This range assigns numerical value of RF is 0.6 or less which means partially damaged of OAR will be expected. High RF: This range assigns RF is 0.6 or higher, means maximum damage OAR will be predicted. The MFs for 2 inputs and 1 output are shown in Figure 4. Fuzzy rules [Annaxure 1] were framed according to knowledge-based OAR rectal volume and its possible toxicity limits. Each CBCT versus CTp was evaluated to estimate subvolume-related NTCP according to the position of the week. If toxicity limits exceed based on NTCP (may be subjected to lack of rectal volume control or any existing comorbidities), re-optimization and planning will be recommended with altered contours of OAR rectum. For analyzing the radiation dose to OARs, the dose tolerance was considered as per the quantitative analysis of normal tissue effects in the clinic (QUANTEC) dose constraints.
Figure 4.
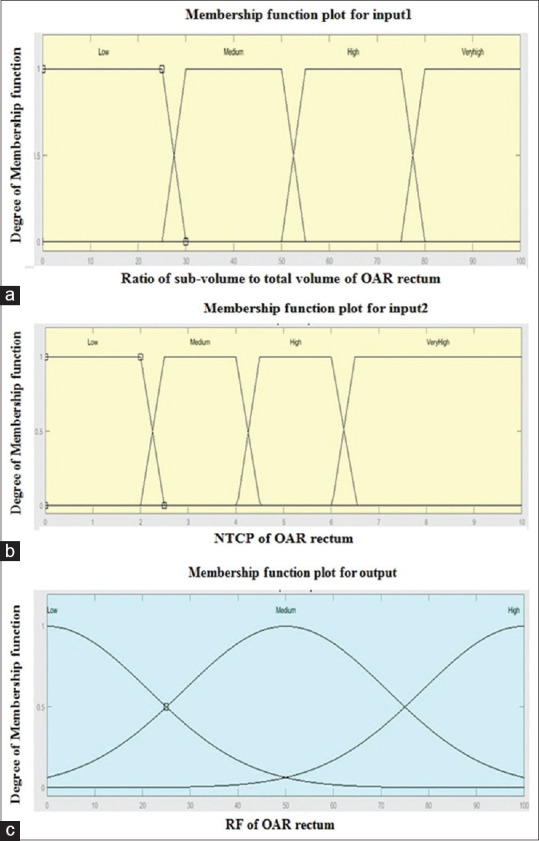
Membership functions for two inputs and one output in second-level fuzzy approach. (a) Four trapezoid membership functions for input 1 as low, medium, high, very high (b) Four trapezoid membership functions for input 2 as low, medium, high, very high (c) Three Gaussian membership functions for RF output as low, medium, and high
RESULTS AND DISCUSSION
Organ-at-risk rectum risk factor estimation
In first-level fuzzy, prostate PTV margin was adopted from Patnaikuni et al.[16] under low error range organ motion displacement of ranging from 0 to 5 mm standard deviation (SD) In the present study, all results were studied in this low error range. At this error range of target motion, the results were found to be more or less similar to conventional margin models when output fuzzy PTV margin compared to conventional van Herk margin methods. The comparative results showed good agreement of results between fuzzy PTV margin and van Herk et al.'s formulation in displacement error range up to 5 mm SD while considering modeling uncertainty 0.5 ± 0.2 mm into account. With the application of fuzzy margin output, the VMAT plans were performed with 6 mm fuzzy PTV margins in this range. All plans of the present study were clinically acceptable so there were no significant changes found in view of PTV conformity and target objectives. The PTV margin selection was based on acceptable values of TCP and NTCP, as mentioned in Figure 2. But at higher organ motion displacement error magnitudes (>5 mm SD error), fuzzy PTV plans were might be favorable clinical endpoints when compared to plans using current treatment margins.[16]
In second-level fuzzy approach, the overlapped rectum volumes and its corresponding NTCP values were considered in fuzzification using weekly CBCT-based rectal volume variations. Rectal RF estimation was generated in MatbalR2018a environment as 3D fuzzy output surface. The output is shown in Figure 5 that indicates combination of subvolume related NTCP of OAR and its corresponding estimated RF value. From this output surface, it was observed that the increase in the NTCP results in an increase in RF. Although it is understood as treatment subvolume is higher, OAR risk is higher according to volume effect. But inter-fractional changes in positions of OAR and their respective volumes can alter dose–volume metrics of OAR considerably, warranting daily or weekly investigation.
Figure 5.
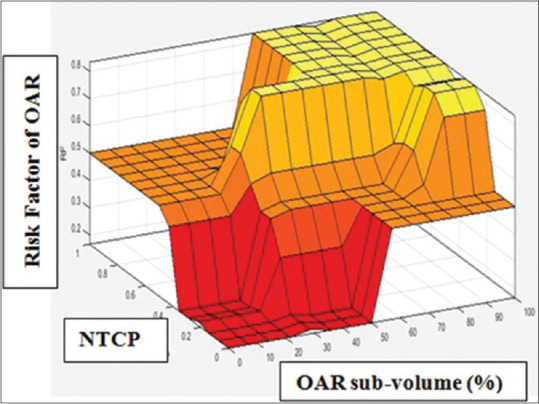
Fuzzy second-level output as three-dimensional surfaces for risk factor estimation of OAR rectum at low organ motion displacement error standard deviation
For each patient, there were notable variations in OAR mean real diameter. The mean, minimum, and maximum rectal volumes over 5 CBCTs were listed and compared to planned CT rectal volume for each patient in Table 2. The results show that each CBCT has a rectal volume which significantly varied compared to CTp volume of OAR rectum. Due to variation of actual and planned volumes of OAR rectum, the sub-volume percentage varies for each patient. The sub-volume % range was 5.40cc to 28.27cc as shown in Table 2. The reason may be confounding nature of OAR rectum such as organ motion and mean rectal diameter. In our study, localized prostate patients were included. If it is for extensive disease, the subvolumes may greatly increase. However due to subvolume variation, the actual rectal mean dose received may significantly vary that affects expected rectal end points. Therefore, it is more appropriate to assess possible risk of OAR rectum based on real time position so that investigator may be focused on expected toxicity using fuzzy approach. Table 3 demonstrates the qualitative observation of possible risk or toxicity (%) of OAR rectum corresponding to NTCP values under low errors SD of target. The results of RF estimation of OAR rectum corresponding to NTCP values for a typical patient case (sample no 08) were mentioned as rectal subvolume varies greatly from 10.6 cc to 28.27 cc. Fuzzy RF was also compared with manual calculated RF. For CBCT1-4 subvolumes, RF was read as low and for CBCT5, RF was read as medium from output surface from Figure 5. Here, all NTCP values were within acceptable limits. This might be due to the selection of localized prostate sampling for RT planning under low SD displacement range. However, it is expected from the observation that if RF is medium or high, then the investigation will be preferred subjected to acceptable limits NTCP values. It was also noticed that though RF is within acceptable limit, this may depend on tumor progression and or mean real diameter while maintaining the target conformity. The selection of RF value may be higher under poor or moderate CBCT image matching conditions and existing comorbidities of rectum if any. The fuzzy method in present study makes such investigations easier as well as possibly biologically significant. Thus, this approach is a possible advantage over current treatment methods, in which all investigations were mainly on physical dose–volume metrics even in adaptive radiotherapy.
Table 3.
Risk factor estimation of organ-at-risk rectum corresponding to normal tissue complication probability values for one patient case (sample number 8): Fuzzy versus manual calculated risk factor
| Parameter | Weekly estimation of subvolumes (cc) and associated risk | ||||
|---|---|---|---|---|---|
|
| |||||
| CBCT1 | CBCT2 | CBCT3 | CBCT4 | CBCT5 | |
| Subvolume (%) | 10.6 | 14.4 | 21.55 | 14.9 | 28.27 |
| NTCP (%) | 0.84 | 1.68 | 1.94 | 1.05 | 2.52 |
| Fuzzy OAR RF | Low (0.16) | Low (0.16) | Low (0.19) | Low (0.166) | Medium (0.46) |
| Calculated RF | 0.14 | 0.17 | 0.20 | 0.17 | 0.38 |
RF: Risk factor, NTCP: Normal tissue complication probability, OAR: Organ at risk, CBCT: Cone-beam computed tomography
A course of radiotherapy of prostate is usually planned on a single CTp scan. Due to onset of geometric uncertainties, the initial planning scan is unlikely to be representative of the position of the prostate or normal structures throughout the course of treatment. From earlier studies,[20,21,22,23] substantial inter- and intra-patient variations were found in measured rectal volumes both at planning and during treatment. Although rectal volumes tend to vary between planning and treatment levels, no systemic change in rectal volume was identified over the course of treatment. The weekly CBCT like IGRT also provides the potential for margin reduction but does not provide subvolume related inter-fractional organ motion and its NTCP until there would be interventions like manual calculations concerned to volume-based NTCP. This may take extra time for analyzing all the CBCTs retrospectively which was proven to be too time-consuming.
The conventional van Herk margin formulations were population based margins for adequate dose coverage of target volumes. But if a larger CTV-PTV margins to better dose coverage of the tumor, then it might be a larger irradiation volume of normal tissue within the high-dose envelope. This, in return, may increase toxicity to neighboring normal tissue.[24] Daily CBCT image guidance was studied by Sveistrup et al. to manage these treatment margins and organ toxicity by helping to provide a precise knowledge of the actual position of the target at treatment.[25] Although this approach may useful in the estimation and reduction of OAR toxicity, normal structure-related biological-based toxicity may not be covered which is significant in correlation of true outcomes of treatment. In case of prostate-like moving organ radiotherapy, the prostate gland is known to be a moving and deformable gland, which can also be influenced by changes in nearby rectal and bladder volumes.[26,27,28] Although dose escalation can be possible with techniques such as IMRT and VMAT, but subvolume-based toxicity is always a challenge in rigid margin model-based treatment planning, particularly where internal organ motions are inevitable during the course of radiotherapy. In such cases, daily CBCT-based IGRT is useful for prostate RT. The daily CBCT, in turn, adds up to a cumulative dose that may differ from the initial planning dose. However, due to concomitant dose in daily CBCT and its image quality are also challenging. Therefore, in the present study, weekly CBCT-based image-guided method was used for toxicity estimation under good image matching conditions at low standard displacement error SD.
Scope for clinical assessment and practical implementation
The rectal volume variation is always significant during the course of PC radiotherapy. During RT, the involvement of fractional of full volume gets irradiated that decides possible toxicity of any normal tissue around target. In patients with low rectal volume (50 cc or less), there might be up to 25% more rectal volume included in the high-dose region at planning level. The CBCT-based adoptive planning dose–volume statistics have shown that not all the patients were able to maintain a stable rectal volume, which is one of the biggest challenges for prostate RT. Based on acceptable limits of NTCP, if toxicity limit exceeds than permissible level, the target volume or normal structure contours may be altered. Therefore re-optimization/planning will be preferred. The possibility of individualization of organ specific margins with new adapted contours and plan re-optimization using off-line strategies[29,30] may be significantly effective and advantageous for tumor conformity. However, the procedure is time consuming and needs approximately 6 to 7 hrs for each patient. Also for OAR like rectum, the biological-based estimation of toxicity level may be challenging. Using current platforms in adaptive re-planning, the generation of patient-specific margins and/or imaging schedules based on analysis of early treatment imaging histories and/or measurements of patient-specific factors are more practical strategies for given short time period in fractionated radiation therapy. Such strategies may allow a reduction in imaging frequency and/or PTV margins. Nonrepresentative patient anatomy (such as distended rectum) at the time of planning may require larger PTV margins to account for systematic error for treatment planning. Similarly, larger body habitus of the patient may lead to more setup uncertainty requiring either larger PTV margins or more frequent imaging. For re-planning using current platforms like physical dose distribution-based adaptive planning, the generation of patient-specific margins and/or imaging schedules based on analysis of treatment histories and/or measurements of patient-specific factors such as body mass index (BMI), rectal, and bladder volumes may be more practical strategies in given time, particularly busy radiotherapy centers. In the present study, the biological-based toxicity level was estimated in simplest approach under low target displacement error range. Physiological behavior of the rectum was considered to explain our results. The selection of fuzzy rules and MFs was mainly based on displacement error SD range, organ type (such as serial organ or parallel organ or serial–parallel organ), and acceptance of QUANTEC dose constraints. RF estimation may be also directly performed in in-house organ motion-based margin formulations, but those must be consistent with van Herk formulations under low displacement error SD in PC RT.
The limitations of the present work include the estimation of toxicity level under low standard errors SD only for localized PC RT. Furthermore, PTV fuzzy margin was obtained with patient realignment performance in all axial views (SI, AP, and LR directions) with small number of samples. In our study, the patient preparation is considered so that rectum was not encroached 50% of its volume across the diameter of the PTV. The identification of relevant factors (comorbidities, lifestyle, etc.) or locally advanced cases may improve the performance of the current study which can affect the normal tissue complications. These factors should be incorporated into fuzzy rules and MFs at the planning level to reflect the true prediction of results. In case of locally advanced cases, the inter- and intra-observer variability in contouring seminal vesicles and its nonuniform deformation than prostate that may offer different margin considerations in rectal toxicity estimation.[27,31] This may be an area of interest for future scope.
CONCLUSION
The currently used rigid margins have their limitations in radiotherapy for moving organs. Today, these margin recipes are still used in most radiotherapy centers for treatment and the survival rates are generally satisfactory. However, the adverse effects on critical organs and normal tissues are variable. Hence, current margin formulations can be clearly improved to get better outcomes. In moving organ radiotherapy, no gold standard method currently exists that addresses all the previously mentioned limitations to be used for comparing the models from this study. The method proposed in this study is a novel approach that allows modification of tumor and normal tissue responses for increased therapeutic benefit using individualized patient-specific margins. In manual setup of currently using margin techniques, it may be very difficult to accomplish biological-based patient-specific acceptable margins. The fuzzy logic approach from the present study may augment personalized treatment from generalized treatment. However, clinical trials are required to fully validate this observation. As a conclusion from this study, fuzzy logic-based RF tool may be expected a comprehensive evaluation tool in deciding the qualitative nature of VMAT plan because of encompasses a wider range of clinically relevant parameters and TD of OARs. The major advantage of current study is it gives possible information about tumor dose conformity as well as severity level of damage of OARs for all other dynamic target radiotherapies at a glance in routine clinical application.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are thankful to Department of radiotherapy, Pt JNM Medical College, and Department of Physics, National Institute of technology, Raipur, for providing support at each level to complete this work.
ANNAXURE 1: KNOWLEDGE BASED FUZZY RULES FOR NORMAL ORGAN RISK ESTIMATION
The MATLAB-based fuzzy rules were generated using clinical expertise and acceptance of NTCP limits. Here 11 numbers of rules were mentioned out of all possible permutations. But it is recommended that rules may be changed according to clinical goals in favor of better treatment outcomes.
If subvolume-to-total volume ratio is low and NTCP is low, then RF is low
If subvolume-to-total volume ratio is low and NTCP is medium, then RF is low
If subvolume-to-total volume ratio is low and NTCP is high, then RF is medium
If subvolume-to-total volume ratio is medium and NTCP is low, then RF is low
If subvolume-to-total volume ratio is medium and NTCP is medium, then RF is medium
If subvolume-to-total volume ratio is medium and NTCP is high, then RF is high
If subvolume-to-total volume ratio is high and NTCP is medium, then RF is medium
If subvolume-to-total volume ratio is high and NTCP is high, then RF is high
If subvolume-to-total volume ratio is high and NTCP is very high, then RF is high
If subvolume-to-total volume ratio is very high and NTCP is medium, then RF is high
If subvolume-to-total volume is very high and NTCP is high then RF is high
If subvolume-to-total volume is very high and NTCP is very high, then RF is high.
REFERENCES
- 1.Byrne TE. A review of prostate motion with considerations for the treatment of prostate cancer. Med Dosim. 2005;30:155–61. doi: 10.1016/j.meddos.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 2.van Herk M, Remeijer P, Rasch C, Lebesque JV. The probability of correct target dosage: Dose-population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–35. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 3.Langen KM, Jones DT. Organ motion and its management. Int J Radiat Oncol Biol Phys. 2001;50:265–78. doi: 10.1016/s0360-3016(01)01453-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Paskalev K, Xu X, Zhu J, Wang L, Price RA, et al. Rectal dose variation during the course of image-guided radiation therapy of prostate cancer. Radiother Oncol. 2010;95:198–202. doi: 10.1016/j.radonc.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Yartsev S, Bauman G. Target margins in radiotherapy of prostate cancer. Br J Radiol. 2016;89:20160312. doi: 10.1259/bjr.20160312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–9. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varadhan R, Hui SK, Way S, Nisi K. Assessing prostate, bladder and rectal doses during image guided radiation therapy-need for plan adaptation? J Appl Clin Med Phys. 2009;10:56–74. doi: 10.1120/jacmp.v10i3.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, Yang Z, Wang J, Hu W. Dosimetric impact of different bladder and rectum filling during prostate cancer radiotherapy. Radiat Oncol. 2016;11:103. doi: 10.1186/s13014-016-0681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ansari S, Zope MK, Yadav N. A new method for risk factor assessment of organs at risk including conformity index in radiotherapy treatment plan. J Radiother Pract. 2020:1–9. [Google Scholar]
- 10.Deasy JO, Mayo CS, Orton CG. Treatment planning evaluation and optimization should be biologically and not dose/volume based. Med Phys. 2015;42:2753–6. doi: 10.1118/1.4916670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi W, Li JG, Zlotecki RA, Yeung A, Newlin H, Palta J, et al. Evaluation of kV cone-beam ct performance for prostate IGRT: A comparison of automatic grey-value alignment to implanted fiducial-marker alignment. Am J Clin Oncol. 2011;34:16–21. doi: 10.1097/COC.0b013e3181d26b1a. [DOI] [PubMed] [Google Scholar]
- 12.Maund IF, Benson RJ, Fairfoul J, Cook J, Huddart R, Poynter A. Image-guided radiotherapy of the prostate using daily CBCT: The feasibility and likely benefit of implementing a margin reduction. Br J Radiol. 2014;87:20140459. doi: 10.1259/bjr.20140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Létourneau D, Martinez AA, Lockman D, Yan D, Vargas C, Ivaldi G, et al. Assessment of residual error for online cone-beam CT-guided treatment of prostate cancer patients. Int J Radiat Oncol Biol Phys. 2005;62:1239–46. doi: 10.1016/j.ijrobp.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 14.Nuver TT, Hoogeman MS, Remeijer P, van Herk M, Lebesque JV. An adaptive off-line procedure for radiotherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1559–67. doi: 10.1016/j.ijrobp.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Góra J, Stock M, Lütgendorf-Caucig C, Georg D. Is there an advantage in designing adapted, patient-specific PTV margins in intensity modulated proton beam therapy for prostate cancer? Int J Radiat Oncol Biol Phys. 2013;85:881–8. doi: 10.1016/j.ijrobp.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 16.Patnaikuni SK, Saini SM, Chandola RM, Chandrakar P, Chaudhary V. Study of asymmetric margins in prostate cancer radiation therapy using fuzzy logic. J Med Phys. 2020;45:88–97. doi: 10.4103/jmp.JMP_110_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prostate Cancer. NCCN Practice. [Last accessed on 2019 Aug 05]. Available from: http://www.nccn.orgguidelines.in.oncology-/professional/physician-g/s/f-guidelines.asp.v. 30.2016 .
- 18.Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Med Phys. 1997;24:103–10. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 19.Mzenda B, Hosseini-Ashrafi ME, Palmer A, Hodgson DF, Liu H, Brown DJ. Determination of target volumes in radiotherapy and the implications of technological advances: A literature review. J Radiother Pract. 2009;8:41–51. [Google Scholar]
- 20.Kupelian PA, Langen KM, Zeidan OA, Meeks SL, Willoughby TR, Wagner TH, et al. Daily variations in delivered doses in patients treated with radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:876–82. doi: 10.1016/j.ijrobp.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Sripadam R, Stratford J, Henry AM, Jackson A, Moore CJ, Price P. Rectal motion can reduce CTV coverage and increase rectal dose during prostate radiotherapy: A daily cone-beam CT study. Radiother Oncol. 2009;90:312–7. doi: 10.1016/j.radonc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 22.Lebesque JV, Bruce AM, Kroes AP, Touw A, Shouman RT, van Herk M. Variation in volumes, dose-volume histograms, and estimated normal tissue complication probabilities of rectum and bladder during conformal radiotherapy of T3 prostate cancer. Int J Radiat Oncol Biol Phys. 1995;33:1109–19. doi: 10.1016/0360-3016(95)00253-7. [DOI] [PubMed] [Google Scholar]
- 23.Engels B, Tournel K, Soete G, Storme G. Assessment of rectal distention in radiotherapy of prostate cancer using daily megavoltage CT image guidance. Radiother Oncol. 2009;90:377–81. doi: 10.1016/j.radonc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Boydev C, Taleb-Ahmed A, Derraz F, Peyrodie L, Thiran JP, Pasquier D. Development of CBCT-based prostate setup correction strategies and impact of rectal distension. Radiat Oncol. 2015;10:83. doi: 10.1186/s13014-015-0386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sveistrup J, af Rosenschöld PM, Deasy JO, Oh JH, Pommer T, Petersen PM, et al. Improvement in toxicity in high risk prostate cancer patients treated with image-guided intensity-modulated radiotherapy compared to 3D conformal radiotherapy without daily image guidance. Radiat Oncol. 2014;9:44. doi: 10.1186/1748-717X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padhani AR, Khoo VS, Suckling J, Husband JE, Leach MO, Dearnaley DP. Evaluating the effect of rectal distension and rectal movement on prostate gland position using cine MRI. Int J Radiat Oncol Biol Phys. 1999;44:525–33. doi: 10.1016/s0360-3016(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 27.Roeske JC, Forman JD, Mesina CF, He T, Pelizzari CA, Fontenla E, et al. Evaluation of changes in the size and location of the prostate, seminal vesicles, bladder, and rectum during a course of external beam radiation therapy. Int J Radiat Oncol Biol Phys. 1995;33:1321–9. doi: 10.1016/0360-3016(95)00225-1. [DOI] [PubMed] [Google Scholar]
- 28.de Crevoisier R, Tucker SL, Dong L, Mohan R, Cheung R, Cox JD, et al. Increased risk of biochemical and local failure in patients with distended rectum on the planning CT for prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:965–73. doi: 10.1016/j.ijrobp.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 29.Ten Haken RK, Forman JD, Heimburger DK, Gerhardsson A, McShan DL, Perez-Tamayo C, et al. Treatment planning issues related to prostate movement in response to differential filling of the rectum and bladder. Int J Radiat Oncol Biol Phys. 1991;20:1317–24. doi: 10.1016/0360-3016(91)90244-x. [DOI] [PubMed] [Google Scholar]
- 30.Schild SE, Casale HE, Bellefontaine LP. Movements of the prostate due to rectal and bladder distension: Implications for radiotherapy. Med Dosim. 1993;18:13–5. doi: 10.1016/0958-3947(93)90021-k. [DOI] [PubMed] [Google Scholar]
- 31.Fiorino C, Reni M, Bolognesi A, Cattaneo GM, Calandrino R. Intra- and inter-observer variability in contouring prostate and seminal vesicles: Implications for conformal treatment planning. Radiother Oncol. 1998;47:285–92. doi: 10.1016/s0167-8140(98)00021-8. [DOI] [PubMed] [Google Scholar]


