Abstract
Background
Chagas disease is a parasitic infection that can insidiously cause non‐ischemic cardiomyopathy. Given the largely silent nature of this progressive disease, asymptomatic blood donors pose potential blood transfusion risk. Blood donation screening has become an unintentional form of Chagas disease surveillance, with thousands of new cases identified since national surveillance was initiated in 2007.
Study Design and Methods
We recruited T. cruzi‐positive blood donors identified from California and Arizona blood centers for confirmatory blood screening and assessment of lifetime infection risk.
Results
Among eight suspected cases, we identified four confirmed US autochthonous infections. The current manuscript details the transmission sources, healthcare‐seeking behaviors post‐blood donation resulting, and clinical course of disease among persons without any history of travel to endemic Latin American countries.
Discussion
This manuscript presents four additional US‐acquired Chagas disease cases and identifies an opportunity for blood centers to assist in confronting barriers surrounding Chagas disease in the US.
Keywords: autochthonous Chagas disease, Chagas disease USA, Trypanosoma cruzi
Abbreviations
- CDC
U. S. Centers for Disease Control and Prevention
- ECG
electrocardiogram
- ECHO
echocardiogram
- ELISA
enzyme‐linked immunosorbent assay
- FDA
U. S. Food and Drug Administration
- TESA
trypomastigote excreted‐secreted antigen immunoblot
- UofSC
University of South Carolina
- US
United States
1. INTRODUCTION
Locally‐acquired Chagas disease has been increasingly recognized as a public health concern in the United States (US). Caused by infection with the protozoan hemoflagellate T. cruzi, Chagas remains a primary cause of non‐ischemic cardiomyopathy in Latin America but is not widely recognized or investigated in the US. 1 True case counts are unknown; however, >76 suspected or confirmed US‐acquired cases have been reported in at least 8 states. 2 , 3 Though cases are likely rare, low physician awareness 4 , 5 and suboptimal surveillance across the country create challenges to understanding the true burden of autochthonous Chagas disease. 6 In Texas, Chagas disease research capacity has led to the identification of locally‐acquired cases, with 34 cases documented between 2013 and 2019 alone. 6 , 7 While a great number of locally‐acquired cases have been identified in Texas, the southwestern US is home to a greater triatomine biodiversity and abundance, 8 giving premise to the potential that locally‐acquired cases might be occurring in these states as well. Further supporting this hypothesis, recent locally‐acquired cases have infrequently been reported in Arizona and California. 9 , 10
In the US, up to 350,000 people are estimated to be infected and those infections are primarily foreign‐acquired. 11 However, a rising interest in the potential for locally‐acquired infection has led to increased identification of suspected autochthonous cases, nearly tripling the number of autochthonous cases previously thought to exist in the nation. 3 , 10 , 12 , 13 The majority of the southwestern states house a high dispersion index and species richness of the triatomine vector with high Trypanosoma cruzi insect infection prevalence. 14 Although the primary area of concern in human exposure to US triatomines is the risk for an anaphylactic reaction to the highly antigenic insect saliva, 14 , 15 , 16 , 17 cardiac disease has been documented among US T. cruzi‐positive blood donors. 18 Working outdoors at night, military training activities, and recreational camping and hunting have been identified as possible risk factors for T. cruzi infection in the US. 9 , 19 Evidence of peridomestic transmission cycles and frequent human‐vector contact has been documented across the southwestern US. 16 , 20
Blood donor screening was implemented in the US starting in 2007, following the approval of a screening test in 2006. 21 , 22 Based on FDA guidance, blood donor first‐time donation screening replaced universal screening in 2012. From 2007 to 2019, nearly 2500 seropositive blood donors were confirmed positive using a two‐step screening approach. 23 During this time, the highest number of confirmed positive donations came from California (N = 890), followed by Florida (N = 325), and then Texas (N = 199). 23 The largest concentration of confirmed positive samples was identified in 2008, shortly after the screening was initiated. 23 Among positive screening donors who reported birth country, individuals were largely from Mexico and the United States. 23 Previous studies in Texas have used blood donation centers to identify locally‐acquired cases, and incidental blood donor screening results in further states have been documented in a limited number of case reports, as shown in Figure 1. 9 , 10 , 12 , 13 , 19 , 24 , 25 , 26 , 27 This study sought to expand this effective, unintended screening mechanism to assess the potential for locally‐acquired cases in other US southwestern states.
FIGURE 1.
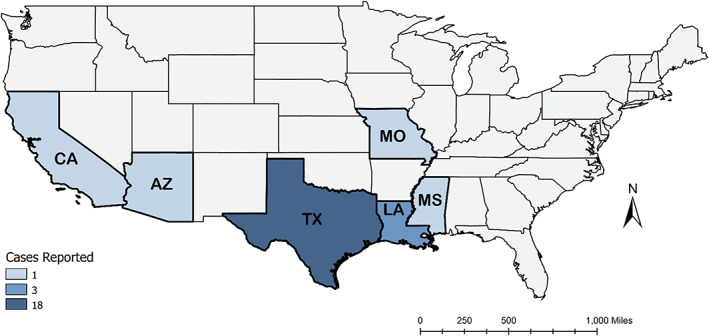
Count of locally‐acquired Chagas disease blood donors identified in the literature since 2010 with known geographic transmission origin. [Color figure can be viewed at wileyonlinelibrary.com]
2. MATERIALS AND METHODS
This study was approved by the University of South Carolina's institutional review board. Approval was also obtained from the American Red Cross, Central California Blood Center, and Vitalant. The U.S. Centers for Disease Control and Prevention (CDC) did not interact or intervene with study participants and did not have access to personal identifiers or links to personal identifiers. Blood donor centers from the aforementioned organizations operating in California, Arizona, New Mexico, and West Texas sent invitation letters on behalf of the study team to individuals in early 2019, who had tested positive between the years 2007 and 2019 and who were ≥ 18 years at the time of the study. Invitation letters and study team personnel were bilingual (Spanish and English). Blood center testing varied across years and blood centers, but all invited participants yielded two or more positive test results on the same sample at the time of donation at their respective blood centers.
All enrolled participants completed a detailed questionnaire to assess lifetime risk factors for infection and donated a small blood sample for confirmatory testing. Participants also underwent electrocardiogram (ECG) to appraise cardiac health. Enrolled persons from the American Red Cross who were willing, signed forms authorizing the release of original T. cruzi testing results to the study team for comparison. All blood samples were tested at the Laboratory of Vector‐Borne and Zoonotic Diseases at the University of South Carolina (UofSC) through Chagas Stat‐Pak (Chembio, Medford, NY) and Hemagen Chagas' Kit enzyme‐linked immunosorbent assay (ELISA) (Hemagen Diagnostics, Inc., Columbia, MD). Blood samples were sent to CDC's Parasitic Diseases Branch for confirmatory testing with Weiner Chagatest ELISA (Weiner Laboratories, Rosario, Argentina) and trypomastigote excreted‐secreted antigen immunoblot (TESA). Test manufacturers report high sensitivity and specificity for each diagnostic: Chagas Stat‐Pak (≥98.5%, ≥94.8%), 28 Hemagen Chagas' Kit ELISA (100%, 98.7%), 29 Weiner Chagatest ELISA (97.9%, 97.8%), 30 and TESA blot (100%, >94%), 31 respectively. Diagnostic performances vary by infected persons' geographic origin. 32 For the purposes of this manuscript, blood donors were considered confirmed positive if two or more of the total four blood tests (performed by CDC or in our laboratory) were positive. Blood donors were otherwise considered suspected, as all enrolled participants were positive through blood bank testing.
3. RESULTS
Approximately 1130 blood donors tested positive from California, Arizona, New Mexico, and Texas in the study's time frame of 2007 to 2019. 33 Attempts to contact these blood donors were made, and a total of 57 seropositive blood donors contacted the study team between May 2019 and July 2020; 46 completed consents and were enrolled. Of the 11 who contacted the study team but never enrolled, lack of trust in having someone visit their home for sample collection or inability to schedule an appointment was the most common cause of failure to enroll. While invitation letters were sent to all previously diagnosed T cruzi seropositive donors from this region, it is unknown how many addresses were current. Therefore, the recruitment of positive donors may have been affected by changes in address. In addition, blood donors who have positive Chagas disease screening test results are deferred from future donations, and the majority of these 1130 screening positive donors were identified before 2010, 33 giving further evidence that letters were likely received by a fraction of the original seropositive group. Authors were unable to call or email potential Chagas disease‐positive donors given a lack of resources and/or lack of information.
Out of 46 initially consented and enrolled study participants, we identified eight donors with evidence of autochthonous Chagas disease in the southwestern US through blood bank screening, Table 1. To the best of our knowledge, none of these donors have been previously reported in the scientific literature. All enrolled participants in this study were previously positive by two or more tests through their respective blood centers; however, prior test results were only available for a limited number of donors (Table 2). Lack of prior blood donor testing results was due to either participants' refusal to sign a consent releasing testing results or the blood bank organization no longer having access to these historical records. Based on serologic testing performed at UofSC and CDC, four of the eight donors with locally‐acquired infections had detectable T. cruzi antibody levels by two or more tests at the time of follow‐up. Therefore, these four donors were considered confirmed autochthonous infections. Donors with evidence of Latin American transmission origin (n = 38) are not discussed in this article, given the focus on autochthonous transmission.
TABLE 1.
Demographics and testing results for suspected, probable, and confirmed cases of autochthonous Chagas disease, may 2019–July 2020
| Study ID | Donor age, sex, state of residence | Ethnicity | Likely state exposure | UofSC serology test results | CDC serology test results | Epidemiologic status | Year treated | ||
|---|---|---|---|---|---|---|---|---|---|
| Stat‐Pak | Hemagen | Weiner EIA | TESA | ||||||
| BSW‐001 | 34, M, CA | N | CA | + | + | + | + | Confirmed | 2017 |
| BSW‐004 | 28, F, CA | N | CA | − | + | − | n/a | Suspected | 2012 |
| BSW‐007 | 67, F, AZ | N | AZ/ TX | + | + | + | + | Confirmed | − |
| BSW‐009 | 56, F, AZ | H | AZ | − | − | − | n/a | Suspected | − |
| BSW‐020 | 69, M, AZ | N | AZ/CA | −/+ | − | − | n/a | Suspected | − |
| BSW‐042 | 65, M, CA | N | CA/TX | + | + | + | + | Confirmed | − |
| BSW‐043 | 20, F, AZ | M | AZ | − | + | + | − | Confirmed | − |
| BSW‐052 | 67, M, AZ | N | AZ/ TX | − | − | − | n/a | Suspected | − |
Abbreviations: AZ, Arizona; CA, California; CDC, United States Center for Disease Control and Prevention; H, latinx; M, multi‐racial (non‐latinx caucasian/native American); N, non‐latinx caucasian; N/A, not administered; TX, Texas; UofSC, University of South Carolina; −/+ indicates a weak positive result.
TABLE 2.
Results of blood donor Chagas disease testing for American Red Cross Chagas donors with available results for analysis
| Study ID | Date of blood bank test | Screening test | Confirmation test | Additional notes |
|---|---|---|---|---|
| BSW‐007 | 9/25/2010 | ELISA | RIPA | ELISA result: positive (average: 4.669;) RIPA result: positive |
| BSW‐009 | 5/30/2016 | PRISM | ESA | PRISM result: positive (average 4.40); ESA result: positive |
| BSW‐020 | 6/23/2008 | ELISA | RIPA | ELISA result: positive (average: 1.291) RIPA result: positive |
| BSW‐042 | 1/3/2008 | ELISA | RIPA | ELISA result: positive (average 5.227); RIPA result: positive |
Abbreviations: ELISA, Ortho T. cruzi Enzyme‐Linked Immunosorbent Assay (Ortho‐Clinical Diagnostics, Inc., Raritan, NJ, US); ESA, Abbott ESA Chagas (Abbott Laboratories, Abbott Park, IL); PRISM, Abbott Prism Chagas EIA (Abbott Laboratories, Abbott Park, IL); RIPA, Radioimmunoprecipitation assay (Quest Diagnostics, Chantilly, VA).
None of these 8 confirmed or suspected autochthonous donors had mothers or maternal grandmothers born in Latin America, had traveled outside of the US for ˃2 weeks in an endemic country, or reported receiving blood, tissue, or organs in their lifetime. All were born in the contiguous US and lived in the US their entire lifetime. To understand potential differences between confirmed and suspected autochthonous donors, Table 3 details each of the eight donors' transmission risk profiles. The specific clinical and epidemiological details of the four confirmed autochthonous donors are presented in the subsequent paragraphs, as these are verified clinical cases. Any medical history or reported participant experience was reported to the study team at the time of the respective study interview.
TABLE 3.
Reported T. cruzi infection risk factors
| Study ID | BSW‐001 | BSW‐004 | BSW‐007 | BSW‐009 | BSW‐020 | BSW‐042 | BSW‐043 | BSW‐052 | |
|---|---|---|---|---|---|---|---|---|---|
| Residential | State(s) where exposure likely occurred | CA | CA | AZ/TX | AZ | AZ/CA | CA/TX | AZ | AZ/TX |
| Reported vector bite exposures | + | + | − | − | + | − | − | − | |
| Known reservoir species around home | + | + | + | + | + | − | − | + | |
| Resided on open land | + | + | + | − | + | − | − | − | |
| Ever resided in a rural area in state with known vector transmission | + | + | + | − | + | + | − | + | |
| Ever resided in TX | − | − | + | − | − | + | − | + | |
| Occupational | Ever worked outdoors | + | − | − | + | + | + | − | + |
| Ever worked outdoors at night | + | − | − | − | − | + | − | − | |
| History of military service | + | − | − | − | − | + | − | − | |
| Reported seeing kissing bug around home | + | + | − | − | + | − | − | − | |
| Recreational | Ever hunted | − | − | − | + | − | − | + | − |
| Ever camped | + | + | + | + | + | + | + | + | |
| Ever gardened | − | − | + | − | − | − | − | − | |
Abbreviations: AZ, Arizona; CA, California; TX, Texas.
3.1. Donor 1 (BSW‐001)
Donor 1 was a 36‐year‐old Caucasian man from Fresno, California with a history of gastrocolitis who was notified by the blood bank of his positive status in August 2017. A month prior, he reported being bitten by an insect at night while sleeping uncovered on a trampoline outside of his home with his children. Reaction to the bite included eyelid swelling and puffiness of the face, diarrhea, nausea, and general malaise. Shortly following notification of his infection status, the man went to his primary care physician who referred him to an infectious disease specialist. The specialist ordered two new tests for T. cruzi infection along with ECG and ECHO. Both tests for T. cruzi were positive and ECHO was normal. ECG at this time found partial branch block; however, a second ECG ordered in 2019 was normal. The specialist obtained Benznidazole and donor 1 completed the full course of treatment in 2017. Reported side effects from treatment included general malaise and persisting peripheral neuropathy two years later at the time of the interview in 2019. Donor 1 reported having no other indication of symptomatic Chagas disease at the time of interview.
Donor 1 reported a bite exposure with inflammatory reaction of the face suggestive of possible Romaña's sign. This feature is pathognomonic of acute Chagas infection, however, Romaña's was never clinically diagnosed and the insect that bit him was not confirmed to be a triatomine. Other possible triatomine exposures could have occurred during the donor's extensive outdoor activities over many years, as sylvatic animals may attract these insects to certain outdoor spaces. The current residence was located in a rural area, in good condition, and recently constructed with cement. The home was well maintained with limited apparent possibility for easy triatomine entry into the home and no evidence of triatomine infestation. Chickens in coops were kept approximately 40 feet from the residence and domestic dogs slept in an open‐air kennel next to the house, both of which might be sources of bloodmeals for triatomines. Donor 1 reported frequent tent camping trips to the Sierra Nevada Mountains approximately five to six times per year. Donor 1 reported employment as a solar technician working mainly at night or before dawn. While serving in the US military, Donor 1 was stationed in both Kentucky and Tennessee. Potential exposures in those two states include known sylvatic cycles and/or history of locally‐acquired human cases. 8 Except for his four‐year military travel history, donor 1 had lived in Central California his entire lifetime.
3.2. Donor 2 (BSW‐007)
Donor 2 was a 67‐year‐old Caucasian woman living outside of Tucson, Arizona with a history of renal disease and nephrectomy who tested positive for T. cruzi antibodies during a routine blood donation in September 2010. The woman reported having seen her primary care physician upon notification of her screening results. She reported her physician had been aware of Chagas disease and ordered diagnostic confirmatory tests with negative results in 2010. Her physician did not order ECG or ECHO and did not refer her to an infectious disease specialist. Donor 2 reported that her physician indicated there were no available medications to treat Chagas disease, and she did not pursue or receive treatment. The woman indicated frequent trouble swallowing, but no other indications of symptomatic Chagas disease. Blood samples collected at the time of interview (July 2019) were positive on all four administered tests and ECG was normal.
Possible domestic exposures included prior history of living in a rural area and a 13‐year history of frequent gardening. However, donor 2 did not report ever gardening at night and reported no notable hunting or camping history. Donor 2 reported having lived in Austin, Texas for four years, along with rural parts of the Boulder, Colorado area.
Donor 2 lived in a small residential area outside of Tucson surrounded by open land with a high amount of wildlife activity. This home had stucco exterior, was built in approximately 2006, and was in a suburban neighborhood. At the time of the interview with the study team, donor 2 reported neighbors complaining of triatomine infestation in their homes during the months prior to the study interview. Specifically, she reported her neighbors' taping doors shut to prevent insect intrusion. However, upon survey administration, donor 2 was not able to correctly distinguish triatomine insects from look‐alikes.
3.3. Donor 3 (BSW‐042)
Donor 3 was a 65‐year‐old Caucasian man, living in Irvine, California. At the time of interview, this donor reported recent‐onset, routine difficulty breathing when lying down, two pillow orthopnea, and diagnosed history of cardiac abnormality. After original donor screening results notification in 2008, donor 3 did not seek medical care. In 2016, he sought the care of his primary care physician for the aforementioned cardiac symptomology onset. During that visit, his blood donor results from eight years prior were discussed, at which point his doctor referred him to an infectious disease specialist. His medical team ordered confirmatory diagnostic testing which yielded a positive result. He then underwent an ECG, which was normal. Blood samples collected at the time of the December 2019 interview had positive results on all four administered tests and his ECG was abnormal. Donor 3 had not received treatment but was seeking treatment through his health care provider at the time of the interview.
Donor 3 reported possibly having a blood transfusion in 2013 following an accident but was not able to remember definitively. Possible domestic exposure included his 3‐year history of rural residence on a livestock ranch in San Benito, Texas where ample evidence of human autochthonous infection in this region of the state has been reported. 28 , 29 Potential occupational exposures included extensive history of in‐field trainings across military instillations in Ft Riley, Kansas; Ft. Ord, California; Ft Irwin, California; and Fort Hood, Texas. Specifically, donor 3 reported overnight stays for military training in tents and in open‐air in Kansas and Texas. The man also reported having been a park ranger in the California mountains for approximately six months.
3.4. Donor 4 (BSW‐043)
Donor 4 was a 20‐year‐old multiracial female from Phoenix, Arizona with history of cardiac abnormality. The woman reported intermittent difficulty breathing while lying down, negative for two pillow orthopnea, and occasional heart racing while resting. She further reported having seen a cardiologist several times for heart palpitations, which her cardiologist deemed normal. Upon donor screening results notification, donor 4 visited her primary care physician who ordered additional testing, which yielded a negative result. No cardiac tests were ordered.
Donor 4 reported little travel outside of the Phoenix area in her lifetime. She reported never working an agricultural job and never having worked outside at night. Donor 4 reported having RV camped approximately ten times in her lifetime in northern Arizona. Donor 4 on rare occasions had been bird hunting in the rural desert with her grandfather. No further occupational or recreational exposures were reported. Reported domestic exposures involved part‐time living with her father in a rural area surrounded by open desert. She reported keeping domestic cats and dogs that were allowed outside and inside the home. Other domestic exposure included monthly stays on her family livestock farm in southern Yuma County, situated on open land and surrounded by brush and wildlife. She reported staying overnight at her family farm and spending considerable time outdoors at night at this location. Donor 4 mentioned frustration with conflicting testing results, the inability to get treatment, and great anxiety for the possibility of cardiac problems later in life.
Blood samples collected during the March 2020 interview yielded positive results on two of four administered tests (Table 1). Prior blood bank test information and date of original positive serologic screening were not available for Donor 4. Her ECG was abnormal with three findings (left axis deviation, low QRS voltage, and lateral infarct); one of which, low QRS voltage, is considered a less specific Chagas cardiac abnormality. 34
4. DISCUSSION
This study provides evidence of eight additional locally‐acquired Chagas disease cases identified via the US blood donor screening system. Four had detectable antibodies by two or more tests at the time of study follow‐up and were considered confirmed cases. Though these positive donors were identified in California and Arizona, two confirmed donors had lived in Texas for multiple years. Therefore, we cannot exclude the possibility that these donors' infections were acquired in Texas, where the majority of US‐acquired cases have been identified. 20 , 35 , 36 California does have a long history of sylvatic enzootic transmission, and the reported frequency of camping and other nocturnal outdoor activity supports the potential for vector‐borne infection in that state. 37 Additional overlapping risk factors among these positive donors were ever living in a rural area in a state with known vector‐human transmission and having lived on property surrounded by open land (suggestive of potential exposure to sylvatic transmission cycles). Lastly, history of military service that included overnight field training was noted, which has previously been described as possible vector‐borne transmission sources. 9 , 38
Among the 8 donors with positive screening test results in this study, only two of the eight had received anti‐trypanosomal therapy. This low number highlights the need for greater physician awareness so that seropositive blood donors may have adequate access to diagnostic screening tests. As blood donation screening is non‐diagnostic in nature, physician awareness and thus the ability to receive a true diagnosis are key components in access to treatment. Sadly, a low anti‐parasitic medication rate is common for all Chagas‐infected persons living in the US, 39 particularly for those without a history of Latin American travel. 12 , 19 Both study participants who had received treatment (donors BSW‐001 & BSW‐004) were from the same hometown in central California, suggesting potentially higher physician knowledge in this area. Interestingly, one of these individuals was seropositive by only one of the study antibody diagnostics at the time of the study. Donor BSW‐004 was a 28‐year‐old non‐Hispanic woman who had never traveled out of the contiguous US and had lived in the same region of California all her life.
Effectiveness of treatment is typically related to promptness of therapy with benznidazole or nifurtimox after initial infection. Treatment is highly effective during acute phase infection, though limited evidence suggests further treatment benefits to those with chronic stage Chagas disease. 40 Blood donors with confirmed indeterminate chronic Chagas disease would benefit from treatment. 41 , 42 Blood donor screening for T. cruzi presents a unique clinical challenge to US physicians that are then responsible for ordering confirmation testing, obtaining treatment when warranted, and patient continuum of care. Thus, positive donors would also benefit from enhanced notification letters with recommendations for physician follow‐up. To ensure prompt treatment, physician awareness of Chagas disease should also be increased, to minimize misinformation surrounding Chagas disease. 43 , 44 , 45 For example, in this investigation, one donor reported being told no treatment was available, and another was informed that local triatomines were not infected with T. cruzi despite contrary scientific evidence. 20
Serologic discrepancies remain a prominent hindrance for Chagas disease. 44 As noted between Tables 1 and 2, considerable inconsistencies occurred in this donor population across time. Prior donor screening results were available for four donors. In two (BSW‐009 & BSW‐020), detectable antibodies may have been lost within a 3‐year and 11‐year interim, respectively. Alternatively, this may be indicative that these individuals were false‐positive during initial blood donor testing. The difference in results from blood donor testing compared to diagnostic testing years later may be due to clearance of parasite or possibly differences in test performance. Clinical diagnostic test discrepancies have been described in US patient populations which may reflect differences in the antibody responses to the T. cruzi DTUs causing infection in those regions. 44 , 46 This highlights the need for more feasible access to rigorous diagnostic testing, as those used by blood centers vary both between and within blood centers over time.
This investigation was not without limitations. The primary limitation was the poor response rate (5%). The majority of blood donors diagnosed with Chagas disease in the US were detected prior to 2010 33 and our follow‐up study occurred nearly a decade later in 2019–2020. Seropositive donors are permanently deferred from donation, and most are lost to follow‐up. Given the nearly ten‐year difference between the initial diagnosis and the current study, we theorize several donors likely never received invitations to participate, which might have resulted in selection bias. Next, the diagnostic tests used by US blood banks are not available to government officials or academic partners, preventing study personnel from being able to run the same tests for prospective evaluation. Despite these limitations, this study has scientific merit and adds to the limited literature on locally‐acquired Chagas disease human cases in the US.
The potential for locally‐acquired Chagas disease in the United States exists. Though cases are likely rare, it is vital to understand the frequency and location of these infections to further clarify vector‐human transmission dynamics across the country. Due to the neglected nature of this parasitic infection in areas not traditionally considered endemic and lacking physician familiarity with Chagas disease, locally‐acquired cases are at particular risk of going undetected. Further, as this manuscript demonstrates, cases initially identified through blood donation present unique clinical management challenges years after initial diagnosis. In this cohort, half of the initially positive autochthonous Chagas disease blood donors were seropositive up to a decade after the initial seropositive diagnosis. Though clear challenges exist with diagnostic performance, physician awareness, and treatment access, treatment is possible and recommended for the majority of individuals under 50. 47
This manuscript identifies an opportunity for enhanced blood center action in regard to T. cruzi positive donors of both US and foreign origin. Blood centers should provide positive donors with information and follow‐up recommendations specifically with an infectious disease specialist to account for the rates of low physician awareness nation‐wide. 4 , 5 T. cruzi testing results and deferral letters should better inform donors of the implications of their T. cruzi positive status and provide Chagas disease education materials. Future qualitative analysis of blood donors is warranted to design more effective notification letters to ensure a proper continuum of care for all donors testing T. cruzi positive.
FUNDING INFORMATION
This research was funded by The Brockman Medical Research Foundation.
CONFLICT OF INTEREST
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
ACKNOWLEDGMENTS
The authors would like to thank all study participants for their time and effort in increasing awareness of Chagas disease in the US.
Lynn MK, Dye‐Braumuller KC, Beatty NL, Dorn PL, Klotz SA, Stramer SL, et al. Evidence of likely autochthonous Chagas disease in the southwestern United States: A case series of Trypanosoma cruzi seropositive blood donors. Transfusion. 2022;62(9):1808–1817. 10.1111/trf.17026
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding information Brockman Foundation, Grant/Award Number: Pro00078573
DATA AVAILABILITY STATEMENT
Data is available upon reasonable request.
REFERENCES
- 1. Bocchi EA, Arias A, Verdejo H, Diez M, Gómez E, Castro P. The reality of heart failure in Latin America. J Am Coll Cardiol. 2013;62(11):949–58. 10.1016/j.jacc.2013.06.013 [DOI] [PubMed] [Google Scholar]
- 2. Forsyth CJ, Manne‐Goehler J, Bern C, Whitman J, Hochberg NS, Edwards M, et al. Recommendations for screening and diagnosis of Chagas disease in the United States. J Infect Dis. 2021;225:1601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lynn MK, Bossak BH, Sandifer PA, Watson A, Nolan MS. Contemporary autochthonous human Chagas disease in the USA. Acta Trop. 2020;205:105361. 10.1016/j.actatropica.2020.105361 [DOI] [PubMed] [Google Scholar]
- 4. Edwards MS, Abanyie FA, Montgomery SP. Survey of Pediatric Infectious Diseases Society members about congenital Chagas disease. Pediatr Infect Dis J. 2018;37(1):e24–e7. 10.1097/inf.0000000000001733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stimpert KK, Montgomery SP. Physician awareness of Chagas disease, USA. Emerg Infect Dis. 2010;16(5):871–2. 10.3201/eid1605.091440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bennett C, Straily A, Haselow D, Weinstein S, Taffner R, Yaglom H, et al. Chagas disease surveillance activities—seven states, 2017. Morb Mortal Wkly Rep. 2018;67(26):738–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DSHS T . Chagas disease data: Texas Department of State Health Services. 2021 [updated may 4, 2021July 20, 2021]. Available from: https://www.dshs.state.tx.us/IDCU/disease/chagas/Chagas-Disease-Data.aspx?terms=chagas.
- 8. Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011;24(4):655–81. 10.1128/CMR.00005-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris N, Woc‐Colburn L, Gunter SM, Gorchakov R, Murray KO, Rossmann S, et al. Autochthonous Chagas disease in the southern United States: a case report of suspected residential and military exposures. Zoonoses Public Health. 2017;64(6):491–3. 10.1111/zph.12360 [DOI] [PubMed] [Google Scholar]
- 10. Beatty NL, Perez‐Velez CM, Yaglom HD, Carson S, Liu E, Khalpey ZI, et al. Evidence of likely autochthonous transmission of Chagas disease in Arizona. Am J Trop Med Hyg. 2018;99(6):1534–6. 10.4269/ajtmh.18-0485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bern C, Messenger LA, Whitman JD, Maguire JH. Chagas disease in the United States: a public health approach. Clin Microbiol Rev. 2019;33(1), pp. e00023–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia MN, Aguilar D, Gorchakov R, Rossmann SN, Montgomery SP, Rivera H, et al. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg. 2015;92(2):325–30. 10.4269/ajtmh.14-0238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turabelidze G, Vasudevan A, Rojas‐Moreno C, Montgomery SP, Baker M, Pratt D, et al. Autochthonous Chagas disease ‐ Missouri, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(7):193–5. 10.15585/mmwr.mm6907a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klotz SA, Smith SL, Schmidt JO. Kissing bug intrusions into homes in the Southwest United States. Insects. 2021;12(7):654. 10.3390/insects12070654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vecchio FL, Van Tran T. Allergic reactions from insect bites. Am J Emerg Med. 2004;22(7):631. [DOI] [PubMed] [Google Scholar]
- 16. Behrens‐Bradley N, Smith S, Beatty NL, Love M, Ahmad N, Dorn PL, et al. Kissing bugs harboring Trypanosoma cruzi, frequently bite residents of the US southwest but do not cause Chagas disease. Am J Med. 2020;133(1):108–114.e13. 10.1016/j.amjmed.2019.06.016 [DOI] [PubMed] [Google Scholar]
- 17. Beatty NL, Klotz SA. Autochthonous chagas disease in the United States: how are people getting infected? Am J Trop Med Hyg. 2020;103(3):967–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia MN, Murray KO, Hotez PJ, Rossmann SN, Gorchakov R, Ontiveros A, et al. Development of chagas cardiac manifestations among Texas blood donors. Am J Cardiol. 2015;115(1):113–7. 10.1016/j.amjcard.2014.09.050 [DOI] [PubMed] [Google Scholar]
- 19. Gunter SM, Murray KO, Gorchakov R, Beddard R, Rossmann SN, Montgomery SP, et al. Likely autochthonous transmission of Trypanosoma cruzi to humans, south Central Texas, USA. Emerg Infect Dis. 2017;23(3):500–3. 10.3201/eid2303.161157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klotz SA, Shirazi FM, Boesen K, Beatty NL, Dorn PL, Smith S, et al. Kissing bug (Triatoma spp.) intrusion into homes: troublesome bites and domiciliation. Environ Health Insights. 2016;10:45–9. 10.4137/ehi.s32834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Blood donor screening for chagas disease–United States, 2006‐2007. MMWR Morb Mortal Wkly Rep. 2007;56(7):141–3. [PubMed] [Google Scholar]
- 22. Bern C, Montgomery SP, Katz L, Caglioti S, Stramer SL. Chagas disease and the US blood supply. Curr Opin Infect Dis. 2008;21(5):476–82. 10.1097/QCO.0b013e32830ef5b6 [DOI] [PubMed] [Google Scholar]
- 23. AABB . Chagas Biovigilance Network Bethesda, Maryland: U.S. AABB. 2021 [updated December 20, 2019; cited 2021 September 26, 2021]. Available from: https://www.aabb.org/news-resources/resources/hemovigilance/chagas-biovigilance-network.
- 24. Cantey PT, Stramer SL, Townsend RL, Kamel H, Ofafa K, Todd CW, et al. The United States Trypanosoma cruzi infection study: evidence for vector‐borne transmission of the parasite that causes Chagas disease among United States blood donors. Transfusion. 2012;52(9):1922–30. 10.1111/j.1537-2995.2012.03581.x [DOI] [PubMed] [Google Scholar]
- 25. Webber MB, Wozniak EJ, Chang D, Bush KN, Wilson MC, Watts JA, et al. A case of Chagas cardiomyopathy following infection in south Central Texas. US Army Med Department J. 2017 Jan‐Jun;(1‐17). 55–9. PMID:28511274. [PubMed] [Google Scholar]
- 26. Hernandez S, Flores CA, Viana GM, Sanchez DR, Traina MI, Meymandi SK. Autochthonous transmission of Trypanosoma Cruzi in Southern California. Open Forum Infect Dis. 2016;3(4):ofw227. 10.1093/ofid/ofw227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LOPH . Chagas Disease‐ American Trypanosomiasis. Baton Rouge (LA): Louisiana Office of Public Health ‐ Infectious Disease Epidemiology Section (US). 2018.
- 28. Luquetti AO, Ponce C, Ponce E, Esfandiari J, Schijman A, Revollo S, et al. Franco da Silveira J. Chagas' disease diagnosis: a multicentric evaluation of Chagas stat‐Pak, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagn Microbiol Infect Dis. 2003;46(4):265–71. 10.1016/s0732-8893(03)00051-8 [DOI] [PubMed] [Google Scholar]
- 29. Hemagen Chagas' Kit (EIA Method). Columbia, MD: Hemagen Diagnostics, Inc; 2009. Report No.: Contract No.: 66101. [Google Scholar]
- 30. Summary of 510(k) safety and effectiveness information is being submitted in accordance with the requirements of SMDA 1990 and 21CFR 807.92. Administration UFaD, editor. Rosario, Argentina: Weiner. 2004: Searching for Safety. Aaron Wildavsky.
- 31. Berrizbeitia M, Ndao M, Bubis J, Gottschalk M, Aché A, Lacouture S, et al. Purified excreted‐secreted antigens from Trypanosoma cruzi trypomastigotes as tools for diagnosis of Chagas' disease. J Clin Microbiol. 2006;44(2):291–6. 10.1128/jcm.44.2.291-296.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Truyens C, Dumonteil E, Alger J, Cafferata ML, Ciganda A, Gibbons L, et al. Geographic variations in test reactivity for the serological diagnosis of Trypanosoma cruzi infection. J Clin Microbiol. 2021;59(12):e0106221. 10.1128/jcm.01062-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. AABB. Chagas biovigilance network. American Association of Blood Banks; [online]. Available at https://www.aabb.org/news-resources/resources/hemovigilance/chagas-biovigilance-network. Accessed September 26, 2021. [Google Scholar]
- 34. Sabino EC, Ribeiro AL, Salemi VM, Di Lorenzo Oliveira C, Antunes AP, Menezes MM, et al. Ten‐year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi‐seropositive former blood donors. Circulation. 2013;127(10):1105–15. 10.1161/circulationaha.112.123612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dye‐Braumuller KC, Waltz H, Lynn MK, Klotz SA, Schmidt JO, Romero A, et al. A southwestern United States pilot investigation of Triatomine–mite prevalence. Insects. 2021;12(9):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klotz SA, Dorn PL, Klotz JH, Pinnas JL, Weirauch C, Kurtz JR, et al. Feeding behavior of triatomines from the southwestern United States: an update on potential risk for transmission of Chagas disease. Acta Trop. 2009;111(2):114–8. 10.1016/j.actatropica.2009.03.003 [DOI] [PubMed] [Google Scholar]
- 37. Dye‐Braumuller KC, Lynn MK, Nolan MS. History of indigenous Trypanosoma cruzi infection in humans, animals and triatomines in California. USA Zoonoses Public Health. 2021;68(4):299–308. 10.1111/zph.12797 [DOI] [PubMed] [Google Scholar]
- 38. Garcia MN, Cropper TL, Gunter SM, Kramm MM, Pawlak MT, Roachell W, et al. Vector‐borne diseases of public health importance for personnel on military installations in the United States. US Army Med Dep J. 2017;1‐17:90–101. [PubMed] [Google Scholar]
- 39. Herwaldt BL, Dougherty CP, Allen CK, Jolly JP, Brown MN, Yu P, et al. Characteristics of patients for whom Benznidazole was released through the CDC‐sponsored investigational new drug program for treatment of Chagas disease ‐ United States, 2011–2018. MMWR Morb Mortal Wkly Rep. 2018;67(29):803–5. 10.15585/mmwr.mm6729a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murcia L, Carrilero B, Ferrer F, Roig M, Franco F, Segovia M. Success of benznidazole chemotherapy in chronic Trypanosoma cruzi‐infected patients with a sustained negative PCR result. Eur J Clin Microbiol Infect Dis. 2016;35(11):1819–27. 10.1007/s10096-016-2733-6 [DOI] [PubMed] [Google Scholar]
- 41. Yun O, Lima MA, Ellman T, Chambi W, Castillo S, Flevaud L, et al. Feasibility, drug safety, and effectiveness of etiological treatment programs for Chagas disease in Honduras, Guatemala, and Bolivia: 10‐year experience of Médecins Sans Frontières. PLoS Negl Trop Dis. 2009;3(7):e488. 10.1371/journal.pntd.0000488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dias JC, Ramos AN Jr, Gontijo ED, Luquetti A, Shikanai‐Yasuda MA, Coura JR, et al. 2 nd Brazilian consensus on Chagas disease, 2015. Rev Soc Bras Med Trop. 2016;49(Suppl 1):3–60. 10.1590/0037-8682-0505-2016 [DOI] [PubMed] [Google Scholar]
- 43. Marcus R, Henao‐Martínez AF, Nolan M, Livingston E, Klotz SA, Gilman RH, et al. Recognition and screening for Chagas disease in the USA. Ther Adv Infect Dis. 2021;8:20499361211046086. 10.1177/20499361211046086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hochberg NS, Wheelock A, Hamer DH, Marcus R, Nolan MS, Meymandi S, et al. Chagas disease in the United States: a perspective on diagnostic testing limitations and next steps. Am J Trop Med Hyg. 2021;104(3):800–4. 10.4269/ajtmh.19-0871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nolan MS, Hochberg NS. Chagas disease in HIV‐infected patients: It's time to consider the diagnosis. Am J Trop Med Hyg. 2021;105(3):545–6. 10.4269/ajtmh.21-0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nolan MS, Aguilar D, Misra A, Gunter SM, Erickson T, Gorchakov R, et al. Trypanosoma cruzi in nonischemic cardiomyopathy patients, Houston, Texas, USA. Emerg Infect Dis. 2021;27(7):1958–1960. 10.3201/eid2707.203244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meymandi S, Hernandez S, Park S, Sanchez DR, Forsyth C. Treatment of Chagas disease in the United States. Curr Treat Options Infect Dis. 2018;10(3):373–88. 10.1007/s40506-018-0170-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon reasonable request.


