Background:
Vedolizumab is a humanized monoclonal antibody that inhibits gut-selective α4β7 integrins on the surface of leukocytes, preventing their trafficking into the gastrointestinal tract, and ultimately achieves the effect of suppressing intestinal inflammation. This study aimed to evaluate the efficacy and safety of vedolizumab in the treatment of inflammatory bowel disease.
Methods:
After a systematic review of relevant studies, the pooled relative risk (RR) and 95% confidence intervals (CIs) were calculated to evaluate the effect. Heterogeneity was explored using sensitivity analysis, univariate meta-regression, and subgroup analysis. Potential publication bias was evaluated using Egger test and trim-and-fill method.
Results:
Nine randomized controlled trials involving 4268 participants were included in the meta-analysis. During induction therapy, vedolizumab was more effective than placebo in treating active ulcerative colitis and Crohn disease in terms of clinical response (RR = 1.55, 95%CI: 1.35–1.78), clinical remission (RR = 1.90, 95%CI: 1.50–2.41), and mucosal healing (RR = 1.53, 95%CI: 1.21–1.95). A superior effect in terms of durable Clinical or Crohn disease Activity Index-100 response (RR = 1.65, 95%CI: 1.20–2.26), clinical remission (RR = 1.92, 95%CI: 1.48–2.50), and glucocorticoid-free remission (RR = 2.22, 95%CI: 1.71–2.90) was found during maintenance treatment. Vedolizumab was not associated with any adverse events and was as safe as placebo in terms of the risk of serious adverse reactions.
Conclusions:
Vedolizumab may be safe and effective as an induction and maintenance therapy for the treatment of inflammatory bowel disease; however, further studies are needed to validate this conclusion.
Keywords: Crohn disease, inflammatory bowel disease, ulcerative colitis, vedolizumab
1. Introduction
Inflammatory bowel disease (IBD) is a gastrointestinal disorder that includes ulcerative colitis (UC) and Crohn disease (CD), and is characterized by abdominal pain, chronic diarrhea, weight loss, and fatigue.[1,2] IBD has become a global public health challenge in the past decade. In North America and Europe, >1.5 million and 2 million people suffer from the disease.[3] Incidence rates have been increasing in newly industrialized countries in Africa, Asia, and South America since 1990. Unemployment, sick leave, and permanent work disability are more common in patients with IBD than in the general population.[4] Moreover, there is a higher risk of adverse health outcomes, including multiple cancers, cardiovascular disease, adverse pregnancy outcomes, and other adverse events.[5]
Currently available medical treatments for IBD include immunosuppressants (e.g., azathioprine, mercaptopurine, and methotrexate), 5-aminosalicylates (5-ASAs), corticosteroids, and biological therapies such as tumor necrosis factor-alpha (TNF-α) antagonists.[6–8] However, these medical therapies have limitations. 5-ASAs are only modestly effective[9]; a meta-analysis showed no statistically significant benefit in IBD patients receiving immunosuppressive therapy compared to placebo[10]; glucocorticoids can cause serious adverse effects and do not benefit from maintenance therapy[11]; TNF-α antagonists are effective but predispose patients to serious infection; and treatment failures may manifest as nonresponse or loss of response to these drugs over time.[12,13] Therefore, new treatment strategies are required.
Vedolizumab is a humanized monoclonal antibody that inhibits the adhesion and migration of lymphocytes into the gastrointestinal tract by binding the alpha4beta7 (α4β7) integrin, which is a protein on the surface of lymphocytes targeted to the gastrointestinal tract.[14,15] This disruption reduces inflammation of the gastrointestinal tract. Vedolizumab is indicated for the treatment of moderately to severely active UC and CD in patients with an inadequate response, loss of response, or intolerance to TNF-α inhibitors or other conventional therapies.[16]
Monitoring the efficacy and safety of vedolizumab is essential due to its relative newness and increasing number of patients being treated worldwide. The purpose of this study was to assess the efficacy and safety of vedolizumab induction and maintenance therapy in patients with IBD.
2. Materials and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were adhered to as a methodological template for this review (Table S1, Supplemental Digital Content, http://links.lww.com/MD/xxx).[17]
2.1. Literature search strategy
Two investigators (B.Q. and J.X.L.) independently searched the MEDLINE (using PUBMED as the search engine), EMBASE, and Cochrane databases. Database were used to identify suitable studies published through April 2022. MeSH terms and keywords were used, and the search terms included: “inflammatory bowel diseases,” “ulcerative colitis,” “Crohn’s disease,” “vedolizumab,” “MLN0002,” “MLN02,” and “LDP-02.” The article type and additional filters did not restrict the search for published work. A manual search was conducted using the references listed in the original articles and the review articles retrieved. Two investigators collected the results separately.
2.1.1. Inclusion criteria.
Randomized clinical trials (RCT);
Patients with active CD or UC;
Patients were treated with vedolizumab or placebo;
2.1.2. Exclusion criteria.
Duplicate reports;
Studies conducted on animals;
Systematic reviews, meta-analyses, or nonrandomized controlled studies.
2.2. Data extraction
For each included study, all data elements uniformly reported across most studies were extracted by 2 reviewers (B.Q. and J.X.L.) and cross-verified by a third reviewer (C.L). When the same population was published in several journals, only the most informative articles or complete studies were retained to avoid duplication. The following information was extracted from each study: first author, year of publication, study design, sample size, diagnosis of enrolled patients, endpoint of the induction and maintenance phase, duration of follow-up, and adverse reactions.
2.3. Definition
Moderate-to-severely active UC was defined as a baseline full Mayo score of 6 to 12 with an endoscopic subscore ≥2.[18] Clinical remission was defined as a total Mayo score of ≤2 and no individual subscore >1. Clinical response was defined as a reduction of ≥3 points and ≥30% from baseline in the full Mayo score. Mucosal healing was defined as an endoscopic subscore ≤1. Durable clinical response/remission was defined as clinical response/remission at the end of both the induction and maintenance phases. Corticosteroid-free remission was defined as clinical remission at the end of the maintenance phase without corticosteroid in patients who received concomitant corticosteroid therapy at week 0.
Moderate-to-severely active CD was defined as a baseline Crohn Disease Activity Index (CDAI) score of 220–450. Clinical response was defined as a ≥70-point decrease in CDAI from baseline. Clinical remission was defined as a CDAI score ≤150. The CDAI-100 response was defined as a ≥100 point reduction in the CDAI score from baseline.
2.4. Risk of bias assessment
The Cochrane risk-of-bias tool was used to assess the risk of bias in randomized trials.[19] Two authors independently assessed each included article using this tool, and disagreements between the 2 authors were resolved by discussion with a third investigator.
2.5. Statistical analysis
Efficacy and safety were analyzed using dichotomous data, and the results were expressed as relative risk (RR) and 95% confidence intervals (CIs). The I2 statistic was used to measure the study heterogeneity, with I2 ≥ 50% indicating significant heterogeneity. If there was no heterogeneity, a Mantel–Haenszel fixed-effects model was used to calculate the pooled RRs, rather than a random-effects model. If heterogeneity was observed, univariate meta-regression or subgroup analysis was performed to explore different sources of heterogeneity. Sensitivity analyses were performed to determine whether there was an undue influence of a single study on the combined study results.[20] We assessed potential publication bias using Egger test, with P > .05 indicating no publication bias. We also performed the Duval and Tweedie nonparametric “trim and fill” procedure to further assess the effect of publication bias in our meta-analysis. All statistical analyses were performed using Stata version 15 (Stata Corp, College Station, TX, USA) and RevMan 5.4 (The Cochrane Collaboration, Oxford, UK).
3. Results
3.1. Characteristics of the included studies
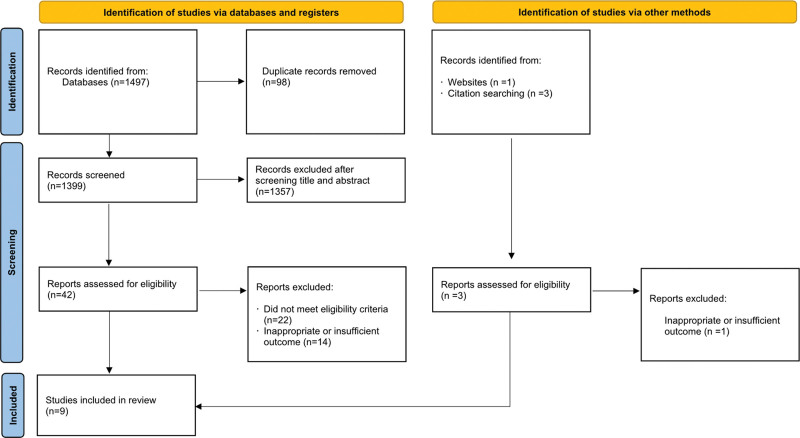
Nine eligible RCTs were identified to evaluate the effectiveness and adverse events of vedolizumab in 4268 patients with active IBD.[21–29] Figure 1 shows the identification and selection process of the study.
Figure 1.
Study identification and selection flowchart.
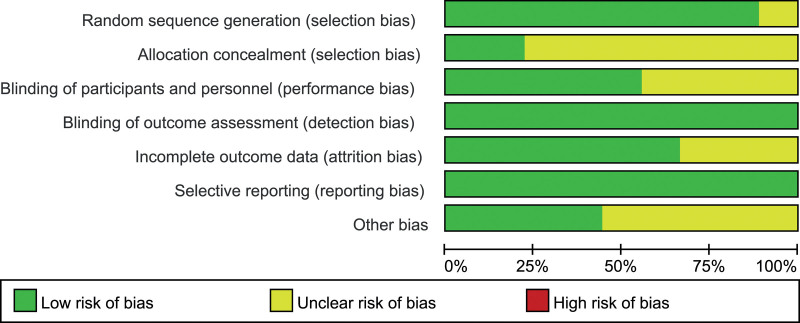
In all studies conducted at multiple medical centers, the follow-up period ranged from 6 to 60 weeks. Four of the 9 studies included patients with active UC[21,23,24,26] and 5 included patients with active CD.[22,25,27–29] Three studies included patients who received open-label vedolizumab (cohort 2) in addition to randomized placebo-controlled trials (cohort 1).[23–25] Eligible patients for inclusion in all studies needed to have evidence of active UC or CD and inadequate response, loss of response, or intolerance to at least 1 other treatment (corticosteroids, immunomodulators, or anti-TNF). Patients previously treated with vedolizumab, natalizumab, efalizumab, or rituximab were excluded from the respective included studies. The main characteristics of the included studies are summarized in Table 1. Before data analysis and synthesis, the Cochrane risk of bias tool was used to assess the quality of the studies, as shown in Figure 2.
Table 1.
Study characteristics.
| Author (yr) | Study design | Diseases | Sample size | Follow-up (wk) | Primary endpoint for the induction phase | Primary endpoint for the maintenance phase | The most common adverse events |
|---|---|---|---|---|---|---|---|
| Feagan 2005[21] | Double-blind | UC | Placebo: 63 VDZ: 118 |
6 | Clinical remission | UC exacerbation, headache, nausea, frequent bowel movement, fatigue | |
| Feagan 2008[22] | Double-blind | CD | Placebo: 58 VDZ: 127 |
8 | Clinical response | CD exacerbation, headache, nausea, fatigue, nasopharyngitis | |
| Feagan 2013[23] | Cohort 1: double-blind Cohort 2: open-label | UC | IP: 895 MP: 373 |
52 | Clinical response | Clinical remission | Headache, UC exacerbation, nasopharyngitis, upper respiratory tract infection |
| Motoya 2019[24] | Cohort 1: double-blind Cohort 2: open-label | UC | IP: 292 MP: 83 |
60 | Clinical response; | Clinical remission | Nasopharyngitis, and upper respiratory tract infection |
| Sandborn 2013[25] | Cohort 1: double-blind Cohort 2: open-label | CD | IP: 1115 MP: 461 |
52 | Clinical response and clinical remission | Clinical remission | CD exacerbation, arthralgia, pyrexia, nasopharyngitis, headache, and nausea |
| Sandborn 2020[26] | IP: open-label MP: double-blind |
UC | IP: 383 MP: 216 |
52 | Clinical remission | Nasopharyngitis, anemia, and upper respiratory tract infection | |
| Sands 2014[27] | Double-blind | CD | Placebo: 207 VDZ: 209 |
6 | Clinical remission | Nausea, upper respiratory tract infection, arthralgia, abdominal pain | |
| Vermeire 2022[28] | IP: open-label MP: double-blind | CD | IP: 644 MP: 410 |
52 | Clinical remission | Nasopharyngitis, upper respiratory infections and gastrointestinal disorders | |
| Watanabe 2020[29] | Double-blind | CD | IP: 157 MP: 42 |
54 | CDAI-100 response | Clinical remission | Exacerbation of CD and upper respiratory tract infection |
CD = Crohn disease, CDAI-100 = reduction in CD activity index score of ≥100 points from baseline, IP = induction phase, MP = maintenance phase, UC = ulcerative colitis, VDZ = vedolizumab.
Figure 2.
Evaluation of study quality.
3.2. Efficacy of vedolizumab for IBD
3.2.1. Induction therapy.
3.2.1.1. Clinical remission.
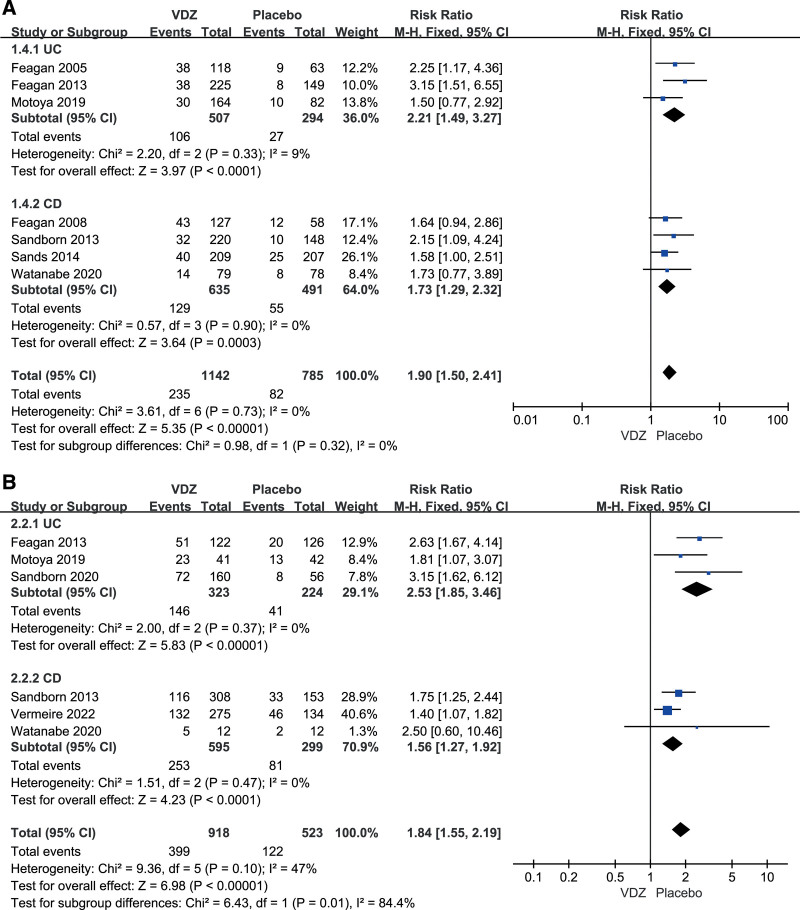
Seven studies assessed the clinical remission after induction therapy.[21–25,27,29] The results of the meta-analysis revealed that the clinical remission rate was significantly higher for patients who received vedolizumab than for those in the control group (RR = 1.90, 95%CI: 1.50–2.41). There was no heterogeneity between studies (I2 = 0%, P = .73). For UC patients as well as CD patients, a statistically significant difference between the vedolizumab and placebo groups was observed in our meta-analysis. Subgroup analyses were performed to assess the differential effects of vedolizumab in patients with UC and CD, and the results were similar to those of the overall analysis (Fig. 3A).
Figure 3.
Subgroup analyses of clinical remission in (A) induction therapy and (B) in maintenance therapy.
3.2.1.2. Clinical or CDAI-100 response.
Three studies reported clinical responses in patients with UC[21,23,24] and 4 reported CDAI-100 responses in patients with CD.[22,25,27,29] The overall analysis showed that patients receiving vedolizumab had significantly higher clinical response rates than those receiving placebo (RR = 1.55, 95%CI: 1.35–1.78). There was no significant heterogeneity after pooling study data (I2 = 2%, P = .41). Similar results to the overall analysis were obtained for the UC and CD subgroups (Table 2).
Table 2.
Efficacy and safety of vedolizumab versus placebo for IBD.
| Category | Subgroup | RR (95% CI) | P | I 2 |
|---|---|---|---|---|
| Induction therapy | ||||
| Clinical/CDAI-100 response | UC | 1.62 (1.33–1.97) | <.05 | 43% |
| CD | 1.49 (1.23–1.80) | <.05 | 2% | |
| Mucosal healing | UC | 1.53 (1.21–1.95) | <.05 | 28% |
| CD | NA | |||
| Maintenance therapy | ||||
| Durable clinical remission | UC | 2.12 (1.06–4.25) | <.05 | 0% |
| CD | 1.31 (0.86–2.01) | .21 | 0% | |
| Clinical/CDAI-100 response | UC | 2.15 (1.56–2.96) | <.05 | 0% |
| CD | 1.34 (1.13–1.59) | <.05 | 59% | |
| Glucocorticoid-free remission | UC | 2.44 (1.61–3.71) | <.05 | 0% |
| CD | 2.09 (1.48–2.94) | <.05 | 0% | |
| Safety | ||||
| Adverse events | UC | 1.03 (0.97–1.10) | .34 | 47% |
| CD | 1.00 (0.92–1.08) | .90 | 13% | |
| Disease exacerbation | UC | 0.90 (0.59–1.37) | .63 | 68% |
| CD | 0.64 (0.40–1.03) | .07 | 70% | |
| Serious adverse events | UC | 1.05 (0.78–1.42) | .08 | 0% |
| CD | 1.22 (0.97–1.52) | .08 | 57% | |
| Serious infection | UC | 0.68 (0.30–1.51) | .34 | 0% |
| CD | 1.12 (0.27–4.71) | .87 | 69% | |
CD = Crohn disease, CDAI = CD Activity Index, CI = confidence interval, IBD = inflammatory bowel disease, NA = not applicable, RR = relative risk, UC = ulcerative colitis.
3.2.1.3. Mucosal healing.
Three studies reported the mucosal healing rate in induction therapy.[21,23,24] A statistically significant difference was observed between the vedolizumab and placebo groups in the overall analysis (RR = 1.53, 95%CI: 1.21–1.95). No heterogeneity was observed among the studies (I2 = 28%, P = .25).
3.2.2. Maintenance therapy.
3.2.2.1. Clinical remission.
Six RCTs evaluated clinical remission in maintenance therapy.[23–26,28,29] The overall analysis showed significantly higher clinical remission rates in patients receiving vedolizumab compared to those in the control group (RR = 1.92, 95%CI: 1.48–2.50). Heterogeneity was observed among these studies (I2 = 47%, P = .10). The results in the UC and CD subgroups were similar to those of the overall analysis (Fig. 3B).
Four studies reported durable clinical remission with maintenance therapy.[24–26,29] A statistically significant difference was found between the vedolizumab and placebo groups in the overall analysis (RR = 1.52, 95%CI: 1.06–2.19, I2 = 0%). However, in the sub-analysis, there was no statistically significant difference in CD patients (RR = 1.31, 95%CI: 0.86–2.01) (Table 2).
3.2.2.2. Durable clinical or CDAI-100 response.
Two studies reported durable clinical responses in patients with active UC,[24,26] and 3 studies reported the durable CDAI-100 response in patients with active CD.[25,28,29] A significant difference was found between the vedolizumab and placebo groups in the overall analysis (RR = 1.65, 95%CI: 1.20–2.26). However, there was heterogeneity between these studies (I2 = 68%, P = .01), therefore, a random-effects model was used. Our meta-analysis revealed statistically significant differences between the vedolizumab and placebo groups in UC patients as well as in CD patients (Table 2).
3.2.2.3. Glucocorticoid-free remission.
Six studies evaluated glucocorticoid-free remission during maintenance therapy.[23–26,28,29] The overall analysis showed significantly higher glucocorticoid-free remission rates in the vedolizumab group than in the control group (RR = 2.22, 95%CI: 1.71–2.90). There was no heterogeneity among the studies (I2 = 0%, P = .92). The results of the UC and CD subgroups were similar to those of the overall analysis (Table 2).
3.3. Clinical remission in subgroups based on prior TNF antagonist use status
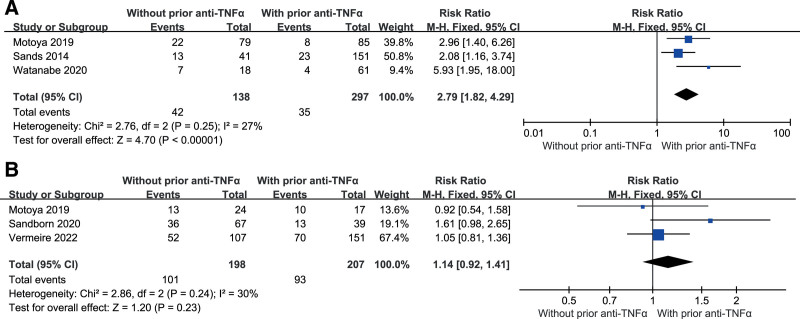
During the induction phase with vedolizumab, clinical remission rates were higher in anti-TNF-naive patients than in patients with prior use of anti-TNF (RR = 2.79, 95%CI: 1.82–4.29, I2 = 27%). However, there was no significant difference between the 2 groups during the maintenance phase (RR = 1.14, 95%CI: 0.92–1.41, I2 = 30%) (Fig. 4).
Figure 4.
Clinical remission in subgroups based on prior TNF antagonist use status in (A) induction therapy and (B) in maintenance therapy. TNF-α = tumor necrosis factor-alpha.
3.4. Safety of vedolizumab for IBD
3.4.1. Adverse events.
Seven studies reported adverse events during the follow-up period.[22–24,26–29] Adverse events included disease exacerbation, nasopharyngitis, upper respiratory tract infection, nausea, vomiting, abdominal pain, headache, arthralgia, and fatigue. No statistically significant difference between the vedolizumab and placebo groups (RR = 1.01, 95%CI: 0.96–1.07, I2 = 25%).
Seven RCTs reported exacerbation events of UC or CD.[21–23,25,27–29] Based on the overall analysis, there was no statistically significant difference between these 2 groups (RR = 0.77, 95%CI: 0.57–1.03, I2 = 64%) (Table 2).
3.4.2. Serious adverse events.
All included studies reported serious adverse events during the follow-up.[21–29] In the overall analysis, there were no statistically significant differences between the vedolizumab and placebo groups (RR = 1.16, 95%CI: 0.97–1.39, I2 = 32%).
Serious infections were reported in 6 studies.[21,23–25,27,28] The difference between the vedolizumab and placebo groups was not statistically significant (RR = 0.91, 95%CI: 0.40–2.08, I2 = 51%) (Table 2).
3.5. Heterogeneity analysis
Significant heterogeneity was observed in “durable clinical or CDAI-100 response in maintenance therapy (I2 = 68%),” “UC or CD exacerbation (I2 = 64%),” and “serious infection events (I2 = 51%).” Hence, we conducted a meta-regression analysis to examine the sources of potential heterogeneity based on the following predefined characteristics: diagnosis (UC vs CD) and study design (open-label vs non-open-label). The results showed that “patient diagnosis,” and “study design” were not factors contributing to heterogeneity (Table 3).
Table 3.
Univariate meta-regression analysis.
| Category | P value | |
|---|---|---|
| Diagnosis | Study design | |
| DC or CDAI-100 response in MP | .85 | .54 |
| Disease exacerbation | .67 | .88 |
| Serious infection | .94 | .45 |
DC = durable clinical, MP = maintenance phase.
Sensitivity analysis was performed by removing 1 study and recalculating the pooled estimates for the remaining studies, which showed that the pooled results were not significantly affected by the individual studies.
3.6. Publication bias
All P values of the Egger statistical tests were >0.05. Although Egger test was not statistically significant, visual inspection of the funnel plot revealed asymmetry, which raised the possibility of publication bias. Hence, we performed a sensitivity analysis using the trim-and-fill method.[30] The difference between the original and corrected effect size estimates was not significant, suggesting that publication bias did not affect the results (Table 4).
Table 4.
The overall effect sizes before/after applying the trim-and-fill methods.
| Category | Result of Egger test | Imputed missing studies | Before trim-and-fill methods | After trim-and-fill methods |
|---|---|---|---|---|
| Clinical remission in IP | P = .33 | 1 | 1.97 (1.57–2.38) | 2.05 (1.66–2.44) |
| CDAI-100/clinical response in IP | P = .93 | 0 | 1.57 (1.43–1.70) | 1.57 (1.43–1.70) |
| Mucosal healing in IP | P = .81 | 1 | 1.53 (1.29–1.77) | 1.46 (1.23–1.69) |
| Clinical remission in MP | P = .20 | 3 | 2.14 (1.61–2.67) | 1.62 (1.06–2.19) |
| DC remission in MP | P = .37 | 1 | 1.69 (1.05–2.33) | 1.94 (1.24–2.64) |
| CDAI-100/DC response in MP | P = .10 | 2 | 1.47 (1.32–1.62) | 1.30 (1.16–1.45) |
| GFR in MP | P = .29 | 1 | 2.32 (1.93–2.72) | 2.29 (1.92–2.67) |
| Adverse events | P = .77 | 1 | 1.04 (0.99–1.08) | 1.04 (1.00–1.09) |
| Serious adverse events | P = .26 | 4 | 1.20 (1.02–1.38) | 1.34 (1.18–1.50) |
| Serious infection | P = .87 | 1 | 1.49 (0.45–2.53) | 1.20 (0.08–2.31) |
| Disease exacerbation | P = .17 | 2 | 0.87 (0.72–1.03) | 0.90 (0.74–1.07) |
CDAI = CD Activity Index, DC = durable clinical, GFR = glucocorticoid-free remission, IP = induction phase, MP = maintenance phase.
4. Discussion
Inflammatory bowel disease (IBD) is a multifactorial chronic disease that may be associated with lifestyle, surgery, living environment, and inappropriate inflammatory responses to intestinal microbes in genetically susceptible individuals.[31,32] Patients with IBD are at a higher risk of adverse health outcomes.[5] Although conventional treatments to induce remission of moderately to severely active UC and CD include sodium 5-ASAs, corticosteroids, and immunomodulators, treatment effects have been unsatisfactory owing to a few limitations or serious adverse events.[33–35] Vedolizumab specifically antagonizes the intestinal α4β7 integrin heterodimer, which prevents lymphocytes from migrating and homing from the blood to intestinal tissues, ultimately inhibiting intestinal inflammation.[36] Vedolizumab is considered a first-line biological treatment for UC and CD.[37]
In this meta-analysis, we identified 9 randomized, placebo-controlled clinical trials that evaluated vedolizumab in the treatment of CD or UC. Our study demonstrated that vedolizumab improved clinical remission and clinical response in patients with active UC, as well as clinical remission and CDAI-100 response in patients with active CD in both induction and maintenance therapy, which indicated that vedolizumab enhances the relief of patient-perceived symptoms. However, it is important to consider that vedolizumab had no effect on durable clinical remission in patients with CD. As reported by Vermeire et al,[28] clinical remission at week 52 was similar between vedolizumab and placebo in the anti-TNF-naive population (P = .59). Watanabe et al[29] also showed no significant difference between the vedolizumab and placebo groups in terms of durable clinical remission (P = .65). In addition, the vedolizumab group exceeded the placebo group at all endpoints, regardless of prior anti-TNF-α use, except for durable remission in patients without prior anti-TNF-α use. Thus, a possible confounding factor is whether the patients had a previous exposure to anti-TNF therapy. Our subgroup analysis based on the status of prior anti-TNF use, demonstrated a higher rate of clinical remission in TNF antagonist-naive patients during induction therapy. A multicenter cohort study by Amiot et al[38] evaluated the efficacy of vedolizumab in IBD patients with prior TNF antagonist failure, resulting in clinical remission rates of 36% and 39% in the CD and UC groups, respectively, after 14 weeks of induction therapy. Kopylov et al[39] evaluated the efficacy of vedolizumab in patients without previous anti-TNF-α use. The results showed that vedolizumab was as effective in IBD patients without previous anti-TNF-α use and had significantly improved efficacy in patients with previous anti-TNF-α failure. Mucosal healing and glucocorticoid-free remission were also significantly higher in the vedolizumab group. Mucosal healing is an important IBD treatment goal that is associated with sustained clinical remission, glucocorticoid-free remission, and reduced incidence of hospitalization and surgery.[40] In our systematic review, more than half of patients with UC achieved mucosal healing, with a significant benefit compared with placebo (55.1% vs 22.8%).
The most frequently observed adverse effects in patients treated with vedolizumab included nasopharyngitis, headache, nausea, arthralgias, pyrexia, fatigue, upper respiratory tract infections, cough, and abdominal pain.[41] Wang et al[42] showed a higher incidence of serious adverse events with vedolizumab in patients with CD (21.7% vs 14.3%) than with placebo which is similar to the results of Sandborn et al[25] (24.4% vs 15.3%). In this study, the incidence of serious adverse events was higher (17.3% vs 11.7%) during induction therapy, but was comparable between the 2 treatment groups during maintenance therapy (11.1% vs 12.3%). In patients with CD, the incidence of serious adverse events was higher compared to patients with UC (19.2% vs 12.4%). This may be because CD is a systemic disease with multi-organ involvement, as it may affect any part of the gastrointestinal tract.[43] Our results showed that vedolizumab was not associated with a greater incidence of adverse events and was as safe as the placebo in terms of the risk of serious adverse reactions, which also included the risk of serious infections. Although the risk analysis of drug-related adverse events in the treatment of IBD has not been well studied, the use of vedolizumab may be a better option than immunosuppressants, corticosteroids, or TNF-α antagonists in treating patients at higher risk for serious infections, such as the elderly or patients with chronic lung disease (e.g., chronic obstructive pulmonary disease).[44] Therefore, a prospective evaluation of this possibility is required.
Sandborn et al[26] showed that a subcutaneous (SC) formulation of vedolizumab (108 mg administered every 2 weeks) is effective and safe as maintenance therapy for patients with moderately to severely active UC. Furthermore, the European Commission has approved a subcutaneous formulation of vedolizumab based on the Sandborn pivotal Phase 3 VISIBLE trial. The subcutaneous formulation of vedolizumab will provide an additional option for patients to maintain a clinical response to vedolizumab. In 2 pilot studies on maintenance therapy, patients who responded to vedolizumab at week 6 were randomly assigned to receive placebo or vedolizumab every 8 or 4 weeks up to 52 weeks.[23,25] Patients treated with vedolizumab every 8 and every 4 weeks differed significantly from those treated with placebo who were in clinical remission at week 52. However, there was no significant difference for the 2 groups treated with vedolizumab.
Although the included studies were multicenter randomized controlled studies, there were some limitations. First, it was not the purpose of the present study to identify the duration of greatest effect of vedolizumab as induction therapy. Extension of induction therapy beyond 6 weeks may result in greater efficacy. Second, the efficacy of the maintenance therapy was not designed to be statistically evaluated in some studies. The relative efficacy and safety of vedolizumab compared with other IBD therapies, particularly the TNF-a antagonists, adalimumab, infliximab, and golimumab, should also be evaluated in future studies. It is difficult to make recommendations on the initial choice of biologic therapy for biologic-naive patients in the absence of direct comparisons. Additionally, the number of included RCTs was small, and most of the included RCTs did not report specific details of drug-related serious adverse events. Moreover, including only English-language studies might lead to better results because studies with positive results are more likely to be accepted by an international journal.
In conclusion, the results of this meta-analysis showed that vedolizumab was significantly more effective than placebo as an induction and maintenance treatment for IBD. Importantly, serious adverse events were not more common in vedolizumab-treated patients than in the control patients. However, the number of studies included for the analysis was significantly smaller, necessitating a reanalysis when more data became available.
Author contributions
Study concept and design: BQ, JXL, CL. Data collections: BQ, JXL. Data analysis and interpretation: BQ, JXL. Drafting of the manuscript: BQ, JXL. Critical revision of the manuscript for important intellectual content: CL. All authors read and approved the final manuscript. Bo Qiu and Jiaxu Liang contributed equally to this work.
Conceptualization: Bo Qiu.
Data curation: Jia-Xu Liang.
Formal analysis: Bo Qiu.
Investigation: Bo Qiu.
Methodology: Bo Qiu, Jia-Xu Liang.
Supervision: Bo Qiu, Cong Li, Jia-Xu Liang.
Validation: Bo Qiu, Cong Li, Jia-Xu Liang.
Visualization: Bo Qiu, Cong Li, Jia-Xu Liang.
Writing – original draft: Bo Qiu.
Writing – review & editing: Bo Qiu, Jia-Xu Liang.
Abbreviations:
- 5-ASAs =
- 5-aminosalicylates
- CD =
- Crohn disease
- CDAI =
- CD Activity Index
- CI =
- confidence interval
- IBD =
- Inflammatory bowel disease
- RCT =
- randomized controlled trial
- RR =
- relative risk
- TNF-α =
- tumor necrosis factor-alpha
- UC =
- ulcerative colitis
- VDZ =
- Vedolizumab
An ethics statement is not applicable because this study was based exclusively on published literature.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Qiu B, Liang J-X, Li C. Efficacy and safety of vedolizumab for inflammatory bowel diseases: A systematic review and meta-analysis of randomized controlled trials. Medicine 2022;101:40(e30590).
Supplemental Digital Content is available for this article.
Contributor Information
Jia-Xu Liang, Email: liangjx0719@foxmail.com.
Cong Li, Email: 745282023@qq.com.
References
- [1].Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- [2].Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–78. [DOI] [PubMed] [Google Scholar]
- [4].Burisch J, Jess T, Martinato M, Lakatos PL; ECCO-EpiCom. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7:322–37. [DOI] [PubMed] [Google Scholar]
- [5].Shen X, Wan Q, Zhao R, et al. Inflammatory bowel diseases and the risk of adverse health outcomes: umbrella review of meta-analyses of observational studies. Dig Liver Dis. 2021;53:809–16. [DOI] [PubMed] [Google Scholar]
- [6].Rietdijk ST, D’Haens GR. Recent developments in the treatment of inflammatory bowel disease. J Dig Dis. 2013;14:282–7. [DOI] [PubMed] [Google Scholar]
- [7].Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644–59, quiz 660. [DOI] [PubMed] [Google Scholar]
- [8].Lam MC, Bressler B. Vedolizumab for ulcerative colitis and Crohn’s disease: results and implications of GEMINI studies. Immunotherapy. 2014;6:963–71. [DOI] [PubMed] [Google Scholar]
- [9].Ford AC, Khan KJ, Achkar JP, Moayyedi P. Efficacy of oral vs. topical, or combined oral and topical 5-aminosalicylates, in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2012;107:167–76; author reply 177. [DOI] [PubMed] [Google Scholar]
- [10].Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:630–42. [DOI] [PubMed] [Google Scholar]
- [11].Waljee AK, Wiitala WL, Govani S, et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS One. 2016;11:e0158017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Billioud V, Ford AC, Tedesco ED, Colombel JF, Roblin X, Peyrin-Biroulet L. Preoperative use of anti-TNF therapy and postoperative complications in inflammatory bowel diseases: a meta-analysis. J Crohns Colitis. 2013;7:853–67. [DOI] [PubMed] [Google Scholar]
- [13].Mocci G, Marzo M, Papa A, Armuzzi A, Guidi L. Dermatological adverse reactions during anti-TNF treatments: focus on inflammatory bowel disease. J Crohns Colitis. 2013;7:769–79. [DOI] [PubMed] [Google Scholar]
- [14].Fedyk ER, Wyant T, Yang LL, et al. Exclusive antagonism of the α4 β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012;18:2107–19. [DOI] [PubMed] [Google Scholar]
- [15].Jovani M, Danese S. Vedolizumab for the treatment of IBD: a selective therapeutic approach targeting pathogenic a4b7 cells. Curr Drug Targets. 2013;14:1433–43. [DOI] [PubMed] [Google Scholar]
- [16].Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–75. [DOI] [PubMed] [Google Scholar]
- [17].Salameh JP, Bossuyt PM, McGrath TA, et al. Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): explanation, elaboration, and checklist. BMJ. 2020;370:m2632. [DOI] [PubMed] [Google Scholar]
- [18].Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Copas JB, Shi JQ. A sensitivity analysis for publication bias in systematic reviews. Stat Methods Med Res. 2001;10:251–65. [DOI] [PubMed] [Google Scholar]
- [21].Feagan BG, Greenberg GR, Wild G, et al. Treatment of ulcerative colitis with a humanized antibody to the alpha4beta7 integrin. N Engl J Med. 2005;352:2499–507. [DOI] [PubMed] [Google Scholar]
- [22].Feagan BG, Greenberg GR, Wild G, et al. Treatment of active Crohn’s disease with MLN0002, a humanized antibody to the alpha4beta7 integrin. Clin Gastroenterol Hepatol. 2008;6:1370–7. [DOI] [PubMed] [Google Scholar]
- [23].Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- [24].Motoya S, Watanabe K, Ogata H, et al. Vedolizumab in Japanese patients with ulcerative colitis: a Phase 3, randomized, double-blind, placebo-controlled study. PLoS One. 2019;14:e0212989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- [26].Sandborn WJ, Baert F, Danese S, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158:562–572.e12. [DOI] [PubMed] [Google Scholar]
- [27].Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–627.e3. [DOI] [PubMed] [Google Scholar]
- [28].Vermeire S, D’Haens G, Baert F, et al. Efficacy and safety of subcutaneous vedolizumab in patients with moderately to severely active Crohn’s disease: results from the VISIBLE 2 randomised trial. J Crohns Colitis. 2022;16:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Watanabe K, Motoya S, Ogata H, et al. Effects of vedolizumab in Japanese patients with Crohn’s disease: a prospective, multicenter, randomized, placebo-controlled phase 3 trial with exploratory analyses. J Gastroenterol. 2020;55:291–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [31].Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–40. [DOI] [PubMed] [Google Scholar]
- [33].Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- [34].Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- [35].Hoentjen F, van Bodegraven AA. Safety of anti-tumor necrosis factor therapy in inflammatory bowel disease. World J Gastroenterol. 2009;15:2067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987–95. [DOI] [PubMed] [Google Scholar]
- [37].Attauabi M, Madsen GR, Bendtsen F, Seidelin JB, Burisch J. Vedolizumab as the first line of biologic therapy for ulcerative colitis and Crohn’s disease—a systematic review with meta-analysis. Dig Liver Dis. 2021:S1590-8658(21)00856-2. [DOI] [PubMed] [Google Scholar]
- [38].Amiot A, Grimaud JC, Peyrin-Biroulet L, et al. ; Observatory on efficacy and of vedolizumab in patients with inflammatory bowel disease study group; Groupe d’Etude Therapeutique des Affections Inflammatoires du tube Digestif. Effectiveness and safety of vedolizumab induction therapy for patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2016;14:1593–1601.e2. [DOI] [PubMed] [Google Scholar]
- [39].Kopylov U, Verstockt B, Biedermann L, et al. Effectiveness and safety of vedolizumab in anti-TNF-naïve patients with inflammatory bowel disease-a multicenter retrospective European study. Inflamm Bowel Dis. 2018;24:2442–51. [DOI] [PubMed] [Google Scholar]
- [40].Pineton de CG, Blanc P, Peyrin-Biroulet L. Current evidence supporting mucosal healing and deep remission as important treatment goals for inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2016;10:915–27. [DOI] [PubMed] [Google Scholar]
- [41].Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629–35. [DOI] [PubMed] [Google Scholar]
- [42].Wang MC, Zhang LY, Han W, et al. PRISMA—efficacy and safety of vedolizumab for inflammatory bowel diseases: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltim). 2014;93:e326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Cominelli F. Inhibition of leukocyte trafficking in inflammatory bowel disease. N Engl J Med. 2013;369:775–6. [DOI] [PubMed] [Google Scholar]
- [44].Mosli MH, MacDonald JK, Bickston SJ, et al. Vedolizumab for induction and maintenance of remission in ulcerative colitis: a Cochrane systematic review and meta-analysis. Inflamm Bowel Dis. 2015;21:1151–9. [DOI] [PubMed] [Google Scholar]