Abstract
Transpositional activity of mobile elements can be induced by different environmental stresses. Here, we present evidence that transposition of Tn4652 is elevated in stationary-phase Pseudomonas putida and suppressed in an isogenic ςS-defective strain. We demonstrate that transcription from the Tn4652 transposase promoter is controlled by the stationary-phase-specific sigma factor ςS. To our knowledge, this is the first example of direct stationary-phase-specific regulation of a mobile element transposase. Data presented in this report support the idea that activation of transposition under stressful conditions could be an inducible process.
Transposons are widespread in genomes and have important roles in evolution. Transpositional activity of a mobile element is generally maintained at a low level, yet a high frequency of transposition may occur in response to certain environmental stimuli. It has been shown that different stresses, such as carbon starvation (17), temperature effects (16, 21), and UV light (7), can enhance transposition of bacterial mobile elements. Moreover, it is hypothesized that activation of transposition under stress conditions might serve as an adaptive response to overcome stress and permit new traits to evolve (4, 24). However, the exact molecular mechanisms that underlie stress-induced transposition remain undefined.
Transposon Tn4652 is a 17-kb-long deletion derivative of the toluene degradation transposon Tn4651. Pseudomonas putida strain PaW85 harbors Tn4652 in the chromosome. Mutational processes in P. putida PaW85 have been previously studied in starving conditions on phenol minimal plates (13). That work showed the emergence of phenol-utilizing mutants due to the activation of transcription of plasmid-encoded promoterless phenol degradation genes pheBA in the plasmid pEST1414. About one-third of the starvation-induced Phe+ mutants appeared as a result of insertion of Tn4652 in front of the phenol monooxygenase gene pheA (13) (Fig. 1A). The transposition resulted in the formation of a fusion promoter between the transposon-inverted repeat and target DNA (13, 19). Interestingly, transposition of Tn4652 seemed to depend upon the physiological state of bacteria: transposition frequency increased with time of starvation, whereas no Tn4652-linked Phe+ mutants were detected among growing cells of P. putida (13). This indicated that starvation might increase transposition activity of Tn4652.
FIG. 1.
(A) Schematic presentation of transposition target region in plasmid pEST1332. Catechol 1,2-dioxygenase (pheB) and phenol monooxygenase (pheA) genes are indicated by grey boxes. Vector DNA of pAYC32 is depicted with a line. Different insertion sites of Tn4652 are indicated with arrows. (B) Accumulation of Phe+ mutants on phenol minimal plates is indicated for P. putida strain PaW85 (wt) and isogenic rpoS-defective strain PKS54 (rpoS) containing plasmid pEST1332. Each point represents the mean of five independent determinations, and error bars represent standard deviations. Dashed lines indicate the theoretical appearance of Tn4652-linked Phe+ mutants deduced from the results of PCR analysis of Phe+ colonies. Up to 30 Phe+ mutants were subjected to analysis on each day. (C) Viability of P. putida PaW85 (wt) and PKS54 (rpoS) carrying plasmid pAYC32 on phenol minimal plates. Each point represents the mean of five independent measurements, and error bars represent standard deviations. 1,0E + 08, e.g., marks 108 viable cells.
By the adaptation of bacteria to limited nutrient availability, changes in gene regulation take place, i.e., several genes are shut down while others are induced. One of the upregulated genes, rpoS, codes for an alternative sigma factor, ςS, which controls expression of multiple stationary-phase genes (10, 18). In order to examine the potential role of ςS in the regulation of Tn4652, we measured transposition of Tn4652 in the wild-type P. putida PaW85 and in an isogenic ςS-defective strain.
Transposition of Tn4652 is decreased in the P. putida rpoS mutant strain.
Transposition of native Tn4652 was examined in a starvation assay as described previously (13), except that target plasmid pEST1332 was used instead of pEST1414. Similar to pEST1414, plasmid pEST1332 (15) contains the promoterless pheBA operon. However, it is more suitable for probing transposition of Tn4652 since most of the Phe+ clones arising on phenol minimal plates emerge from the insertion of Tn4652 (19). Plasmid pEST1332 contains a specific target site that is preferred over the other sites present in both pEST1332 and pEST1414. To study the effects of ςS on transposition of Tn4652, plasmid pEST1332 was introduced into P. putida PaW85 and into its rpoS-defective derivative PKS54. Bacteria were grown overnight (ON) in Luria-Bertani medium at 30°C and washed with M9 solution. Approximately 109 cells of ON cultures of PaW85 and PKS54 were spread onto five phenol minimal plates, and the accumulation of mutant Phe+ colonies was monitored upon incubation of the plates at 30°C for 7 days. Results presented in Fig. 1B demonstrate that the emergence of Phe+ mutants in the rpoS-defective P. putida was strongly suppressed. Appearance of Phe+ mutants in the rpoS-defective strain was reduced 5 to 10 times compared to that in the wild-type P. putida. In order to test the insertions of transposon Tn4652 into pEST1332, Phe+ mutants were analyzed by PCR with oligonucleotides pheA, TnR, and TnL (Table 1). PCR analysis of Phe+ colonies of the P. putida wild-type strain revealed that more than 95% of these mutants contained a Tn4652 insertion upstream of the pheA coding region. In contrast, only about 20 to 30% of the Phe+ colonies that appeared in the P. putida ςS-defective strain carried a Tn4652 insertion in pEST1332 (Fig. 1B). Thus, the absence of ςS protein decreased transposition substantially, by more than 1 order of magnitude, but did not prevent it entirely. Here, we want to point out that the Phe+ colonies revealed similar patterns of insertions in both the wild-type and rpoS-defective strains. Also, previous results indicate that RpoS is not obligatory for transcription from the fusion promoters created by Tn4652 insertions (20).
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides
| Strain, plasmid, or oligonucleotide | Description | Reference |
|---|---|---|
| Strains | ||
| P. putida | ||
| PaW85 | Tn4652 | 3 |
| PKS54 | Tn4652 rpoS::Km | 20 |
| Plasmids | ||
| pAYC32 | Broad-host-range vector (Apr) | 6 |
| pEST1332 | Plasmid pAYC32 carrying promoterless pheBA operon | 15 |
| pKT240 | Cloning vector (Apr Kmr) | 2 |
| pKTtnpA(D/H) | Tn4652 tnpA gene cloned into pKT240 | 12 |
| pKTlacZS/C | 122-bp Tn4652 tnpA promoter region with IHF binding site cloned into pKTlacZ | 11 |
| pKTlacZD/C | 65-bp Tn4652 tnpA promoter region lacking IHF binding site cloned into pKTlacZ | 11 |
| Oligonucleotides | ||
| pheA | 5′-TGCTCAAGATTATCATTACGCT-3′ (positions 11–32 in the pheA coding region) | |
| TnR | 5′-ATCAGCATAGACGGCTAGCCAG-3′ (positions 101–122 from Tn4652 right end) | |
| TnL | 5′-CTTCCTCAATGGATGGCTGAAG-3′ (positions 111–132 from Tn4652 left end) |
RpoS is known to contribute to the maintenance of bacterial cell viability during the stationary phase of growth and during nutrient starvation (18, 22). Survival of rpoS-defective P. putida strain KT2440 has been demonstrated to decrease by 2 orders of magnitude during 1 week in liquid minimal medium (22; our unpublished results). Therefore, we estimated the viability of starving P. putida PaW85 and PKS54 on phenol minimal plates. In this experiment, P. putida PaW85 and PKS54 carrying plasmid pAYC32 (which differs from pEST1332 by its lack of the pheBA genes) were used in order to avoid the accumulation of Phe+ mutants. About 5 × 108 to 8 × 108 bacteria of PaW85(pAYC32) and PKS54(pAYC32) were plated onto five phenol minimal plates, and small plugs were cut from the agar on each starvation day. Bacteria from the plugs were suspended in M9 solution, and the number of colony-forming units was determined on glucose minimal plates supplied with carbenicillin. Data in Fig. 1C show that viability of the ςS-defective strain decreases slowly during 14 days of starvation on phenol plates; by the end of the second week, the number of viable cells of PKS54(pAYC32) had decreased by 2 orders of magnitude. However, during the first 6 days of starvation, survival of the ςS-defective strain dropped only twofold. This cannot explain how Tn4652-linked Phe+ mutants had an accumulation rate in PKS54(pEST1332) that was more than 10-fold lower than that in PaW85(pEST1332) (Fig. 1B). Therefore, we conclude that ςS can act as a positive regulator in transposition of Tn4652.
Expression of transposase of Tn4652 is ςS dependent.
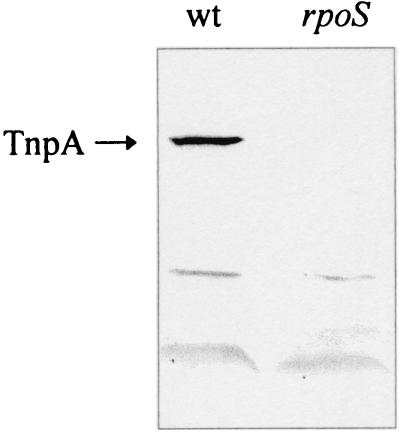
How can RpoS control transposition of Tn4652? Transposition is mostly regulated by the amount and activity of transposase, the protein that performs the transposition reaction. Therefore, we evaluated the amount of transposase (TnpA) of Tn4652 in a ςS-defective background by Western blot analysis with an anti-TnpA polyclonal antiserum. TnpA is downregulated by the Tn4652-encoded TnpC, and therefore, the concentration of TnpA in the Tn4652 background is not detectable by Western blot analysis (12). Yet, TnpA protein can be shown by this method if the copy number of the tnpA gene is increased by cloning the tnpA into plasmid pKT240 [plasmid pKTtnpA(D/H)] (12). Thus, we performed Western blot analysis with cell lysates prepared from ON cultures of P. putida PaW85 and PKS54 carrying plasmid pKTtnpA(D/H). We found that expression of plasmid-encoded TnpA was substantially decreased in the ςS-defective strain; no TnpA protein could be detected by Western blot analysis in PKS54 (Fig. 2).
FIG. 2.
Western immunoblot analyses of Tn4652 TnpA in P. putida strain PaW85 (wt) and rpoS-defective strain PKS54 (rpoS) containing TnpA-expressing plasmid pKTtnpA(D/H). About 40 μg of crude cell lysate was loaded per lane.
Transcription from the transposase tnpA promoter of Tn4652 is growth phase controlled and ςS dependent.
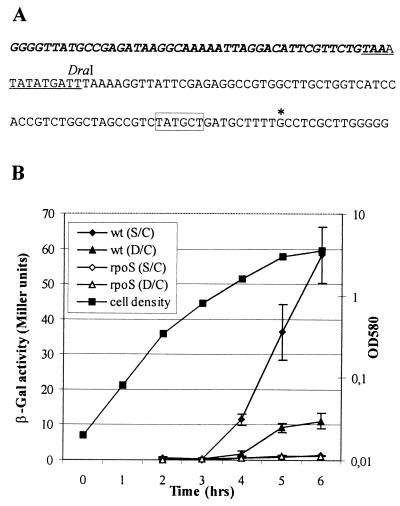
In order to test whether the Tn4652-encoded transposase could be under the control of ςS, the transcriptional activity of the tnpA promoter (Fig. 3A) was examined in P. putida strains PaW85 and PKS54. Previously, transcriptional fusions of the tnpA promoter region with the reporter gene lacZ have been constructed, and it has been demonstrated that the tnpA promoter is positively affected by integration host factor (IHF) (11). It has been shown that ςS is involved in the regulation of the expression of IHF in Escherichia coli (1). Therefore, the tnpA promoter constructs either containing or lacking the IHF binding site (plasmids pKTlacZS/C and pKTlacZD/C, respectively) were tested in the ςS-defective background. The results presented in Fig. 3B demonstrate that the transcription from the tnpA promoter is entirely dependent on the growth phase of the bacteria. Both reporter plasmids, pKTlacZS/C and pKTlacZD/C, tested in PaW85 exhibited stationary-phase-specific induction of the tnpA promoter. Also, as demonstrated previously (11), an about five- to sixfold-higher positive effect became apparent in the presence of the IHF binding site upstream of the tnpA promoter (Fig. 3B). Measurement of the β-galactosidase expression in the ςS-defective P. putida strain PKS54 revealed that no obvious increase could be detected with either pKTlacZS/C or pKTlacZD/C during growth (Fig. 3B). Bacteria harboring either plasmid pKTlacZS/C or pKTlacZD/C showed similar and only slightly detectable levels of β-galactosidase activity that remained 50- or 10-fold lower, respectively, than that estimated in the wild-type strain, and no positive effects of the IHF binding site could be detected. Thus, these data indicate that stationary-phase-specific activation of the tnpA promoter specifically requires ςS.
FIG. 3.
(A) Sequence of right end of Tn4652 containing promoter region of tnpA. The 46-bp inverted repeat is in boldface italics. The −10 hexamer of the tnpA promoter is boxed, and the transcription start of tnpA (11) is indicated by an asterisk. The potential IHF binding site is underlined. (B) Growth-dependent expression of tnpA promoter. P. putida wild-type strain PaW85 (wt) and its rpoS mutant PKS54 (rpoS) carrying either pKTlacZS/C or pKTlacZD/C were grown on Luria-Bertani medium. Plasmid pKTlacZD/C lacks the 57 nucleotides (up to the DraI restriction site; for details, see the description for panel A) of the Tn4652 right end sequence. Data (mean ± standard deviation) of at least four independent experiments are presented. OD580, optical density at 580 nm.
RpoS may act either directly on the tnpA promoter or indirectly by activation of some transcription factor operating on the tnpA promoter. Although ςS- and ς70-dependent promoters are generally quite similar, some subtle but essential differences in promoter sequences exist to ensure the selectivity between these two major sigma factors. ςS-dependent promoters harbor mostly the sequence CTATACT in the conserved −10 region (8), while ς70 preferentially recognizes promoters with the sequence TATAAT. The −10 region CTATGCT of the tnpA promoter of Tn4652 contains the sequence determinants suggested to be important for ςS-dependent transcription, the C nucleotide upstream of the −10 hexamer and the C at the fifth position in the −10 hexamer (Fig. 3A). Therefore, we suppose that RpoS recognizes the tnpA promoter and is directly involved in the stationary-phase-specific expression of TnpA.
Up to now the role of ςS in regulation of transposition has been studied only in experiments with the mutant bacteriophage Mu. It has been shown that carbon starvation conditions trigger induction of mutant Mu prophage, resulting in formation of the araB-lacZ coding sequence fusions (17). Appearance of the araB-lacZ fusion clones on lactose-selective plates was completely abolished in a ςS-negative E. coli strain (9). Since the transposase promoter of Mu was demonstrated to be not under the direct control of ςS, it was supposed that ςS could regulate Mu activation indirectly (17). Thus, according to our knowledge, ςS-dependent upregulation of the transposase of Tn4652 is the first example of direct stationary-phase-specific regulation of a mobile element transposase.
It is well known that plenty of mutations and other types of genetic variation are associated with the activity of mobile elements. Transpositional activity of most mobile elements is greatly suppressed, yet there are several examples of transposons that are activated under the conditions in which fast genetic changes are needed, i.e., under different stresses (5, 14, 23). An interesting question arises: does the activation occur due to malfunction of host defense mechanisms or is this an induced process to promote mutations that may potentially contribute to survival in unfavorable conditions? According to the results presented in this report, we prefer the latter version. Our results demonstrate that transposition of Tn4652 is regulated by physiological conditions of the host. In starving bacteria, transposition of Tn4652 is elevated due to direct control of the stationary-phase sigma factor ςS that is induced just for better survival of cells in stressed conditions. Therefore, we believe that Tn4652 serves as a good example of transposons that are activated under stressful conditions to increase the overall mutation rate and to generate new and potentially useful mutations.
Acknowledgments
We thank T. Alamäe and A. Tamm for critically reading the manuscript.
This work was supported by grants 4481 and 4482 from the Estonian Science Foundation and by grant no. HHMI 55000316 from the Howard Hughes Medical Institute International Research Scholars Program.
REFERENCES
- 1.Aviv M, Giladi H, Schreiber G, Oppenheim A B, Glaser G. Expression of the genes coding for the Escherichia coli integration host factor are controlled by growth phase, rpoS, ppGpp and by autoregulation. Mol Microbiol. 1994;14:1021–1031. doi: 10.1111/j.1365-2958.1994.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 2.Bagdasarian M M, Amann E, Lurz R, Ruckert B, Bagdasarian M. Activity of the hybrid trp-lac (tac) promoter of Escherichia coli in Pseudomonas putida. Construction of broad-host-range, controlled-expression vectors. Gene. 1983;26:273–282. doi: 10.1016/0378-1119(83)90197-x. [DOI] [PubMed] [Google Scholar]
- 3.Bayley S A, Duggleby C J, Worsey M J, Williams P A, Hardy K G, Broda P. Two modes of loss of the Tol function from Pseudomonas putida mt-2. Mol Gen Genet. 1977;154:203–204. doi: 10.1007/BF00330838. [DOI] [PubMed] [Google Scholar]
- 4.Capy P, Gasperi G, Biemont C, Bazin C. Stress and transposable elements: co-evolution or useful parasites? Heredity. 2000;85:101–106. doi: 10.1046/j.1365-2540.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- 5.Chao L, Vargas C, Spear B B, Cox E C. Transposable elements as mutator genes in evolution. Nature. 1983;303:633–635. doi: 10.1038/303633a0. [DOI] [PubMed] [Google Scholar]
- 6.Chistoserdov A Y, Tsygankov Y D. Broad host range vectors derived from an RSF1010::Tn1 plasmid. Plasmid. 1986;16:161–167. doi: 10.1016/0147-619x(86)90053-3. [DOI] [PubMed] [Google Scholar]
- 7.Eichenbaum Z, Livneh Z. UV light induces IS10 transposition in Escherichia coli. Genetics. 1998;149:1173–1181. doi: 10.1093/genetics/149.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa-Urgel M, Chamizo C, Tormo A. A consensus structure for ςS-dependent promoters. Mol Microbiol. 1996;21:657–659. doi: 10.1111/j.1365-2958.1996.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Gómez J M, Blázquez J, Baquero F, Martínez J L. H-NS and RpoS regulate emergence of Lac Ara+ mutants of Escherichia coli MCS2. J Bacteriol. 1997;179:4620–4622. doi: 10.1128/jb.179.14.4620-4622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hengge-Aronis R. Interplay of global regulators and cell physiology in the general stress response of Escherichia coli. Curr Opin Microbiol. 1999;2:148–152. doi: 10.1016/S1369-5274(99)80026-5. [DOI] [PubMed] [Google Scholar]
- 11.Hõrak R, Kivisaar M. Expression of the transposase gene tnpA of Tn4652 is positively affected by integration host factor. J Bacteriol. 1998;180:2822–2829. doi: 10.1128/jb.180.11.2822-2829.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hõrak R, Kivisaar M. Regulation of the transposase of Tn4652 by the transposon-encoded protein TnpC. J Bacteriol. 1999;181:6312–6318. doi: 10.1128/jb.181.20.6312-6318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasak L, Hõrak R, Kivisaar M. Promoter-creating mutations in Pseudomonas putida: a model system for the study of mutation in starving bacteria. Proc Natl Acad Sci USA. 1997;94:3134–3139. doi: 10.1073/pnas.94.7.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidwell M G, Lisch D. Transposable elements as sources of variation in animals and plants. Proc Natl Acad Sci USA. 1997;94:7704–7711. doi: 10.1073/pnas.94.15.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kivisaar M, Hõrak R, Kasak L, Heinaru A, Habicht J. Selection of independent plasmids determining phenol degradation in Pseudomonas putida and the cloning and expression of genes encoding phenol monooxygenase and catechol 1,2-dioxygenase. Plasmid. 1990;24:25–36. doi: 10.1016/0147-619x(90)90022-5. [DOI] [PubMed] [Google Scholar]
- 16.Kretschmer P J, Cohen S N. Effect of temperature on translocation frequency of the Tn3 element. J Bacteriol. 1979;139:515–519. doi: 10.1128/jb.139.2.515-519.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamrani S, Ranquet C, Gama M J, Nakai H, Shapiro J A, Toussaint A, Maenhaut-Michel G. Starvation-induced Mucts62-mediated coding sequence fusion: a role for ClpXP, Lon, RpoS and Crp. Mol Microbiol. 1999;32:327–343. doi: 10.1046/j.1365-2958.1999.01352.x. [DOI] [PubMed] [Google Scholar]
- 18.Loewen P C, Hengge-Aronis R. The role of the sigma factor ςS (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 19.Nurk A, Tamm A, Hõrak R, Kivisaar M. In-vivo-generated fusion promoters in Pseudomonas putida. Gene. 1993;127:23–29. doi: 10.1016/0378-1119(93)90612-7. [DOI] [PubMed] [Google Scholar]
- 20.Ojangu E-L, Tover A, Teras R, Kivisaar M. Effects of combination of different −10 hexamers and downstream sequences on stationary-phase-specific sigma factor ςS-dependent transcription in Pseudomonas putida. J Bacteriol. 2000;182:6707–6713. doi: 10.1128/jb.182.23.6707-6713.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfeifer F, Blaseio U. Transposition burst of the ISH27 insertion element family in Halobacterium halobium. Nucleic Acids Res. 1990;18:6921–6925. doi: 10.1093/nar/18.23.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos-González M I, Molin S. Cloning, sequencing, and phenotypic characterization of the rpoS gene from Pseudomonas putida KT2440. J Bacteriol. 1998;180:3421–3431. doi: 10.1128/jb.180.13.3421-3431.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Skaliter R, Eichenbaum Z, Shwartz H, Ascarelli-Goell R, Livneh Z. Spontaneous transposition in the bacteriophage lambda cro gene residing on a plasmid. Mutat Res. 1992;267:139–151. doi: 10.1016/0027-5107(92)90118-l. [DOI] [PubMed] [Google Scholar]
- 24.Wessler S R. Turned on by stress. Plant retrotransposons. Curr Biol. 1996;6:959–961. doi: 10.1016/s0960-9822(02)00638-3. [DOI] [PubMed] [Google Scholar]