Abstract
Maternal sepsis results in poor outcomes such as fetal or maternal death. The incidence and mortality rates of maternal sepsis vary in different places because of differences in economic development, race and medical conditions. Identifying the clinical features and determining possible mechanisms for avoiding morbidity and preventing poor outcomes would benefit committed patients. Therefore, this was an epidemiological study at a maternity transfer center in Southeast China that aimed to identify local disease features of maternal sepsis. To investigate the incidence and risk factors associated with maternal sepsis and its progression to severe sepsis in a large population-based birth cohort. This local epidemiological study was conducted in at a tertiary care center in Guangzhou, China, from 2015 to 2019. A total of 74,969 pregnant women experiencing childbirth were included in this study; Of these, 74 patients with maternal sepsis were diagnosed according to the sepsis criterion, and 118 patients without sepsis in the same period were selected randomly as the control group to study possible reasons for postpartum sepsis. This retrospective analysis covered the entire period from the first trimester to puerperium. Clinical data were collected using the hospital’s electronic medical record system. Multivariate logistic regression was used to analyze risk factors for maternal sepsis. The incidences of maternal sepsis, the maternal mortality, and the fetal mortality were 0.099%, 0.004%, and 0.007%, respectively. Septic shock was associated with a higher severity of illness. All poor outcomes (maternal or fetal death) occurred during pregnancy. Postpartum sepsis had the longest onset period, and was associated with premature rupture of fetal membranes and preeclampsia. Sepsis is an important cause of both maternal and fetal mortality. Herein, we describe an epidemiological study that evaluated the incidence, development, and prognosis of local maternal sepsis. Furthermore, the characteristics of maternal sepsis are likely due to unknown pathological mechanisms, and patients would benefit from identifying more effective treatments for maternal sepsis.
Keywords: bad outcome, epidemiology, maternal sepsis, organism isolation, Southeast China
Key Points
What is Already Known on this Subject?
Sepsis has a high incidence and mortality rate in the general population.
What do the Results of this Study Add?
We studied the incidence, risk factors, and types of pathogenic microorganisms associated with sepsis throughout pregnancy in a tertiary referral center in southern China
What are the Implications of these Findings for Clinical Practice and/or Further Research?
We demonstrated the clinical characteristics of maternal sepsis in Guangzhou and isolated the organisms at different times of infection. Furthermore, some useful data were identified, including poor outcomes during pregnancy and the associated reasons for postpartum sepsis. This study will help affected patients by demonstrating the pathological features of the local maternal sepsis process and identifying effective treatment options. It also helps reduce the incidence rate and mortality of sepsis in pregnant women.
1. Introduction
Sepsis is defined as “a severe, potentially fatal, organic dysfunction caused by an inadequate or dysregulated host response to infection” by the latest consensus,[1] and it seriously threatens global human health because of its high incidence and mortality.[2] According to ongoing epidemiological investigations, the incidence and mortality of sepsis continue to decline annually, and the severity of sepsis is associated with economic development of the affected population.[2] Maternal sepsis develops during pregnancy and puerperium, and poor outcomes include maternal or fetal mortality. In the literature, global epidemiological descriptions of maternal sepsis are lacking, and the incidences in different countries are similar (0.1%–0.3%).[3,4] It is alarming that the incidences is consistently increasing yearly in the United Kingdom[5] and the United States of America.[6] Compared to the case fatality rate of sepsis (nearly 20%),[7,8] the fatality rate of maternal sepsis is extremely low. Epidemiological reports have suggested that future studies should focus on a long time period (minimum 5-year time span) to define epidemiological characteristics,[9] but the mortality rate was reported to have decreased in the latest study.[10] At the same time, there are few data covering the entire pregnancy and puerperium period. However, complex factors that affect maternal sepsis initiation, development and prognosis, including race, climate, economic development, and medical and healthcare conditions, still required further study. Our retrospective epidemiological study could benefit both local and regional populations.
To analyze maternal sepsis in our location, this retrospective study focused on the characteristics and factors associated with maternal sepsis. According to the latest definitions and classifications of sepsis, the diagnoses are sepsis and septic shock[1] and maternal sepsis.[11] Microbial culture and isolation can be obtained from body fluids,[12] and the microbial isolates were the most concerning factor in a previous maternal sepsis study.[3] The organisms vary during different onset times, leading to different disease outcomes. The most serious risk factor was caused by Group A Streptococcus.[6,13] Previous epidemiological research on maternal sepsis has focused on a single period in the pregnancy (i.e., the intrapartum or antenatal period)[14] or on the puerperium (i.e., the postnatal period).[15,16] Some demographics have also been statistically analyzed, such as age, obesity, mode of delivery and complications, which increase the incidence of maternal sepsis.[3,17]
In this study, we focused on maternal sepsis in a maternity transfer center. Clinical data on the affected cases were collected and summarized to identify their characteristics, and poor outcomes of maternal or fetal death occurred mainly during the antepartum and intrapartum periods.
2. Methods
2.1. Study population
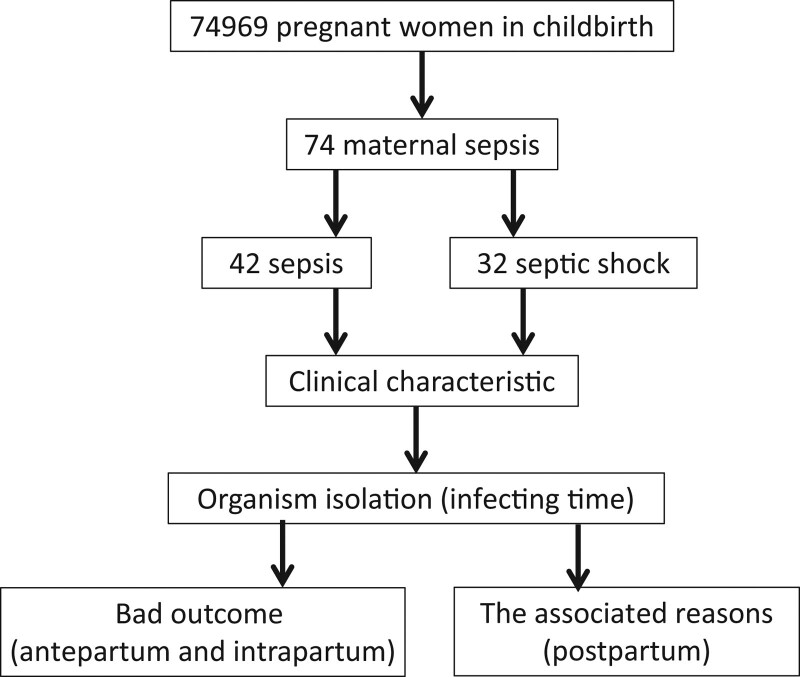
This was a retrospective case-control study conducted at Guangdong Women’s and Children’s Hospital. Ethical approval was obtained from the Institutional Review Board of Guangdong Women and Children’s Hospital, Guangzhou, Guangdong, China. We confirmed that all research was performed in accordance with the relevant guidelines and regulations, and informed consent was obtained from all participants and/or their legal guardians. There were 74,969 pregnant women treated as inpatients for labor and delivery in the hospital from 2015 to 2019. Seventy-four patients with maternal sepsis were diagnosed using the sepsis criterion, and 118 patients without sepsis in the same period were selected randomly as the control group to study the possible reasons for postpartum sepsis. Clinical data were collected during this period, including for all of the pregnant women with microbial infection-induced sepsis (septic shock) and randomly selected control patients. Patients with acute pulmonary embolism, amniotic fluid embolism, adverse drug reactions, drug fever, viral infection, autoimmune conditions, or transfusion reactions were excluded from this study. Patient enrollment flow chart is shown in Figure 1.
Figure 1.
Flow diagram of patient enrollment.
2.2. Clinical data
Sepsis and septic shock were diagnosed according to the criteria of the Third International Consensus Definitions for Sepsis and Septic Shock[1] and SOMANZ guidelines.[18] Sepsis was defined as an acute change in the total Obstetrically Modified Sequential Organ Failure Assessment (omSOFA) score (Table 1) of ≥2 points on sequent to infection. Sepsis-induced shock is characterized by severe clinical symptoms and poor outcomes, and the clinical symptoms could be evaluated by the obstetric-modified quick sequential organ failure assessment (omSOFA) score[18] and the acute physiology and chronic health evaluation II (APACHE II) score.[19] The clinical criteria validated to identify septic shock in pregnant patients include: hypotension requiring vasopressor therapy to maintain a mean arterial pressure ≥65 mm Hg (despite adequate fluid resuscitation) and a serum lactate level >2 mmol/L after adequate fluid resuscitation. Bacterial agents were confirmed by cultures of the blood and other sites as clinically indicated, including urine cultures, vaginal swabs, episiotomy wound swabs, cerebrospinal fluid, respiratory swabs and cesarean wound swabs. Onset timing was classified as antepartum, intrapartum and postpartum. Antepartum was defined as the time from the confirmation of pregnancy to the beginning of labor defined by contractions, intrapartum as the time from the onset of labor to the delivery of the placenta, and postpartum as the 42-day period following the delivery of the placenta, and the intrapartum and antenatal periods are the periods most associated with poor fetal outcomes affecting the fetus. The obstetric information obtained included age, infection source, infection site, gestation/stage at delivery, mode of delivery, maternal complications, maternal ICU (intensive care unit) admission time, and maternal outcome as well as neonatal data including gestational age at delivery, fetal and neonatal outcomes and mode of delivery. APACHE II and omSOFA scores on the first day of ICU admission were recorded to evaluate the severity of illness.
Table 1.
Obstetrically modified SOFA score.
| System parameter | Score | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Respiration | |||
| PaO2/FiO2 | ≥400 | 300 to <400 | <300 |
| Coagulation | |||
| Platelets, ×106/L | ≥150 | 100–150 | <100 |
| Liver | |||
| Bilirubin (μmol/L) | ≤20 | 20–32 | >32 |
| Cardiovascular | |||
| Mean arterial pressure (mm Hg) | MAP ≥ 70 | MAP < 70 | Vasopressors required |
| Central nervous system | Alert | Rousable by voice | Rousable by pain |
| Renal | |||
| Creatinine (μmol/L) | ≤90 | 90–120 | >120 |
SOFA = Sequential Organ Failure Assessment.
The factors associated with postpartum delivery after statistical analysis included mode of delivery, age, body mass index (BMI), primipara, in vitro fertilization (IVF), premature rupture of membrane (PROM), gestational diabetes, and preeclampsia/eclampsia. PROM refers to the disruption of fetal membranes before delivery and is characterized by a painless surge of fluid that leaks out of the vagina. The diagnosis of gestational diabetes was based on the fasting 75 g oral glucose tolerance test during pregnancy and was diagnosed if fasting glucose was ≥5.8, 1-hour glucose was ≥10 mmol/L, or 2-hour glucose was ≥11.1 mmol/L. The diagnosis of preeclampsia required the confirmation of hypertension arising after 20 weeks of gestation on 2 or more occasions and 1 or more of the organ/system features related to the mother and/or fetus.
2.3. Statistical analyses
Demographic, antenatal and intrapartum complications in women with postpartum sepsis were compared with in women without sepsis to identify risk factors. Descriptive statistical analyses were performed using means with standard deviations and medians with range and frequency. Parameters were analyzed using the Fisher exact test, chi-square test, and t test. All P values were 2-sided, and statistical significance was set at P < .05. Risk factors were analyzed using multivariate logistic regression. Multivariable regression results were adjusted for age, BMI, DM, IVF, and all other factors. Results are reported as adjusted odds ratio with 95% confidence intervals. Statistical analyses were performed with SPSS version 22.0.
3. Results
3.1. The incidence of septic shock was associated with the clinical score when sepsis occurred in pregnant women
There were 74969 confined women included in this study from 2015 to 2019. According to the criteria for sepsis and septic shock,[1] 42 pregnant women had sepsis (the incidence was 5.6 per 10,000), and 32 had septic shock (4.3 per 10,000). There were no significant differences between the sepsis and septic shock groups, in terms of age, maternal ICU admission (days), IVF, and poor outcomes (Table 2). There were more cases of septic shock in the antenatal period than in the puerperium period sepsis group (P < .05) (Table 2). Pregnant women with septic shock had higher APACHE II and omSOFA scores than those with sepsis (P < .05) (Table 2).
Table 2.
Clinical information of maternal sepsis.
| Sepsis (n = 42) | Septic shock (n = 32) | OR | 95% CI | P value | Total (n = 74) | ||
|---|---|---|---|---|---|---|---|
| Age (yr) | Mean ± SD | 30.76 ± 5.63 | 30.41 ± 6.549 | 0.99 | 0.916–1.07 | 0.803 | 30.61 ± 6.004 |
| Range | 18–44 | 18–49 | 18–49 | ||||
| Maternal ICU admission (days) | Mean ± SD | 5.48 ± 2.391 | 6.22 ± 6.656 | 1.035 | 0.932–1.15 | 0.516 | 5.8 ± 4.708 |
| Range | 2–11 | 1–40 | 1–40 | ||||
| IVF | n (%) | 5 | 3 | 1.306 | 0.288–5.923 | 0.518 | 8 |
| APACHE II score | Mean ± SD | 10.5 ± 6.05 | 14.22 ± 5.999 | 1.111 | 1.02–1.211 | 0.016 | 12.11 ± 6.267 |
| Range | 1–35 | 2–27 | 1–35 | ||||
| omSOFA score | Mean ± SD | 1.48 ± 1.851 | 2.44 ± 1.848 | 1.339 | 1.016–1.766 | 0.038 | 1.89 ± 1.899 |
| Range | 0–10 | 0–6 | 0–10 | ||||
| Period of onset | |||||||
| Antenatal | n (%) | 12 (28.6) | 19 (59.4) | 9.135 | 2.212–8.207 | 0.004 | 31 (41.9) |
| puerperium | n (%) | 30 (71.4) | 13 (40.6) | ||||
| Poor outcome | |||||||
| Fetal death | n (%) | 3 (7.1) | 6 (18.8) | 3 | 0.688–13.075 | 0.125 | 9 (12.2) |
| Maternal death | n (%) | 1 (2.4) | 2 (6.3) | 2.733 | 0.237–31.555 | 0.398 | 3 (4.1) |
APACHE II = acute physiology and chronic health evaluation II, CI = confidence intervals, ICU = intensive care unit, IVF = in vitro fertilization, omSOFA = obstetric-modified quick sequential organ failure assessment.
3.2. The pathogens were associated with period of onset
Among all isolates, there were 33 postnatal (44.59%), 10 intrapartum (13.51%), and 31 antenatal (41.89%) isolates (Table 3). Two pathogens, were detected at all periods of onset: Escherichia coli and Klebsiella pneumonia (Table 3). The organisms causing sepsis differed over time. Four pathogens were detected in 2 periods, whereas the others were detected in only 1 period. Five pathogens were detected only in the postpartum period, 2 in the intrapartum period, and 8 in the antepartum period, as the organisms of antenatal infection were more varied than those in the postpartum and intrapartum periods (Table 3).
Table 3.
Pathogens of maternal sepsis.
| Organism | Postpartum (n) | Intrapartum (n) | Antepartum (n) | All isolates (n) |
|---|---|---|---|---|
| Escherichia coil | 21 | 3 | 14 | 37 |
| Klebsiella pneumonia | 3 | 2 | 2 | 7 |
| Hemolytic Staphylococcus | 1 | 0 | 1 | 2 |
| Streptococcus gallolyticus | 1 | 0 | 0 | 2 |
| Enterococcus faecalis | 5 | 0 | 0 | 5 |
| Enterococcus avium | 1 | 0 | 0 | 1 |
| Prevotella oralis | 1 | 0 | 0 | 1 |
| Listeria | 0 | 1 | 1 | 2 |
| Mycoplasma pneumoniae | 0 | 1 | 2 | 3 |
| Streptococcus agalactiae | 0 | 1 | 1 | 2 |
| Gardnerella vaginalis | 0 | 1 | 0 | 1 |
| Stenotrophomonas maltophilia | 0 | 1 | 0 | 1 |
| Morganella morganii | 0 | 0 | 1 | 1 |
| Enterobacter cloacae | 0 | 0 | 2 | 2 |
| Pseudomonas putida | 0 | 0 | 1 | 1 |
| Brucella maltese | 0 | 0 | 1 | 1 |
| Streptococcus pneumoniae | 0 | 0 | 1 | 1 |
| Salmonella typhi | 0 | 0 | 1 | 1 |
| Pseudomonas aeruginosa | 0 | 0 | 1 | 1 |
| Aspergillus | 0 | 0 | 1 | 1 |
| Streptococcus hemolyticus | 0 | 0 | 1 | 1 |
| Total | 33 | 10 | 31 | 74 |
3.3. Poor outcomes of sepsis in pregnant women were associated with infection time and organism
Poor outcomes in pregnant women with antepartum infection-induce sepsis included maternal or fetal death. In our study, there were 3 antepartum cases resulting in maternal death (maternal mortality ratio was 0.004%), all of which occurred after 28 weeks, and the fetuses survived by cesarean section. The infection sites and organisms were different in these cases, and the organisms were unique to our study (Table 4). Five antepartum cases resulted in fetal death (fetal mortality ratio: 0.007%), and infection occurred in the first and second trimesters. Three pregnant women with blood infections had unique organisms cultured in our study, and the pathogens of 2 pregnant women with genital tract infections resulted in fetal survival (Table 4).
Table 4.
Poor outcome of maternal sepsis.
| Maternal outcome/fetal outcome | Infecting time | Mode of delivery | Delivery weeks | Infecting weeks | Infection site | Infecting bacteria | Singleton/multiple pregnancy |
|---|---|---|---|---|---|---|---|
| Died/survival | Antepartum | CS | 36+ | 36+ | Blood | Streptococcus hemolyticus | Singleton |
| CS | 34+ | 30+ | Intracranial infection | Streptococcus pneumoniae | Singleton | ||
| CS | 32+ | 32+ | Respiratory tract | Aspergillus | Singleton | ||
| Survival/died | Antepartum | Abortion | 8+ | 8+ | Genital tract | Escherichia coil | Twin |
| Abortion | 24+ | 24+ | Blood | Pseudomonas putida | Singleton | ||
| CS | 22+ | 21+ | Blood | Morganella morganii | Singleton | ||
| CS | 21+ | 21+ | Genital tract | Streptococcus agalactiae | Singleton | ||
| Abortion | 14+ | 12+ | Blood | Brucella maltese | Singleton | ||
| Intrapartum | CS | 25+ | 25+ | Blood | Streptococcus gallolyticus | Singleton | |
| Abortion | 35+ | 36+ | Blood | Enterococcus faecalis | Singleton | ||
| Postpartum | Abortion | 23+ | 23+ | Blood | Streptococcus agalactiae | Singleton | |
| Abortion | 13+ | 13+ | Blood | Escherichia coil | Singleton |
CS = caesarean section.
3.4. Postpartum sepsis cases did not result in maternal death and were associated with pregnancy factors
In the included postpartum sepsis cases, no maternal deaths occurred. Statistical analysis was conducted to determine the correlation between the occurrence of postpartum sepsis and pregnancy factors. Factors that showed no association were cesarean section, age, BMI, IVF, and gestational diabetes. The control group was randomly selected cases by clinical characteristic matched in the same period. There were 46 cases of cesarean section, 12 cases of obesity (BMI ≥ 30 kg/m), 24 cases of age ≥34 years old. In addition, there were 34 primiparas, 10 cases of IVF, 8 cases of PROM, 18 cases of gestational diabetes, and 5 cases of preeclampsia. The factors that showed a significant association were PROM and preeclampsia or eclampsia (Table 5).
Table 5.
Correlation of pregnancy factors and postpartum sepsis.
| Covariate | Postpartum sepsis (n = 33) | Control population (n = 118) | Adjusted odds ratio (95% CI) | P value* |
|---|---|---|---|---|
| Caesarean section | 25 (75.8) | 46 (39.0) | 1.747 (0.684–7.041) | .186 |
| Age ≥ 34 yr | 10 (30.3) | 24 (20.3) | 1.094 (−0.070 to 0.245) | .276 |
| BMI ≥ 30 kg/m2 | 4 (12.1) | 12 (10.2) | 0.320 (−0.182 to 0.252) | .749 |
| Primipara | 22 (66.7) | 34 (28.8) | 2.107 (0.688–6.449) | .192 |
| IVF | 3 (9.1) | 10 (8.5) | 0.111 (−0.225 to 0.252) | .912 |
| PROM | 11 (33.3) | 8 6.8) | 5.498 (1.737–17.407) | .004 |
| Gestational diabetes | 9 (27.3) | 18 (15.3) | 1.596 (−0.033 to 0.313) | .113 |
| Preeclampsia/eclampsia | 2 (6.1) | 5 (4.2) | 3.326 (1.099–10.072) | .033 |
BMI = body mass index, CI = confidence intervals, CS = caesarean section, IVF = in vitro fertilization, PROM = premature rupture of fetal membranes.
Adjusted P value.
4. Discussion
The incidence and mortality of maternal sepsis in different locations shared similarities but were different. The maternal mortality ratio has continued to decrease globally in recent decades, especially in Southeast Asia[20]; however, data on maternal sepsis are lacking. Pregnant women who met the criteria for sepsis had a significant increase in the mortality rate compared with those without sepsis,[10] and a previous study focusing on race, ethnicity, and cases of maternal sepsis indicated that disparities existed.[21] In this study, we documented the incidence (0.099%) and mortality rate (0.004%).The incidence was consistent with that in developed countries but higher than that in West China, and the mortality was the highest.[5,6,10,22] The differences in the clinical manifestations of sepsis in the study population could be due to several reasons. In our previous study, we indicated that genetics was an important factor affecting the incidence, development and prognosis of sepsis,[23] and that genetic differences could be the primary reason for the difference in maternal sepsis by race. China has the largest population in the world; however, epidemiological studies covering the entire scope of maternal sepsis are lacking. Differences in maternal sepsis in various places exist owing to differences in economic development, climate, people’s habits, and customs. Collectively, we demonstrated the clinical characteristics of maternal sepsis in Southeast China, detailed the clinical features of cases with poor outcomes, and determined the possible reasons for the increased incidence of postnatal sepsis. Over seventy thousand clinical data points were collected over 5 years in our study, but this study was limited to transfer center for pregnant women in Southeast China. Further studies are needed to organize a larger study.
The gestation time of maternal sepsis is associated with sepsis severity and isolated pathogenic microorganisms. The maternal period was divided into antepartum, intrapartum, and postpartum periods, as bodily changes in pregnant women may mask signs of sepsis.[24] Rapid diagnosis and management are important to address maternal sepsis.[25] Antepartum was defined as the initial part of pregnancy before birth and was the longest period of the 3; and infections isolated in this period showed a diversity of affected sites and types of microorganisms isolated.[26] In our study, patients with antepartum sepsis had a higher risk of septic shock, and evidence showed that all maternal deaths and most fetal deaths occurred during this period. The puerperium includes the intrapartum and postpartum periods, which is shorter than the antepartum period. Sepsis in this period could be referred to as delivery-associated sepsis, and the risk of fatality in mothers with sepsis was the highest in this period.[10] In our study, the poor outcome of this period was the high loss of fetuses. Gram-negative bacteria are the most common isolates during the whole maternal period, and E coli is a frequently isolated pathogenic microorganism in maternal sepsis.[3,4] The most common gram-positive bacteria isolated were Streptococcus,[27] and group A Streptococcus is regarded as the top risk factor for maternal mortality.[13] In our study, the 3 most common isolates were E coli, K pneumoniae, and Enterococcus faecalis. Two Streptococcus isolates and 1 fungal isolate were found in 3 maternal mortality cases. Fetal loss is a more common issue in maternal sepsis than maternal mortality, and microorganism isolation is diverse.
In our study, obstetric sepsis is most likely to occur in the postpartum period but did not lead to poor outcomes. There were some reasons why maternal sepsis was high during this period, including PROM,[15] diabetes,[28] and mode of delivery.[3] The consistent results of this period suggest a higher risk factor, such as diabetes, being a significant risk factor associated with postpartum sepsis,[15,28] but the opposite also existed, such as cesarean delivery,[3,15] which is a possible reason for the different populations. In the current study, 2 factors were associated with postpartum sepsis: PROM and preeclampsia/eclampsia. PROM is 1 of the common reasons for preterm birth, which can be caused by infection or as a consequence of infection.[29] PROM-associated infections may be the reason for the high incidence of sepsis. Preeclampsia/eclampsia and maternal sepsis are both leading causes of abortion,[30] and additional studies are needed to interpret the causal relationship between them and to develop management protocols to protect mothers against these conditions.
Patients with highly suspected sepsis were given effective antibiotics within 1 hour of diagnosis, in line with the guidelines of SOMANZ guidelines.[18] Patients with septic shock who received aggressive fluid resuscitation also had a good prognosis. However, 3 pregnant women were given effective antibiotics and relevant rescue measures and still died. This suggests that sepsis deaths may also be related to other causes, such as the timing of antibiotic use, the underlying disease of the patient and the type of pathogenic bacteria.
Pregnancy is a miraculous experience; it is the process of birthing a new life, and there could be a rejuvenating effect on the mother.[31] Evidence to confirm this includes the fact that fetal cells transfer to the mother[32] in humans and participate in maternal wound healing[33] in animal studies. Maternal sepsis has a lower mortality rate than other causes of sepsis, even in matched controls[34]; however, due to the characteristics of affected individuals, the precise mechanism is unknown. Pregnancy and childbirth provide medical means to treat sepsis. In a previous study, we demonstrated that mesenchymal stem cells (MSCs) derived from amniotic fluid of the second trimester and Wharton jelly of the umbilical cord showed a curative effect in an experimental sepsis animal model.[35] In maternal sepsis, the hypothesis that internal MSCs associated with the pregnancy and childbirth process relieve the acute inflammatory response requires furth experimental evidence. MSC cyto-therapy is widely accepted as a potential therapeutic strategy to treat acute injury inflammatory diseases, including COVID-19.[36] Although MSCs derived from amniotic fluid of the second trimester have been shown to improve the survival of neonatal sepsis in a rat model,[37] evidence that MSCs from different sources are effective in relieving maternal sepsis is lacking, and further studies are necessary before the clinical use of MSC cryotherapy to treat maternal sepsis.
5. Conclusion
In summary, we demonstrated the clinical characteristics of maternal sepsis in Guangzhou and isolated organisms at different infection times. Furthermore, some useful data were identified, including poor outcomes during pregnancy and associated reasons for postpartum sepsis. This study will help affected patients by demonstrating the pathological features of the local maternal sepsis process and identifying effective treatment options.
Author contributions
Conceptualization: Xuan Zhong.
Data curation: Xuan Zhong, Wenni Zhang, Ding Wang.
Formal analysis: Xuan Zhong.
Funding acquisition: Shan Huang.
Methodology: Xuan Zhong, Rongfeng Lin, Yiping Luo.
Project administration: Yiping Luo.
Resources: Xuan Zhong, Yiping Luo.
Software: Rongfeng Lin.
Writing – original draft: Xuan Zhong, Ding Wang.
Writing – review & editing: Xuan Zhong, Ding Wang.
Abbreviations:
- APACHE II =
- acute physiology and chronic health evaluation II
- BMI =
- body mass index
- ICU =
- intensive care unit
- IVF =
- in vitro fertilization
- MSCs =
- mesenchymal stem cells
- omSOFA =
- obstetrically modified SOFA failure assessment
- PROM =
- premature rupture of membrane
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study. All data generated or analyzed during this study are included in this published article [and its supplementary information files].
This study was supported by Guangdong Education Department Project (2021KTSCX095), the Guangzhou Education Bureau-funded project (No. 202032868), Guangzhou Science and Technology Project (No. 202102010105), Traditional Chinese Medicine Bureau of Guangdong Province (No. 20211045), and Medical Science and Technology Research Fund of Guangdong Province (No. B2019114). The funding sources and sponsors did not participate in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
This study was approved by the institutional review board of Women’s and Children’s Hospital. Written informed consent from participants (or patient family members) was obtained.
Oral and written informed consent was obtained from the participants.
The authors read the final version of the manuscript and approved its submission for publication.
The authors have no conflicts of interest to disclose.
How to cite this article: Zhong X, Lin R, Zhang W, Huang S, Luo Y, Wang D. Epidemiology and clinical features of maternal sepsis: A retrospective study of whole pregnancy period. Medicine 2022;101:40(e30599).
Contributor Information
Xuan Zhong, Email: 290642013@qq.com.
Rongfeng Lin, Email: linrongfeng@gzucm.edu.cn.
Shan Huang, Email: 427941842@qq.com.
Yiping Luo, Email: doctorluoyiping@126.com.
References
- [1].Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Knowles SJ, O’Sullivan NP, Meenan AM, et al. Maternal sepsis incidence, aetiology and outcome for mother and fetus: a prospective study. BJOG. 2015;122:663–71. [DOI] [PubMed] [Google Scholar]
- [4].Surgers L, Valin N, Carbonne B, et al. Evolving microbiological epidemiology and high fetal mortality in 135 cases of bacteremia during pregnancy and postpartum. Eur J Clin Microbiol Infect Dis. 2013;32:107–13. [DOI] [PubMed] [Google Scholar]
- [5].Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203. [DOI] [PubMed] [Google Scholar]
- [6].Bauer ME, Bateman BT, Bauer ST, et al. Maternal sepsis mortality and morbidity during hospitalization for delivery: temporal trends and independent associations for severe sepsis. Anesth Analg. 2013;117:944–50. [DOI] [PubMed] [Google Scholar]
- [7].Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–74. [DOI] [PubMed] [Google Scholar]
- [8].Kaukonen KM, Bailey M, Suzuki S, et al. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–16. [DOI] [PubMed] [Google Scholar]
- [9].Bouvier-Colle MH, Mohangoo AD, Gissler M, et al. What about the mothers? An analysis of maternal mortality and morbidity in perinatal health surveillance systems in Europe. BJOG. 2012;119:880–889; discussion 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kendle AM, Salemi JL, Tanner JP, et al. Delivery-associated sepsis: trends in prevalence and mortality. Am J Obstet Gynecol. 2019;220:391 e391–16. [DOI] [PubMed] [Google Scholar]
- [11].Greer O, Shah NM, Sriskandan S, et al. Sepsis: precision-based medicine for pregnancy and the puerperium. Int J Mol Sci. 2019;20:5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kankuri E, Kurki T, Carlson P, et al. Incidence, treatment and outcome of peripartum sepsis. Acta Obstet Gynecol Scand. 2003;82:730–5. [DOI] [PubMed] [Google Scholar]
- [13].Phillips C, Walsh E. Group A streptococcal infection during pregnancy and the postpartum period. Nursing for women’s health. 2020;24:13–23. [DOI] [PubMed] [Google Scholar]
- [14].Barinov SV, Tirskaya YI, Kadsyna TV, et al. Pregnancy and delivery in women with a high risk of infection in pregnancy. J Matern Fetal Neonatal Med. 2020;35:1–6. [DOI] [PubMed] [Google Scholar]
- [15].Bakhtawar S, Sheikh S, Qureshi R, et al. Risk factors for postpartum sepsis: a nested case-control study. BMC Pregnancy Childbirth. 2020;20:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Woodd SL, Montoya A, Barreix M, et al. Incidence of maternal peripartum infection: a systematic review and meta-analysis. PLoS Med. 2019;16:e1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sriskandan S. Severe peripartum sepsis. J R Coll Physicians Edinb. 2011;41:339–46. [DOI] [PubMed] [Google Scholar]
- [18].Bowyer L, Robinson HL, Barrett H, et al. SOMANZ guidelines for the investigation and management sepsis in pregnancy. Aust N Z J Obstet Gynaecol. 2017;57:540–51. [DOI] [PubMed] [Google Scholar]
- [19].Zabolotskikh IB, Musaeva TS, Denisova EA. [Validity of APACHE II, APACHE III, SAPS 2, SAPS 3 and SOFA scales in obstetric patients with sepsis]. Anesteziol Reanimatol. 2012:55. [PubMed] [Google Scholar]
- [20].Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grobman WA, Bailit JL, Rice MM, et al. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstet Gynecol. 2015;125:1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Duan R, Xu X, Wang X, et al. Perinatal outcome in women with bacterial sepsis: a cross-sectional study from West China. Medicine. 2019;98:e17751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang D, Zhong X, Huang D, et al. Functional polymorphisms of interferon-gamma affect pneumonia-induced sepsis. PLoS One. 2014;9:e87049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guinn DA, Abel DE, Tomlinson MW. Early goal directed therapy for sepsis during pregnancy. Obstet Gynecol Clin North Am. 2007;34:459–479, xi. [DOI] [PubMed] [Google Scholar]
- [25].Galvao A, Braga AC, Goncalves DR, et al. Sepsis during pregnancy or the postpartum period. J Obstet Gynaecol. 2016;36:735–43. [DOI] [PubMed] [Google Scholar]
- [26].Adorno M. Sepsis in the obstetric client. Crit Care Nurs Clin North Am. 2018;30:415–22. [DOI] [PubMed] [Google Scholar]
- [27].Seale AC, Bianchi-Jassir F, Russell NJ, et al. Estimates of the burden of Group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis. 2017;65(suppl_2):S200–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Acosta CD, Knight M, Lee HC, et al. The continuum of maternal sepsis severity: incidence and risk factors in a population-based cohort study. PLoS One. 2013;8:e67175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2001;347:CD001058. [DOI] [PubMed] [Google Scholar]
- [30].Oppong SA, Bakari A, Bell AJ, et al. Incidence, causes and correlates of maternal near-miss morbidity: a multi-centre cross-sectional study. BJOG. 2019;126:755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Falick Michaeli T, Bergman Y, Gielchinsky Y. Rejuvenating effect of pregnancy on the mother. Fertil Steril. 2015;103:1125–8. [DOI] [PubMed] [Google Scholar]
- [32].Mahmood U, O’Donoghue K. Microchimeric fetal cells play a role in maternal wound healing after pregnancy. Chimerism. 2014;5:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nassar D, Droitcourt C, Mathieu-d’Argent E, et al. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J. 2012;26:149–57. [DOI] [PubMed] [Google Scholar]
- [34].Kidson KM, Henderson WR, Hutcheon JA. Case fatality and adverse outcomes are reduced in pregnant women with severe sepsis or septic shock compared with age-matched comorbid-matched nonpregnant women. Crit Care Med. 2018;46:1775–82. [DOI] [PubMed] [Google Scholar]
- [35].Chen R, Xie Y, Zhong X, et al. MSCs derived from amniotic fluid and umbilical cord require different administration schemes and exert different curative effects on different tissues in rats with CLP-induced sepsis. Stem Cell Res Ther. 2021;12:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Coelho A, Alvites RD, Branquinho MV, et al. Mesenchymal stem cells (MSCs) as a potential therapeutic strategy in COVID-19 patients: literature research. Front Cell Dev Biol. 2020;8:602647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sato Y, Ochiai D, Abe Y, et al. Prophylactic therapy with human amniotic fluid stem cells improved survival in a rat model of lipopolysaccharide-induced neonatal sepsis through immunomodulation via aggregates with peritoneal macrophages. Stem Cell Res Ther. 2020;11:300. [DOI] [PMC free article] [PubMed] [Google Scholar]