Abstract
Context
The emergence of COVID-19 caused a new public health crisis, leading to major changes in daily life routines, often including physical activity (PA) levels. The main goal of this study was to analyze the differences in self-reported physical activity of people with complete spinal cord injuries between the time prior to the COVID-19 lockdown and the lockdown period itself.
Methods
A sample of 20 participants with complete thoracic spinal cord injuries completed the Physical Activity Scale for Individuals with Physical Disabilities before and during the COVID-19 lockdown.
Results
The results showed differences between the pre-lockdown and lockdown measurements in total self-reported PA (z=−3.92; P<0.001; d=1.28), recreational PA (z=−3.92; P<0.001; d=1.18) and occupational PA (z=−2.03; P=0.042; d=0.55). Nevertheless, no differences were found in housework PA between the two time periods. Furthermore, the results showed differences in total minutes (z=−3.92; P<0.001; d=1.75), minutes spent on recreational activities (z=−3.82; P<0.001; d=1.56) and minutes spent on occupational activities (z=−2.032; P=0.042; d=0.55) of moderate/vigorous intensity.
Conclusions
Individuals with thoracic spinal cord injuries who were full-time manual wheelchair users displayed lower levels of PA during the pandemic than in the pre-pandemic period. The results suggest that the prohibition and restrictions on carrying out recreational and/or occupational activities are the main reasons for this inactivity. Physical activity promotion strategies should be implemented within this population to lessen the effects of this physical inactivity stemming from the COVID-19 pandemic.
Keywords: COVID-19, Physical activity, Spinal cord injury
Introduction
The world is currently experiencing an extraordinary situation caused by the COVID-19 pandemic, which has led to changes in the lifestyles of the worldwide population, and possibly also in their physical activity (PA) levels. This may lead to some negative health effects. Maintaining adequate PA levels produces health benefits and can protect the general population from diseases such as type 2 diabetes, strokes or coronary heart disease.1–3 In the case of people with spinal cord injuries (SCI), most of the scientific literature stresses the importance of PA in this population, as it is associated with improvements in fitness, cardiometabolic health and quality of life, lower levels of comorbidity and reductions in other negative factors such as fatigue or pain.4–8
Even though society is aware of the benefits of PA, a large part of the population remains physically inactive, and this is especially true of people with SCI.9,10 SCI can lead to a lack of physical exercise, which can increase the impact of this injury by adding a higher risk of secondary chronic health complications.11 Furthermore, the lack of physical fitness is a serious problem for the autonomy of SCI population, and it may place them in a situation of dependency.12
Currently, the worldwide pandemic unleashed by the Coronavirus (COVID-19) has led to the imposition of lockdown measures in a large number of countries such as China, Italy and Spain.13 This quarantine is viewed as the best way to stop the rapid spread of infections.13 but it entails certain restrictions (e.g. limited PA and/or the inability to move around outside the home at will)14 which may lead to in adverse health outcomes. These limitations affect patterns of daily life and can substantially reduce energy expenditure.15 In Spain, the lockdown was ordered on 15 March 2020, and it lasted until 21 June 2020, coinciding with the end of the state of alarm decreed by the national government. However, on 4 May 2020, a plan to ease the lockdown in four phases began to be implemented with the aim of making the conditions more flexible until the country reached what has been called the “new normality”.
Individuals with SCI may be particularly affected by the difficulties and consequences associated with COVID-19,16,17 and the reduction in PA levels can be one of them. This population faces different types of barriers and demotivating factors (e.g. lack of knowledge, mobility problems, equipment problems and/or a lack of financial resources for exercise programs or equipment), often resulting in a lower frequency of exercise.18 Taking in account that during the lockdown it was impossible to attend gyms and it was more difficult to receive specific instructions from professionals as to how to exercise at home, we can hypothesize that the PA levels of people with SCI were reduced during this period.
For this reason, it is important to obtain information about the changes that may have occurred by comparing the pre-quarantine PA of people with SCI with the PA undertaken during the lockdown. Thus, the main objective of this study was to analyze the differences in self-reported measurements of PA among a sample made up of people with complete thoracic SCI prior to and during the lockdown.
Material and methods
Participants
The participants in this study were individuals living with complete thoracic SCI from Spain who undergo regular medical monitoring at authors institution. A convenience sample of 20 participants with complete motor SCI with injury levels between T2 and T12 (classified as American Spinal Injury Association Impairment Scale A) and full-time manual wheelchair users were enrolled in this study.
Instruments
The instrument used to assess the participants’ self-reported PA was the Spanish version of Physical Activity Scale for Individuals with Physical Disabilities (PASIPD).19,20 In a study by Pérez-Tejero et al.19 the Spanish version of PASIPD was used in manual wheelchair users, and the scores on the scale were correlated with the accelerometer variables. This study found positive and significant correlations between the PASIPD and MET average (r=0.52; P<0.01), energy expenditure (r=0,35; P<0.05) and duration of PA (r=0.53; P<0.01).
This scale is made up of a total of 13 items, gathering data on the recreational activities (6 items), housework (6 items) and occupational activities (1 item) that a respondent has carried out over the preceding seven days. Participants indicate how many days per week and hours each day they do a list of activities. The score was obtained by multiplying the average hours per day for each item by a MET value associated with the intensity of the activity. Thus, the energy expenditure on recreational, housework and occupational activities was obtained, as well as the overall energy expenditure.
Additionally, this study used the PASIPD data to calculate the time spent on activities considered to be of moderate/vigorous intensity (all questionnaire activities except 1, 7 and 12). These data were calculated because the SCI scientific exercise guidelines indicate that moderate to vigorous physical activity is the intensity of activity needed to achieve fitness and health benefits.21
Procedure
The pre-lockdown PASIPD data were collected in December 2019. These data were collected as part of a previous study whose aim was to assess self-reported PA and other variables in people with complete SCI who were full-time manual wheelchair users. In this case, the scale was completed by the participants in person on the premises of the hospital.
However, the PASIPD data during the lockdown were collected via telephone between May 21 and 25. As the questionnaire refers to the previous seven days, the data obtained reflect activities performed during the days 14–18 to 20–24 May. During this period, the participants were in phase 0 of the deconfinement plan. In this phase, citizens could only travel outside their homes to go shopping, go to the doctor or pharmacy, or travel to work (where possible). In addition, they could also go for a walk or exercise individually (only one hour, maximum one kilometer from home and within a very reduced time range).
For the purposes of data collection, the same researcher phoned each participant, read them both the questions for each item and all the possible answers, and wrote down the participants’ choices. All participants completed the pre- and post-lockdown questionnaire.
This study was approved by authors institution ethical committee and all the participants signed a written informed consent.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) software Version 24 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Firstly, the Shapiro–Wilk test was applied to test the normality assumption. The results showed that the variables did not meet the criteria for the normality assumption, and therefore non-parametric tests were used.
The Wilcoxon signed rank test was applied to analyze the differences between pre-quarantine and quarantine measurements in terms of total PASIPD and of the scores for recreational activities, housework and occupational activities both using METS and minutes of moderate-to-vigorous physical activity. Cohen’s d was calculated as effect size being the values 0.2 as small, 0.5 as moderate and 0.8 as large effect.22 The level of significance was set at P = 0.05.
Results
The mean age of the participants in the sample was 45.4 (9.48) years, and they had a mean weight of 74.32 (13.35) kg, height of 174.35 (8.49) cm and time period of 15.85 (10.09) years elapsed since injury.
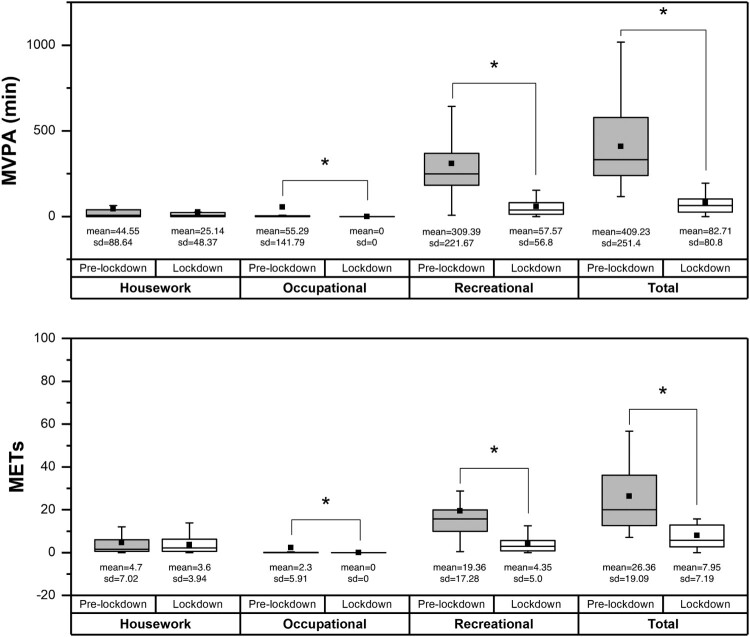
The pairwise comparisons showed differences between the pre-lockdown and lockdown measurements in total PASIPD (z=−3.92; P<0.001; d=1.28), recreational activities (z=−3.92; P<0.001; d=1.18) and occupational activities (z=−2.03; P=0.042; d=0.55). Nevertheless, no differences were found in housework scores between the two moments (z=−0.39; P=0.69; d=0.19).
With regard to pairwise comparisons between the pre-lockdown and lockdown time spent on moderate/vigorous intensity activities, the results showed differences in total minutes spent (z=−3.92; P<0.001; d=1.75), minutes spent on recreational activities of moderate/vigorous intensity (z=−3.82; P<0.001; d=1.56) and minutes spent on occupational activities of moderate/vigorous intensity (z=−2.03; P=0.042; d=0.55). There were not differences in minutes spent in housework MVPA between tests (z=−1.38; P=0.16; d=0.28) (Fig. 1).
Figure 1.
Boxplots of physical activity variables before lockdown and during lockdown. The boxes represent the 25th and 75th percentiles, the lines represent the 95% confidence interval and the black squares represent the mean.
Discussion
The results of this study showed differences between the pre-lockdown and lockdown measurements in terms of total self-reported PA and in PA carried out through recreational and occupational activities, but no differences were revealed with regard to housework activities. These results clearly show that the bans or restrictions placed on recreational and occupational activities outside the home during the quarantine have affected self-reported PA in people with paraplegia.
The new public health crisis caused by the emergence of COVID-19 has produced changes in the everyday routines of a large part of the population, affecting PA levels. Authors like Hall et al.23 have suggested the existence of a secondary pandemic related to physical inactivity and sedentary behaviors. These low levels of PA and the associated sedentary lifestyles have adverse health effects on the population in general, but the impact can be even greater among the population with SCI.24 This inactivity can lead to an increased risk for chronic secondary health complications in this population,11 but promoting active lifestyles and increasing their PA levels can mitigate or prevent some of these complications, can increase their quality of life7 and prevent the emergence of the risk factors associated with metabolic and heart diseases.25,26
At present, it is difficult to predict the evolution and duration of the COVID-19 pandemic and the effects it will have on the behavior of the population even after the pandemic is over.23 For this reason, is important to pay attention to the PA levels of people with SCI during this period of isolation, because this period of inactivity might have serious consequences for their physical fitness, which could lead to subsequent health and independence problems such as an increased probability of developing a cardiovascular disease or type II diabetes.26 This population is particularly inactive, and as the results of this study show, during the pandemic their PA levels have decreased, which is likely to damage their health. Against this background, health-related communities must plan specific strategies to promote the practice of physical exercise in this population during the pandemic (e.g. performing tele-exercise interventions, providing material and guidelines of home-based exercise for people with SCI) in order to avoid the consequences of sedentary behavior and inactivity, which, as has been explained, can be especially hard on them. Currently, due to the major access to new technologies (e.g. computers, tablets or smartphones),27 tele-exercise interventions can be key to fight the inactivity produced by COVID-19 pandemic. However, there are still few studies that develop this type of intervention in people with SCI, although in the last years the number of manuscripts focused on this topic have been increased. The current situation can be a great opportunity to implement and increase knowledge around the effectiveness of tele-exercise in people with SCI. In this way, it will be possible to offer to health professionals a clear guideline to fight against inactivity in this population.
The main limitations of this study lie in the small sample size, the fact that quarantine data were collected by telephone, and the capacity of PASIPD to assess the PA. Firstly, the sample was limited by the data obtained in the previous study mentioned above. On the other hand, the choice to carry out the questionnaire via telephone was made due to the impossibility of administering it in person and because some of the participants did not have sufficient digital competence to complete the questionnaire on-line. Finally, although the PASIPD is an instrument with limitations when it comes to assessing PA, it does have similar properties to other self-report PA questionnaires used among the general population,28 and it can provide valuable information about the PA performed by the SCI population.
In conclusion, the results of this study show that self-reported PA levels of people with complete SCI who are full-time manual wheelchair users may have decreased during the quarantine. Furthermore, the findings of this study suggest that the prohibition or restrictions on carrying out recreational and/or occupational activities are the main reason for this inactivity.
Supplementary Material
Acknowledgment
The authors wish to thank Xurxo Segura Navarro for his support in this study.
Funding Statement
This work was supported by the Fundació la Marató de la TV3 under [grant number 201720-10].
Disclaimer statements
Contributors None.
Declaration of interest None.
Conflicts of interest There are no conflicts of interest to declare.
References
- 1.Warburton DE, Charlesworth S, Ivey A, Nettlefold L, Bredin SS.. A systematic review of the evidence for Canada’s physical activity guidelines for Adults. Int J Behav Nutr Phys Act 2010;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee I-M, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT.. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet 2012;380(9838):219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahid A, Manek N, Nichols M, Kelly P, Foster C, Webster P, et al. Quantifying the association between physical activity and cardiovascular disease and diabetes: a systematic review and meta-analysis. J Am Heart Assoc 2016;5(9):e002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Scheer JW, Martin Ginis KA, Ditor DS, Goosey-Tolfrey VL, Hicks AL, West CR, et al. Effects of exercise on fitness and health of adults with spinal cord injury: a systematic review. Neurology 2017;89(7):736–745. [DOI] [PubMed] [Google Scholar]
- 5.Nash MS. Exercise as a health-promoting activity following spinal cord injury. J Neurol Phys Ther 2005;29(2):87–103. [DOI] [PubMed] [Google Scholar]
- 6.Montesinos-Magraner L, Serra-Añó P, García-Massó X, Ramírez-Garcerán L, González L-M, González-Viejo M.. Comorbidity and physical activity in people with paraplegia: a descriptive cross-sectional study. Spinal Cord 2018;56(1):52–56. [DOI] [PubMed] [Google Scholar]
- 7.Tomasone JR, Wesch NN, Ginis KAM, Noreau L.. Spinal cord injury, physical activity, and quality of life: a systematic review. Kinesiology Review 2013;2(2):113–129. [Google Scholar]
- 8.Farrow M, Nightingale TE, Maher J, McKay CD, Thompson D, Bilzon JLJ.. Effect of exercise on cardiometabolic risk factors in adults with chronic spinal cord injury: a systematic review. Archives of Physical Medicine and Rehabilitation [Internet] 2020;101(12):2177–2205. [DOI] [PubMed] [Google Scholar]
- 9.Kerstin W, Gabriele B, Richard L.. What promotes physical activity after spinal cord injury? An interview study from a patient perspective. Disabil Rehabil 2006;28(8):481–488. [DOI] [PubMed] [Google Scholar]
- 10.Ginis KAM, Latimer AE, Arbour-Nicitopoulos KP, Buchholz AC, Bray SR, Craven BC, et al. Leisure time physical activity in a population-based sample of people with spinal cord injury part I: demographic and injury-related correlates. Arch Phys Med Rehabil 2010;91(5):722–728. [DOI] [PubMed] [Google Scholar]
- 11.Ginis KAM, Hicks AL, Latimer AE, Warburton DER, Bourne C, Ditor DS, et al. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord 2011;49(11):1088–1096. [DOI] [PubMed] [Google Scholar]
- 12.Noreau L, Shephard RJ.. Spinal cord injury, exercise and quality of life. Sports Med 1995;20(4):226–250. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez-Pavón D, Carbonell-Baeza A, Lavie CJ.. Physical exercise as therapy to fight against the mental and physical consequences of COVID-19 quarantine: special focus in older people. Prog Cardiovasc Dis 2020;63(3):386–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G, Henry BM, Sanchis-Gomar F.. Physical inactivity and cardiovascular disease at the time of coronavirus disease 2019 (COVID-19). Eur J Prev Cardiolog 2020. doi: 10.1177/2047487320916823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King AJ, Burke LM, Halson SL, Hawley JA.. The challenge of maintaining metabolic health during a global pandemic. Sports Med 2020;50(7):1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connell CM, Eriks-Hoogland I, Middleton JW.. Now, more than ever, our community is needed: spinal cord injury care during a global pandemic. Spinal Cord Series and Cases 2020;6(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas FP, Murphy C.. COVID-19 and spinal cord injury professionals: maintaining a scholarly perspective. J Spinal Cord Med 2020;43(3):279–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fekete C, Rauch A.. Correlates and determinants of physical activity in persons with spinal cord injury: a review using the international classification of functioning, disability and health as reference framework. Disabil Health J 2012;5(3):140–150. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Tejero J, García-Hernández JJ, Coterón-López J, Benito-Peinado PJ, Sampedro-Molinuevo J.. Medición de los niveles de actividad física en personas con discapacidad física mediante acelerometría u cuestionario. Archivos de Medicina del Deporte: Revista de la Federación Española de Medicina del Deporte y de la Confederación Iberoamericana de Medicina del Deporte 2012;19(147):517–526. [Google Scholar]
- 20.Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF.. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil 2002;83(2):193–200. [DOI] [PubMed] [Google Scholar]
- 21.Martin Ginis KA, van der Scheer JW, Latimer-Cheung AE, Barrow A, Bourne C, Carruthers P, et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: an update and a new guideline. Spinal Cord 2018;56(4):308–321. [DOI] [PubMed] [Google Scholar]
- 22.Coolican H. Research Methods and Statistics in Psychology. London: Routledge; 2018. p. 827. [Google Scholar]
- 23.Hall G, Laddu DR, Phillips SA, Lavie CJ, Arena R.. A tale of two pandemics: How will COVID-19 and global trends in physical inactivity and sedentary behavior affect one another? Prog Cardiovasc Dis 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferri-Caruana A, Millán-González L, García-Massó X, Pérez-Nombela S, Pellicer-Chenoll M, Serra-Añó P.. Accelerometer assessment of physical activity in individuals with paraplegia who do and do not participate in physical exercise. J Spinal Cord Med 2020;43(2):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hetz SP, Latimer AE, Buchholz AC, Martin Ginis KA, SHAPE-SCI Research Group . Increased participation in activities of daily living is associated with lower cholesterol levels in people with spinal cord injury. Arch Phys Med Rehabil. 2009;90(10):1755–1759. [DOI] [PubMed] [Google Scholar]
- 26.Buchholz AC, Martin Ginis KA, Bray SR, Craven BC, Hicks AL, Hayes KC, et al. Greater daily leisure time physical activity is associated with lower chronic disease risk in adults with spinal cord injury. Appl Physiol Nutr Metab 2009;34(4):640–647. [DOI] [PubMed] [Google Scholar]
- 27.Jee H. Review of researches on smartphone applications for physical activity promotion in healthy adults. J Exerc Rehabil 2017;13(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Ploeg HP, Streppel KRM, van der Beek AJ, van der Woude LHV, Vollenbroek-Hutten M, van Mechelen W.. The physical activity Scale for Individuals with physical Disabilities: test-retest reliability and comparison with an accelerometer. J Phys Act Health 2007;4(1):96–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.