Background:
This study was performed to update the current evidence and evaluate the effects of robot-assisted rehabilitation (RAR) in comparison with conventional rehabilitation (CR) in patients following total knee (TKR) or hip replacements (THR).
Methods:
PubMed Central, OVID Medline, Cochrane Collaboration Library, and EMBASE for a comprehensive search for all relevant studies, from database inception to July 2022. The following inclusion criteria were used to determine eligibility for studies: randomized and matched controlled trials recruiting men and women who underwent TKR and THR; and studies examining the effect of RAR on outcome measures of physical function and pain.
Results:
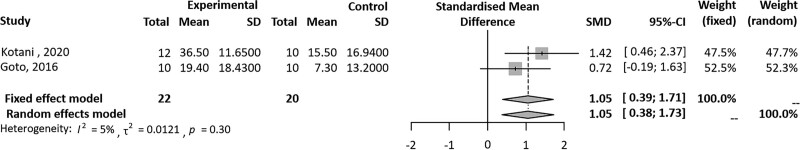
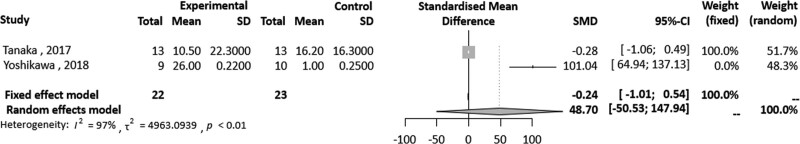
A total of 9 studies (230 patients) were included in this review and 4 were included in the meta-analysis. The meta-analysis of 2 studies showed that Hybrid Assistive Limb (HAL) training for 5 days, significantly improved pain measured on a visual analogue scale, compared to CR in patients following TKR (SMD = 1.05, 95% confidence interval [Cl] 0.39–1.71). Heterogeneity for I2 value was lower than moderate (tau^2 = 0.0121; I2 = 5%; P = .30). There were 2 studies that assessed self-selected walking speed. The meta-analysis of these studies showed that HAL training was significantly superior to CR in patients following TKR (SMD = 48.70, 95% Cl -50.53 to 147.94) at 2 months. A high heterogeneity was detected (P < .01; I2 = 97%).
Conclusion:
The result of this systematic review and meta-analysis suggest that RAR may be an effective treatment in TKR and THR patients. However, high-quality studies are needed to verify the long-term effect on their recovery.
Keywords: meta-analysis, rehabilitation, robot, systematic review, total hip replacement, total knee replacement
1. Introduction
Total knee replacement (TKR) and total hip replacements (THR) are common surgical procedures for patients suffering from end-stage arthritis, with an accelerating number of TKR and THR performed in recent years.[1,2] These procedures are reportedly effective in alleviating pain, enabling functional recovery, and improving quality of life for patients.[2,3] Considering the economic cost of these procedures, improving the efficiency of management models for arthroplasty patients is a matter of considerable policy interest. Rehabilitation, as a multidisciplinary approach, can perform the majority of required management for TKR and THR patients.[4] However, previous studies reported various barrier to using rehabilitation services, including structural, personal, financial, and social, which limit access.[5]
Although traditional postoperative rehabilitation for TKR and THR patients improves patient’s gait effectively, it is time- and energy-consuming. It is also difficult to maintain consistency of training intensity among different patients. Rehabilitation using innovative technologies – such as eHealth, telemedicine, wearables, virtual reality, and online education tools – are being applied on a trial basis in clinical trials.[6] Among these technologies, a type of wearable consisting of a robotic support structure is being tested. Robotic devices are suited to make intensive, task-oriented motor training for moving the patient’s limbs, supervised by physical therapists, with a promising approach to rehabilitation and reducing the burden on caregivers.[7,8] Lower limbs robot-assisted training has become of particularly interest as it can effectively resolve the problems traditional rehabilitation currently faces. Most studies using robot-assisted training have focused on central nervous system diseases, such as stroke,[9] spinal cord injury,[10] and cerebral palsy.[11] The study by Carda et al[12] showed a progression of the improvements up to 6 months in patients with early-stage Parkinson’s disease through robotic gait training. Like this, development of robotic technologies to restore the functionality of impaired neurological circuits has extended to the neural rehabilitation field for restoration of sensorimotor control and functions.[13] Recently, a growing body of literature supports the use of robotic devices for improving the pain and function of patients following TKR and THR.[14–22] Several forms of robotic devices are commercially available at this time, and are typically classified as either exoskeletons or end-effector robots, depending on the motion they apply.[23] In all of the studies in which patients undergoing TKR or THR were followed, exoskeletons not only moved joints such as knees and hips, in coordination with phases of gait but also increased the range of motion of joints. Robot-assisted rehabilitation (RAR) is used either in combination with conventional rehabilitation (CR) or alone and is believed to have satisfied the necessary external requirements for improving motor function.[14–22] In the meantime, the quantification of gait training intensity is needed to allow the standardization of gait parameters, because the applied robots are merely tools in the hands of physical therapists and therapists themselves, ultimately provide unique benefits to the treatment protocols in patients following TKR and THR. Indeed, outcome measurements for evaluating the functional recovery of TKR and THR were all different.[14–22] Consequently, the quantification and standardization of RAR through review of existing studies is clearly needed.
A small number of systematic reviews of rehabilitation using any technology-based interventions, have been conducted using a variety of different technologies such as education, monitoring or treatment delivering via telecommunication technologies, internet, software or virtual reality devices.[24–27] However, currently no study has systematically analyzed the effects of RAR for TKR and THR. Therefore, the aim of the present systematic review and meta-analysis is to update the current evidence and evaluate the effectiveness of RAR in comparison with CR in patients following TKR and THR.
2. Methods
2.1. Design and registration
This systematic review and meta-analysis was done according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[28] It has been registered in the PROSPERO database, an international prospective register of systematic reviews in health and social care (National Institute for Health Research, CRD 42020218723).
2.2. Literature search
We used PubMed Central, OVID Medline, Cochrane Collaboration Library, and EMBASE for a comprehensive search for all relevant studies, from database inception to July 2022. We used the following search terms: ((rehabilitation) AND hybrid) AND total hip arthroplasty)) OR (((rehabilitation) AND total knee arthroplasty) AND hybrid)) OR (((hybrid) AND total knee arthroplasty) AND robot rehabilitation)) OR ((hybrid) AND robot rehabilitation) (Supplemental File 1, http://links.lww.com/MD/H557). We also did a manual search of possibly related references. Two researchers (CH and JI) reviewed the titles, abstracts, and full texts of all potentially relevant studies independently, as recommended by the Cochrane Collaboration.[29] Any disagreement was resolved by the third reviewer. We reviewed full-texts of the screened articles and then selected eligible articles. The reviewers were not blinded to authors or institutions.
2.3. Inclusion and exclusion criteria
Studies were selected by means of the following criteria: study design: prospective comparative studies, randomized controlled studies (RCTs); study population: patients who underwent TKA or THR; intervention: RAR with CR, and outcomes: demographic factors, clinical results during postoperative follow-up. Studies, which did not meet above criteria, posters, letters, and review articles were excluded.
2.4. Assessment of risk of bias
Two authors (CH and JI) independently evaluated the risk of bias of each study. In RCTs and crossover study designs, selection, performance, detection, attrition, and reporting biases were assessed using the Cochrane Risk-of-Bias Tool.[30] For assessing the risk of bias in non-RCTs, we used the Newcastle-Ottawa scale for case-control studies.[31] Studies were considered to have a low risk of bias when the quality assessment was 50% of, or above the checklist criteria. A funnel plot was used for estimating and adjusting publication bias.[32]
2.5. Study selection and data extraction
Data extraction was independently performed by 2 investigators (C.H. and J.I.) using a structured table that included the study characteristics (author, date of publication, study design, and country, participants characteristics (sample size, mean age, and numbers of male and female participants), diagnosis and follow-up period, specific interventions; rehabilitation using robot or Hybrid Assistive Limb (HAL), and primary outcome measures. The primary outcomes pooled in this analysis included visual analogue scale (VAS) score and self-selected walking speed (SWS).
2.6. Data synthesis and statistical analysis
In the studies by Kotani et al[15] and Goto et al,[16] the change in VAS was evaluated using meta-analysis (Table 1). We aggregated the data obtained from 2 studies reporting the effects of HAL in comparison to CR in the form of mean (standard deviation) to produce an overall mean effect (standardized mean effect [SMD]). VAS score was summarized as mean plus/minus standard deviation, when provided or calculated. Also the change in SWS was evaluated using meta-analysis in the studies by Yoshikawa et al[14] and Tanaka et al.[19] These 2 studies had different units, m/s[14] and rate of change,[19] so we unified them to rate of change. We used a pooled meta-analysis to analyze the dislocation rate. For dichotomous results, we calculated the risk ratio (RR) and 95% confidence interval (CI). For continuous outcomes, SMD and 95% CI were calculated.[33,34] The heterogeneity of each study was estimated using Higgins I2 statistics and the Chi square test.[35] Studies with a P < .10 and I2 > 50% were considered as having heterogeneity. We employed a fixed-effect model in studies without heterogeneity and a random-effects model in studies with heterogeneity. The trim and fill method was used to estimate and adjust the number and outcomes of missing studies in the meta-analysis.[36] Statistical analysis was done using R software 3.02 (R Foundation for Statistical Computing, Vienna, Austria) and the meaningful value was set to P < .05.
We had no choice but to descriptive analysis, as most of studies had various outcomes that did not overlap. We not only analyzed the secondary outcome measures for studies following TKR and THR separately, after categorizing according to the interventions used in included studies, but also classified and re-analyzed these secondary outcome measures according to the purpose of the assessment. The secondary outcome measures could be divided into 3 groups. Firstly, outcome measures related to walking ability were as follows: maximum walking speed (MWS), step length in the SWS and MWS, cadence at SWS and MWS, cadence, single/double support time, velocity, stride length, anterior/posterior/lateral variability, and joint moment. Secondly, extension lag, quadriceps strength during knee extension, hamstring muscle strength during knee flexion, knee range of motion (ROM), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), knee kinesthesia grade, knee proprioception grade, functional ambulation category, Berg balance score, 10-m sitting-standing time, 6-minute walking distance, modified functional reach test, manual muscle test, 10 meter walk test, timed up and go test, strength (hip flexion/extension), and hip ROM were used in the assessments related to knee or hip function. Lastly, Harris hip score, hospital for special surgery, 36-Item Short Form Health Survey score were used in the assessments for clinical evaluation.
Seven studies[14–16,19–22] of 7 investigated rehabilitation with HAL. Another study[18] showed the efficacy of the robot-assisted walking training (AVATAR-M; Shanghai Zhanghe Corporation, China) following TKR, and the remaining study[17] was performed by using trunk control rehabilitation robot training (TCRRT, 3DBT-33, Man and tel, Gumi, Korea) following THR.
In studies using HAL, 6[14–16,19,21,22] included patients having post-TKR rehabilitation and one[20] included patients undergoing post-THR rehabilitation. First of all, among 6[14–16,19,21,22] studies, the outcome measures were analyzed descriptively (with exception of VAS) to evaluate the knee function in the studies by Kotani et al,[15] Goto et al.[16] Improvement in active and passive knee ROM and strength of knee flexion were assessed for the evaluation of function in the study by Kotani et al.[15] Goto et al[16] reported the effectiveness of HAL assisted rehabilitation through the extension lag. VAS and extension lag were analyzed descriptively as the specific data of VAS was not reported in the study by Yoshioka et al.[21] In the other 2 studies, Yoshikawa et al[14] used the following outcome measures: muscle strength of knee extension and flexion, the knee ROM, and WOMAC to evaluate the knee function at the pre-surgery, and at weeks 1, 2, 3, 4, and 8 after training. As well, MWS, step length in the SWS and MWS, cadence at SWS and MWS were used to assess the walking ability. The quadriceps strength for knee function and NRS were analyzed prior to surgery and on postoperative weeks 1, 2, and 3 in remaining study by Tanaka colleagues.[19] Fukaya et al[22] performed the gait analysis to verify the effect of HAL on the kinematic and kinetic variables of lower limb joint function at 5 weeks after TKA. In studies using HAL, one[20] included patients undergoing post-THR rehabilitation. Gait analysis was performed before and after surgery, and comparisons were made between the 2 groups. This study used several outcome measures. They consisted of waling ability parameters such as single support time, double support time, cadence, velocity, stride length, and anterior/posterior/lateral variability as well as hip function through hip and knee ROM and clinical assessments such as Harris hip score, hospital for special surgery, and 36-Item Short Form Health Survey score.
The study by Li et al[18] were also analyzed descriptively, because the intervention program and outcome measures were completely different when compared to other studies which were conducted in the patients following TKR. Li colleagues[18] used AVATAR-M that could help the patients take up early rehabilitation, while the other studies were performed in patients following TKR through HAL assisted training. In addition to the hospital for special surgery score for clinical evaluation, assessment related to knee function such as knee kinesthesia grade, knee proprioception grade, functional ambulation category, Berg balance score, 10-m sitting-standing time, and 6-minute walking distance were examined, and the effects of robot-assisted walking training were compared with those of CR.
Lastly, one study[17] was performed by using TCRRT following THR. The modified functional reach test, manual muscle test of lower extremity, 10 meter walk test, timed up and go test, and strength of hip and knee as well as VAS were analyzed to evaluate of function and pain.
3. Results
3.1. Identification of eligible studies
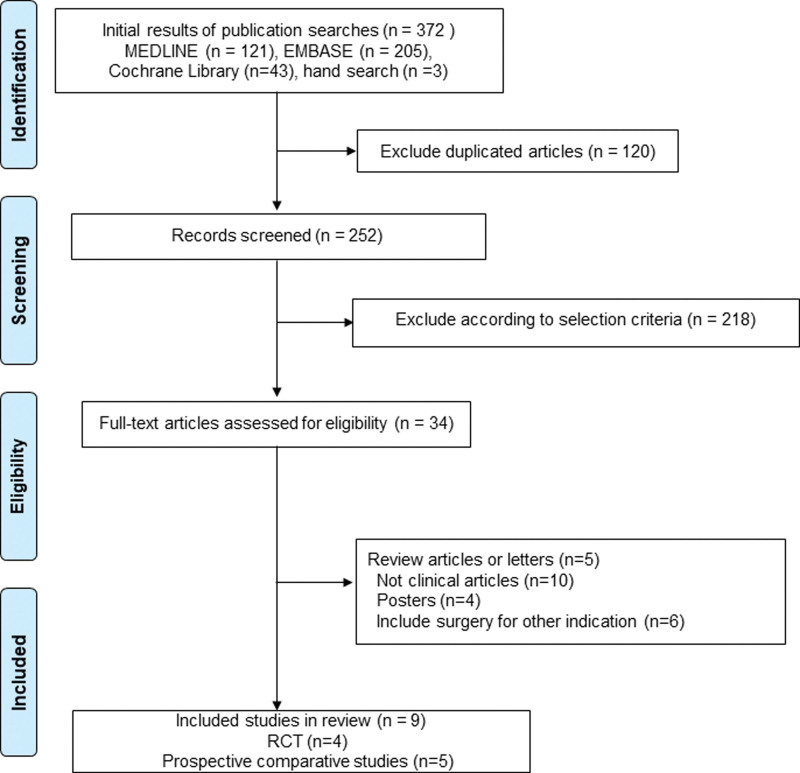
The initial search identified 372 articles from the selected databases. After screening titles and abstracts for duplicates, unrelated articles, and case reports, 338 articles were excluded. The remaining 34 studies underwent full-text review and 25 studies among them were excluded. This left 9 studies, which were included in the final analysis (Fig. 1). There were 4 RCTs and 5 prospective comparative studies.
Figure 1.
PRISMA flow of information through the different phases of meta-analysis. PRISMA = preferred reporting items for systematic reviews and meta-analyses.
3.2. Study characteristics
Table 1 summarizes the characteristics of study participants. These 9 studies included 230 patients (116 in the experimental group and 114 in the control group). Among them, 4 studies[15,17–19] were RCTs with 133 patients and the other 5 were prospective comparative studies with 97 patients.
Table 1.
Characteristics of the included studies.
| Study | Participant characteristics | Intervention program | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Reference/country | N (e.g./CG) | Female, n (%) (e.g./CG) | Disease | Mean age ± SD (yr) (e.g./CG) | EG | CG | Length of intervention | Outcomes | Time point |
| Total knee replacement | |||||||||
| Yoshikawa[14] Japan Kotani[15] Japan Goto[16] Japan Li[18] China Tanaka[19] Japan Yoshioka[21] Japan Fukaya[22] Japan |
9/10 | 8 (88.9)/8 (80) | OA and RA | 61.2 ± 8.9/59.1 ± 9.8 | Training with HAL + CR | CR | e.g.:10 to 12 times HAL training (14.4 min/time) over 4 weeks + 60 to 80 min a day of CR. CG: 60 to 120 min a day of CR Total times were 26.5 (e.g.) hours and 28.2 hours (CG) respectively. |
SWS, MWS, SL-SWS, SL-MWS, cadence at SWS, cadence at MWS, strength of knee extension and flexion, knee ROM, WOMAC-P, WOMAC-F | Pre-surgery, 1, 2, 3, 4, 8 weeks |
| 12/10 | 10 (83.3)/8 (80) | OA | 77.3 ± 3.8/75.0 ± 5.1 | Use of HAL-SJ in knee flexion + CR | CR | e.g.: 50 active knee flexion at each session every other day using HAL-SJ (starting on POD 5) + CR (starting on POD 1). 5 to 10 min of average time over 5 days training CG: 50 active knee flexion at each session every other day (starting on POD 5). + CR (starting on POD 1). 10 to 15 min of average time over 5 days training |
VAS, Strength of knee flexion, knee ROM | Pre-intervention, 5 days | |
| 10/10 | 10 (100)/8 (80) | NR | 74.2 ± 7.5/73.2 ± 8.0 | Use of HAL-SJ in active assistive knee exercise + CR | CR | e.g.: 50 active assistive knee exercise at each session (HAL-SJ applied every other day, starting on POD 5) + CR (starting on POD 1) over 5 days training CG: CR (starting on POD 1) over 5 days training |
VAS, extension lag | Post-surgery 5 and 10 days | |
| 30/30 | 41 (68.3) | OA | NR | Robot-assisted walking training + CR | CR | e.g.: 5 times a week, 10 times in total (30 min each and twice a day, starting POD 7) over 2-weeks training CG: CPM training, peri-knee neuromuscular, electrical stimulation, isometric contraction of peri-knee muscle (starting POD 7) over 2-weeks training |
HSS, knee kinesthesia grade, knee proprioception grade, FAC, BBS, 10-m sitting-standing time, 6m walking distance | Pre-intervention, 1, 2 weeks, 1, 3, 6, 12 months | |
| 13/13 12/12 9/9 |
10 (76.9)/10 (76.9) 8 (66.7)/11 (91.7) 8 (88.9)/8 (88.9) |
OA OA OA and RA |
75.5 ± 5.4/75.6 ± 4.5 71.3 ± 6.2/74.9 ± 8.7 74.1 ± 5.7/76.4 ± 7.6 |
Training with HAL Use of HAL-SJ in knee exercise and flexion + CR Training with HAL |
CR CR CR |
e.g.: 5 times a week, 10 times in total (HAL training for 40 min) over 2-weeks training CG: 5 times a week, 10 times in total (CR for 40 min once a day) over 2-weeks training e.g.: knee exercise with HAL-SJ (10 extensions per set, 5 sets, twice per week, starting on POD 8) + CR (starting on POD 1) over 5 days per week training until discharge CG: CR (starting on POD 1) over 5 days per week training until discharge e.g.: 10 to 12 times in total over 4-weeks training (starting on POD 1 to 5 weeks) CG: no significant difference in the total physical therapy time compared to the e.g. |
SWS, strength of knee extension, NRS VAS, extension lag Step length, hip ROM, knee ROM, ankle ROM, joint moment |
Pre-surgery, 1, 2, 3 weeks Pre-intervention, 8,10, 15 days, Post-surgery 5 weeks |
|
| Total hip replacemet | |||||||||
| Yang[17] South korea | 13/12 | 11 (84.6)/10 (83.3) | Femur neck and introchanteric fracture | 79.3 ± 6.7/78.5 ± 5.4 | TCRRT + CR | CR | e.g.: 1 time a day (TCRRT for 15 min + CR for 20 min, total 35 min, starting POD 4) over 15 days CG: 1 time a day (CR for 35 min, starting POD 4) over 15 days |
MFRT, 10MWT, TUG, VAS, Strength (hip flexion/extension, knee flexion/extension) | Pre-intervention, 5, 10, 15 days |
| Setoguchi[20] Japan | 8/8 | 6 (75)/6 (75) | OA | 74 ± 4.4/63.8 ± 7.7 | Gait training with HAL | CR | e.g.: gait training with HAL (40 min/time, 3 times a week, 6 times in total) (On the day without HAL, 40 min/time, CR once/day) over 2 weeks CG: CR1 time a day, 40 min/1 time over 2 weeks |
HHS, SF-36 score, single support time, double support time, cadence, velocity, stride length, anterior/posterior/lateral variability, hip ROM, knee ROM | Pre-surgery, 1, 3 weeks |
10MWT = 10 meter walk test, BBS = Berg balance score, CG = control group, CR = conventional rehabilitation, FAC = functional ambulation category, HAL = hybrid assistive limb, HAL-SJ = single-joint hybrid assistive limb, HHS = Harris hip score, HSS = hospital for special surgery, e.g. = experimental group, MFRT = modified functional reach test, MWS = maximum walking speed, NR = not reported, NRS = numerical rating scale, OA = osteoarthritis, POD = postoperative day, RA = rheumatoid arthritis, ROM = range of motion, SF-36 = score 36-Item Short Form Health Survey score, SL-MWS = step length-maximum walking speed, SL-SWS = step length- self-selected walking speed, SWS = self-selected walking speed, TCRRT = trunk control rehabilitation robot training, TUG = timed up and go test, WOMAC-F/P = Western Ontario and McMaster Universities Osteoarthritis Index-function/pain, VAS = visual analogue scale.
According to the type of robots used in 9 studies, 7 studies[14–16,19–22] investigated rehabilitation with HAL. In these 7 studies, 6 studies[14–16,19,21,22] included patients having post-TKR rehabilitation and one study[20] included patients undergoing post-THR rehabilitation. Another study[18] reported the efficacy of AVATAR-M following TKR and the remaining one study[17] was conducted by using TCRRT following THR.
3.3. Meta-analysis of primary outcome measured
Our meta-analysis of 2 studies[15,16] showed that HAL training significantly improved pain measured on an 0 to 10-point VAS, compared to CR in patients who underwent TKR (SMD = 1.05, 95% Cl 0.39–1.71) after 5 days of training. Heterogeneity for I2 value was lower than moderate (tau^2 = 0.0121; I2 = 5%; P = .30) (Fig. 2). There were 2 studies[14,19] that assessed SWS. These studies showed that HAL training was significantly superior to CR in patients following TKR (SMD = 48.70, 95% Cl -50.53 to 147.94) at 8 weeks. A high heterogeneity was detected (P < .01; I2 = 97%) (Fig. 3).
Figure 2.
Forest plot of overall effects of robot-assisted rehabilitation on TKR based on VAS score for standardized mean difference. TKR = total knee replacement, VAS = visual analog scale.
Figure 3.
Forest plot of overall effects of robot-assisted rehabilitation on TKR based on SWS for standardized mean difference. SWS = self selected walking speed, TKR = total knee replacement.
3.4. Descriptive analysis of secondary outcome measured
3.4.1. Type of intervention technologies with their purpose of assessment
Hal
A total of 7 studies[14–16,19–22] investigated rehabilitation with HAL. Among them, 6[14–16,19,21,22] included patients undergoing post-TKR rehabilitation. The other[20] included patients having post-THR rehabilitation. In patients following TKR, HAL combined with CR was conducted in the experimental group and only CR was applied in the control group in 3 of the studies, while in the studies conducted by Tanaka et al[19] and Fukaya et al,[22] training with HAL and CR were provided to experimental and control groups, respectively.
Walking ability
Yoshikawa et al[14] reported that the treatment given to the experimental group was more effective than that given to control group in patients following TKR. The patients in the HAL group underwent HAL training (average 14.4 ± 5.9 minute a session) and CR (60–80 minute a day). Total number of HAL interventions ranged from 10 to 12 during the 4-week period. The patients in the control group underwent only CR (60–120 minute a day) after TKR. In addition to the SWS used in the meta-analysis, various outcome measurements were obtained. The SWS, step length in the SWS, and the MWS were greater in the HAL group than in the control group at 4 and 8 weeks (P < .05). The step length in the MWS was greater in the HAL group than in the control group at 2, 4, and 8 weeks (P < .05). HAL training was initiated 1 to 5 weeks after TKA in the HAL group and CR was performed in the control group in the study by Fukaya et al.[22] In the HAL group, the odds ratio of hip extension was as large as 1.741, while that of knee swing was as large as 1.501 at post-surgery 5 weeks. These 2 variables were significant between the 2 groups. They suggest that HAL training increased the mobility of the knee and hip joints and that walking ability was improved by increasing the step length because knee swing and varus significantly affected step length.[22]
Knee function
In the study by Yoshikawa et al,[14] the muscle strength of knee extension in the HAL group was greater than in the control group at 8 weeks (P < .05). Also the extension lag and knee pain (WOMAC-P) were lower in the HAL group than in the control group at 2 weeks (P < .05). In the study conducted by Tanaka et al,[19] the outcome measures were evaluated prior to surgery and at weeks 1, 2, and 3 postoperatively. Rehabilitation using HAL resulted in a significantly better improvement in quadriceps strength compared to CR (weeks 1 and 2; weeks 1 and 3: P < .001). In the studies by Kotani et al[15] and Goto et al,[16] other outcome measures except VAS were analyzed descriptively to evaluate the function. RAR through HAL was performed to assist knee ROM exercise every other day in the experimental group in the single knee joint. Significant improvement occurred in both groups between postoperative days 5 and 10. Improvement in active knee ROM, passive knee ROM, and strength of knee flexion were significantly greater in the HAL assisted rehabilitation group than in the control group (P < .01).[15] Goto et al[16] reported that the HAL assisted rehabilitation group showed 89.4%±15.7% improvement (P < .01), while the control group showed 34.8% ± 32.1% improvement (P = .016) in the extension lag. Yoshioka et al[21] also assessed extension lag to report the effectiveness of HAL. The extension lag significantly improved in the HAL group after the 2nd and 3rd sessions.
Hip function
Lastly, in the study by Setoguchi et al,[20] HAL was used for training over 2 weeks (3 times a week) after THA. Gait analysis was performed before and after surgery, and comparisons were made between the 2 groups. Several outcome measures were used in this study, the extension angle in the sagittal full range of hip motion were better in the HAL group than control group at 3 weeks postoperatively.
AVATAR-M Knee function
The study by Li et al[18] was also analyzed descriptively, because the intervention program and outcome measures were completely different from other studies and was conducted in patients following TKR. In it the authors used AVATAR-M over 2 weeks (5 times a week) to help the patients start rehabilitation early, while the other studies were performed in patients following TKR through HAL assisted training. Berg balance score, 10-m sitting-standing time, and 6-minute walking distance of RAR group were significantly higher than the control group (P < .05). Also, knee kinesthesia grade, knee proprioception grade, and functional ambulation category of experimental group were better than the control group although there was not significant (P > .05).
Clinical evaluation
The HSS score of RAR group were significantly higher than the control group (P < .05) in the study by Li et al.[18]
TCRRT Knee function
There was one study by Yang et al[17] which was conducted in patients who had THR. The experimental group received the TCRRT for 15 minutes and CR for 20 minutes, while the control group received only the CR for 20 minutes once a day over 15 days. The experimental group had significantly more improvement in the modified functional reach test, manual muscle test of lower extremity, 10 meter walk test, and timed up and go test compared to the control group (P < .05).
3.5. Risk of bias within individual studies
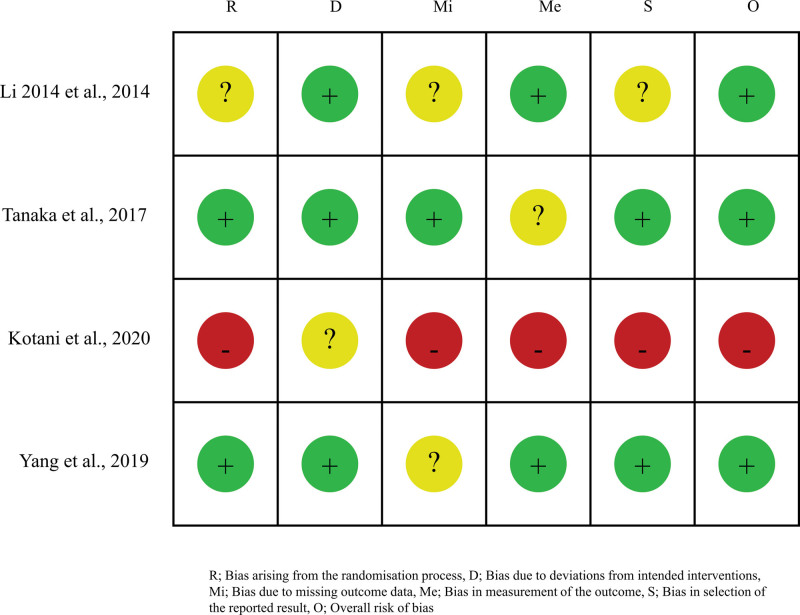
Study quality assessment of RCTs by the Cochrane Risk-of-Bias Tool is summarized in Figure 4. Newcastle-Ottawa scale was used to assess the quality of the selected studies. All included studies scored 5–8 points, indicating relatively high quality.[31]
Figure 4.
Risk of bias assessment in the included studies.
3.6. Publication bias
Since the number of included studies were less than 10, funnel plot analysis was not performed.
4. Discussion
Our review focused on the effects of RAR following TKR or THR in patients with osteoarthritis, rheumatoid arthritis, or hip fracture. This is a unique review that considered the implication of RAR on several outcome measures.
Of the 9 studies included, only 5[15–17,19,21] (4[15,16,19,21] for TKR and 1[17] for THR) analyzed the change of pain and the VAS and NRS were used to those. NRS is the simplest and most commonly used scale. The numerical scale is most commonly 0 to 10, with 0 being: no pain: and 10 being “the worst pain imaginable.”[37] NRS could have been used as a substitute for VAS in the study by Tanaka colleagues.[19] However, the NRS score was not summarized as mean plus/minus standard deviation, as instead, the change in the number of participants with high NRS scores (6 points or higher) was identified. Thus, the NRS score was not able to be included in the meta-analysis. Although the details were not described separately in the results, we were able to identify through descriptive analysis that rehabilitation using HAL resulted in a significantly greater improvement in NRS scores (week 1: P = .03) when compared to CR.[19] In the study by Yoshioka et al,[21] there was a reduction in the VAS for pain after training, which was not significant. Two[15,16] of the remaining 3 studies evaluated pain through meta-analysis and the other studies[17] using TCRRT was analyzed through descriptive analysis as it was performed in patients following THR. The TCRRT group had significantly more improvement in the VAS compared to that before intervention (P < .05) at the 5, 10, and 15 days.[17] In sum, all 4 studies[15,16,19,21] using HAL showed superior effect of RAR over those of the control group. The superior therapeutic effects of RAR in the reduction of pain can be attributed to the following reasons: in TKR patients, training with HAL improved active and passive ROMs. This is partly because knee movements are less painful with the HAL system than CR, as knee flexion is possible without excessive effort. Also, pain from the surgical incision and damage to the soft tissues inhibit normal hip joint movement in THR patients.[38] These can induce emotional and psychological pain as well as chronic pain.[39] Also, it is believed that TCRRT’s consistent repetitive muscle strengthening exercises in the lower extremities may have had a positive effect on pain reduction by increasing the ROM, reducing adhesion, and promoting muscle strengthening.
SWS, one of the principle findings of our meta-analysis, were improved more by RAR than CR at 8 weeks. The change in walking speed after TKA is also considered an indicator of the effectiveness of rehabilitation.[40] In the study by Tanaka et al,[19] the difference in change of walking speed between the 2 groups was significant during the early part of the postoperative recovery phase and this difference diminished over time. On the other hand, the walking speed in the RAR group was better than in the CR group at weeks 4 and 8 in the study by Yoshikawa et al.[14] The difference in timing of walking improvements was likely due to differences in the experimental and conventional groups in terms of the length of time that training was applied. In the study by Tanaka et al,[19] the experimental group using HAL performed one 40-minutes session once a day and also undertook a single 20-minutes rehabilitation session consisting of ROM exercises and walking. In the control group, CR was done 40 minutes once a day, and the same 20 minutes session of ROM exercises and walking as the experimental group was performed. The training time was 6 hours because both groups received 10 times in total. Meanwhile, total physical therapy time using HAL was 26.5 ± 4.2 and 28.2 ± 5.2 hours in the experimental and control group, respectively, in the study by Yoshikawa et al.[14]
For pain after total joint arthroplasty, Jonathan et al[41] investigated the minimal clinically important difference (MCID) for VAS in patients following TKA. The authors reported that the MCID for pain improvement in TKA was 2.26 cm. The VAS is a horizontal line, 10cm in length, with 0 cm labeled “no pain” and 10 cm labeled “worst pain I have ever had.” Patients mark the point on the line based on their perception of their current state.[42] In our meta-analysis, we found that the average change on the VAS was lower than MCID value for TKA in the experimental groups. When considering control groups, all control groups in the studies underwent CR. Therefore, the SMDs in 2 studies were small. The average change of the VAS was lower than MCID, may have been caused by effects of CR in 2 studies. The difference of SWS did not exceed the MCID in our review,[43,44] which is also thought to be the result of CR used in all control groups, as was the case with VAS.
We showed that various outcomes that did not overlap were significantly improved in the experimental groups compared to the control groups through descriptive analysis. This review found sufficient evidence to support the conclusion that RAR had significant clinical effects for TKR and THA surgery patients.
Among the 9 studies of our review, HAL was used in the 7 studies.[14–16,19–22] Six studies[14–16,19,21,22] included patients having post-TKR rehabilitation. The other study[20] included patients following THR. HAL is a wearable robot designed to facilitate movement and interactively provides motion according to the wearer’s voluntary drive.[45] Still, the mechanism through which the use of HAL improves gait function after TKA and THA remains unclear. Movement of HAL is triggered by bioelectric signals from the muscles accompanying the exercise of intention, correctly supporting spontaneous movement of impaired limb and generating sensory feedback.[16] In patients following TKA, quadriceps arthrogenic muscle inhibition may be one reason for the effect of rehabilitation and is the phenomenon of inhibition of the quadriceps femoris muscle after surgery.[46] This inhibition is believed to result from pain or swelling of the knee, or damage to pressure receptors.[46] The suggested mechanisms for quadriceps arthrogenic muscle inhibition include possible inhibition of α motor neurons via the spinal reflex[47] and involvement of pathways engaging upper motor neurons.[46] Using the HAL for post-THA therapy would improve hip extension and lead to a correct gait. This improvement could be attributed to assistance provided by HAL during the extension of the hip. The motion assist feature has the effect of motor learning, by feedback from HAL to the muscle, nerve, spinal cord, and brain.[48,49] Several studies have shown the effect and feasibility of HAL-assisted rehabilitation and single joint HAL-assisted rehabilitation for stroke, spinal cord injury, and post-TKA knee extension failure.[50–52]
In 2 studies[17,18] not using HAL, it was shown that AVATAR after TKR can provide patients with a safe and comfortable driving force to assist them in initiating walking training as early as possible and thus speed their walking recovery in a manner maximally close to a physiological condition as shown in the study by Li colleagues.[18] As they reported, the periodical movement of the hip, knee, and ankle during a normal walking process was simulated in a partial weight-supported condition, and a tolerable and comfortable walking speed was adjusted. TCRRT was used in the experimental group following THR in the study by Yang and colleagues.[17] TCRRT was feasible and effective for improving dynamic balance, lower extremity strength, gait ability, and pain after THR. It was believed that TCRRT’s consistent repetitive muscle strengthening exercise of lower extremities might have had a positive effect on pain reduction by increasing ROM, decreasing adhesion, and enhancement of muscle strength.
This review had some limitations. First, only 2 studies for each outcome measure were included in meta-analysis. We examined VAS and SWS to determine the effect of RAR in our meta-analysis. However, all of the included studies were prospective comparative studies, and we performed the quality assessment of the risk of bias to overcome this limitation. Second, most trials used various outcome measures which limited the pooling of results. Future RCTs should demonstrate the effect of RAR on consensus on a set of suitable outcome measures. Third, most studies (except the study by Tanaka et al[19]) did not perform an a priori sample size calculation, which can increase the risk of underpowered results. This has been caused by a lack of reports on the use of robot in rehabilitation after TKR and THR. Fourth, it could not be described the standardization of antalgic therapy because that there was no description of antalgic therapy in the papers included in this study. Lastly, our review cannot provide the long-term effects (more than 8 weeks) of RAR.
5. Conclusion
The result of this meta-analysis of primary outcome and descriptive analysis of secondary outcome suggest that RAR may be an effective treatment for each TKR and THR patients. However, high-quality studies are needed to verify the long-term effects of RAR for TKR and THR patients. Further, it is important to consider a careful stratification of patients’ characteristics such as cognitive function and sarcopenia.
Acknowledgments
We give our sincere thanks to Harrisco (translation company) for providing language help.
Authors’ contributions
Conceptualization: Yoo JI, Lee CH.
Data curation: Yoo JI, Oh MK.
Formal analysis: Yoo JI.
Investigation: Oh MK, Lee SU.
Methodology: Yoo JI.
Supervision: Oh MK.
Validation: Oh MK.
Writing-original draft: Yoo JI.
Writing-review & editing: Lee CH.
Supplementary Material
Abbreviations:
- CI =
- confidence interval
- CR =
- conventional rehabilitation
- HAL =
- hybrid assistive limb
- MCID =
- minimally clinically important difference
- MWS =
- maximum walking speed
- NRS =
- numerical rating scale
- RAR =
- robot-assisted rehabilitation
- RCT =
- randomized clinical trial
- ROM =
- range of motion
- SMD =
- standardized mean difference
- SWS =
- self-selected walking speed
- TCRRT =
- trunk control rehabilitation robot training
- THR =
- total hip replacements
- TKR =
- total knee replacement
- VAS =
- visual analog scale
- WOMAC =
- Western Ontario and McMaster universities osteoarthritis index
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Yoo J-I, Oh M-K, Lee S-U, Lee CH. Robot-assisted rehabilitation for total knee or hip replacement surgery patients: A systematic review and meta-analysis. Medicine 2022;101:40(e30852).
Contributor Information
Jun-Il Yoo, Email: furim@hanmail.net.
Min-Kyun Oh, Email: solioh21@hanmail.net.
Shi-Uk Lee, Email: ychkhk1407@gmail.com.
References
- [1].Sanna M, Sanna C, Caputo F, et al. Surgical approaches in total knee arthroplasty. Joints. 2013;1:34–44. [PMC free article] [PubMed] [Google Scholar]
- [2].Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet (London, England). 2007;370:1508–19. [DOI] [PubMed] [Google Scholar]
- [3].Callahan CM, Drake BG, Heck DA, et al. Patient outcomes following tricompartmental total knee replacement: a meta-analysis. JAMA. 1994;271:1349–57. [PubMed] [Google Scholar]
- [4].Ibrahim MS, Khan MA, Nizam I, et al. Peri-operative interventions producing better functional outcomes and enhanced recovery following total hip and knee arthroplasty: an evidence-based review. BMC Med. 2013;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ottenbacher KJ, Graham JE. The state-of-the-science: access to postacute care rehabilitation services. A review. Arch Phys Med Rehabil. 2007;88:1513–21. [DOI] [PubMed] [Google Scholar]
- [6].Christensen CM. The innovator’s dilemma: when new technologies cause great firms to fail. Harvard Business Review Press, 2013. [Google Scholar]
- [7].Calafiore D, Negrini F, Tottoli N, et al. Efficacy of robotic exoskeleton for gait rehabilitation in patients with subacute stroke: a systematic review with meta-analysis. Eur J Phys Rehab Med. 2021;58:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bhardwaj S, Khan AA, Muzammil M. Lower limb rehabilitation robotics: The current understanding and technology. Work 2021(Preprint):1-19. [DOI] [PubMed]
- [9].Mehrholz J, Thomas S, Werner C, et al. Electromechanical-assisted training for walking after stroke: a major update of the evidence. Stroke. 2017;48:e188–9. [DOI] [PubMed] [Google Scholar]
- [10].Cheung EY, Ng TK, Kevin K, et al. Robot-assisted training for people with spinal cord injury: a meta-analysis. Arch Phys Med Rehabil. 2017;98:2320–2331. e2312. [DOI] [PubMed] [Google Scholar]
- [11].Carvalho I, Pinto SM, das Virgens Chagas D, et al. Robotic gait training for individuals with cerebral palsy: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2017;98:2332–44. [DOI] [PubMed] [Google Scholar]
- [12].Carda S, Invernizzi M, Baricich A, et al. Robotic gait training is not superior to conventional treadmill training in parkinson disease: a single-blind randomized controlled trial. Neurorehabil Neural Repair. 2012;26:1027–34. [DOI] [PubMed] [Google Scholar]
- [13].Nizamis K, Athanasiou A, Almpani S, et al. Converging robotic technologies in targeted neural rehabilitation: a review of emerging solutions and challenges. Sensors. 2021;21:2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yoshikawa K, Mutsuzaki H, Sano A, et al. Training with Hybrid Assistive Limb for walking function after total knee arthroplasty. J Orthop Surg Res. 2018;13:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kotani N, Morishita T, Saita K, et al. Feasibility of supplemental robot-assisted knee flexion exercise following total knee arthroplasty. J Back Musculoskeletal Rehab. 2020;33:413–421. [DOI] [PubMed] [Google Scholar]
- [16].Goto K, Morishita T, Kamada S, et al. Feasibility of rehabilitation using the single-joint hybrid assistive limb to facilitate early recovery following total knee arthroplasty: a pilot study. Assist Technol. 2017;29:197–201. [DOI] [PubMed] [Google Scholar]
- [17].Yang H, Lim H. Effects of trunk control rehabilitation robot training on dynamic balance, lower extremity strength, gait ability and pain in bipolar hemiarthroplasty. J Korean Phys Therapy. 2019;31:94–102. [Google Scholar]
- [18].Li J, Wu T, Xu Z, et al. A pilot study of post-total knee replacement gait rehabilitation using lower limbs robot-assisted training system. Eur J Orthop Surg Traumatol. 2014;24:203–8. [DOI] [PubMed] [Google Scholar]
- [19].Tanaka Y, Oka H, Nakayama S, et al. Improvement of walking ability during postoperative rehabilitation with the hybrid assistive limb after total knee arthroplasty: a randomized controlled study. SAGE Open Med. 2017;5:2050312117712888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Setoguchi D, Kinoshita K, Kamada S, et al. Hybrid assistive limb improves restricted hip extension after total hip arthroplasty. Assist Technol. 2020;1:9. [DOI] [PubMed] [Google Scholar]
- [21].Yoshioka T, Kubota S, Sugaya H, et al. Feasibility and efficacy of knee extension training using a single-joint hybrid assistive limb, versus conventional rehabilitation during the early postoperative period after total knee arthroplasty. J Rural Med. 2021;16:22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fukaya T, Mutsuzaki H, Yoshikawa K, et al. Effect of training with the hybrid assistive limb on gait cycle kinematics after total knee arthroplasty. Geriatr Orthop Surg Rehabil. 2021;12:21514593211049075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Morone G, Paolucci S, Cherubini A, et al. Robot-assisted gait training for stroke patients: current state of the art and perspectives of robotics. Neuropsychiatr Dis Treat. 2017;13:1303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pastora-Bernal JM, Martín-Valero R, Barón-López FJ, et al. Evidence of benefit of telerehabitation after orthopedic surgery: a systematic review. J Med Internet Res. 2017;19:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cottrell MA, Galea OA, O’Leary SP, et al. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31:625–38. [DOI] [PubMed] [Google Scholar]
- [26].Shukla H, Nair S, Thakker D. Role of telerehabilitation in patients following total knee arthroplasty: Evidence from a systematic literature review and meta-analysis. J Telemed Telecare. 2017;23:339–46. [DOI] [PubMed] [Google Scholar]
- [27].Wang X, Hunter DJ, Vesentini G, et al. Technology-assisted rehabilitation following total knee or hip replacement for people with osteoarthritis: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2019;20:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [29].Beaudet K. The cochrane collaboration and meta-analysis of clinical data. Am Orthoptic J. 2010;60:6–8. [DOI] [PubMed] [Google Scholar]
- [30].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [32].Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [33].Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, 2011. [Google Scholar]
- [34].Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- [37].Alghadir AH, Anwer S, Iqbal A, et al. Test–retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res. 2018;11:851–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lavernia C, D’apuzzo M, Hernandez VH, et al. Patient-perceived outcomes in thigh pain after primary arthroplasty of the hip. Clin Orthop Related Res. (1976-2007). 2005;441:268–73. [DOI] [PubMed] [Google Scholar]
- [39].Erlenwein J, Müller M, Falla D, et al. Clinical relevance of persistent postoperative pain after total hip replacement–a prospective observational cohort study. J Pain Res. 2017;10:2183–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Barthuly AM, Bohannon RW, Gorack W. Gait speed is a responsive measure of physical performance for patients undergoing short-term rehabilitation. Gait Posture. 2012;36:61–4. [DOI] [PubMed] [Google Scholar]
- [41].Danoff JR, Goel R, Sutton R, et al. How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. J Arthroplasty. 2018;33:S71–S75.e2. [DOI] [PubMed] [Google Scholar]
- [42].McCormack HM, David JL, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18:1007–19. [DOI] [PubMed] [Google Scholar]
- [43].Abbasi-Bafghi H, Fallah-Yakhdani HR, Meijer OG, et al. The effects of knee arthroplasty on walking speed: a meta-analysis. BMC Musculoskelet Disord. 2012;13:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].White DK, Felson DT, Niu J, et al. Reasons for functional decline despite reductions in knee pain: the Multicenter Osteoarthritis Study. Phys Ther. 2011;91:1849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kawamoto H, Kamibayashi K, Nakata Y, et al. Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol. 2013;13:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rice DA, McNair PJ. Quadriceps arthrogenic muscle inhibition: neural mechanisms and treatment perspectives. 2010. Elsevier. p 250–266. [DOI] [PubMed] [Google Scholar]
- [47].Rice DA, McNair PJ, Lewis GN, et al. Quadriceps arthrogenic muscle inhibition: the effects of experimental knee joint effusion on motor cortex excitability. Arthritis Res Therapy. 2014;16:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kawamoto H, Sankai Y. Power assist method based on phase sequence and muscle force condition for HAL. Adv Robot. 2005;19:717–34. [Google Scholar]
- [49].Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19:84–90. [DOI] [PubMed] [Google Scholar]
- [50].Morishita T, Inoue T. Interactive bio-feedback therapy using hybrid assistive limbs for motor recovery after stroke: current practice and future perspectives. Neurol Med Chir (Tokyo). 2016;56:st. 2016-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ueba T, Hamada O, Ogata T, et al. Feasibility and safety of acute phase rehabilitation after stroke using the hybrid assistive limb robot suit. Neurol Med Chir (Tokyo). 2013;53:287–90. [DOI] [PubMed] [Google Scholar]
- [52].Sczesny-Kaiser M, Höffken O, Aach M, et al. HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J Neuroeng Rehabil. 2015;12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.