Abstract
Individuals considering living kidney donation face geographic, financial, and logistical challenges. Telemedicine can facilitate healthcare access/care coordination. Yet difficulties exist in telemedicine implementation and sustainability. We sought to examine centers' practices and providers' attitudes toward telemedicine to improve services for donors. We surveyed multidisciplinary providers from 194 active adult US living donor kidney transplant centers; 293 providers from 128 unique centers responded to the survey (center representation rate = 66.0%), reflecting 83.9% of practice by donor volume and 91.5% of US states/territories. Most centers (70.3%) plan to continue using telemedicine beyond the pandemic for donor evaluation/follow‐up. Video was mostly used by nephrologists, surgeons, and psychiatrists/psychologists. Telephone and video were mostly used by social workers, while video or telephone was equally used by coordinators. Half of respondent nephrologists and surgeons were willing to accept a remote completion of physical exam; 68.3% of respondent psychiatrists/psychologists and social workers were willing to accept a remote completion of mental status exam. Providers strongly agreed that telemedicine was convenient for donors and would improve the likelihood of completing donor evaluation. However, providers (65.5%) perceived out‐of‐state licensing as a key policy/regulatory barrier. These findings help inform practice and underscore the instigation of policies to remove barriers using telemedicine to increase living kidney donation.
Keywords: access to health care, attitudes, health services, kidney transplantation, living donors, telehealth
Short abstract
A national survey of multidisciplinary providers regarding the use of telemedicine services for living kidney donor evaluation and care delineates center practices, provider attitudes, and perceived barriers including regulatory limitations of state licensing requirements.
Abbreviations
- AST
American Society of Transplantation
- ASTS
American Society of Transplant Surgeons
- COVID‐19
Coronavirus 2019
- ILDAs
independent living donor advocates
- NATCO
North American Transplant Coordinators Organization
- OPTN
Organ Procurement and Transplantation Network
- SIG
Special Interest Group
- STSW
Society for Transplant Social Workers
- US
United States
1. INTRODUCTION
Individuals who consider living kidney donation face geographic, financial, and logistical challenges to complete donor evaluation. 1 , 2 , 3 , 4 In a single‐center study, 50% of potential kidney donors had to travel at least 50 miles to access a transplant center to begin donor evaluation. 5 Longer distance from the transplant center is associated with higher costs of transportation, lodging, missed work, and care expenses for dependents. 6 , 7 As a result, potential donors may disengage from the evaluation process, which comprises complex multiphase activities. 5 , 8 This involves care coordination with nephrologists, surgeons, nurse coordinators, social workers or independent living donor advocates (ILDAs), and psychologists or psychiatrists for assessment and counseling of potential donors, 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 a population that has declined over much of the last two decades. 21 , 22 Furthermore, those who have donated a kidney have been found to have suboptimal follow‐up care. 23 , 24 , 25 , 26 The Coronavirus 2019 (COVID‐19) pandemic has augmented the obstacles for potential donors to complete donor evaluation and follow‐up. 27
Telemedicine can help donors more easily access transplant centers and can facilitate care coordination. 28 , 29 , 30 , 31 The transformation of healthcare delivery during the pandemic has accelerated the adoption of telemedicine services across the United States and created opportunities and challenges in light of policy and regulatory changes. 32 , 33 , 34 , 35 , 36 , 37 , 38 Telemedicine using a live‐video and/or ‐telephone visit has helped sustain access to transplant centers and maintain follow‐up care. 28 , 39 , 40 However, telemedicine as a practice has challenged care providers given that no evidence‐based practice exists to guide implementation and sustainability of telemedicine services. In a survey of US transplant centers, 81% reported difficulties in implementing telemedicine including staff training, technology limitations, and infrastructure deficiency. 40 In primary and other specialty care practices, telemedicine has achieved provider and patient satisfaction with similar outcomes compared to in‐person visits. 41 , 42 , 43 , 44 , 45 Efforts are needed to characterize telemedicine practice among multidisciplinary providers to improve care of donors.
To better understand center practices and provider attitudes and perceived barriers to using telemedicine services for living kidney donation, we conducted a national survey of multidisciplinary providers as an essential step to advance the use of telemedicine for the evaluation and follow‐up care of donors.
2. METHODS
2.1. Survey design
The survey instrument was developed by the American Society of Transplantation (AST) Living Donor Community of Practice Telemedicine Workgroup. The workgroup consists of experts in living kidney donation from 10 academic US transplant centers. We identified key constructs of interest via conference calls. Survey items were developed and refined iteratively through direct discussion and email, external feedback, and pilot testing. The pilot test was conducted entailing multidisciplinary providers of the target population. The final survey comprised 20 questions, with skip logic for respondents whose centers have not used telemedicine (Table S1). Skip logic was also used to ensure that certain roles of providers were asked role‐specific questions. Survey items included multiple‐choice, multiple select, free‐text responses, and 5‐point Likert scale to allow respondents to express how much they agree or disagree with a specific point on a scale from 5 (strongly agree or very willing) to 1 (strongly disagree or not willing at all). The survey instrument was designed to understand center practices, procedures, and provider perceived barriers and attitudes regarding telemedicine services for living kidney donation across the United States. Telemedicine was defined as a healthcare delivery platform using synchronous video and/or telephone visit that permits real‐time communications between the patient and provider at a distant site.
2.2. Participants and survey administration
We surveyed multidisciplinary providers at adult US living donor kidney transplant centers. Pediatric transplant centers (n = 19) were excluded given that living kidney donors are adults (≥18 years) and are typically evaluated at adult transplant centers. The target population included nephrologists, surgeons, nurse coordinators, social workers or ILDAs, and psychiatrists or psychologists. To clarify, the involvement of a psychiatrist or psychologist in the donor evaluation process is not routinely used at many centers and is referred to when needed, given that the psychosocial evaluation can be performed by a social worker or an ILDA.
We used nonprobability sampling methods to recruit our study population, 46 specifically purposive and snowball sampling: (1) by collaborating with multiple professional societies to facilitate distributing the survey to their listservs via email, including the AST Living Donor Community of Practice, AST Kidney Pancreas Community of Practice, AST Advanced Practice Providers Community of Practice, American Society of Transplant Surgeons (ASTS), North American Transplant Coordinators Organization (NATCO), Society for Transplant Social Workers (STSW), Academy of Consultation‐Liaison Psychiatry Transplant Psychiatry Special Interest Group (SIG), AST Psychosocial and Ethics Community of Practice, and AST Newsletter; (2) by sending the survey via email to the AST Living Donor Community of Practice Telemedicine Workgroup's professional connections who are directly involved with living kidney donation, such as living kidney donor program directors. These connections had the opportunity to email the survey via anonymous link to potential participants involved in living kidney donation.
The survey was created and distributed through Qualtrics Survey Software with an online link. The survey was distributed between February 18, 2021 and May 13, 2021, and up to two reminders were provided. Participation in this study was not compensated, and responses were anonymously analyzed. This study was approved by the Johns Hopkins Institutional Review Board.
2.3. Statistical Analysis
We used descriptive statistics to analyze survey data. Responses to each survey question were described by either proportions and percentages or means and ranges, where appropriate. We used analysis of variance to determine whether mean responses varied across multidisciplinary providers. We excluded respondents who provided substantial incomplete information (i.e., did not answer ≥66.7% of non‐skip logic survey items) from the analysis (n = 60); there were no patterns of respondents who were excluded by center volume, center state, or provider role. For free text responses, we inductively created mutually exclusive categories based on the content of text and the analysis team agreed upon the categories through consensus. For type of questions that “select all that apply,” the total number of responses for each answer choice exceeded the total number of centers or respondents who were eligible to respond to the specific question (i.e., column totals added up to more than 100.0%).
Given our sampling methods, we calculated a center representation rate which is equal to the number of unique centers that were represented in the survey divided by the total number of all adult US living donor kidney transplant centers that were active in 2020 (n = 194). We also computed the proportion of living donor transplant volume represented by the participating centers based on data from the Scientific Registry on Transplant Recipients. 47 To ensure that we did not overestimate the center representation rate, we verified the number of adult US living donor kidney transplant centers in 2020 to those in 2019 (n = 201) and 2018 (n = 210) given that some centers suspended their living donation procedures in response to the pandemic.
For survey items pertaining to center practices (Q3‐Q11), each center was represented only once in the analysis. To obtain proportions, we divided the number of unique centers that selected the same answer by the total number of represented centers. For centers with multiple respondents, a center would have been recorded as not having used telemedicine if all respondents from that same center selected “no” for survey items Q3, Q5, Q8, and Q11 (Table S1) since an aim of the survey was to understand center practices using telemedicine; for example, all respondents of the same center selected “no” for the item Q5: “has your center ever used telemedicine (video and/or telephone for a clinical visit) for living kidney donor evaluation and/or follow‐up.” For survey items that were “select all that apply” with respect to telemedicine modalities Q8, Q11 (Table S1), a center would have been recorded as “video and telephone” if at least one respondent either selected: (i) video and the remaining respondents from the same center selected “telephone” or vice versa, or (ii) “telephone and video” irrespective of the remaining responses from the same center. In other words, a center would have been recorded as either video or telephone if all respondents from the same center selected the same answer.
For survey items pertaining to provider perceived barriers and attitudes (Q12‐Q20), each respondent was represented once in the analysis. To obtain proportions, we divided the total number of individual responses that selected the same answer by the total number of respondents. For 5‐point Likert scale questions, we calculated a mean and standard deviation to characterize overall perceptions and attitudes. All analyses were performed using Stata 17.0/MP for Linux.
3. RESULTS
3.1. Center and provider characteristics
Of 194 active adult US living donor kidney transplant centers in 2020, 293 providers from 128 unique centers responded to the survey (median center volume: 26; interquartile range [IQR]: 12–40) (Figure S1). The center representation rate was 66.0%, which reflects 83.9% of national living kidney donation practice by center volume and 91.5% (43/47) of US states and territories (including D.C.) that have performed living donor kidney transplantation in 2020 (Table S2). Centers that were not represented in our survey (N = 66) had a small volume of living donor kidney transplants (median center volume: 6; IQR: 2–14).
Of 293 respondents, 65 (22.2%) were nephrologists, 51 (17.4%) were surgeons, 56 (19.1%) were coordinators, 58 (19.8%) were social workers or ILDAs, 24 (8.2%) were psychiatrists or psychologists, and 39 (13.3%) had other roles (Figure 1).
FIGURE 1.
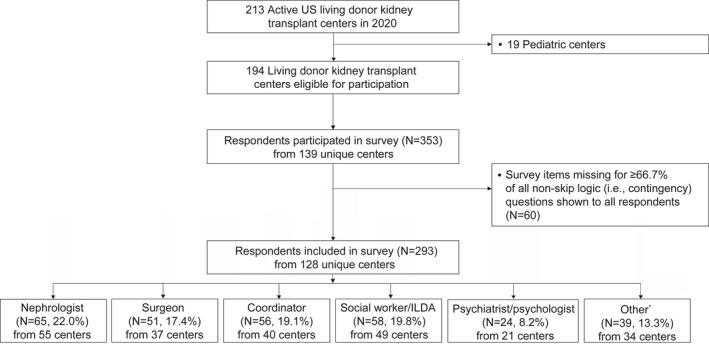
Flowchart representative of surveys eligible for inclusion
3.2. Center trends using telemedicine for living kidney donation
Of 128 represented centers, 91.4% reported having ever used telemedicine for living kidney donor evaluation and/or follow‐up. The 10 centers that have never used telemedicine for living kidney donation reported the following reasons: not needed, in‐person visit preferences, evaluation requirements, uses limited to transplant recipients, and regulatory restrictions.
Most centers (70.3%) plan to continue using telemedicine beyond the pandemic for donor evaluation and/or follow‐up, while 16.4% of centers did not know if they will continue to use telemedicine beyond the pandemic. Conversely, only 28.1% of centers used telemedicine prior to the pandemic. The six centers that will not use telemedicine beyond the pandemic reported the following reasons: difficulty for donors to use telemedicine, convenience for patients to complete testing in an in‐person visit at a single time, preference of providers to see donors in an in‐person visit, and willingness to use for the donor follow‐up but not for the evaluation (Table 1).
TABLE 1.
Center practices regarding telemedicine usage for living kidney donor evaluation and/or follow‐up
| Center (N = 128) | % | |
|---|---|---|
| Center ever used telemedicine for living kidney donor evaluation and/or follow‐up | ||
| Yes | 117 | 91.4 |
| No a | 10 | 7.8 |
| Do not know | 1 | 0.8 |
| Center used telemedicine for living kidney donor evaluation and/or follow‐up prior to COVID‐19 pandemic | ||
| Yes | 36 | 28.1 |
| No | 81 | 63.3 |
| Do not know | 0 | 0.0 |
| Missing | 11 | 8.6 |
| Center will continue to use telemedicine for living kidney donor evaluation and/or follow‐up beyond COVID‐19 pandemic | ||
| Yes | 90 | 70.3 |
| No b | 6 | 4.7 |
| Do not know | 21 | 16.4 |
| Missing | 11 | 8.6 |
Abbreviation: ILDA, independent living donor advocate.
Reasons included: not needed, in‐person preference or evaluation requirement, telemedicine for recipients but not living donors, regulatory restrictions, temporary hold of center during the height/initial months of the pandemic, unknown, miscellaneous.
Reasons included: in‐person preference, convenience for patients to complete testing in‐person at a single time, difficulty for donors to use telemedicine, willingness to use for follow‐up but not for evaluation.
3.3. Center practices using telemedicine for the evaluation of potential donors
Centers' usage of telemedicine modalities for the evaluation of potential donors varied across specialty. Video was mostly used by nephrologists, surgeons, and psychiatrists or psychologists. Telephone and video were mostly used by social workers or ILDAs. However, either video or telephone were equally used by coordinators (Table 2).
TABLE 2.
Center practices regarding telemedicine modalities used for living kidney donor evaluation and/or follow‐up
| Center practices | Centers, N (%) | ||||
|---|---|---|---|---|---|
| No telemedicine | Video | Telephone | Telephone and video | Missing | |
| Donor evaluation a | |||||
| Medical (N = 55 centers) | 8 (14.6) | 27 (49.1) | 0 (0.0) | 13 (23.6) | 7 (12.7) |
| Surgical (N = 37 centers) | 6 (16.2) | 19 (51.4) | 0 (0.0) | 6 (16.2) | 6 (16.2) |
| Coordinator (N = 40 centers) | 8 (20.0) | 13 (32.5) | 11 (27.5) | 5 (12.5) | 3 (7.5) |
| Social work/ILDA (N = 49 centers) | 0 (0.0) | 11 (22.4) | 9 (18.4) | 23 (46.9) | 6 (12.2) |
| Psychiatric/psychological (N = 21 centers) | 1 (4.8) | 9 (42.8) | 0 (0.0) | 6 (28.6) | 5 (23.8) |
| Donor follow‐up (N = 76 centers) b | |||||
| 6 months | 4 (5.2) | 23 (30.3) | 12 (15.8) | 27 (35.5) | 10 (13.2) |
| 1 year | 7 (9.2) | 16 (21.1) | 18 (23.7) | 24 (31.6) | 11 (14.4) |
| 2 years | 9 (11.8) | 15 (19.7) | 16 (21.1) | 23 (30.3) | 13 (17.1) |
| Beyond the 2‐year OPTN mandate | 14 (18.4) | 12 (15.8) | 14 (18.4) | 16 (21.1) | 20 (26.3) |
Abbreviations: ILDA, independent living donor advocate; OPTN, Organ Procurement and Transplantation Network.
Items were restricted to centers who had at least one respondent of the specific role type of the evaluation in question (e.g., medical evaluation was restricted to centers who had at least one nephrologist respond).
Items were restricted to centers who had at least one nephrologist or surgeon respond.
Centers used different methods to obtain vital signs and weight and to complete physical exam when using telemedicine for donor evaluation. Among 76 centers with a respondent nephrologist or surgeon, 76.3% used self‐reported measures to obtain vital signs and weight, 46.1% used local providers or primary care physicians, 13.2% used laboratory testing facilities, 3.9% used pharmacies, and 23.7% used other methods (e.g., subsequent in‐person visit for testing or evaluation) to obtain these measurements. Moreover, most of these centers opted to complete a physical exam at an in‐person visit before the committee donor approval (69.7%) or waited until after the committee donor approval (32.9%); for example, during preoperative visit. Few centers opted to complete a physical exam by a local provider or primary care physician (9.2%), at another transplant center (5.3%), or other methods (9.2%) (Table 3).
TABLE 3.
Center practices in how to complete physical exam when using telemedicine and provider willingness to accept a remote completion of a physical or mental status exam
| Center practices | Centers a (N = 76) | % a |
|---|---|---|
| How centers obtain vital signs and weight when using telemedicine | ||
| Local provider/PCP | 35 | 46.1 |
| Laboratory testing facility | 10 | 13.2 |
| Pharmacy | 3 | 3.9 |
| Self‐reported | 58 | 76.3 |
| Other b | 18 | 23.7 |
| Missing | 7 | 9.2 |
| How centers complete a physical exam when using telemedicine | ||
| Local provider/PCP | 7 | 9.2 |
| Other transplant center | 4 | 5.3 |
| Your center in‐person visit, before committee donor approval | 53 | 69.7 |
| Your center in‐person visit, after committee donor approval | 25 | 32.9 |
| Other c | 7 | 9.2 |
| Missing | 7 | 9.2 |
| Provider willingness | Providers | % |
|---|---|---|
| Nephrologist or surgeon willingness to accept remote completion of a physical exam | N = 116 | |
| Yes | 58 | 50.0 |
| No d | 54 | 46.6 |
| Missing | 4 | 3.4 |
| Psychologist/psychiatrist or social worker/ILDA willingness to accept remote completion of a mental status exam for potential donors | N = 82 | |
| Yes | 56 | 68.3 |
| No e | 17 | 20.7 |
| Missing | 9 | 11.0 |
Abbreviations: ILDA, independent living donor advocate; PCP, primary care physician.
Categories were not mutually exclusive, and percentages may exceed more than 100%.
Other included: subsequent in‐person visit for testing or evaluation, miscellaneous.
Other included: medical examination via video, in‐person medical visit, other.
Reasons included: inadequate information (n = 35, 64.8%), personal preferences (n = 32, 59.3%), communication issues between PCP/local providers and transplant centers (n = 20, 37.0%), and other reasons (e.g., ethical, legal, or quality concerns; patient‐focused concerns) (n = 15, 27.8%).
Reasons included: inadequate information (n = 12, 70.6%), personal preferences (n = 9, 52.9%), communication issues between PCP/local providers and transplant centers (n = 6, 35.3%), and other reasons (e.g., quality concerns, miscellaneous) (n = 4, 23.5%).
3.4. Center practices using telemedicine for post‐donation follow‐up
Centers' usage of telemedicine modalities to conduct post‐donation follow‐up care varied over the first 2 years as mandated by the Organ Procurement and Transplantation Network (OPTN) and beyond this mandated policy. 48 Among 76 centers with a respondent nephrologist or surgeon, telephone, and video were mostly used for post‐donation follow‐up at 6 months (35.5%), at 1 year (31.6%), at 2 years (30.3%), and beyond 2 years (21.1%) (Table 2).
3.5. Provider willingness to accept remote completion of a physical or mental status exam
Regarding the acceptance of remote completion of a physical exam for potential donors (e.g., via local provider/PCP or other transplant center): 50% (n = 58/116) of nephrologists and surgeons were willing to accept a remote completion of a physical exam. Of the remaining half who were not willing to accept a remote completion of a physical exam, reasons included inadequate information (64.8%), personal preferences (59.3%), communication issues between local providers or primary care physicians (37.0%), or other reasons (27.8%) (e.g., ethical, legal or quality concerns, and patient‐focused concerns) (Table 3).
Regarding the acceptance of remote completion of a mental status exam for potential donors (e.g., via local psychiatrist/psychologist or other transplant center): 68.3% (n = 56/82) of psychologists and psychiatrists and social workers or ILDAs were willing to accept a remote completion of a mental status exam. Of the remaining 20.7% (n = 17/82) who were not willing to accept a remote completion of a mental status exam, reasons included inadequate information (70.6%), personal preferences (52.9%), communication issues between local providers or primary care physicians (35.3%), or other reasons (23.5%) (e.g., quality concerns) (Table 3).
3.6. Provider perceived barriers to starting or expanding telemedicine
Perceived policy and regulatory barriers that were reported by multidisciplinary providers (N = 293) included: out‐of‐state licensing (65.5%), Medicare reimbursement (34.8%) and private payor reimbursement (29.0%), legal regulations (29.0%), restrictions regarding new or established patients (27.0%), Medicare geographic restrictions (22.2%), and other factors (6.8%) (e.g., donor‐patient‐related concerns, regulatory restrictions) (Table 4).
TABLE 4.
Provider perceived barriers and challenges regarding telemedicine, according to role
| Barriers and challenges | Providers N (%) a | |||||
|---|---|---|---|---|---|---|
| Overall (N = 293) | Nephrologist (N = 65) | Surgeon (N = 51) | Coordinator (N = 56) | Social worker/ILDA (N = 58) | Psychiatrist/psychologist (N = 24) | |
| Perceived policy/regulatory barriers to starting or expanding telemedicine at center's living kidney donor center | ||||||
| Out‐of‐state licensing | 192 (65.5) | 52 (80.0) | 32 (62.7) | 34 (60.7) | 31 (53.4) | 21 (87.5) |
| Medicare geographic restrictions | 65 (22.2) | 26 (40.0) | 9 (17.6) | 9 (16.1) | 6 (10.3) | 6 (25.0) |
| Medicare reimbursement | 102 (34.8) | 26 (40.0) | 24 (47.1) | 19 (33.9) | 9 (15.6) | 11 (45.8) |
| Private payor reimbursement | 85 (29.0) | 25 (38.5) | 21 (41.2) | 14 (25.0) | 7 (12.1) | 9 (37.5) |
| Restrictions regarding new or established patients | 79 (27.0) | 23 (35.4) | 16 (31.4) | 12 (21.4) | 9 (15.6) | 6 (25.0) |
| Legal regulations (e.g., institutional risk management) | 85 (29.0) | 26 (40.0) | 17 (33.3) | 16 (28.6) | 10 (17.2) | 7 (29.2) |
| Other b | 20 (6.8) | 3 (4.6) | 3 (5.9) | 6 (10.7) | 5 (8.6) | 0 (0.0) |
| Missing | 37 (12.6) | 2 (3.1) | 4 (7.8) | 6 (10.7) | 16 (27.6) | 2 (8.3) |
| Perceived logistical barriers to starting or expanding telemedicine at center's living kidney donor center | ||||||
| Lack of institutional incentives | 44 (15.0) | 13 (20.0) | 11 (21.6) | 5 (8.9) | 7 (12.1) | 6 (25.0) |
| Cost of telemedicine infrastructure | 19 (6.5) | 3 (4.6) | 5 (9.8) | 4 (7.1) | 3 (5.2) | 1 (4.2) |
| Insufficient staff/administrative support | 51 (17.4) | 12 (18.5) | 18 (35.3) | 4 (7.1) | 8 (13.8) | 4 (16.7) |
| Communication technology issues | 117 (39.9) | 25 (38.5) | 20 (39.2) | 23 (41.1) | 24 (41.4) | 12 (50.0) |
| Provider comfort with using telemedicine | 71 (24.2) | 18 (27.7) | 14 (27.5) | 19 (33.9) | 9 (15.5) | 4 (16.7) |
| Patient privacy | 22 (7.5) | 1 (1.5) | 3 (5.9) | 5 (8.9) | 9 (15.5) | 1 (4.2) |
| Patient language barrier | 63 (21.5) | 13 (20.0) | 14 (27.5) | 14 (25.0) | 12 (20.7) | 1 (4.2) |
| Patient access to internet/electronic device | 194 (66.2) | 43 (66.2) | 35 (68.6) | 35 (62.5) | 41 (70.7) | 17 (70.8) |
| Other c | 70 (23.9) | 19 (29.2) | 8 (15.7) | 17 (30.4) | 13 (22.4) | 6 (25.0) |
| Missing | 18 (6.1) | 2 (3.1) | 5 (9.8) | 6 (10.7) | 2 (3.4) | 1 (4.2) |
Categories were not mutually exclusive, and percentages may exceed more than 100%.
Categories included: personal preference, donor‐patient–related concerns, regulatory restrictions, logistical issues, lack of interest, no barriers, unknown.
Categories included: in‐person exam needed or preferred, regulatory and licensing concerns, patient preference and willingness, patient needs to come in for an in‐person evaluation, ease of access of telemedicine, lack of technology allocated to telemedicine, no barriers, miscellaneous.
Perceived logistical barriers that were reported by multidisciplinary providers (N = 293) included: patient access to internet/electronic device (66.2%), communication technology issues (39.9%), provider comfort with using telemedicine (24.2%), patient language barriers (21.5%), and other factors (23.9%) (e.g., in‐person exam needed or preferred, patient preference) (Table 4).
3.7. Provider attitudes toward using telemedicine
Respondents strongly agreed that telemedicine was convenient for donors (mean response on 5‐point Likert scale, standard deviation [SD]: 4.5 [0.7]), and somewhat agreed that telemedicine was accessible for donors (mean response: 4.2 [0.8]), equitable for donors (mean response: 3.6 [1.2]), and efficient for transplant centers (mean response: 4.1 [0.9]).
Moreover, respondents strongly agreed that telemedicine will improve the likelihood of completing donor evaluation and counseling for donors who have limited access to a transplant center (mean response: 4.5 [0.8]). Respondents somewhat agreed that telemedicine will improve the likelihood of completing donor evaluation and counseling for donors who reside out‐of‐state (mean response: 4.3 [1.0]) or in the same state (mean response: 3.8 [1.0]) where the transplant center is located, donors who have limited financial/job support (mean response: 3.8 [1.1]) or limited social/caregiving support (mean response: 3.5 [1.2]). However, respondents neither agreed nor disagreed that telemedicine will help donors who have relative contraindications, that is, marginal donors (mean response: 3.3 [1.2]). Overall, attitudes were consistent across provider roles (p > .05) (Table 5).
TABLE 5.
Provider attitudes regarding telemedicine, according to role
| Attitudes | Providers, mean (SD) | |||||
|---|---|---|---|---|---|---|
| Overall (N = 293) | Nephrologist (N = 65) | Surgeon (N = 51) | Coordinator (N = 56) | Social worker/ILDA (N = 58) | Psychiatrist/psychologist (N = 24) | |
| Regarding whether telemedicine is a : | ||||||
| Accessible for donors | 4.2 (0.8) | 4.3 (0.7) | 4.3 (0.8) | 4.1 (0.9) | 4.2 (0.8) | 4.2 (0.9) |
| Convenient for donors | 4.5 (0.7) | 4.6 (0.6) | 4.5 (0.6) | 4.6 (0.7) | 4.5 (0.7) | 4.7 (0.6) |
| Equitable for donors (i.e., care that does not vary in quality because of age, gender, ethnicity, SES, etc.) | 3.6 (1.2) | 3.5 (1.2) | 3.5 (1.1) | 4.1 (1.0) | 3.6 (1.2) | 3.5 (1.3) |
| Efficient for transplant centers | 4.1 (0.9) | 4.0 (0.9) | 4.2 (0.8) | 4.0 (1.0) | 4.1 (1.0) | 4.4 (0.9) |
| Regarding whether telemedicine will improve the likelihood of completing donor evaluation and counseling for potential donors who a : | ||||||
| Have limited access to a transplant center (e.g., distance) | 4.5 (0.8) | 4.5 (0.7) | 4.6 (0.6) | 4.5 (0.8) | 4.5 (0.9) | 4.3 (1.0) |
| Have limited social/caregiving support | 3.5 (1.2) | 3.7 (1.2) | 3.6 (1.1) | 3.6 (1.2) | 3.3 (1.2) | 3.5 (1.2) |
| Have limited financial/job support | 3.8 (1.1) | 3.9 (1.0) | 3.9 (0.9) | 4.0 (1.0) | 3.7 (1.2) | 3.9 (1.0) |
| Reside in the same state as the transplant center | 3.8 (1.0) | 3.7 (1.0) | 3.9 (0.8) | 3.8 (1.0) | 3.9 (1.0) | 4.0 (1.1) |
| Reside out‐of‐state of the transplant center | 4.3 (1.0) | 4.4 (0.9) | 4.4 (0.9) | 4.6 (0.8) | 4.2 (1.2) | 4.0 (1.2) |
| Have relative contraindications (i.e., marginal donors) | 3.3 (1.2) | 3.3 (1.2) | 3.2 (1.1) | 3.6 (1.2) | 3.2 (1.2) | 3.2 (1.2) |
Abbreviations: SD, standard deviation; SES, socioeconomic status.
Five‐point Likert scale: 1 = Strongly disagree, 2 = Somewhat disagree, 3 = Neither disagree nor agree, 4 = Somewhat agree, 5 = Strongly agree.
3.8. Provider willingness to use telemedicine beyond the COVID‐19 pandemic
Respondents were very willing to use telemedicine beyond the pandemic for counseling of potential donors (mean response: 4.5 [0.8]) and post‐donation follow‐up care (mean response: 4.6 [0.7]), and were somewhat willing to use telemedicine for the initial evaluation (mean response: 4.1 [1.2]) or psychiatric or psychological evaluation (mean response: 4.1 [1.1]). However, they were undecided whether they would use telemedicine to conduct a limited physical exam (mean response: 3.4 [1.3]) (Figure 2).
FIGURE 2.
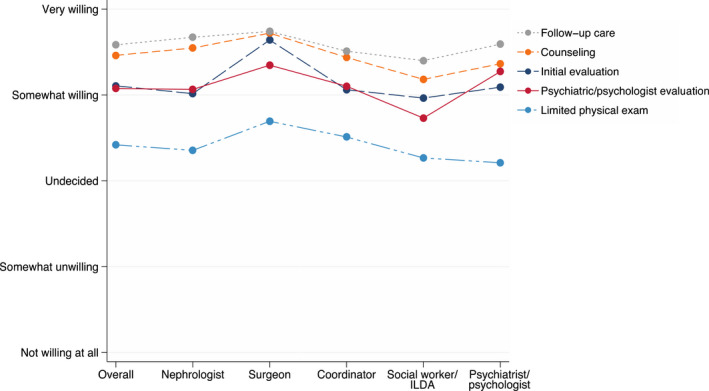
Mean response of multidisciplinary providers regarding their willingness to use telemedicine services for living kidney donation beyond the COVID‐19 pandemic. Overall, respondents were very willing to use telemedicine for counseling of potential donors (mean response [standard deviation]: 4.5 [0.8]) and post‐donation follow‐up care (mean response: 4.6 [0.7]). Respondents were somewhat willing to use telemedicine for the initial evaluation (mean response: 4.2 [1.2]) or psychiatric or psychological evaluation (mean response: 4.1 [1.1]). However, respondents were undecided whether they would use telemedicine to conduct a limited physical exam (mean response: 3.4 [1.3]). ILDA, independent living donor advocate. [Color figure can be viewed at wileyonlinelibrary.com]
Across specialties, surgeons compared to other respondents were the most willing to use telemedicine for the initial evaluation (mean response: 4.6 [0.7], p = .02) and counseling (mean response: 4.7 [0.5], p = .02). Otherwise, attitudes were consistent across provider roles (p > .05) (Figure 2).
4. DISCUSSION
In this US survey of multidisciplinary providers, we found heterogeneity in current practice using telemedicine for living kidney donation. For donor evaluation and counseling, video was mostly used by nephrologists, surgeons, and psychiatrists/psychologists. Telephone and video were mostly used by social workers, while video or telephone was equally used by coordinators. Furthermore, centers employed various methods to complete a physical exam of a potential donor. Remarkably, more than two thirds of living donor kidney transplant centers plan to continue using telemedicine for donor evaluation and/or follow‐up care beyond the pandemic. Providers across roles agreed that telemedicine was convenient, accessible, and equitable for donors and efficient for transplant centers. Our results highlight the potential donors that may benefit most from telemedicine services, including those who have limited access (e.g., distance) to a transplant center, have limited financial or caregiving support, or reside out‐of‐state. While providers were favorably disposed to use telemedicine beyond the pandemic, they noted out‐of‐state licensing as a key barrier limiting the scope of this practice. These results provide insights to inform clinical practice and policy to help advance telemedicine services for living kidney donation.
Our study underlines that telemedicine visits are not a complete replacement for in‐person visits for donor evaluation because of the limited physical exam. Nearly half of respondent nephrologists and surgeons were not willing to accept a remote completion of a physical exam. Conversely, nearly 70% of respondent psychiatrists or psychologists and social workers or ILDAs were willing to accept a remote completion of a mental status exam, which parallels the increasing adoption of telemedicine by psychiatrists. 49 Additionally, we found that providers across their roles were undecided as to whether they would use telemedicine to conduct a limited physical exam. This finding is relatively consistent with primary care providers' view that they have doubt about the video examination; however, unlike donors, this view was especially for patients with more complicated chief symptoms where diagnosis is largely based on the physical exam. 41 Although donors are typically healthy, providers still prefer an independent personally conducted physical exam. This reflects historical perspectives of medical practices regarding provider comfort levels, and perhaps the avoidance of medical‐legal issues. It is worth noting that potential donors undergo rigorous laboratory and imaging screenings, including electrocardiogram, chest X‐ray, and abdomen and pelvic imaging (e.g., computed tomography [CT] scan). Nevertheless, there are proposed strategies to conduct a limited physical exam virtually or involve a primary care provider locally. 37 , 43 , 50 Some educational programs are available to learn best practices for performing virtual physical exams. 51 Training to perform a virtual physical exam can to be part of the medical education curriculum for students, and continuing medical education for providers. Innovations are needed to overcome the telemedicine constraint of a physical exam.
Our results are consistent with prior studies about logistical barriers to telemedicine and that patient access to internet/electronic device remains a common hurdle. 27 , 40 , 41 Herein, we add that policy and regulatory restrictions are key barriers that limit expanding telemedicine services, particularly insurer policy and out‐of‐state licensing. Historically, Medicare restricted services with respect to telemedicine services prior to the COVID‐19 pandemic. It was required that the beneficiary be located in a rural area and travel to specific types of originating sites such as a provider's clinic, hospital, or skilled nursing facility to receive telemedicine services from a provider in a remote location. In 2019, Medicare allowed for few exceptions regarding geographic and originating site requirements for certain telemedicine services, which included monthly kidney failure clinical assessment visits for patients receiving home dialysis. 52 , 53 On March 6, 2020, the Centers for Medicare and Medicaid Services (CMS) broadened access to telemedicine services under the Coronavirus Preparedness and Response Supplemental Appropriations Act and Section 1135 waiver authority. 54 , 55 , 56 As such, patients outside rural areas and in their homes became eligible for telemedicine services. Moreover, Medicare now reimburses telemedicine visits across states and at the same rate as if these visits were in‐person, irrespective of patient location and prior existing relationship with the provider. 32 Various private payors have adopted a similar policy to CMS. 57 In the context of transplantation, centers can lose the facility fee when using telemedicine for clinic visits; however, this financial disincentive is not applicable to pre‐transplant evaluations since the insurer reimburses transplant centers for organ acquisition costs. Specifically, for the medical evaluation of potential donors in anticipation of a kidney donation, costs of all hospital and physician services are considered kidney acquisition costs. 58
Furthermore, the individual state's licensing requirements strikingly overrule the CMS waivers to broadening telemedicine services across states; therefore, out‐of‐state licensing becomes a major barrier hindering the evaluation of potential donors. 32 Taken together with the increased mortality risk for waitlisted kidney transplant candidates, it is vital to expedite the evaluation process for living donor kidney transplantation given that potential donors and recipients may come from different states. 28 , 32 Additionally, kidney paired donation is increasingly adopted to allow the exchange of kidneys between two or more ABO‐ or tissue‐incompatible donor/recipient pairs across the United States. 22 , 59 , 60 Although several states reactively issued waivers for out‐of‐state providers to practice telemedicine under the Public Health Emergency (PHE), many of these waivers have expired affecting new and established patients. 32 Our study calls for legislations to allow out‐of‐state transplant providers to practice telemedicine across states, aligning with the Advancing American Kidney Health initiative to increase access to kidney transplantation. 61
Our findings must be framed in context of their limitations. Our sampling methods could have introduced non‐response bias. However, (1) we achieved a high center representation rate, reflecting the majority of living kidney donation practice nationally and corresponds to centers located in nearly all regions of the United States; (2) centers that were not represented in our survey performed a small volume of living donor kidney transplants; (3) participants who were excluded due to inadequate responses (N = 60) were similar to the study respondents; (4) recognizing that there was a disproportion in the representation of different providers involved in the evaluation process of living kidney donors, respondents reported attitudes were consistent across provider roles. We acknowledge that telemedicine does not remove all barriers that have traditionally sidelined many of disadvantaged individuals who lack the means (e.g., technology literacy, reliable internet connection, and sufficient electronic device) to take advantage of this option to access healthcare providers. That said, the major strengths of our study are the inclusion of multidisciplinary providers involved in the complex multiphase process of living kidney donation and the high representation of centers, allowing for broadly generalizable findings across the country. To emphasize, our findings systematically capture providers’ perceptions at a national level to provide scientific evidence as the first step to effectively understand how we can advance the use telemedicine in clinical practice and inform policy to expand telemedicine services for kidney transplantation.
In conclusion, our findings provide the most comprehensive assessment of telemedicine practice for living kidney donation in the United States. and offer critical information to help inform telemedicine practices and policies to overcome geographic, financial, and logistical challenges that face prospective living kidney donors. Our results underscore the instigation of policies and regulations that support expanding telemedicine services beyond the PHE to enhance access to living donor kidney transplantation and improve care of donors. In light of the multidisciplinary nature of living kidney donation, our findings may extend to other medical specialties.
Funding information
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (grants K23DK129820 [Al Ammary] and R01DK120551 [Lentine]). The funding sources had no role in the design, conduct, or reporting of the study or the decision to publish the manuscript.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This manuscript is a work product of the American Society of Transplantation's Living Donor Community of Practice. The authors are volunteer members of the AST Living Donor Community of Practice. The authors thank the AST Executive Committee, the AST Education Committee, and the AST Board of Directors for review and feedback. We also thank survey respondents, including members of the following professional society listservs: AST Living Donor Community of Practice, AST Kidney‐Pancreas Community of Practice, AST Advanced Practice Providers Community of Practice, AST Psychosocial and Ethics Community of Practice, ASTS, NATCO, STSW, and Academy of Consultation‐Liaison Psychiatry Transplant Psychiatry SIG.
Al Ammary F, Motter JD, Sung HC, et al. Telemedicine services for living kidney donation: A US survey of multidisciplinary providers. Am J Transplant. 2022;22:2041‐2051. doi: 10.1111/ajt.17093
REFERENCES
- 1. Moore DR, Serur D, Rudow DL, Rodrigue JR, Hays R, Cooper M. Living donor kidney transplantation: improving efficiencies in live kidney donor evaluation–recommendations from a consensus conference. Clin J Am Soc Nephrol. 2015;10(9):1678‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lentine KL, Kasiske BL, Levey AS, et al. KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Transplantation 2017;101(8S Suppl 1):S1‐S109, 1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tushla L, Rudow DL, Milton J, Rodrigue JR, Schold JD, Hays R. Living‐donor kidney transplantation: reducing financial barriers to live kidney donation—recommendations from a consensus conference. Clin J Am Soc Nephrol. 2015;10(9):1696‐1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gill J, Dong J, Gill J. Population income and longitudinal trends in living kidney donation in the United States. J Am Soc Nephrol. 2015;26(1):201‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar K, Tonascia JM, Muzaale AD, et al. Racial differences in completion of the living kidney donor evaluation process. Clin Transpl. 2018;32(7):e13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodrigue JR, Schold JD, Morrissey P, et al. Predonation direct and indirect costs incurred by adults who donated a kidney: findings from the KDOC study. Am J Transplant. 2015;15(9):2387‐2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Przech S, Garg AX, Arnold JB, et al. Financial costs incurred by living kidney donors: a prospective cohort study. J Am Soc Nephrol. 2018;29(12):2847‐2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Habbous S, Arnold J, Begen MA, et al. Duration of living kidney transplant donor evaluations: findings from 2 multicenter cohort studies. Am J Kidney Dis. 2018;72(4):483‐498. [DOI] [PubMed] [Google Scholar]
- 9. Mandelbrot DA, Reese PP, Garg N, et al. KDOQI US commentary on the 2017 KDIGO clinical practice guideline on the evaluation and care of living kidney donors. Am J Kidney Dis. 2020;75(3):299‐316. [DOI] [PubMed] [Google Scholar]
- 10. Garg N, Lentine KL, Inker LA, et al. The kidney evaluation of living kidney donor candidates: US practices in 2017 Am J Transplant. 2020;20:3379‐3389. [DOI] [PubMed] [Google Scholar]
- 11. Shukhman E, Hunt J, LaPointe‐Rudow D, et al. Evaluation and care of international living kidney donor candidates: strategies for addressing common considerations and challenges. Clin Transpl. 2020;34(3):e13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long‐term survival following live kidney donation. JAMA. 2010;303(10):959‐966. [DOI] [PubMed] [Google Scholar]
- 13. Muzaale AD, Massie AB, Wang MC, et al. Risk of end‐stage renal disease following live kidney donation. JAMA. 2014;311(6):579‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mjoen G, Hallan S, Hartmann A, et al. Long‐term risks for kidney donors. Kidney Int. 2014;86(1):162‐167. [DOI] [PubMed] [Google Scholar]
- 15. Lentine KL, Schnitzler MA, Xiao H, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363(8):724‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garg AX, McArthur E, Lentine KL. Donor nephrectomy outcomes research N. gestational hypertension and preeclampsia in living kidney donors. N Engl J Med. 2015;372(15):1469‐1470. [DOI] [PubMed] [Google Scholar]
- 17. Al Ammary F, Luo X, Muzaale AD, et al. Risk of ESKD in older live kidney donors with hypertension. Clin J Am Soc Nephrol. 2019;14(7):1048‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doshi MD, Ortigosa‐Goggins M, Garg AX, et al. APOL1 genotype and renal function of black living donors. J Am Soc Nephrol. 2018;29(4):1309‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duerinckx N, Timmerman L, Van Gogh J, et al. Predonation psychosocial evaluation of living kidney and liver donor candidates: a systematic literature review. Transpl Int. 2014;27(1):2‐18. [DOI] [PubMed] [Google Scholar]
- 20. Dew MA, Jacobs CL, Jowsey SG, et al. Guidelines for the psychosocial evaluation of living unrelated kidney donors in the United States. Am J Transplant. 2007;7(5):1047‐1054. [DOI] [PubMed] [Google Scholar]
- 21. Al Ammary F, Bowring MG, Massie AB, et al. The changing landscape of live kidney donation in the United States from 2005 to 2017. Am J Transplant. 2019;19(9):2614‐2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al Ammary F, Yu Y, Ferzola A, et al. The first increase in live kidney donation in the United States in 15 years. Am J Transplant. 2020;20(12):3590‐3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waterman AD, Dew MA, Davis CL, et al. Living‐donor follow‐up attitudes and practices in U.S. kidney and liver donor programs. Transplantation. 2013;95(6):883‐888. [DOI] [PubMed] [Google Scholar]
- 24. Schold JD, Buccini LD, Rodrigue JR, et al. Critical factors associated with missing follow‐up data for living kidney donors in the United States. Am J Transplant. 2015;15(9):2394‐2403. [DOI] [PubMed] [Google Scholar]
- 25. Henderson ML, Thomas AG, Shaffer A, et al. The National Landscape of living kidney donor follow‐up in the United States. Am J Transplant. 2017;17(12):3131‐3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Al Ammary F, Thomas AG, Massie AB, et al. The landscape of international living kidney donation in the United States. Am J Transplant. 2019;19(7):2009‐2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lentine KL, Vest LS, Schnitzler MA, et al. Survey of US living kidney donation and transplantation practices in the COVID‐19 era. Kidney Int Rep. 2020;5(11):1894‐1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al Ammary F, Concepcion BP, Yadav A. The scope of telemedicine in kidney transplantation: access and outreach services. Adv Chronic Kidney Dis. 2021;28(6):542‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Forbes RC, Concepcion BP. Use of telehealth to expand living kidney donation and living kidney donor transplantation. Curr Transplant Rep. 2020;7(2):56‐61. [Google Scholar]
- 30. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154‐161. [DOI] [PubMed] [Google Scholar]
- 31. Reed ME, Parikh R, Huang J, Ballard DW, Barr I, Wargon C. Real‐time patient‐provider video telemedicine integrated with clinical care. N Engl J Med. 2018;379(15):1478‐1479. [DOI] [PubMed] [Google Scholar]
- 32. Al Ammary F, Sidoti C, Segev DL, Henderson ML. Health care policy and regulatory challenges for adoption of telemedicine in kidney transplantation. Am J Kidney Dis. 2021;77(5):773‐776. [DOI] [PubMed] [Google Scholar]
- 33. Shachar C, Engel J, Elwyn G. Implications for telehealth in a postpandemic future: regulatory and privacy issues. JAMA. 2020;323(23):2375‐2376. [DOI] [PubMed] [Google Scholar]
- 34. Mehrotra A, Bhatia RS, Snoswell CL. Paying for telemedicine after the pandemic. JAMA. 2021;325(5):431‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yadav A, Caldararo K, Singh P. Optimising the use of telemedicine in a kidney transplant programme during the coronavirus disease 2019 pandemic. J Telemed Telecare 2020:1357633X20942632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Concepcion BP, Forbes RC. The role of telemedicine in kidney transplantation: opportunities and challenges. Kidney360. 2020;1(5):420‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yadav A, Singh P. Telehealth use by living kidney donor transplant programs during the COVID‐19 pandemic and beyond: a practical approach. Curr Transplant Rep. 2021;8:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel SY, Mehrotra A, Huskamp HA, Uscher‐Pines L, Ganguli I, Barnett ML. Trends in outpatient care delivery and telemedicine during the COVID‐19 pandemic in the US. JAMA Intern Med. 2020;181:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abuzeineh M, Muzaale AD, Crews DC, et al. Telemedicine in the Care of Kidney Transplant Recipients with Coronavirus Disease 2019: case reports. Transplant Proc. 2020;52(9):2620‐2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boyarsky BJ, Ruck JM, Chiang TP, et al. Evolving impact of COVID‐19 on transplant center practices and policies in the United States. Clin Transpl. 2020;34(12):e14086. [DOI] [PubMed] [Google Scholar]
- 41. Srinivasan M, Asch S, Vilendrer S, et al. Qualitative assessment of rapid system transformation to primary care video Visits at an Academic Medical Center. Ann Intern Med. 2020;173:527‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patient–provider video telemedicine integrated with clinical care: patient experiences. Ann Intern Med. 2019;171(3):222‐224. [DOI] [PubMed] [Google Scholar]
- 43. Policy recommendations to guide the use of telemedicine in primary care settings: an American College of Physicians Position Paper. Ann Intern Med. 2015;163(10):787‐789. [DOI] [PubMed] [Google Scholar]
- 44. Ahmed S, Kelly YP, Behera TR, et al. Utility, appropriateness, and content of electronic consultations across medical subspecialties. Ann Intern Med. 2020;172(10):641‐647. [DOI] [PubMed] [Google Scholar]
- 45. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364(23):2199‐2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burns KE, Duffett M, Kho ME, et al. A guide for the design and conduct of self‐administered surveys of clinicians. Canadian Med Assoc J. 2008;179(3):245‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014;14(8):1723‐1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Organ Procurement and Transplantation Network (OPTN) Policies. Health Resources and Services Administration, U.S. Department of Health & Human Services. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Accessed on November 13, 2021.
- 49. Mansour O, Tajanlangit M, Heyward J, Mojtabai R, Alexander GC. Telemedicine and office‐based care for behavioral and psychiatric conditions during the COVID‐19 pandemic in the United States. Ann Intern Med. 2021;174(3):428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. DeJong C, Lucey CR, Dudley RA. Incorporating a new technology while doing no harm, virtually. JAMA. 2015;314(22):2351‐2352. [DOI] [PubMed] [Google Scholar]
- 51. Video Visits Beyond simple cases. https://www.acponline.org/practice‐resources/business‐resources/telehealth/telemedicine‐201‐education‐program‐moving‐beyond‐adoption‐to‐optimal‐integration‐into‐practice/video‐visits‐beyond‐simple‐cases. Accessed April 9, 2022.
- 52. Lew SQ, Sikka N. Operationalizing telehealth for home dialysis patients in the United States. Am J Kid Dis. 2019;74(1):95‐100. [DOI] [PubMed] [Google Scholar]
- 53. H.R . 1892. Bipartisan budget act of 2018. https://www.congress.gov/bill/115th‐congress/house‐bill/1892/text. Accessed April 9, 2022.
- 54. Public Health Emergency Declarations . https://www.phe.gov/emergency/news/healthactions/phe/Pages/default.aspx. Accessed on April 9, 2022.
- 55. Medicare Telemedicine Health Care Provider Fact Sheet . Expansion of telehealth with 1135 waiver, March 6, 2020. https://www.cms.gov/newsroom/fact‐sheets/medicare‐telemedicine‐health‐care‐provider‐fact‐sheet. Accessed March 20, 2022.
- 56. Coronavirus Preparedness and Response Supplemental Appropriations Act , 2020. https://www.congress.gov/116/plaws/publ123/PLAW‐116publ123.pdf. Accessed May 17, 2020.
- 57. Telehealth Coverage Policies in the time of COVID‐19. The Center for Connected Health Policy. https://www.cchpca.org/resources/covid‐19‐telehealth‐coverage‐policies. Accessed March 17, 2022.
- 58. Centers for Medicare & Medicaid Services (CMS) released a second fiscal year (FY) 2022. Inpatient Prospective Payment System (IPPS) final rule. https://www.govinfo.gov/content/pkg/FR‐2021‐12‐27/pdf/2021‐27523.pdf. Accessed April 10, 2022.
- 59. Chipman V, Cooper M, Thomas AG, et al. Motivations and outcomes of compatible living donor‐recipient pairs in paired exchange. Am J Transplant. 2021;22:266‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Treat E, Chow EKH, Peipert JD, et al. Shipping living donor kidneys and transplant recipient outcomes. Am J Transplant. 2018;18(3):632‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Executive order on Advancing American Kidney Health . https://www.federalregister.gov/documents/2019/07/15/2019‐15159/advancing‐american‐kidney‐health. Accessed January 10, 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


