Abstract
This study evaluated the optimal timing of a primary three‐dose hepatitis B vaccination and postvaccination serologic testing (PVST) among a large group of healthy naïve adults in the Netherlands. Data were collected from the Ease Travel Clinic hepatitis B vaccination database. The study population consisted of 22,997 adults who received three hepatitis B vaccinations. Seroprotection was attained in 97.3% individuals. When compared with PVST performed at 1–2 months (98.2%) after the final dose, lower seroprotection rates were observed with <1 (97.3%, p = 0.128), 3–6 (90.6%, p < 0.001), and ≥7 (88.4%, p < 0.001) months after vaccination. Among the subpopulation with a PVST 1–2 months, no statistically significant difference was observed for the various intervals between the first and second vaccination (<1, 1–2, 3–4, or ≥5 months). When compared with 4–5 months between the second and third vaccine dose, lower seroprotection rates were observed with <4 (odds ratio [OR]: 0.29, p = 0.020) and ≥12 (OR: 0.22, p < 0.001) months, although comparable rates were observed with 6–11 months interval (OR: 0.85, p = 0.262). Our data indicate that PVST should be obtained 1–2 months after the last vaccination and a delayed PVST was the major determinant of a lower seroprotection rate after primary three‐dose hepatitis B vaccination schedule. Based on our data, the hepatitis B vaccination also leaves room for flexibility for the second dose and the third dose without the necessity of restarting the vaccination series or confirmation of the immune response to the vaccine.
Keywords: antibody response, hepatitis B, immunization, vaccination, vaccine
1. INTRODUCTION
Hepatitis B is an important global health problem and a life‐threatening liver infection caused by the hepatitis B virus (HBV). Worldwide, an estimated 292 million people were living with chronic HBV infection in 2016 and each year hepatitis B resulted in 887 000 deaths, mostly from cirrhosis and hepatocellular carcinoma. 1 , 2
Despite advances in antiviral therapy, a vaccine against HBV is the gold measure for prevention and is the most cost‐effective. 3 Since its discovery in 1982, more than 1 billion doses of hepatitis B vaccine have been administered. 3
Primary vaccination in healthy naïve adults generally consists of three intramuscular doses of recombinant HBV vaccine on a 0‐, 1‐ and 6‐month schedule. Seroprotection rate is documented by a hepatitis B surface antibody (anti‐HBs) level of ≥10 mIU/ml and is achieved in 90–95% of the immunocompetent adult population after completion of the vaccination schedule. 4 , 5 Recently, various strategies have been developed to elicit higher rates of protective anti‐HBs levels: increased antigen dose, intradermal vaccination, adjuvanted vaccines, and other strategies (hepatitis A + B vaccine, Sci‐B‐Vac®, DNA vaccines, polypeptide micelle vaccine). 6 , 7 However, adhering to the optimal timing of a primary three‐dose recombinant hepatitis B vaccination and postvaccination serologic testing (PVST) might be a relatively easy way to ensure seroprotection. Among immunocompetent adults, most studies investigated the optimal interval between the second and third hepatitis B vaccine dose on immune response to the vaccine, with only two studies reporting on the interval between the first and second vaccine. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 However, these studies are hampered by a low number of subjects (n < 500), a lack of multivariate analyses and a poor definition of nonresponder based on delayed PVST. In line with the recent recommendations of the Advisory Committee on Immunization Practices (ACIP), PVST should be determined within 1–2 months after the completion of the hepatitis B vaccine series as anti‐HBs levels wane over time. 4 Notwithstanding that the evidence for this recommendation of PVST within 1–2 months instead of 1–6 months or 1–12 months is largely limited to studies in pediatric patients. 22 , 23 , 24
This study evaluated the optimal timing of a primary three‐dose hepatitis B vaccination and PVST among a large group of healthy naïve adults in the Netherlands, a country located in Western Europe.
2. PATIENTS AND METHODS
2.1. Study design
This retrospective study included hepatitis B naïve adults from the Ease Travel Clinic at the Maastricht University Medical Centre+ (MUMC+; Maastricht, The Netherlands). Data of individuals aged 18 years or older initiating a primary three‐dose hepatitis B vaccination between 1983 and 2017 were eligible. Exclusion criteria were missing demographic data, not receiving all three hepatitis B vaccine doses and no information on PVST. According to the local guidelines, the commercially available HBVax© 20 μg/ml (from the year 1983 to 1987) or Engerix‐B© 20 μg/ml (from 1988 to 2017) vaccines were injected into the deltoid muscle with adequate 23‐gauge needle with a length of 25 mm. All individuals were born before the introduction of the universal infant hepatitis B vaccination in the Dutch immunization program on August 1, 2011.
The study was approved (number 2017‐0308) by the medical research ethics committee of MUMC+ and the need of informed consent was waived seen the noninterventional character of our study.
2.2. Measurement of outcome and exposures
The primary endpoint of the current study was the proportion of individuals with seroprotection (i.e., anti‐HBs levels ≥10 mIU/ml) according to the PVST. First, we assessed the relation between time from the third vaccination to PVST and seroprotection according to the PVST. Time to PVST was categorized in <1 (too early), 1–2 (on time), 3–6 (late), and ≥7 months (very late), in part adapted from the recommendations of the ACIP. 4 Secondly, this study assessed whether the interval between the first and second vaccination (first and second vaccine interval) and between the second and third vaccination (second and third vaccine interval) were independent predictors of seroprotection in patients with PVST at 1–2 months. Time between the first and second vaccination was categorized into <1 (too early), 1–2 (on time), 3–4, and ≥5 months, adapted from ACIP guidelines. 4 Accordingly, the interval between the second and third vaccination was grouped into <4 (too early), 4–5 (on time), 6–11, and ≥12 months. 4
2.3. Statistical analysis
Categorical data were expressed as frequencies and percentages, and continuous variables were expressed as mean ± SD or median ± interquartile range as appropriate. For the comparison of categorical variables, either χ 2 test or Fisher's exact test was used, as appropriate.
Among the total study population, odds ratios (ORs) of having seroprotection by age, gender, race, time between the third vaccination and PVST, time between the first and second vaccination, and time between the second and third vaccination were calculated using univariable and multivariable‐adjusted logistic regression analyses.
Potential interactions between the number of months of PVST after the final vaccine dose, first and second vaccine interval or second and third vaccine interval, and age (≥40 year vs. <40 year), sex (male vs. female), and race (Caucasian vs. non‐Caucasian) were explored using a Wald test. In case of a statistical significant interaction, statistical analyses were stratified accordingly. All statistical analyses were performed using SPSS (version 25.0, 2017). p < 0.05 was considered statistically significant.
3. RESULTS
Out of 30 344 adults initiating hepatitis B vaccination in our clinic, 22 997 were included according to the inclusion and exclusion criteria (Figure 1). The study population consisted to a lesser degree of individuals aged ≥40 years (29.4%) and males (28.3%). Median age was 25 ± 24.0 years. Caucasians made up 98.8% of the study population, with 0.4% of Asian and 0.8% of African race. Median interval first and second vaccine was 1 ± 0.2 months, this was 5 ± 0.5 months for interval second and third vaccine and 1 ± 0.5 months for PVST after final dose. Out of 22 997 individuals, 22 377 (97.3%) had achieved seroprotection after primary three‐dose hepatitis B vaccination schedule.
Figure 1.

Flowchart of the study.
3.1. Seroprotection rate: PVST after final vaccine dose
Figure 2 shows the proportion of individuals with seroprotection with increasing interval from final vaccine dose to PVST. When compared with PVST performed at 1–2 months (98.2%, 19 640/19 991) after the final dose, lower seroprotection rates were observed with <1 (97.3%, 509/523, p = 0.128), 3–6 (90.6%, 1336/1474, p < 0.001), and ≥7 months (88.4%, 892/1009, p < 0.001) after vaccination. Among those individuals with a PVST < 1 month after the final dose, the median ± interquartile range of PVST was 25 ± 6 days after completion of the vaccination schedule. This number was 18 ± 38 months for individuals with serologic testing ≥7 months after the final vaccine dose.
Figure 2.

Proportion of individuals with anti‐HBs levels <10 and ≥ 10 mIU/ml with increasing interval from final vaccine dose to postvaccination serologic testing (n = 22 997).
Interactions between PVST and age ≥ 40 years (yes vs. no, p = 0.117), male gender (yes vs. no, p < 0.001), or Caucasian race (yes vs. no, p = 0.162) on the seroprotection rates were determined. Considering the observed interaction of gender on the relationship between time between the third vaccination and PVST on seroprotection rates, the analysis was performed for males and females separately. Among males, when compared with PVST performed at 1–2 months (96.9%, 5267/5437) after the final dose, lower seroprotection rates were observed with <1 (92.2%, 119/129, p = 0.009), 3–6 (87.8%, 468/533, p < 0.001), and ≥7 (months 87.2%, 362/415, p < 0.001) after vaccination. Among females, seroprotection was found in 98.8% (14 373/14 554) when PVST was performed at 1–2 months and seroprotection rates were 99.0% with <1 (390/394, p = 0.821), 92.2% with 3–6 (868/941, p < 0.001), and 89.2% with ≥7 months (530/594, p < 0.001) after the final dose.
Among the total population, age ≥ 40 years (OR: 0.25, p < 0.001) and male gender (OR: 0.43, p < 0.001) were associated with a lower seroprotection rate in multivariable logistic regression analysis (Table 1). When compared with PVST 1–2 months, there was an advancing decline in OR for seroprotection with increasing interval from final vaccine dose: OR 0.71 for PVST < 1 months (p = 0.213), OR: 0.21 for PVST 3–6 months (p < 0.001), and OR: 0.16 for PVST ≥ 7 months (p < 0.001). Table 1 also shows the predictive factors of seroprotection among males and females separately.
Table 1.
Factors predictive of seroprotection in multivariable logistic regression analysis among the total population, males, and females
|
Total population n = 22 997 |
Males n = 6514 |
Females n = 16 483 |
||||
|---|---|---|---|---|---|---|
| Variables | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p |
| Age ≥ 40 years (yes vs. no) | 0.25 (0.21 – 0.30) | p < 0.001 | 0.26 (0.21 – 0.33) | p < 0.001 | 0.24 (0.19 – 0.31) | p < 0.001 |
| Male gender (yes vs. no) | 0.43 (0.36 – 0.51) | p < 0.001 | – | – | – | – |
| Caucasian race (yes vs. no) | 1.16 (0.58 – 2.31) | p = 0.679 | 1.32 (0.56 – 3.1) | p = 0.531 | 0.93 (0.29 – 3.0) | p = 0.909 |
| PVST | p < 0.001 | p < 0.001 | p < 0.001 | |||
| <1 mo vs. 1–2 mo | 0.71 (0.41 – 1.22) | p = 0.213 | 0.40 (0.20 – 0.78) | p = 0.007 | 1.43 (0.53 – 3.89) | p = 0.479 |
| 3–6 mo vs. 1–2 mo | 0.21 (0.17 – 0.26) | p < 0.001 | 0.27 (0.20 – 0.36) | p < 0.001 | 0.17 (0.13 – 0.23) | p < 0.001 |
| ≥7 mo vs. 1–2 mo | 0.16 (0.13 – 0.20) | p < 0.001 | 0.23 (0.17 – 0.32) | p < 0.001 | 0.11 (0.08 – 0.15) | p < 0.001 |
Abbreviations: CI, confidence interval; mo, months; OR, odds ratio; PVST, postvaccination serologic testing.
3.2. Seroprotection rates: Interval between the first and second vaccine and interval between the second and third vaccine among subgroup with a timely PVST 1–2 months after the third dose
Figure 3 shows the seroprotection rates of increasing interval between the first and second vaccine in a subgroup of individuals with a timely PVST 1–2 months after the third dose. When compared to the seroprotection rate of 98.2% (18 270/18 601) with an interval of 1–2 months between the first and second vaccine, comparable rates were observed with <1 month (98.6%, 1161/1177, p = 0.287), 3–4 (97.5%, 118/121, p = 0.728), and ≥5 (98.9%, 91/92, p = 0.736) months between the first and second vaccine dose. Median ± interquartile range was 26 ± 3 days between the first and second vaccination for those individuals with <1 month between the first and second vaccine dose, and 10 ± 9 months for those with ≥5 months between the first and second vaccine dose.
Figure 3.
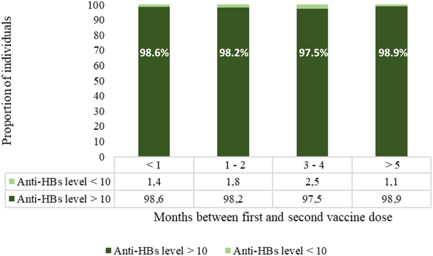
Proportion of individuals with anti‐HBs levels <10 and ≥10 mIU/ml with different intervals between the first and second vaccine dose among subgroup with a timely postvaccination serologic testing 1–2 months after the third dose (n = 19 991).
Figure 4 shows the seroprotection rates of increasing interval between the second and third vaccine. Statistically significant differences in seroprotection rates were observed: when compared with 4–5 months between the second and third vaccine dose (98.4%, 17 064/17 344), lower seroprotection rates were observed with <4 (94.7%, 71/75, p = 0.034), 6–11 (97.6%, 2338/2396, p = 0.004), and ≥12 (94.9%, 167/176, p = 0.003) months after vaccination.
Figure 4.

Proportion of individuals with anti‐HBs levels <10 and ≥10 mIU/ml with different intervals between the second and third vaccine dose among subgroup with a timely postvaccination serologic testing 1–2 months after the third dose (n = 19 991).
Among those individuals with <4 months between the second and third vaccine, the median ± interquartile range was 3 ± 3 months between the second and third vaccination. This number was 22 ± 21 months for individuals with third vaccine dose ≥12 months after the second vaccine dose.
Interactions between interval first and second vaccine or interval second and third vaccine and demographics were assessed with multivariate logistic regression analyses (p = 0.754 and p = 0.732 for age, p = 0.983 and p = 0.246 for male gender, and p = 0.680 and p = 0.994 for Caucasian race, respectively).
Table 2 illustrates the predictive factors of seroprotection in this subgroup with a timely PVST 1–2 months after the final dose. Age ≥ 40 years (OR: 0.26, p < 0.001) and male gender (OR: 0.36, p < 0.001) were independent predictors of seroprotection. When compared with 4–5 months between the second and third vaccine dose, <4 months (OR: 0.29, p = 0.020) and ≥12 months (OR: 0.22, p < 0.001) were associated with a lower seroprotection rate. There was a comparable seroprotection rate with 6–11 months (OR: 0.85, p = 0.262). No significant associations were observed for interval between first and second vaccine dose.
Table 2.
Factors predictive of seroprotection in multivariable logistic regression analysis (n = 19 991)
|
Subgroup with timely PVST n = 19 991 |
||
|---|---|---|
| Variables | OR (95% CI) | p |
| Age ≥ 40 years (yes vs. no) | 0.26 (0.21 – 0.32) | p < 0.001 |
| Male gender (yes vs. no) | 0.36 (0.29 – 0.44) | p < 0.001 |
| Caucasian race (yes vs. no) | 1.50 (0.65 – 3.43) | p = 0.342 |
| Interval first and second vaccine | p = 0.939 | |
| <1 mo vs. 1–2 mo | 1.10 (0.66 – 1.83) | p = 0.708 |
| 3–4 mo vs. 1–2 mo | 0.94 (0.29 – 3.04) | p = 0.918 |
| ≥5 mo vs. 1–2 mo | 1.67 (0.23 – 12.27) | p = 0.612 |
| Interval second and third vaccine | p < 0.001 | |
| <4 mo vs. 4–5 mo | 0.29 (0.10 – 0.82) | p = 0.020 |
| 6–11 mo vs. 4–5 mo | 0.85 (0.63 – 1.13) | p = 0.262 |
| ≥12 mo vs. 4–5 mo | 0.22 (0.11 – 0.44) | p < 0.001 |
Abbreviations: CI, confidence interval; mo, months; OR, odds ratio; PVST, postvaccination serologic testing.
4. DISCUSSION
This is the first study assessing the optimal interval of three hepatitis B vaccine doses and PVST among a large cohort of healthy hepatitis B naïve adults. Our data indicate that PVST should be obtained 1–2 months after the last vaccination and a delayed PVST was the major determinant of a lower seroprotection rate after primary three‐dose hepatitis B vaccination schedule. Whenever the PVST is obtained at 1–2 months, the interval between first and second vaccine might be as low as 26 days and as high as 10 months without considerable changes in seroprotection rates. Although the first and second vaccine interval had minimal/no influence on seroprotection rates, the second and third vaccine interval was shown to moderately affect the seroprotection rates.
As expected, age >40 years and male gender were associated with a lower seroprotection rate in our study cohort, as has been described previously. 25 We found no effect of race, this might be related to the fact that the majority of our population was of Caucasian race.
The results of this study are important in respect to Public Health. Primary hepatitis B vaccination generally consists of three vaccinations at 0, 1, and 6 months, and elicits seroprotection in 95% of the general adult population. 4 , 5 However, the remaining 5% of the population with an inadequate immune response are called hepatitis B vaccine nonresponders and remain susceptible for HBV infection and its serious sequelae such as cirrhosis and hepatocellular carcinoma. 6 , 26
Nonetheless, one should acknowledge the importance of a timely PVST for the definition of a hepatitis B vaccine nonresponder. As illustrated by our study, when compared with the high 98.2% seroprotection rate after a timely PVST (1–2 months after completion of the primary vaccination schedule), seroprotection rates were as low as 90.6% for PVST at 3–6 months and 88.4% for PVST at ≥7 months after vaccination. This study highlights that intervals >3 months between final hepatitis B vaccine dose and PVST are associated with waning anti‐HBs levels, which might fail to confirm seroprotection and results in unnecessary revaccination. Our results are therefore in line with previous findings in infants and dictate the importance of defining hepatitis B vaccine nonresponder based on a timely PVST 1–2 months after the last vaccination. 22 , 23 , 24
An important result of this study was the demonstration that the interval between the first and second vaccine might be as low as 26 days and as high as 10 months without considerable changes in seroprotection rates. Similar findings were reported by Hadler et al. 17 in a study of hepatitis B vaccine doses in Yucpa Indians. On the other hand, a study on hepatitis B vaccination in Spanish prisoners suggested that a delay in administration of the second hepatitis B vaccine dose induced a lower seroprotection rate. 27 Nonetheless, both studies are hampered by the low number of subjects (n < 500), lack of multivariate analyses, and suboptimal definition of nonresponse. Our finding has a practical implication in the fact that delaying the second dose for several months will not necessitate restarting the vaccination series or confirmation of the immune response to the vaccine.
We illustrated that a 4–5 months interval induced comparable seroprotection rates as a 6–11 months interval between the second and third hepatitis B vaccine dose, as was demonstrated by others. 8 , 9 , 10 , 11 , 16 , 17 , 28 An important implication of this finding is that the hepatitis B vaccination leaves some room for flexibility in case of disease, holiday, and so on. Interestingly, a lower seroprotection rate was observed if the third dose was given <4 months or ≥12 months after the second dose. The reasons for this are uncertain, but it might be that immune responses to a third dose <4 months or ≥12 months after the second dose show different kinetics than the typical booster reactions observed with a 4–5 or 6–11 months interval. 17
There are some limitations. First, due to the retrospective design of the current study, we were unable to collect additional data on immunodeficiency, smoking, and body mass index. Nevertheless, only a few people are expected to have an underlying immunodeficient condition in this apparently healthy group of employees. Second, the study population could be biased towards Western European individuals and caution should be taken when extrapolating the results to other populations. Third, employees are not routinely screened for viral hepatitis markers before the initiation of primary hepatitis B vaccination in the Netherlands. However, the universal infant hepatitis B vaccination in the Netherlands has been implemented just recently on August 1, 2011, and the Netherlands is classified as a low‐prevalent country for hepatitis B surface antigen (0.2%), antibody against hepatitis C virus (0.7%), and antibody against HIV (0.2%). 29 , 30 , 31 Accordingly, we do not expect that the implementation of routine screening for hepatitis B, hepatitis C, and HIV before the initiation of hepatitis B vaccination would have had a major impact on the current results.
In conclusion, our study demonstrated that PVST should be obtained 1–2 months after the last vaccination for the definition of hepatitis B vaccine nonresponder and initiating revaccination. Based on our data, the hepatitis B vaccination also leaves room for flexibility for the second dose (28 days to 10 months between first and second dose) and the third dose (4–11 months between the second and third dose) without the necessity of restarting the vaccination series or confirmation of the immune response to the vaccine.
AUTHOR CONTRIBUTIONS
Özgür M. Koc, Eva van Oorschot, Lloyd Brandts, and Astrid Oude Lashof contributed to the conception and design of the study. Astrid Oude Lashof supervised Özgür M. Koc to collect data. Following statistical analysis of data, Özgür M. Koc drafted the first version of the paper; the co‐authors critically revised the article and approved the final version to be submitted, including the authorship list.
CONFLICTS OF INTEREST
Özgür M. Koc received travel grants from Gilead Sciences and his institution received grants from Gilead Sciences, AbbVie, MSD, and CyTuVax B.V. Eva van Oorschot, Lloyd Brandts, and Astrid Oude Lashof have no conflicts of interest to report.
ETHICS STATEMENT
The study was approved (number 2017‐0308) by the medical research ethics committee of MUMC+ and the need of informed consent was waived seen the noninterventional character of our study.
ACKNOWLEDGEMENT
This study is part of the School of Nutrition and Translational Research in Metabolism (NUTRIM).
Koc ÖM, Oorschot E, Brandts L, Oude Lashof A. Timing of primary three‐dose hepatitis B vaccination and postvaccination serologic testing among a large cohort of healthy adults. J Med Virol. 2022;94:4433‐4439. 10.1002/jmv.27848
DATA AVAILABILITY STATEMENT
Proposals should be directed to the corresponding author; to gain access, data requestors will need to sign a data access agreement. Only individual participant data that underlie the results reported in this article, after de‐identification, will be shared.
REFERENCES
- 1. World Health Organization . Hepatitis B. 2020. Accessed August 20, 2020. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b#:%7E:text=Hepatitis%20B%20is%20a%20potentially,from%20cirrhosis%20and%20liver%20cancer
- 2. Polaris Observatory Collaboarators . Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383‐403. [DOI] [PubMed] [Google Scholar]
- 3. Meireles LC, Marinho RT, Van Damme P. Three decades of hepatitis B control with vaccination. World J Hepatol. 2015;7(18):2127‐2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2018;67(1):1‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koc ÖM, Menart C, Theodore J, et al. Ethnicity and response to primary three‐dose hepatitis B vaccination in employees in the Netherlands, 1983 through 2017. J Med Virol. 2020;92(3):309‐316. [DOI] [PubMed] [Google Scholar]
- 6. Walayat S, Ahmed Z, Martin D, Puli S, Cashman M, Dhillon S. Recent advances in vaccination of non‐responders to standard dose hepatitis B virus vaccine. World J Hepatol. 2015;7(24):2503‐2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan HX, Zeng Y, Song XF, et al. Immune response to hepatitis B vaccine with high antigen content in non‐responders after standard primary vaccination in Chinese adults. Vaccine. 2014;32(29):3706‐3712. [DOI] [PubMed] [Google Scholar]
- 8. Jilg W, Schmidt M, Deinhardt F. Vaccination against hepatitis B: comparison of three different vaccination schedules. J Infect Dis. 1989;160(5):766‐769. [DOI] [PubMed] [Google Scholar]
- 9. Wouters K, Leuridan E, Van Herck K, et al. Compliance and immunogenicity of two hepatitis B vaccination schedules in sex workers in Belgium. Vaccine. 2007;25(10):1893‐1900. [DOI] [PubMed] [Google Scholar]
- 10. Yao J, Li J, Chen Y, et al. The response of hepatitis B vaccination on seronegative adults with different vaccination schedules. Hum Vaccines Immunother. 2015;11(5):1102‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yao J, Qiu Y, Chen Y, et al. Optimal vaccination program for healthy adults in China. Hum Vaccines Immunother. 2015;11(10):2389‐2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Asboe D, Rice P, de Ruiter A, Bingham JS. Hepatitis B vaccination schedules in genitourinary medicine clinics. Genitourin Med. 1996;72(3):210‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marsano LS, Greenberg RN, Kirkpatrick RB, et al. Comparison of a rapid hepatitis B immunization schedule to the standard schedule for adults. Am J Gastroenterol. 1996;91(1):111‐115. [PubMed] [Google Scholar]
- 14. Hess G, Hingst V, Cseke J, Bock HL, Clemens R. Influence of vaccination schedules and host factors on antibody response following hepatitis B vaccination. Eur J Clin Microbiol Infect Dis. 1992;11(4):334‐340. [DOI] [PubMed] [Google Scholar]
- 15. Harries AD, Clark M, Beeching NJ, Lavelle J, Mutton KJ. Early anti‐HBs antibody response to accelerated and to conventional hepatitis B vaccination regimens in healthy persons. J Infect. 1991;23(3):251‐254. [DOI] [PubMed] [Google Scholar]
- 16. De Schryver A, Verstrepen K, Vandersmissen L, et al. Comparative immunogenicity of two vaccination schedules of a combined hepatitis A and B vaccine in healthy volunteers. J Viral Hepatitis. 2011;18(4):e5‐e10. [DOI] [PubMed] [Google Scholar]
- 17. Hadler SC, de Monzon MA, Lugo DR, Perez M. Effect of timing of hepatitis B vaccine doses on response to vaccine in Yucpa Indians. Vaccine. 1989;7(2):106‐110. [DOI] [PubMed] [Google Scholar]
- 18. Bryan JP, Sjogren MH, Macarthy P, Cox B, Kao TC, Perine PL. Dosing schedule for recombinant hepatitis B vaccine. J Infect Dis. 1991;163(6):1384‐1385. [DOI] [PubMed] [Google Scholar]
- 19. Marocho L, Vildózola H, Valencia E, et al. [Comparative study on the protective effect of two immunization schemes using a hepatitis B recombinant vaccine in vulnerable health science students]. Rev Gastroenterol Peru. 2005;25(4):313‐319. [PubMed] [Google Scholar]
- 20. Wiström J, Ahlm C, Lundberg S, Settergren B, Tärnvik A. Booster vaccination with recombinant hepatitis B vaccine four years after priming with one single dose. Vaccine. 1999;17(17):2162‐2165. [DOI] [PubMed] [Google Scholar]
- 21. Sabidó M, Gavaldà L, Olona N, Ramon JM. Timing of hepatitis B vaccination: its effect on vaccine response in health care workers. Vaccine. 2007;25(43):7568‐7572. [DOI] [PubMed] [Google Scholar]
- 22. Schillie S, Murphy TV, Fenlon N, Ko S, Ward JW. Update: shortened interval for postvaccination serologic testing of infants born to hepatitis B‐infected mothers. MMWR Morb Mortal Wkly Rep. 2015;64(39):1118‐1120. [DOI] [PubMed] [Google Scholar]
- 23. Euler GL, Copeland JR, Rangel MC, Williams WW. Antibody response to postexposure prophylaxis in infants born to hepatitis B surface antigen‐positive women. Pediatr Infect Dis J. 2003;22(2):123‐129. [DOI] [PubMed] [Google Scholar]
- 24. Niu MT, Targonski PV, Stoll BJ, Albert GP, Margolis HS. Prevention of perinatal transmission of the hepatitis B virus. Outcome of infants in a community prevention program. Am J Dis Child. 1992;146(7):793‐796. [DOI] [PubMed] [Google Scholar]
- 25. Koc ÖM, Smedt P, Kremer C, et al. Immunogenicity and safety of HBAI20 hepatitis B vaccine in non‐responders: double‐blinded, randomised, controlled phase 2 trial. Liver Int. 2021;41:2318‐2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koc ÖM, Damoiseaux J, van Loo IHM, Masquillier HIL, Oude Lashof AML. Case report of delayed seroprotection rather than non‐response after primary three‐dose hepatitis B vaccination. Vaccine. 2020;38(2):112‐114. [DOI] [PubMed] [Google Scholar]
- 27. Bayas JM, Bruguera M, Martin V, Vidal J, Rodes J, Salleras LY. Hepatitis B vaccination in prisons: the Catalonian experience. Vaccine. 1993;11(14):1441‐1444. [DOI] [PubMed] [Google Scholar]
- 28. Jilg W, Schmidt M, Deinhardt F. Prolonged immunity after late booster doses of hepatitis B vaccine. J Infect Dis. 1988;157(6):1267‐1269. [DOI] [PubMed] [Google Scholar]
- 29. Hahné SJ, De Melker HE, Kretzschmar M, et al. Prevalence of hepatitis B virus infection in The Netherlands in 1996 and 2007. Epidemiol Infect. 2012;140(8):1469‐1480. [DOI] [PubMed] [Google Scholar]
- 30. Baaten GG, Sonder GJ, Dukers NH, Coutinho RA, Van den Hoek JA. Population‐based study on the seroprevalence of hepatitis A, B, and C virus infection in Amsterdam, 2004. J Med Virol. 2007;79(12):1802‐1810. [DOI] [PubMed] [Google Scholar]
- 31. van Veen MG, Presanis AM, Conti S, et al. National estimate of HIV prevalence in the Netherlands: comparison and applicability of different estimation tools. AIDS. 2011;25(2):229‐237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Proposals should be directed to the corresponding author; to gain access, data requestors will need to sign a data access agreement. Only individual participant data that underlie the results reported in this article, after de‐identification, will be shared.


