Abstract
Changes in diastolic blood pressure (DBP) are common in patients with acute myocardial infarction (AMI). The relationship between the dynamic change of DBP and in-hospital mortality among patients with AMI remains unclear. This study aimed to explore the importance of DBP during disease development among patients with AMI. We performed a retrospective cohort study involving patients from the Medical Information Mart for Intensive Care III database, which included > 40,000 patients admitted to the intensive care unit (ICU). Overall, 3209 adult AMI admissions were identified. We extracted the clinical and laboratory information in the patients with AMI. Cox proportional hazards models were used to evaluate the prognostic values of baseline DBP. We used the generalized additive mixed model (GAMM) to compare trends in DBP over time among survivors and non-survivors, after adjusting for potential confounders. During the ICU stay, 189 patients died (mortality rate, 6.36%). The age of each non-survivor together with the variations in DBP over time from admission to the time of death is of great importance to the scientific community. Cox multivariable regression analysis displayed that after adjusting for confounding factors, ascended baseline DBP was an important hazard factor for hospital deaths (hazard ratio, 1.02; 95% confidence interval, 1.01–1.03; P = .003). Based on GAMM, DBP in the death group was markedly lower than that of the surviving group. Moreover, the difference between the two groups showed an increasing trend within 3 days after ICU admission. After adjusting for various variables, the results were stable. DBP significantly contributed to in-hospital mortality among patients with AMI. There was a nonlinear correlation between baseline DBP and in-hospital mortality among patients with AMI, and the DBP of the non-survivors decreased within the first 3 days after ICU admission. However, the causality cannot be deduced from our data.
Keywords: acute myocardial infarction, diastolic blood pressure, generalized additive mixed model, in-hospital mortality, repeated-measures analysis
1. Introduction
Blood pressure is the pressure that the blood circulation puts on the walls of the arteries, the main blood vessels in the body. It is universally acknowledged that hypertension is a primarily independent hazard factor for atherosclerotic vascular diseases, such as coronary arterial disease and carotid atherosclerosis.[1,2] Thresholds for high blood pressure and popular concepts about hypertension have shifted over time. Isolated diastolic blood pressure (DPB), isolated systolic blood pressure (SBP), and the consequences of systolic and DPB combined with hypertension emerged in the 1960s when the prevailing view was that only DPB affected the outcome.[3,4] Nonetheless, according to data from the Framingham Heart Study,[5] it has been suggested that measuring SBP alone could improve the treatment of hypertension. Another study suggested that this was inappropriate; although SBP did have a larger effect, SBP and DBP independently affected cardiovascular outcomes; therefore, DBP should not be ignored. Flint et al also found that regardless of the definition of hypertension (≥140/90 mm Hg or ≥ 130/80 mm Hg), both systolic and diastolic hypertension independently affected the risk of adverse cardiovascular matters.[4] Similar relationships between systolic and diastolic hypertension and bad consequences were observed in models stratified by baseline cardiovascular disease or by the American College of Cardiology and American Heart Association risk assessments.[6]
Acute myocardial infarction (AMI) is the pathological definition of myocardial cell death due to prolonged myocardial ischemia (inadequate myocardial oxygen supply). Eight million Americans visit a doctor each year for signs and symptoms suggestive of AMI.[7] About 700,000 people were eventually diagnosed with myocardial infarction.[8] Cardiovascular diseases are the leading cause of death all over the world (http://www.who.int/), and AMI is the leading cardiovascular disease leading to high mortality and morbidity.[9] Previous studies have shown that the death of patients with acute ST-segment elevation myocardial infarction mainly occurs in the first month after AMI,[10] and may be affected by age, severe arrhythmia, cardiogenic shock or heart failure,[10] and other factors. Although many factors, including baseline characteristics such as blood pressure, have been shown to be independent predictors of acute mortality in patients with AMI,[11] the relationship between the dynamic profile of DBP and the prognosis of patients with AMI is unclear.
As we all know, no previous studies have reported the connection between repeated measurement of DBP and in-hospital mortality in patients with AMI. This study aimed to investigate the difference in DBP between survivors and non-survivors and the relationship between baseline and early dynamic changes within the first 3 days after intensive care unit (ICU) admission in DBP and in-hospital prognosis among patients with AMI.
2. Methods
2.1. Study design
This was a retrospective cohort study and data were obtained from the Medical Information Mart for Intensive Care III database, which included > 40,000 patients admitted to the ICU treated in many sorts of ICUs (medical, surgical, coronary care, and neonatal) at the Beth Israel Deaconess Medical Center (Boston, MA, USA) from 2001 to 2012.[12,13] The Medical Information Mart for Intensive Care III database is an openly available critical care database. The requirement for written informed consent was waived owing to the retrospective nature of the study. One author obtained access and was responsible for data extraction (certification number: 6182750).
2.2. Study population
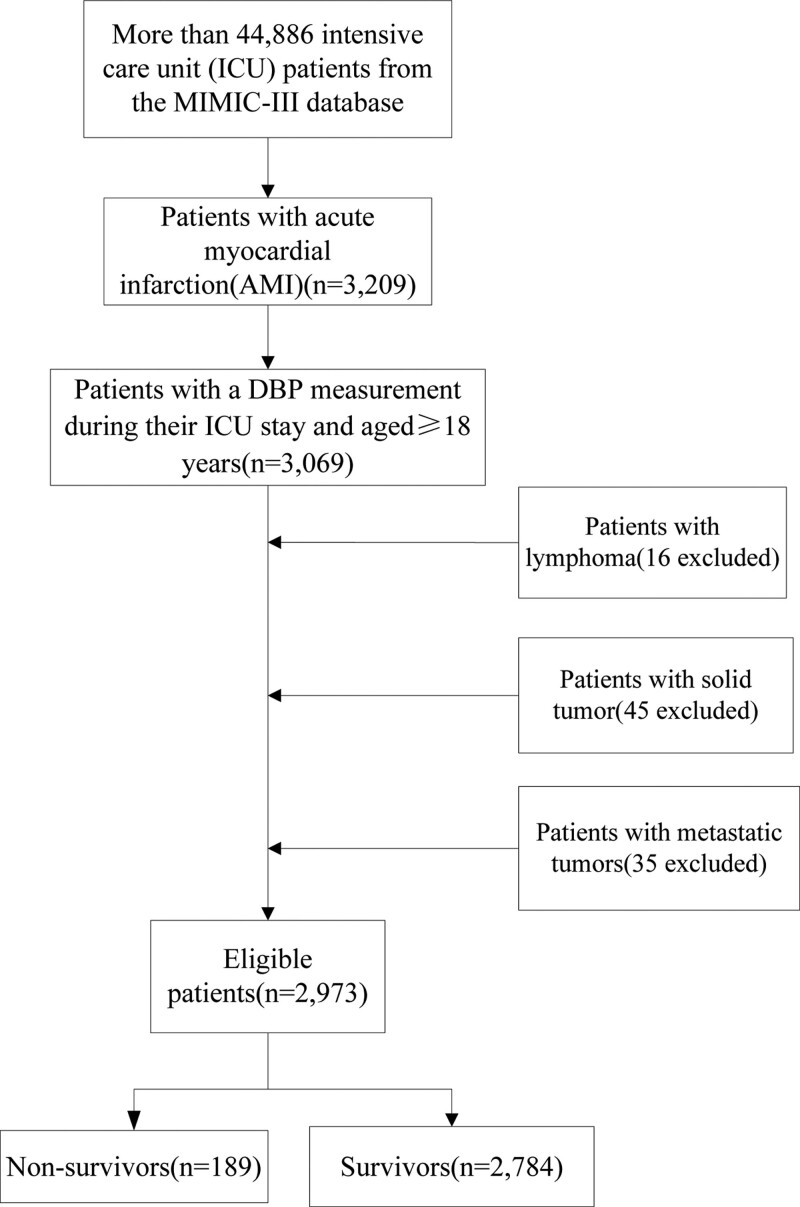
Study participants were persons aged ≥ 18 years who had at least one BP measurement at baseline and at least two BP measurements during the admission period. The participants were observed until death. We restricted the search to adult patients aged ≥ 18 years with AMI using International Classification of Diseases-9codes, which are collectively 140. In total, 3209 adult AMI admissions were identified. The exclusion criteria included: those without a DBP measurement during their hospital admission (n = 140); those with lymphoma (n = 16); those with solid tumors (n = 45); and those with metastatic tumors (n = 35).The study population consisted of 2973 patients with AMI (Fig. 1). Data within 24 hours of admission were extracted as baseline data. Changes of DBP data during hospitalization were also collected. This study complies with the relevant requirements of the “Declaration of Helsinki of the World Medical Association.” The study was approved by the Ethic Committee of the First People’s Hospital of Changde City (No. 2022-037-01). The requirement for written informed consent was waived owing to the retrospective nature of the study.
Figure 1.
Flow chart of patient selection.
2.3. Clinical endpoint measurements
In-hospital mortality was defined as all-cause mortality during the admission period. The patients were divided into 2 groups: survivors and non-survivors. Intergroup differences in the parameters measured at the hospital were evaluated.
2.4. Definition of AMI
Clinical evidence of acute myocardial injury that was evident from detection of a rise and/or fall in troponin T (cTnT) values with at least one value > 99th percentile upper reference limit, and at least one of the following symptoms of myocardial ischemia: new ischemic electrocardiograph changes, development of pathological Q wave imaging evidence of new loss of viable myocardium, or new regional wall motion abnormality in a pattern consistent with an ischemic etiology.[14,15]
2.5. Definition of DBP
Blood pressure is represented by 2 values. The first value SBP represents the pressure within the blood vessels when the heart contracts or beats. The second value DBP represents the pressure within the blood vessels when the heart is resting between beats. The differences in baseline DBP were the differences in the median DBP at admission. The differences on the 2nd to 4th day and 6th to 8th day were the differences in the median DBP on the 2nd to 4th day and 6th to 8th day. The change in DBP between the baseline and maximum values was calculated as the delta RDW value (ΔDBP). We computed ΔDBP as follows: ΔDBP = the maximum DBP reading during hospitalization – the DBP reading on admission. The mean of daily DBP measurements were 24 (25th–75th percentile,19–31) times, see Table S1, http://links.lww.com/MD/H530. DBP measurement frequency was about once an hour. Doctors may adjust the number of measurements based on changes in the patient’s condition. Two physicians checked all data.
2.6. Statistical analysis
First, we revealed the discrepancy in DBP between survivors and non-survivors stratified by sex, and the baseline characteristics of all hospitalized patients were compared using t test or Kruskal–Wallis rank sum test for measurement data, while Chi-squared tests were used for classification data. We also analyzed the age distribution character of the 189 non-survivors. We used Cox regression and smooth curve fitting to identify whether baseline DBP was independently related with hospital deaths in patients with AMI, and the results are shown by hazard ratios (HRs) and 95% confidence intervals (CIs).We chose the lower-DBP group as the reference group. Only age, sex, and ethnicity were adjusted in Model I. In Model II, we adjusted covariables including age; sex; ethnicity; length of ICU stay; levels of glucose, creatinine, and troponin T; white blood cell count; heart rate; SpO2; hypothyroidism; liver disease; diabetes with complications; hypertension; renal failure; chronic pulmonary disease; acquired immunodeficiency syndrome; AMI; coronary artery bypass grafting (CABG); percutaneous transluminal coronary angioplasty; and sequential organ failure assessment. The median value of DBP at baseline was 64 mm Hg, and the median value of ΔDBP was 19 mm Hg. Then, we applied a generalized additive mixed model (GAMM) to analyze the changes in DBP over time in the 2 groups. It is more appropriate to choose GAMM analysis for repeated measures data.[16]
Meanwhile, we performed a subgroup analysis to determine whether the effects of DBP varied between the subgroups.[17] Chronic obstructive pulmonary disease (COPD), congestive heart failure, cardiac arrhythmias, and hypothyroidism were also included.
Empower Stats version 2.17.8 (http://www.empowerstats.com/cn/) and R software (version 3.42) were selected for data analysis. P < 5% was considered statistically significant.
3. Results
3.1. Differences in DBP between survivors and non-survivors
It presents the differences in baseline DBP between survivors and deaths of different sexes duration of hospital stay, as well as on the 2nd to 4th day and 6th to 8th day. The results displayed that the mean value of DBP of deaths was obviously higher than that of survivors at the time points mentioned above, especially on days 6th to 8th day of all patients (female, P = .002; male, P = .028) (Table 1).
Table 1.
The difference in DBP between survivors and non-survivors stratified by sex.
| Time | DBP, mm Hg, median (25th–75th percentile) | ||
|---|---|---|---|
| Male | |||
| Survivors, n = 1757 | Non-survivors, n = 124 | P value | |
| On admission | 66.00(56.00–77.00) | 67.00(58.00–77.25) | .161 |
| On 2nd–4th day | 56.00(49.00–65.00) | 55.00(49.00–61.75) | .069 |
| On 6th–8th day | 55.00(47.00–65.00) | 59.00(54.00–62.00) | .028 |
| Female | |||
| Survivors, n = 1027 | Non-survivors, n = 65 | P value | |
| On admission | 61.00(52.00–72.00) | 63.00(53.00–72.00) | .734 |
| On 2nd–4th day | 52.00(45.00–59.00) | 54.00(46.00–64.00) | .005 |
| On 6th–8th day | 50.00(44.00–57.00) | 54.00(49.75–59.00) | .002 |
P value: as for the difference between survivors and non-survivors; Kruskal–Wallis test was applied for the variables with a skewed distribution.
DBP = diastolic blood pressure.
3.2. Baseline data of all AMI patients
The study population included 2784 survivors and 189 non-survivors. There were no significant differences in age, sex, ethnicity, and length of stay in the ICU between the 2 groups. The SBP, mean blood pressure, and Simplified Acute Physiology Score II were significantly lower in the surviving group than in the non-surviving group (P < .05).The pulse pressure, respiratory rate, and temperature of the surviving group were obviously higher than those of the death group (P < .05). There was no statistically significant difference in baseline DBP between the survival group and the death group in hospital stay. In addition, the surviving group had a higher part of subjects with hypothyroidism compared to the non-surviving group (7.94% vs 3.70%, P = .034). The proportion of patients with AMI was obviously different between the 2 groups, and the non-surviving group had more ST-segment elevation myocardial infarction patients. Patients in the non-surviving group had a higher rate of norepinephrine infusion than the survival group, but the difference was not statistically significant (14.29% vs 12.68%, P = .522) (Table 2).
Table 2.
Baseline characteristics and clinical outcomes of patients with AMI.
| Characteristic | Survivors (n = 2784) | Non-survivors (n = 189) | P value |
|---|---|---|---|
| Age, years | 70.59 (59.76–80.00) | 68.11 (57.62–78.94) | .138 |
| Sex, n(%) | .491 | ||
| Female | 1027 (36.89%) | 65 (34.39%) | |
| Male | 1757 (63.11%) | 124 (65.61%) | |
| Ethnicity, n(%) | .399 | ||
| White | 1832 (65.80%) | 119 (62.96%) | |
| Black | 107 (3.84%) | 5 (2.65%) | |
| Other | 845 (30.35%) | 65 (34.39%) | |
| LOS ICU, days | 2.18 (1.27–3.98) | 2.16 (1.16–4.38) | .336 |
| Glucose, mg/mL | 170.00 (137.00–225.00) | 175.00 (137.00–232.00) | .438 |
| Platelet, 109/L | 195.00 (153.00–245.00) | 197.00 (158.00–252.00) | .496 |
| Potassium, mmol/L | 3.70 (3.50–4.00) | 3.70 (3.50–4.00) | .640 |
| Serum sodium, mmol/L | 136.00 (134.00–139.00) | 137.00 (134.00–139.00) | .679 |
| WBC, 109/l | 12.60 (9.80–16.40) | 12.80 (10.35–16.00) | .840 |
| Creatinine, mEq/L | 1.10 (0.80-1.50) | 1.10 (0.90–1.30) | .065 |
| Troponin T, ng/mL | 1.06 (0.31–3.21) | 1.49 (0.38–5.48) | .126 |
| Heart rate, beats/min | 96.00 (85.00–109.00) | 97.00 (86.00–109.00) | .926 |
| SBP, mm Hg | 141.00 (128.00–156.00) | 154.00 (145.00–167.00) | <.001 |
| DBP,mm Hg | 64.00 (54.00–75.00) | 65.00 (56.00–77.00) | .185 |
| PP, mm Hg | 62.00 (49.00–76.00) | 54.00 (44.00–63.00) | <.001 |
| MBP, mm Hg | 99.00 (90.00–108.75) | 115.00 (108.00–127.00) | <.001 |
| Respiratory rate, beats/min | 26.00 (23.00–29.00) | 25.00 (22.00–28.00) | .005 |
| Temperature, °C | 37.33 (37.00–37.83) | 37.17 (36.89–37.56) | <.001 |
| SOFA | 3.00 (1.00–5.00) | 3.00 (1.00–5.00) | .326 |
| SAPSII | 32.00 (25.00–40.00) | 51.00 (40.00–60.00) | <.001 |
| Diabetes with complication, n(%) | .095 | ||
| No | 2539 (91.20%) | 179 (94.71%) | |
| Yes | 245 (8.80%) | 10 (5.29%) | |
| Hypothyroidism, n(%) | .034 | ||
| No | 2563 (92.06%) | 182 (96.30%) | |
| Yes | 221 (7.94%) | 7 (3.70%) | |
| Renal failure, n(%) | .076 | ||
| No | 2340 (84.05%) | 168 (88.89%) | |
| Yes | 444 (15.95%) | 21 (11.11%) | |
| AIDS, n(%) | .476 | ||
| No | 2777 (99.75%) | 188 (99.47%) | |
| Yes | 7 (0.25%) | 1 (0.53%) | |
| Liver disease, n(%) | .789 | ||
| No | 2691 (96.66%) | 182 (96.30%) | |
| Yes | 93 (3.34%) | 7 (3.70%) | |
| Chronic pulmonary diseases, n(%) | .097 | ||
| No | 2234 (80.24%) | 161 (85.19%) | |
| Yes | 550 (19.76%) | 28 (14.81%) | |
| PTCA, n(%) | .061 | ||
| No | 2021 (72.59%) | 149 (78.84%) | |
| Yes | 763 (27.41%) | 40 (21.16%) | |
| AMI group, n(%) | .002 | ||
| Non-STEMI | 1486 (53.38%) | 76 (40.21%) | |
| STEMI | 1173 (42.13%) | 106 (56.08%) | |
| Other AMI | 112 (4.02%) | 7 (3.70%) | |
| Post-AMI | 13 (0.47%) | 0 (0.00%) | |
| Norepinephrine infusion | .522 | ||
| No | 2431 (87.32%) | 162 (85.71%) | |
| Yes | 353 (12.68%) | 27 (14.29%) |
AMI = acute myocardial infarction, DBP = diastolic blood pressure, LOS ICU = length of stay intensive care unit, MBP = mean blood pressure, PP = pulse pressure, PTCA = percutaneous transluminal coronary angioplasty, SAPSII = simplified acute physiology score II, SBP = systolic blood pressure, SOFA = sequential organ failure assessment, STEMI = ST-segment elevation myocardial infarction, WBC = white blood cell.
3.3. Age distribution of 189 non-survivors
The mean age of the death group was 64.7 ± 13.1 years, which was obviously higher than that of the surviving group (48.2 ± 14.4 years, P < .001). The age of each non-survivor, as well as the change in DBP from admission to death, was of great significance to the scientific community. Therefore, we illustrated the age distribution of 189 deaths for further study (Fig. 2).
Figure 2.
Age distribution of 189 non-survivors.
3.4. Association between baseline DBP and in-hospital mortality
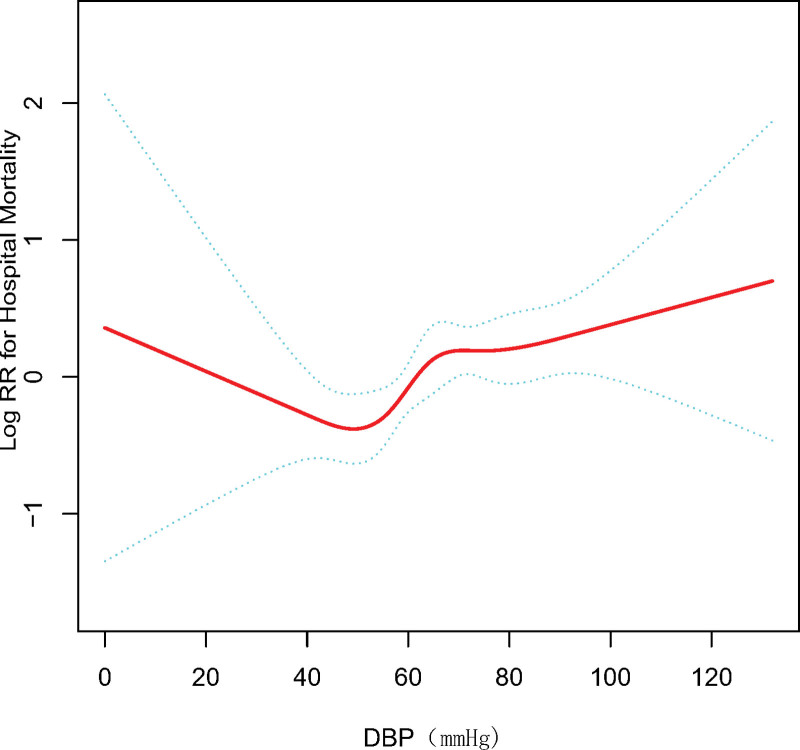
Smooth curve fitting was used to represent the relationship between baseline DBP and in-hospital mortality risk, which is presented in Figure 3. In general, plotting in-hospital mortality against baseline DBP ranges showed a U-shaped relationship.
Figure 3.
Smooth curve fit of the association between the baseline DBP and risk of in-hospital death. The resulting data show the risk of death on the y-axis and the DBP (continuous variable) on the X-axis. The red line is the dose-response curve between DBP and in-hospital mortality, and the two blue lines are 95%CIs. After adjusting for age, sex, and race, a nonlinear relationship between DBP and short-term mortality risk was observed. CI = confidence interval, DBP = diastolic blood pressure.
We estimated this discovery using Cox multivariable regression analysis. As a successive variable, after adjusting for the clinical confounders listed, an SD increase in baseline DBP was related with a 28% increased risk of in-hospital death after adjusting for the listed clinical confounders (HR, 1.28; 95%CI, 1.09–1.52; P = .003). When DBP was assessed in three groups, we found that patients with high baseline DBP (DBP ≥ 90 mm Hg) also had significantly higher risk of in-hospital mortality (HR, 1.97; 95%CI, 1.07–3.63; P = .0291) than patients in the low baseline DBP group (DBP < 60 mm Hg) in the adjusted model. There was a similar result for ΔDBP (Table 3).
Table 3.
Multivariable Cox regression analysis of baseline DBP for in-hospital mortality.
| DBP, mm Hg | Non-adjusted | Adjust I | Adjust II | |||
|---|---|---|---|---|---|---|
| HR(95%CIs) | P value | HR(95%CIs) | P value | HR(95%CIs) | P value | |
| DBP | 1.02 (1.01,1.02) | .0005 | 1.01 (1.00,1.02) | .0040 | 1.02 (1.01,1.03) | .0030 |
| DBP per SD increase | 1.27 (1.11,1.45) | .0005 | 1.23 (1.07,1.41) | .0040 | 1.28 (1.09,1.52) | .0030 |
| DBP group | ||||||
| <60 | 1.0 | 1.0 | 1.0 | |||
| >=60, <90 | 1.50 (1.10,2.05) | .0106 | 1.43 (1.05,1.97) | .0252 | 1.60 (1.11,2.29) | .0107 |
| >=90 | 2.50 (1.51,4.14) | .0004 | 2.23 (1.33,3.74) | .0024 | 1.97 (1.07,3.63) | .0291 |
| ΔDBP | 1.03 (1.02,1.03) | <.0001 | 1.03 (1.02,1.03) | <.0001 | 1.03 (1.02,1.04) | <.0001 |
| ΔDBP group | ||||||
| <19 | 1.0 | 1.0 | 1.0 | |||
| >=19 | 3.95 (2.71,5.75) | <.0001 | 4.19 (2.87,6.11) | <.0001 | 4.06 (2.61,6.32) | <.0001 |
Models were derived from Cox proportional hazards regression models.
Non-adjusted model adjust for: None.
Adjust I model adjust for: age; gender; ethnicity.
Adjust II model adjust for: age; gender; ethnicity; length of stay ICU; glucose; creatinine; troponin T; WBC; heart rate; SpO2; hypothyroidism; liver disease; diabetes with complications; hypertension; renal failure; chronic pulmonary; AIDS; AMI group; CABG; PTCA; SOFA.
DBP = diastolic blood pressure.
3.5. Relationship between early changes in DBP and in-hospital death in AMI patients
We present the link between early changes in DBP and death in 2973 patients with AMI. Based on GAMM, the DBP in the surviving group was importantly higher than that in the death group. Moreover, the difference between the 2 groups displayed a rising trend within 3 days after ICU admission, which decreased by a daily average of 3.7 mm Hg. After adjustment for various variables, the drop value remained at 3.7 mm Hg, indicating that the results were stable (Table 4 and Fig. 4).
Table 4.
Relationship between early (1–3 days) changes in DBP (mm Hg) and in-hospital death in AMI patients derived from a generalized additive mixed model (GAMM).
| Outcome | Model I | Model II | ||
|---|---|---|---|---|
| β(95%CI) | P value | β(95%CI) | P value | |
| Intercept | 73.1465(67.9625,78.3306) | <.0001 | 70.1234(64.4388,75.8080) | <.0001 |
| Day | –0.6450(–0.9487, –0.3412) | <.0001 | –0.6590(–0.9633, –0.3547) | <.0001 |
| In-hospital mortality | 9.4339(4.7434, 14.1244) | <.0001 | 9.6874(4.9830,14.3918) | <.0001 |
| Day × in-hospital Mortality | –3.6722(–5.2105, –2.1338) | <.0001 | –3.7281(–5.2667, –2.1895) | <.0001 |
Intercept, the mean of DBP at day = 0 and in-hospital mortality = 0; Day, the mean of the decreasing of DBP at death = 0 over time (daily); In-hospital mortality, the difference of DBP at day = 0 between the group of in-hospital mortality = 1 and the group of in-hospital mortality = 0; Day × death, the average decreasing in DBP daily under the condition of the group of in-hospital mortality = 1 compared with the group of in-hospital mortality = 0; Model I: adjusted for gender; age, ethnicity; Model II: adjusted for gender; age, ethnicity, length of stay ICU, diabetes with complication, hypertension, AMI group, CABG, PTCA.
AMI = acute myocardial infarction, DBP = diastolic blood pressure.
Figure 4.
Dynamic changes in DBP of patients with AMI. Generalized additive model results suggest a nonlinear relationship between changes in DBP and mortality. Timeline plot shows DBP in patients with AMI (2784 non-survivors and 189 survivors) in the ICU. The solid line shows the DBP of the survivors(0), while the dotted line shows the DBP of the non-survivors(1). P < .05 for non-survivors versus survivors. AMI = acute myocardial infarction, DBP = diastolic blood pressure, ICU = intensive care unit.
3.6. Subgroup analyses
The relationship between baseline DBP and in-hospital mortality was similar in some strata. Patients in the subgroups did not differ in terms of the risk of in-hospital mortality according to DBP. Significant differences were observed in sex, ethnicity, heart rate, COPD, hypothyroidism, and CABG subgroups. Male and white patients had a higher risk of in-hospital mortality among patients with AMI. Meanwhile, in patients with high heart rates only, without COPD, hypothyroidism, or CABG, 3 were higher mortality rates, whereas if a patient had COPD, hypothyroidism, or CABG, DBP had little effect on in-hospital mortality (Table S2, http://links.lww.com/MD/H531).
4. Discussion
In this study, we cover a retrospective cohort study that researched the variation in DBP among deaths and survivors of AMI. We demonstrated that there was a nonlinear association between baseline DBP and in-hospital death among patients with AMI, with increased risk observed at both low and high BP values.[18] Moreover, the DBP that was dynamic during the first 3 days of ICU stay also seems to be a meaningful prognostic factor in patients with AMI. We discovered that DBP in the deaths decreased within the first 3 days after ICU stay, but the changes in DBP of the surviving group were smaller and slower. This trend persisted despite adjustment for potential confounders. Early reduction in DBP in patients with AMI may be associated with poor prognosis. This present study further expanded the effect of changes in DBP. Because AMI is complicated by poor cardiovascular outcomes worldwide, finding a simple and useful prognostic parameter to predict short- and long-term mortality is very important for patients with AMI.[19,20] BP has been shown to be an important determinant of adverse cardiovascular events in patients.[18] Data from a small retrospective study of patients with cardiogenic shock concluded that, of several hemodynamic parameters, only DPB (particularly DPB < 40 mm Hg in the 24 hours prior to ICU admission) was independently associated with 28-day mortality in patients with cardiogenic shock.[21] Then, in another study of 3943 patients with AMI treated at a tertiary hospital, DBP < 60 mm Hg was associated with a poorer outcome.[22] Wong et al analyzed the prognostic value of the last blood pressure value before discharge in 1053 patients hospitalized with acute coronary syndrome.[23] At 5 years of follow-up, an inverse J-shaped relationship between DPB and mortality was observed, even after accounting for inpatient revascularization, cardiac medications, and risk scores. However, no additional benefit was observed when DBP was greater than 90 mm Hg. The significance of DBP is exemplified in the study of Rankin et al,[24] who found that in patients with coronary artery disease, DBP < 70 mm Hg was associated with more patients with near-zero left anterior descending coronary blood flow. Our results of our study are in line with those of the previous studies.[24,25] However, no study has explored the association between an early change in DBP and in-hospital mortality in patients with AMI, which may embody the comprehensive dynamic changes of the patient’s condition and disease evolution.
Therefore, we first used the GAMM model to explore the temporal changes in DBP in and its association with in-hospital mortality in patients in AMI. As a first step, our study displayed that DBP varied over time in patients with AMI during the first 3 days of ICU stay. Next, we compared the trends of DBP over time in the survivor and non-survivor groups. In the last step, the GAMM model was used to explore the connection between the early changes of DBP and short-term prognosis in patients with AMI. We found that the diversity between the 2 groups increased within 3 days of ICU admission.
In this study, we found that the baseline DBP value and change in DBP could predict short-term cardiovascular mortality in patients with AMI.[20] Low DPB can lead to reduced diastolic coronary perfusion during the cardiac cycle, especially in patients with coronary artery disease. Therefore, low DBP can cause significant myocardial ischemia and myocardial injury.[20,25,26] The coronary circulation has relatively high peripheral resistance.[20] Therefore, coronary circulation is more susceptible to decreased perfusion pressure, especially in the presence of obstructive coronary artery disease.[27] In our study, we provide evidence of the existence of a “U-shaped curve phenomenon,” which is similar to the J-shaped curve of SBP and DBP, for secondary level of prevention of adverse events after AMI. The reasons why our results are different from those of other studies include some methodological faults, the observational nature of the study, and several confounding factors, such as patient age, ethnicity, underlying comorbidities, and varying prevalence of cardiovascular risk factors.
Our results have several potential clinical implications. Because our data were collected from comprehensive electronic medical records[28] rather than a billing and administrative database, we used rigorously identified covariates and outcomes. Repeated measures of various DBP provide opportunities for us to enhance the understanding of many important clinical aspects of AMI, including patterns of disease progression and the degree of heterogeneity in disease manifestations across patients. We hold the opinion that repeat measurement of DBP may be helpful in the management of patients with AMI, leading to earlier, more effective measures. Moreover, DBP can be got by physical examination at no additional cost and is more convenient for clinical use. Early variations in DBP may be a sensitive index of disease severity. We find that frequent measurements of DBP may be helpful for the treatment and evaluation of AMI, bringing more effective measures.
Our study has certain limitations. First, data were retrospectively obtained from populated clinical databases. Additionally, we did not include data on antihypertensive treatments and measures such as diet modification. Third, while adjusting for multiple potential confounding variables, residual confounding issues may not be fully resolved. Fourth, as a single-center study, caution should be exercised in interpreting findings from other populations and regions.
5. Conclusions
In conclusion, DBP significantly contributes to in-hospital mortality among patients with AMI. It can also serve as an easy and reproducible clinical index. Changes in DBP occur earlier and are easier to identify than clinical symptoms; therefore, clinicians should keep a watchful eye on the changes in individualized therapeutic interventions and treatment procedures.
Author contributions
SH acquired data, performed the statistical analyses, interpreted data, and drafted the manuscript. NG, XD, and QZ acquired data and revised the manuscript. LG, YL, and LL interpreted data and revised the manuscript. All authors have read and approved the manuscript.
Data curation: Sulan Huang.
Formal analysis: Sulan Huang.
Writing – original draft: Sulan Huang, Liangqing Ge.
Writing – review & editing: Sulan Huang, Yanlan Luo, Li Liang, Ning Guo, Xiangjie Duan, Quan Zhou, Liangqing Ge.
Acknowledgments
The authors thank all the researchers who created and managed the MIMIC III database.
Supplementary Material
Abbreviations:
- AMI =
- acute myocardial infarction
- CABG =
- coronary artery bypass grafting
- CI =
- confidence interval
- COPD =
- chronic obstructive pulmonary disease
- DBP =
- diastolic blood pressure
- GAMM =
- generalized additive mixed model
- HR =
- hazard ratio
- ICU =
- intensive care unit
- SBP =
- systolic blood pressure
SH, YL, LL, and NG contributed equally to this work.
This current project was supported by Technical Research and Development Funding Program of Changde Bureau Science and Technology office (Nos. 2019S191 and 2020S025), the Natural Science Foundation of Hunan Province (No. 2022JJ30086), and Scientific research Project of Hunan Provincial Health Commission (Nos. 20200224, 202103010091, and 202203012734).
The study was approved by the Ethic Committee of the First People’s Hospital of Changde City (No. 2022-037-01). The formulation of this research protocol complied with the relevant requirements of the Declaration of Helsinki of the World Medical Association. The requirement for written informed consent was waived owing to the retrospective nature of the study.
The clinical data used to support the findings of this study were provided by the Intensive Care Database III version 1.4 (MIMIC-III v.1.4). Although the database is public and free, researchers must complete a web-based course from the National Institutes of Health to protect human research participants from applying for permission to access the database. The MIMIC-III database used in this research is available for access, in part or in total, by relevant parties subject to their abiding by usage policies.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
How to cite this article: Huang S, Luo Y, Liang L, Guo N, Duan X, Zhou Q, Ge L. The baseline and repeated measurements of DBP to assess in-hospital mortality risk among critically ill patients with acute myocardial infarction: A retrospective cohort study. Medicine 2022;101:40(e30980).
Contributor Information
Sulan Huang, Email: huangsulanjiayou3@126.com.
Yanlan Luo, Email: 707041909@qq.com.
Li Liang, Email: 35520288@qq.com.
Ning Guo, Email: 1920298@qq.com.
Xiangjie Duan, Email: 739838207@qq.com.
Quan Zhou, Email: zhouquan402@163.com.
References
- [1].Rosendorff C, Lackland DT, Allison M, et al. Treatment of hypertension in patients with coronary artery disease: a scientific statement from the American heart association, American college of cardiology, and American society of hypertension. J Am Coll Cardiol. 2015;65:1998–2038. [DOI] [PubMed] [Google Scholar]
- [2].Park H, Hong YJ, Cho JY, et al. Blood pressure targets and clinical outcomes in patients with acute myocardial infarction. Korean Circ J. 2017;47:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kannel WB. Historic perspectives on the relative contributions of diastolic and systolic blood pressure elevation to cardiovascular risk profile. Am Heart J. 1999;138:205–10. [DOI] [PubMed] [Google Scholar]
- [4].Flint AC, Conell C, Ren X, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243–51. [DOI] [PubMed] [Google Scholar]
- [5].Kannel WB, Dawber TR, McGee DL. Perspectives on systolic hypertension. the framingham study. Circulation. 1980;61:1179–82. [DOI] [PubMed] [Google Scholar]
- [6].Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with Type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140:1661–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Go AS, Mozaffarian D, Roger VL, et al. American heart association statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics-2014 update: a report from the American heart association. Circulation. 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang R, Mei B, Liao X, et al. Determination of risk factors affecting the in-hospital prognosis of patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention. BMC Cardiovasc Disord. 2017;17:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pedersen F, Butrymovich V, Kelbæk H, et al. Short-and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64:2101–8. [DOI] [PubMed] [Google Scholar]
- [11].Mello BH, Oliveira GB, Ramos RF, et al. Validation of the Killip-Kimball classification and late mortality after acute myocardial infarction. Arq Bras Cardiol. 2014;103:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnson AEW, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Johnson AE, Stone DJ, Celi LA, Pollard TJ. The MIMIC code repository: enabling reproducibility in critical care research. J Am Med Inform Assoc. 2018;25:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thygesen K, Alpert JS, Jaffe AS, et al., Executive group on behalf of the Joint European society of cardiology (ESC)/American college of cardiology (ACC)/American heart association (AHA)/World heart federation (WHF) Task Force for the universal definition of myocardial infarction. Fourth universal definition of myocardial infarction. Circulation. 2018;138:e618–51.30571511 [Google Scholar]
- [15].Schiele F, Aktaa S, Rossello X, et al. Update of the quality indicators for acute myocardial infarction: a position paper of the association for acute cardiovascular care: the study group for quality indicators from the ACVC and the NSTE-ACS guideline group. Eur Heart J Acute Cardiovasc Care. 2021;10:224–33. [DOI] [PubMed] [Google Scholar]
- [16].Zhao X, Wang K, Zuo P, et al. Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients – indications for predictive, preventive, and personalized medical approach. EPMA J. 2020;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li J, Yang X, Ma J, et al. Relationship of red blood cell distribution width with cancer mortality in hospital. Biomed Res Int. 2018;2018:8914617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hu AM, Hai C, Wang HB, et al. Associations between elevated systolic blood pressure and outcomes in critically ill patients: a retrospective cohort study and propensity analysis. Shock. 2021;56:557–63. [DOI] [PubMed] [Google Scholar]
- [19].Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (dalys) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- [20].Hsu P-C, Lee W-H, Chiu C-A, et al. Usefulness of ankle-brachial index calculated using diastolic blood pressure for prediction of mortality in patients withacute myocardial infarction. J Clin Hypertens (Greenwich). 2020;22:2044–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rigamonti F, Graf G, Merlani P, et al. The short-term prognosis of cardiogenic shock can be determined using hemodynamic variables: a retrospective cohort study*. Crit Care Med. 2013;41:2484–91. [DOI] [PubMed] [Google Scholar]
- [22].Roth D, Van Tulder R, Heidinger B, et al. Admission blood pressure and 1-year mortality in acute myocardial infarction. Int J Clin Pract. 2015;69:812–9. [DOI] [PubMed] [Google Scholar]
- [23].Wong CK, Herbison P, Tang EW. Relation between blood pressure at hospital discharge after an acute coronary syndrome and long-term survival. Am J Cardiol. 2008;101:1239–41. [DOI] [PubMed] [Google Scholar]
- [24].Rabkin SW, Shiekh IA, Wood DA. The impact of left ventricular mass diastolic blood pressure targets for patients with coronary artery disease. Am J Hypertens. 2016;29:1085–93. [DOI] [PubMed] [Google Scholar]
- [25].Ikonomidis I, Makavos G, Lekakis J. Arterial stiffness and coronary artery disease. Curr Opin Cardiol. 2015;30:422–31. [DOI] [PubMed] [Google Scholar]
- [26].McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol. 2016;68:1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tzeng YC, Ainslie PN. Blood pressure regulation ix: cerebral auto regulation under blood pressure challenges. Eur J Appl Physiol. 2014;114:545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Flint AC, Conell C, Klingman JG, et al. Impact of increased early statin administration on ischemic stroke outcomes: a multicenter electronic medical record intervention. J Am Heart Assoc. 2016;5:e003413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.