Abstract
Background and purpose
A dose‐dependent association between the use of cyproterone acetate (CPA) and intracranial meningioma has been identified but data for other potent progestogens are scarce. The association was assessed between intracranial meningioma surgery and exposure to three potent progestogens: CPA (≥25 mg/day), nomegestrol acetate (NOMAC) (3.75–5 mg/day) and chlormadinone acetate (CMA) (2–10 mg/day).
Methods
In this nationwide population‐based case–control study, cases underwent surgery for intracranial meningioma in France from 2009 to 2018. They were matched to five control subjects for sex, year of birth and area of residence. Progestogen exposure was defined as progestogen use within the year before surgery for cases or the same date for their controls.
Results
In total, 25,216 cases were included (75% women, median age 58 years). Progestogen exposure was noted for 9.9% of cases (2497/25,216) and 1.9% (2382/126,080) of controls, with an odds ratio (OR) of 6.7 (95% confidence interval [CI] 6.3–7.1). The OR was 1.2 (1.0–1.4) for short‐term use (<1 year) and 9.5 (8.8–10.2) for prolonged use. A strong association was identified for prolonged use of CPA (OR = 22.7, 95% CI 19.5–26.4), NOMAC (OR = 6.5, 95% CI 5.8–7.2) and CMA (OR = 4.7, 95% CI 4.5–5.3). Progestogen exposure increased the risk of meningioma for all histological grades and anatomical sites, particularly for the anterior and middle skull base: OR = 35.7 (95% CI 26.5–48.2) and 23.9 (95% CI 17.8–32.2) for CPA. The estimated number of attributable cases was 2124 (95% CI 2028–2220) (212/year).
Conclusion
A strong association between prolonged exposure to potent progestogens and surgery for meningioma was observed. The risk increased from CMA to NOMAC to CPA. Individuals should be informed of this risk.
Keywords: chlormadinone acetate, cyproterone acetate, meningioma, nomegestrol acetate, progestogens
Short abstract
This study highlights a strong association between prolonged use of nomegestrol and chlormadinone acetate (two potent progestogens) and intracranial meningioma, although weaker than that of cyproterone acetate. The estimated number of cases was higher than 2000 in France over 10 years.
INTRODUCTION
Meningiomas are the most frequently occurring intracranial tumours, accounting for 39% of primitive central nervous system tumours [1]. The main risk factors for meningioma are age, being female, exposure to ionizing radiation and neurofibromatosis type 2 (NF2) [2]. A number of observations have long suggested an association between endogenous sex hormones and meningioma based on epidemiological evidence and histopathological studies: there is a higher incidence among women (ratio 2.5/1), particularly of childbearing age [1]; case reports have suggested that the size of meningiomas increases during pregnancy and decreases after delivery [3, 4]; and biological studies have shown that progesterone receptor expression may be involved in the occurrence of certain types of meningioma [5, 6, 7, 8].
No association has been identified for oral contraceptive drugs, for which progestogen doses are low. Concerning hormonal replacement therapy (HRT) for menopausal women, a number of epidemiological studies appear to support a slightly increased risk of meningioma but evidence is limited [9, 10]. By contrast, a high risk of meningioma has been observed with the use of high doses of cyproterone acetate (CPA), a potent progestogen with antiandrogen activity, among women, men and transwomen [11, 12, 13]. Furthermore, the withdrawal of long‐term CPA treatment induces tumour regression, which has also been observed after the withdrawal of two other potent progestogens—nomegestrol acetate (NOMAC) and chlormadinone acetate (CMA) [14, 15, 16, 17, 18, 19, 20, 21, 22, 23]—suggesting that these two progestogens are also associated with the risk of meningioma. However, unlike CPA, no large epidemiological studies have yet been published on the risk of meningioma associated with exposure to NOMAC or CMA. Thus, the aim was to assess, in real life, the association between intracranial meningioma surgery and exposure to three potent progestogens for which regression in the volume of the meningioma after the discontinuation of treatment has been described: CPA, NOMAC and CMA.
METHODS
Data source
In this nationwide population‐based case–control study, data were extracted from the French National Health Data System (SNDS, for Système National des Données de Santé), which covers 99% of the population living in France—67 million residents. The SNDS includes demographic data, outpatient drug dispensations and inpatient care information (hospitalization diagnostic codes according to the International Classification of Diseases, Tenth Revision [ICD‐10], and procedures performed during the hospital stay coded according to the French medical classification for clinical procedures [CCAM]). These data are all recorded prospectively at the individual level and anonymized. This database is a useful and reliable source for the assessment of drug efficacy and safety [24, 25]. The study was performed within the framework of the French data protection agency regulatory decision CNIL‐2016‐316.
Cases and controls
The eligible cases were all individuals living in France who underwent surgery for intracranial meningioma between 1 January 2009 and 31 December 2018 in France. Surgery for intracranial meningioma was defined by the following combination recorded for the same hospital stay: a meningioma neoplasm (ICD‐10 codes D32, D42 or C70) coded as the main diagnosis for hospitalization and a surgical procedure corresponding to intracranial surgery (Table S1). The first surgical intervention for intracranial meningioma during the study period was included and the absence of a hospital stay for intracranial meningioma surgery since June 2007 was verified. The index date was defined as the date of admission to hospital for a first meningioma surgery. For simplicity, ‘intracranial meningioma surgery’ is referred to hereafter as ‘meningioma’ in the results.
Five control individuals were matched to each case for year of birth, sex at birth and area of residence (100 geographical administrative areas) as age and sex are two major confounders in the association between exposure to progestogens and meningioma. Control individuals were randomly selected with at least one reimbursement for out‐of‐hospital care during the study period, excluding cases. Controls were assigned the same index date as their corresponding case and were alive at this index date.
Exposure
For both cases and controls, exposure to a progestogen was defined as at least one delivery of one of the following drugs (coded according to the Anatomical Therapeutic Chemical Classification System [ATC]) during the year preceding the index date: CPA (G03HA01), NOMAC (G03DB04) and CMA (G03DB06). CPA is a synthetic progestogen with antiandrogenic activity indicated for inoperable prostate cancer or paraphilias for men (50–100 mg/day) and for various hirsutism or hyperandrogenism spectrum disorders for women (50 mg/day). In addition, CPA is used off‐label as feminizing hormone therapy for transwomen. CPA can also be used at a dose of 25 mg/day, as the tablets are divisible. NOMAC (3.75–5 mg/day) is a synthetic progestogen prescribed mostly for HRT and contraception. Finally, CMA (2–10 mg/day) is another progestogen indicated for the treatment of menstrual disorders, HRT, endometrial hyperplasia and endometriosis. NOMAC and CMA are not indicated for men in France.
Exposure to progestogens was defined with three indicators: ‘current use’ was defined as exposure to progestogens with at least one delivery of the drug during the 365 days preceding the index date, regardless of whether the subject had been exposed earlier; ‘short‐term use’ as exposure to progestogens during the 365 days preceding the index date, without exposure during the period between 366 and 730 days before the index date; and ‘prolonged use’ as exposure to progestogens both during the 365 days preceding the index date and between 366 and 730 days before the index date, regardless of prior exposure.
Prolonged use was investigated by evaluating exposure to progestogens for the 2013–2018 period, for which cases and controls had at least 6 years of history in the database. ‘Prolonged use’ was defined as explained above for cases and controls and five supplemental prolonged‐use indicators were added: ‘prolonged use for 2 years’ was defined as at least one delivery of a progestogen per year for 2 years (i.e., before the 365 days preceding the index date and between 366 and 730 days before the index date, but not between 731 days and 1096 days before the index date); ‘prolonged use for 3 years’, ‘4 years’, ‘5 years’ and ‘6 years or more’ were defined using the same logic.
Covariates
The study population was described according to the following baseline socio‐demographic characteristics: sex at birth, age and area of residence (six groups). Information about the meningiomas included the year of surgery, anatomical site (five main sites and 16 detailed sites, described in Table S1), tumour grade according to the ICD‐10, and radiotherapy associated with surgery. All‐cause mortality was estimated at 2 years after meningioma surgery for the entire population of cases and at 5 years for the subset of cases for whom sufficient follow‐up data were available, that is, for those undergoing meningioma surgery before 1 January 2016.
Statistical analyses
Incidence rates of intracranial meningioma surgery per 100,000 person‐years for the entire French population were estimated by age group with the publicly available data of the National Institute of Statistics and Economic Studies.
Logistic regression models conditioned on matched pairs (to control for matching variables) were used to estimate odds ratios (ORs) and their 95% confidence intervals (CIs) for the association between meningioma and prior progestogen exposure. The risk of meningioma associated with progestogen exposure was estimated considering exposure either to at least one of the three progestogens or to each progestogen separately, according to current, short‐term and prolonged use. Analyses were further stratified by age group and sex, tumour grade and anatomical site. The association between NF2 (ICD‐10 code Q851) and meningioma was also estimated to determine whether this well‐documented association [2] was also found in our study population.
The population‐attributable fraction (PAF) of cases was calculated from the OR obtained for overall exposure, assuming adequate control of all confounders [26, 27]. This attributable fraction was applied to the total number of cases during the study period to estimate the number of meningioma cases attributable to the three potent progestogens, assuming a causal association between exposure to potent progestogens and meningioma. This estimation was also performed for men and women separately. Finally, the number of meningioma cases attributable to NF2 was estimated.
RESULTS
In total, 25,216 cases who underwent surgery for an intracranial meningioma and 126,080 control individuals were included (Figure 1). Their characteristics (women 75%, median age 58 years [Q1–Q3 48–67]) are presented in Table 1. The crude incidence of intracranial meningioma surgery was 4/100,000 person‐years, with a maximal female‐to‐male ratio (4.6) attained at 45–54 years of age (Figure 2). Most intracranial meningiomas were located in the convexity area (37.9%) and the anterior skull base (20.9%). Ninety‐one per cent of tumours were graded as benign (Table 1). Approximately 7% of the patients died within 5 years of surgery, with higher mortality (25.5%) for those with malignant tumours.
FIGURE 1.
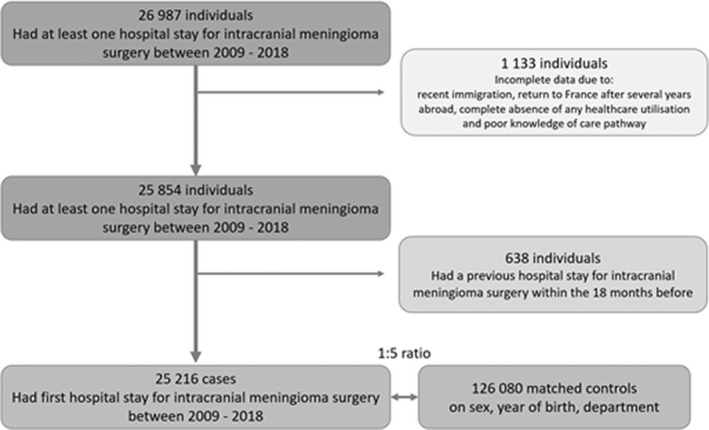
Flowchart
TABLE 1.
Characteristics of the case and control groups
| Characteristics |
Cases N = 25,216 n (%) |
Controls N = 126,080 n (%) |
|---|---|---|
| Sex at birth, female | 18,892 (74.9) | 94,460 (74.9) |
| Age, years, mean (SD) | 57.5 (13.5) | 57.5 (13.5) |
| Age group | ||
| 0–19 | 128 (0.5) | 640 (0.5) |
| 20–34 | 1021 (4.1) | 5105 (4.1) |
| 35–44 | 3132 (12.4) | 15,660 (12.4) |
| 45–54 | 6132 (24.3) | 30,660 (24.3) |
| 55–64 | 6552 (26.0) | 32,760 (26.0) |
| 65–74 | 5570 (22.1) | 27,850 (22.1) |
| 75–84 | 2421 (9.6) | 12,105 (9.6) |
| ≥85 | 260 (1.0) | 1300 (1.0) |
| Area of residence a | ||
| Paris area (Ile‐de‐France) | 4331 (17.2) | 21,655 (17.2) |
| Northeast | 4842 (19.2) | 24,210 (19.2) |
| Northwest | 5000 (19.8) | 25,000 (19.8) |
| Southeast | 5755 (22.8) | 28,775 (22.8) |
| Southwest | 4802 (19.0) | 24,010 (19.0) |
| French overseas area | 486 (1.9) | 2430 (1.9) |
| Year of surgery | ||
| 2009 | 2070 (8.2) | |
| 2010 | 2220 (8.8) | |
| 2011 | 2299 (9.1) | |
| 2012 | 2464 (9.8) | |
| 2013 | 2469 (9.8) | |
| 2014 | 2639 (10.5) | |
| 2015 | 2628 (10.4) | |
| 2016 | 2762 (10.9) | |
| 2017 | 2742 (10.9) | |
| 2018 | 2923 (11.6) | |
| Anatomic location of the meningioma | ||
| Anterior skull base | 5285 (20.9) | |
| Middle skull base | 4790 (19.0) | |
| Posterior skull base | 2770 (11.0) | |
| Convexity | 9554 (37.9) | |
| Falx and tentorium | 2608 (10.3) | |
| Other locations | 209 (0.8) | |
| Tumour grade ICD‐10 | ||
| Benign (D32) | 23,010 (91.3) | |
| Sex, female | 17,429 (75.7) | |
| Atypical (D42) | 1587 (6.3) | |
| Sex, female | 1101 (69.4) | |
| Malignant (C70) | 619 (2.4) | |
| Sex, female | 362 (58.5) | |
| Adjuvant radiation therapy | ||
| All grades | 2274 (9.0) | |
| Benign (D32) | 1917 (8.3) | |
| Atypical (D42) | 145 (9.1) | |
| Malignant (C70) | 212 (34.2) | |
| Death, according to tumour grade, within 2 years (N = 25,216) b | ||
| All grades | 854 (3.4) | |
| Benign (D32) | 692 (3.0) | |
| Atypical (D42) | 70 (4.4) | |
| Malignant (C70) | 92 (14.8) | |
| Within 5 years (N = 16,789) c | ||
| All grades | 1152 (6.8) | |
| Benign (D32) | 971 (6.3) | |
| Atypical (D42) | 91 (8.5) | |
| Malignant (C70) | 90 (25.5) | |
Abbreviations: ICD‐10, International Classification of Diseases, Tenth Revision; Sex, female, individuals born female.
Northeast: Grand Est, Bourgogne Franche‐Comté, Hauts‐de‐France. Paris area (Ile‐de‐France): Paris city and Ile‐de‐France area. Northwest: Bretagne, Centre Val de Loire, Normandie, Pays de la Loire. Southeast: Auvergne‐Rhône‐Alpes, Provence‐Alpes‐Côte d'Azur, Corse. Southwest: Nouvelle‐Aquitaine, Occitanie. French overseas area: Guadeloupe, Martinique, French Guiana, Reunion Island.
Death within 2 years: for the entire cohort, deaths from any cause were identified from the index date until 2 years after this date.
Death within 5 years: deaths from any cause were identified from the index date until 5 years later for all cases undergoing meningioma surgery before 1 January 2016.
FIGURE 2.
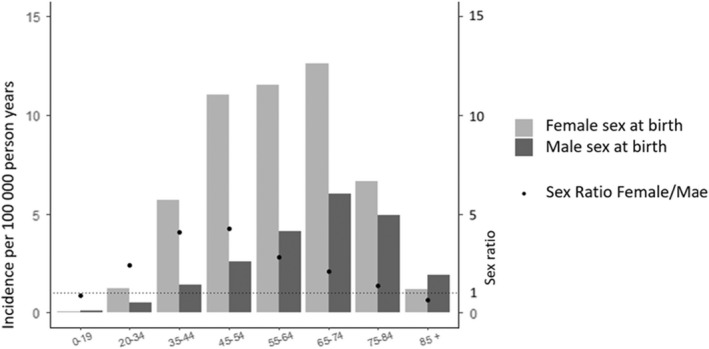
Incidence of surgically treated meningioma according to age group and sex at birth
Current use of at least one of the progestogens the year before the index date was found for 2497 cases (9.9%) and 2382 (1.9%) controls (Table 2). Most current users of progestogens had prolonged use (91.8% [2291/2497] in the case group and 63.9% [1521/2382] in the control group). For subjects included in the 2013–2018 period with prolonged progestogen use, 79.0% of the cases (1130/1430) and 43.4% of the controls (392/903) had been exposed each year for at least 6 years (Table S2). Cases and controls used similar drug dosages (Table S3): 50 mg for CPA (95%), 5 mg for NOMAC (86%) and 10 mg for CMA (81%).
TABLE 2.
Numbers and proportions of cases and controls exposed and estimated ORs, controlling for matching variables, with confidence intervals
| Exposure | Cases N = 25,216 | Controls N = 126,080 | OR (95% CI) |
|---|---|---|---|
| Neurofibromatosis type II | 117 (0.5) | 30 (0.02) | 19.5 (13.1–29.1) |
| Exposure to at least one of the progestogens | |||
| Current use | 2497 (9.9) | 2382 (1.9) | 6.7 (6.3–7.1) |
| Short‐term use <1 year | 206 (0.8) | 861 (0.7) | 1.2 (1.0–1.4) |
| Prolonged use ≥1 year | 2291 (9.1) | 1521 (1.2) | 9.5 (8.8–10.2) |
| Cyproterone acetate | |||
| Current use | 961 (3.8) | 290 (0.2) | 18.3 (16.0–21.1) |
| Short‐term use <1 year | 30 (0.1) | 63 (0.05) | 2.4 (1.5–3.7) |
| Prolonged use ≥1 year | 931 (3.7) | 227 (0.2) | 22.7 (19.5–26.4) |
| Nomegestrol acetate | |||
| Current use | 969 (3.8) | 1149 (0.9) | 4.7 (4.3–5.1) |
| Short‐term use <1 year | 105 (0.4) | 421 (0.3) | 1.3 (1.0–1.6) |
| Prolonged use ≥1 year | 864 (3.4) | 728 (0.6) | 6.5 (5.8–7.2) |
| Chlormadinone acetate | |||
| Current use | 683 (2.7) | 1096 (0.9) | 3.3 (3.0–3.6) |
| Short‐term use <1 year | 80 (0.3) | 416 (0.3) | 0.9 (0.8–1.2) |
| Prolonged use ≥1 year | 603 (2.4) | 680 (0.5) | 4.7 (4.5–5.3) |
Note: Current use: exposed at least once within 365 days before the index date, regardless of former exposure. Short‐term use: exposed within 365 days before the index date, excluding the period between 365 and 730 days before the index date. Prolonged use: exposed both within 365 days and between 365 and 730 days before the index date.
Abbreviations: CI, confidence interval; OR, odds ratio, controlling for matching factors (sex at birth, year of birth and area of residence).
The estimated ORs of meningioma for the exposure of interest are presented in Table 2. NF2 was associated with an increased risk of meningioma: OR = 19.5 (95% CI 13.1–29.1). Exposure to at least one of the progestogens for current use, short‐term use or prolonged use was associated with an increased risk of meningioma: OR = 6.7 (6.3–7.1), 1.2 (1.0–1.4) and 9.5 (8.8–10.2), respectively. The magnitude of the risk decreased from CPA to NOMAC to CMA: OR = 22.7 (19.5–26.4), 6.5 (5.8–7.2) and 4.7 (4.5–5.3), respectively.
Current use of progestogens was associated with a risk of meningioma for women, with an OR of 6.6 (6.3–7.1) (Tables 3 and S4). The OR decreased from CPA to NOMAC to CMA: OR = 19.7 (17.0–22.7), 4.7 (4.3–5.1) and 3.3 (3.0–3.6), respectively. The OR among men, who were exposed only to CPA, was 8.0 (5.2–12.3). The magnitude of the association increased with age for women but decreased for men. Exposure to a progestogen was associated with an increased risk of meningioma in women for benign, atypical and malignant tumours: OR = 6.6 (6.2–7.1), 7.0 (5.4–9.1) and 6.6 (4.0–10.8), respectively. Exposure to a progestogen was also significantly associated with benign and atypical meningiomas in men.
TABLE 3.
Association between surgically treated meningioma and exposure to progestogens according to age, sex at birth, tumour grade and site; estimated OR and 95% CI
| Exposure | Any of the three progestogens | Cyproterone acetate | Nomegestrol acetate | Chlormadinone acetate |
|---|---|---|---|---|
| Sex, female | ||||
| Overall | 6.6 (6.3–7.1) | 19.7 (17.0–22.7) | 4.7 (4.3–5.1) | 3.3 (3.0–3.6) |
| 0–19 | 5.0 (0.3–79.9) | 5.0 (0.3–79.9) | ||
| 20–34 | 5.4 (4.0–7.2) | 12.6 (8.0–19.8) | 3.1 (1.8–5.5) | 2.0 (1.1–3.5) |
| 35–44 | 6.0 (5.2–6.8) | 21.9 (16.4–29.2) | 3.5 (2.9–4.3) | 2.6 (2.1–3.3) |
| 45–54 | 6.3 (5.8–6.9) | 18.7 (15.0–23.4) | 4.6 (4.1–5.2) | 3.6 (3.2–4.0) |
| 55–64 | 8.9 (7.5–10.6) | 21.8 (14.8–32.0) | 7.1 (5.5–9.0) | 4.1 (3.0–5.7) |
| ≥65 years old | 10.6 (7.7–14.6) | 27.9 (15.1–51.6) | 7.7 (4.8–12.3) | 1.8 (0.7–4.5) |
| Sex, male | ||||
| Overall | 8.0 (5.2–12.3) | 8.0 (5.2–12.3) | ||
| <65 years old | 12.5 (5.5–28.4) | 12.5 (5.5–28.4) | – | |
| ≥65 years old | 6.6 (4.0–11.0) | 6.6 (4.0–11.0) | ||
| Tumour grade ICD‐10 | ||||
| Benign (D32) | 6.7 (6.2–7.1) | 18.4 (15.9–21.2) | 4.7 (4.3–5.1) | 3.3 (3.0–3.6) |
| Sex, female | 6.6 (6.2–7.1) | 19.5 (16.7–22.7) | 4.7 (4.3–5.1) | 3.3 (3.0–3.6) |
| Sex, male | 9.1 (5.7–14.6) | 9.1 (5.7–14.6) | ||
| Atypical (D42) | 7.1 (5.5–9.2) | 21.6 (12.1–38.6) | 4.6 (3.2–6.7) | 3.3 (2.2–4.9) |
| Sex, female | 7.0 (5.4–9.1) | 21.7 (11.9–39.7) | 4.6 (3.2–6.7) | 3.3 (2.2–4.9) |
| Sex, male | 20.0 (2.2–178.9) | 20.0 (2.2–178.9) | ||
| Malignant (C70) | 6.1 (3.8–9.8) | 14.3 (6.0–33.8) | 4.9 (2.2–10.8) | 2.8 (1.3–5.9) |
| Sex, female | 6.6 (4.0–10.8) | 23.7 (8.1–69.8) | 4.9 (2.2–10.8) | 2.8 (1.3–5.9) |
| Sex, male | 1.7 (0.2–16.0) | 1.7 (0.2–16.0) | ||
| Anatomic location | ||||
| Anterior skull base | 10.2 (8.9–11.6) | 35.7 (26.5–48.2) | 6.2 (5.2–7.4) | 3.5 (2.9–4.4) |
| Middle skull base | 9.7 (8.6–11.1) | 23.9 (17.8–32.2) | 6.8 (5.7–8.1) | 4.7 (3.9–5.7) |
| Posterior skull base | 2.9 (2.4–3.6) | 6.4 (3.9–10.4) | 2.6 (1.9–3.5) | 2.1 (1.5–2.9) |
| Convexity | 5.1 (4.6–5.7) | 12.4 (9.9–15.5) | 3.5 (3.0–4.2) | 2.9 (2.4–3.4) |
| Falx and tentorium | 3.5 (2.8–4.5) | 10.4 (6.2–17.4) | 2.8 (1.9–3.9) | 2.0 (1.3–3.0) |
Abbreviations: CI, confidence interval; ICD‐10, International Classification of Diseases, Tenth Revision; OR, odds ratio, controlling for matching factors (sex at birth, year of birth and area of residence); Sex, female, individuals born female.
The anatomical sites for which the risk of meningioma associated with progestogens was the highest were the anterior and middle skull base: OR = 10.2 (8.9–11.6) and 9.7 (8.6–11.1), respectively, for all progestogens; OR = 35.7 (26.5–48.2) and 23.9 (17.8–32.2), respectively, for CPA; OR = 6.2 (5.2–7.4) and 6.8 (5.7–8.1), respectively, for NOMAC; and OR = 3.5 (2.9–4.4) and 4.7 (3.9–5.7), respectively, for CMA (Table 3). Following a more precise assessment of this association by anatomical site (Tables S5 and S6), the sites with the highest ORs were the optochiasmatic area (OR = 12.6, 95% CI 10.0–15.8) and the medial third of the middle skull base involving the spheno‐orbital angle (OR = 12.0, 95% CI 10.2–14.1). The risk associated with CPA was particularly high for the optochiasmatic area (OR = 49.1, 95% CI 28.9–83.5).
The PAF of surgically treated meningiomas for current use of at least one of the three potent progestogens studied in France between 2009 and 2018 was 8.4% (8.0%–8.8%). The corresponding number of attributable meningiomas was 2124 (2008–2220) (212 per year, on average), assuming the hypothesis of a causal association between exposure to potent progestogens and meningioma. The PAF of meningiomas was 11.0% (10.5%–11.5%) for women and 0.8% (0.5%–1.0%) for men, with a corresponding number of attributable cases of 2072 (1978–2165) and 48 (33–63). The PAF of meningiomas for NF2 was 0.4% (0.3%–0.5%) and the estimated number of attributable meningiomas was 111 (90–132).
DISCUSSION
This study revealed a strong association between intracranial meningioma requiring surgery and prolonged exposure to potent progestogens, with an increasing gradient from CMA to NOMAC to CPA. This increased risk concerned all grades and anatomical sites but was highest for the anterior and middle skull base for each progestogen. The estimated number of cases attributable to potent progestogens was higher than 2000 in France between 2009 and 2018, that is, approximately 20 times that of cases attributable to NF2.
Our results showing a strong association between long exposure to CPA and the risk of operated meningioma (OR = 22.7, 95% CI 19.5–26.4) are in accordance with those of other studies. A strong dose–response relationship between CPA and intracranial meningioma has already been reported for women in France (hazard ratio 21.7, 95% CI 10.8–43.5, for cumulative doses >60 g) [13], for men in Denmark (hazard ratio 18.5, 95% CI 9.2–37.1, for cumulative doses >10 g) [28] and for transwomen in the Netherlands (standardized incidence ratio 11.9, 95% CI 5.5–22.7) [12]. On the other hand, no published epidemiological studies to date have shown an association between exposure to CMA or NOMAC and meningioma. Case reports of meningiomas have been published for NOMAC [18, 19] and CMA [16] or included in larger series that included CPA [20, 29]. Long exposure of several years, locations at the skull base (frequently with ophthalmological symptoms) and non‐systematic regression upon the discontinuation of treatment in the absence of surgery have been reported. In a recent review, Hage et al. [10] showed that current evidence for the association between HRT and meningioma is conflicting but appears to favour an increased risk. Two unpublished cohort studies on CMA and NOMAC are being discussed at the European Medicine Agency [30]. In our study, a significant association between prolonged use of CMA and NOMAC was found, although the ORs were lower than that for CPA. Such diverse results for the association between meningioma and progestogens probably reflect the use of different types of progestogen and doses and different durations of exposure, with prolonged use and high dose appearing to contribute to the association with meningioma. In particular, the use of these three progestogens (CPA, CMA and NOMAC) in France has been higher in terms of frequency and dose than in other western European countries. CPA, CMA and NOMAC are not currently marketed in the United States. Such an association between potent progestogens and meningiomas could therefore not be demonstrated in the past in the United States.
The risk of operated meningioma was found to be highest in the anterior and middle skull base for patients exposed to progestogens. This result is consistent with those of previous observational studies and with known biological mechanisms. In a study of 300 patients who underwent surgery for meningioma, a higher rate of progesterone receptor expression >50% was observed for meningiomas of the medial skull base than for other sites [7]. Moreover, progestin‐associated meningiomas showed significantly higher levels of progesterone receptor expression and were more frequently located at the skull base than other meningiomas [8]. Another specific feature of progestin‐related meningiomas are mutations affecting the PIK3CA/AKT1 pathway, which have been more frequently observed in CPA‐related meningiomas than in a control population [8, 21]. Such a preferential mutational landscape of CPA‐induced meningiomas appears to have been confirmed by an observational study showing the coexistence of regressing meningiomas harbouring a PIK3CA mutation and growing meningiomas harbouring NF2 mutations within the same patient after drug cessation for four women exposed to CPA [21]. An independent clonal origin, associated with a predisposition of certain meningeal cells (principally located in the anterior and middle skull base) to develop meningiomas, could explain the pathogenesis of these progestin‐related meningiomas [21]. A French epidemiological study also reported that the risk of meningioma of the anterior skull base was 47 times higher for patients exposed to CPA [13]. Interestingly, a strong association with medial skull base meningiomas involving the spheno‐orbital angle was highlighted (OR = 12.0, 95% CI 10.2–14.1). Only one previous study has focused on this specific site and found that a high proportion of women with such tumours had been exposed to high doses of CPA, NOMAC or CMA for at least 2 years [29]. Furthermore, our results showed that the increased risk concerns operated meningiomas of all grades. Although case reports and case series of grade 2 meningiomas associated with CPA, NOMAC or CMA have been published, no epidemiological evidence of an increased risk has been available until now [23].
Strengths of this study
The major strength of the study is the register‐based design and the size of the population (more than 25,000 surgeries for meningioma over 10 years and 125,000 controls). The use of prospective records of dispensed drugs in the SNDS made it possible to prevent recall bias, a major limitation of case–control studies. Moreover, the accuracy of the data on progestogen exposure and operated meningioma location according to the surgical procedure can be emphasized. Population‐attributable fractions were also calculated, which help to support the assessment of the disease burden of a causal factor in a population.
Our short‐term (<1 year) versus long‐term (>1 year) approach highlighted the absence of or very reduced risk with short‐term use for each of the three potent progestogens. The risk of meningioma is mostly driven by very long‐term use (>5 years), which has not been assessed in previous studies.
Limitations of this study
First, patients who underwent surgery for intracranial meningioma were included, whereas exclusive radiotherapy is also indicated. However, surgery is the first option for the management of meningioma of any grade [31]. In a French study, only 4% of patients who developed meningioma after exposure to CPA were treated by radiotherapy [13].
Patients who underwent surgery, regardless of the grade of the tumour, were included. Tumour grading did not precisely follow the latest World Health Organization grade classification [32] due to the data source, but the ICD‐10 codes were reliable as they were recorded by surgeons. As expected, rates of mortality and radiotherapy associated with surgery increased with tumour grade in our study.
Anatomical sites were classified according to the information available using the French medical classification for clinical procedures. Various classifications have been used in previous studies, as no consensual surgical anatomical classification is currently available. Our classification made it possible to consider a large number of sites (16 groups). In addition to diagnostic codes, clinical procedures are recorded by surgeons and may thus be considered reliable.
In terms of exposure, the indication for progestogen treatment was not available. However, the three progestins have, in part, different indications and were all associated with an increased risk of meningioma. All age groups were affected by the increased risk and there was no differential risk for CPA for men, women or transwomen. The indication is thus not likely to be a confounder in the assessment of the risk of meningioma associated with progestogens. Concerning the duration of exposure, current use was considered, even though meningioma is generally a slow‐growing tumour. Despite this limitation, a significant association is shown, in particular for prolonged use (>1 year). It is hypothesized that prolonged use reflects a longer duration of exposure to progestogens, with repeated drug prescription, delivery and intake. The description of prolonged exposure for the 2013–2018 period tends to confirm this hypothesis: 80% of prolonged use corresponded to exposure each year for at least the 6 years preceding the index date. The effect of discontinuing progestogen treatment was not assessed. Several studies concluded that the withdrawal of CPA, NOMAC or CMA resulted in a decreased risk of meningioma or in tumour regression [13, 14, 15, 16, 17, 18, 19]. Ionizing radiation was not considered, as this risk factor applies to only a few cases [33, 34] and is unlikely to confound exposure to progestogens.
Finally, the possibility that the indication for surgery for exposed patients has changed over time cannot be formally excluded. The labelling of CPA was modified in 2011 and 2013 to provide information about reported meningioma cases associated with use of the drug. Nevertheless, these updates had little impact on clinical care [13] and it was assumed that the choice of neurosurgical management was based on current European guidelines for most cases and that asymptomatic patients were managed by observation. The labelling of CMA and NOMAC was changed in 2018, that is, during the last year of the study period.
Clinical implications and future research
In France, meningioma screening by magnetic resonance imaging for patients receiving a progestogen for long‐term treatment has already been implemented for CPA and will soon be recommended for CMA and NOMAC [35, 36]. Individuals who have used potent progestogens for many years could benefit from such screening. Future research could explore the association between very long‐term exposure to the low‐dose progestogens contained in oral contraceptives and the risk of meningioma.
CONCLUSION
In this nationwide case–control study, a strong and significant association was observed between exposure to three progestogens and surgically treated meningioma. The risk was high for patients with prolonged use and for operated meningiomas of the anterior and middle skull base. The surgical removal of such tumours is among the most challenging forms of intracranial surgery and is associated with a much higher risk. Our data should encourage the informing of individuals using potent progestogens for prolonged periods about such a risk and the assessment of the individual benefit‐to‐risk ratio.
AUTHOR CONTRIBUTIONS
Lea Hoisnard: Conceptualization (equal); data curation (lead); formal analysis (lead); methodology (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Moussa Laanani: Conceptualization (equal); methodology (equal); supervision (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Thibault Passeri: Writing – review and editing (supporting). Lise Duranteau: Writing – review and editing (supporting). Joel Coste: Methodology (supporting); writing – review and editing (supporting). Mahmoud Zureik: Supervision (supporting); validation (supporting); writing – review and editing (supporting). Sebastien Froelich: Writing – review and editing (supporting). Alain Weill: Conceptualization (lead); methodology (lead); supervision (lead); validation (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTEREST
The authors report no competing interests.
REGULATORY AND ETHICAL ASPECTS
The French public institution performing this study, the Scientific Interest Group EPI‐PHARE, has permanent access to the SNDS database in accordance with the provisions of Articles R. 1461–12 et seq. of the French Public Health Code and the French data protection authority decision CNIL‐2016‐316. This study was recorded in the EPI‐PHARE Scientific Interest Group study register. No informed consent was therefore required. This research received no specific funding.
Supporting information
Table S1‐S6
Hoisnard L, Laanani M, Passeri T, et al. Risk of intracranial meningioma with three potent progestogens: A population‐based case–control study. Eur J Neurol. 2022;29:2801‐2809. doi: 10.1111/ene.15423
DATA AVAILABILITY STATEMENT
Data sharing is not applicable. According to French laws governing data protection and French regulations, the authors cannot publicly release the data from the French National Health Data System (SNDS). However, any person or structure, public or private, for‐profit or nonprofit, is able to access SNDS data upon authorization from the French Data Protection Office (CNIL Commission Nationale de l Informatique et des Libertes) to carry out a study, research, or an evaluation of public interest (https://www.snds.gouv.fr/SNDS/Processus‐d‐acces‐aux‐donnees and https://www.snds.gouv.fr/).
REFERENCES
- 1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz‐Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021;23(Suppl_3):iii1‐iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307‐314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casabella AM, Urakov TM, Basil G, Morcos JJ. Management of foramen magnum meningioma during pregnancy: literature review and case report. World Neurosurg. 2017;97:752.e15‐752.e18. [DOI] [PubMed] [Google Scholar]
- 4. Chakravarthy V, Kaplan B, Gospodarev V, Myers H, De Los RK, Achiriloaie A. Houdini tumor: case report and literature review of pregnancy‐associated meningioma. World Neurosurg. 2018;114:e1261‐e1265. [DOI] [PubMed] [Google Scholar]
- 5. Omulecka A, Papierz W, Nawrocka‐Kunecka A, Lewy‐Trenda I. Immunohistochemical expression of progesterone and estrogen receptors in meningiomas. Folia Neuropathol. 2006;44(2):111‐115. [PubMed] [Google Scholar]
- 6. Ülgen E, Bektaşoğlu PK, Sav MA, et al. Meningiomas display a specific immunoexpression pattern in a rostrocaudal gradient: an analysis of 366 patients. World Neurosurg. 2019;123:e520‐e535. [DOI] [PubMed] [Google Scholar]
- 7. Maiuri F, Mariniello G, Guadagno E, Barbato M, Corvino S, Del Basso De Caro M. WHO grade, proliferation index, and progesterone receptor expression are different according to the location of meningioma. Acta Neurochir (Wien). 2019;161(12):2553‐2561. [DOI] [PubMed] [Google Scholar]
- 8. Peyre M, Gaillard S, de Marcellus C, et al. Progestin‐associated shift of meningioma mutational landscape. Ann Oncol. 2018;29(3):681‐686. [DOI] [PubMed] [Google Scholar]
- 9. Michaud DS, Gallo V, Schlehofer B, et al. Reproductive factors and exogenous hormone use in relation to risk of glioma and meningioma in a large European cohort study. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2562‐2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hage M, Plesa O, Lemaire I, Raffin Sanson ML. Estrogen and progesterone therapy and meningiomas. Endocrinology. 2022;163(2):bqab259. [DOI] [PubMed] [Google Scholar]
- 11. Gil M, Oliva B, Timoner J, Maciá MA, Bryant V, de Abajo FJ. Risk of meningioma among users of high doses of cyproterone acetate as compared with the general population: evidence from a population‐based cohort study. Br J Clin Pharmacol. 2011;72(6):965‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nota NM, Wiepjes CM, de Blok CJM, et al. The occurrence of benign brain tumours in transgender individuals during cross‐sex hormone treatment. Brain. 2018;141(7):2047‐2054. [DOI] [PubMed] [Google Scholar]
- 13. Weill A, Nguyen P, Labidi M, et al. Use of high dose cyproterone acetate and risk of intracranial meningioma in women: cohort study. BMJ. 2021;372:n37. [DOI] [PubMed] [Google Scholar]
- 14. Bernat AL, Oyama K, Hamdi S, et al. Growth stabilization and regression of meningiomas after discontinuation of cyproterone acetate: a case series of 12 patients. Acta Neurochir (Wien). 2015;157(10):1741‐1746. [DOI] [PubMed] [Google Scholar]
- 15. Voormolen EHJ, Champagne PO, Roca E, et al. Intracranial meningiomas decrease in volume on magnetic resonance imaging after discontinuing progestin. Neurosurgery. 2021;89(2):308‐314. [DOI] [PubMed] [Google Scholar]
- 16. Shimizu J, Matsumoto M, Yamazaki E, Yasue M. Spontaneous regression of an asymptomatic meningioma associated with discontinuation of progesterone agonist administration. Neurol Med Chir (Tokyo). 2008;48(5):227‐230. [DOI] [PubMed] [Google Scholar]
- 17. Vadivelu S, Sharer L, Schulder M. Regression of multiple intracranial meningiomas after cessation of long‐term progesterone agonist therapy. J Neurosurg. 2010;112(5):920‐924. [DOI] [PubMed] [Google Scholar]
- 18. Passeri T, Champagne PO, Bernat AL, et al. Spontaneous regression of meningiomas after interruption of nomegestrol acetate: a series of three patients. Acta Neurochir (Wien). 2019;161(4):761‐765. [DOI] [PubMed] [Google Scholar]
- 19. Champagne PO, Passeri T, Froelich S. Combined hormonal influence of cyproterone acetate and nomegestrol acetate on meningioma: a case report. Acta Neurochir (Wien). 2019;161(3):589‐592. [DOI] [PubMed] [Google Scholar]
- 20. Malaize H, Samoyeau T, Zanello M, et al. Evolution of the neurosurgical management of progestin‐associated meningiomas: a 23‐year single‐center experience. J Neurooncol. 2021;152(2):279‐288. [DOI] [PubMed] [Google Scholar]
- 21. Passeri T, Giammattei L, Le Van T, et al. Atypical evolution of meningiomatosis after discontinuation of cyproterone acetate: clinical cases and histomolecular characterization. Acta Neurochir (Wien). 2021;164(1):255‐263. [DOI] [PubMed] [Google Scholar]
- 22. Devalckeneer A, Aboukais R, Bourgeois P, et al. Preliminary report of patients with meningiomas exposed to cyproterone acetate, nomegestrol acetate and chlormadinone acetate—monocentric ongoing study on progestin related meningiomas. Clin Neurol Neurosurg. 2021;210:106959. [DOI] [PubMed] [Google Scholar]
- 23. Devalckeneer A, Aboukais R, Faisant M, et al. Progestin‐related WHO grade II meningiomas behavior—a single‐institution comparative case series. Neurosurg Rev. 2021;45(2):1691‐1699. [DOI] [PubMed] [Google Scholar]
- 24. Weill A, Dalichampt M, Raguideau F, et al. Low dose oestrogen combined oral contraception and risk of pulmonary embolism, stroke, and myocardial infarction in five million French women: cohort study. BMJ. 2016;353:i2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318(17):1679‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Lippincott Williams & Wilkins; 2008:776. [Google Scholar]
- 27. Greenland S. Bias in methods for deriving standardized morbidity ratio and attributable fraction estimates. Stat Med. 1984;3(2):131‐141. [DOI] [PubMed] [Google Scholar]
- 28. Mikkelsen AP, Greiber IK, Scheller NM, Hilden M, Lidegaard Ø. Cyproterone acetate and risk of meningioma: a nationwide cohort study. J Neurol Neurosurg Psychiatry. 2021;93(2):222‐223. [DOI] [PubMed] [Google Scholar]
- 29. Apra C, Roblot P, Alkhayri A, Le Guérinel C, Polivka M, Chauvet D. Female gender and exogenous progesterone exposition as risk factors for spheno‐orbital meningiomas. J Neurooncol. 2020;149(1):95‐101. [DOI] [PubMed] [Google Scholar]
- 30.Nomegestrol and chlormadinone, article 31 referral notification to the PRAC/EMA. Accessed November 19, 2021. https://www.ema.europa.eu/en/documents/referral/nomegestrol‐chlormadinone‐article‐31‐referral‐notification_en.pdf
- 31. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383‐e391. [DOI] [PubMed] [Google Scholar]
- 32. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803‐820. [DOI] [PubMed] [Google Scholar]
- 33. Taylor AJ, Little MP, Winter DL, et al. Population‐based risks of CNS tumors in survivors of childhood cancer: the British childhood cancer survivor study. J Clin Oncol. 2010;28(36):5287‐5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Braganza MZ, Kitahara CM, Berrington de González A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 2012;14(11):1316‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Actualité—Acétate de cyprotérone (Androcur, Diane 35, et génériques) et vasoconstricteurs (pseudoéphédrine): retour d'information sur le PRAC de février 2020—ANSM [Internet]. Accessed June 28, 2021. https://ansm.sante.fr/actualites/acetate‐de‐cyproterone‐androcur‐diane‐35‐et‐generiques‐et‐vasoconstricteurs‐pseudoephedrine‐retour‐dinformation‐sur‐le‐prac‐de‐fevrier‐2020
- 36. Actualité—Lutényl et Lutéran: documents à venir pour renforcer l'information des patientes—ANSM [Internet]. Accessed June 28, 2021. https://ansm.sante.fr/actualites/lutenyl‐et‐luteran‐documents‐a‐venir‐pour‐renforcer‐linformation‐des‐patientes
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S6
Data Availability Statement
Data sharing is not applicable. According to French laws governing data protection and French regulations, the authors cannot publicly release the data from the French National Health Data System (SNDS). However, any person or structure, public or private, for‐profit or nonprofit, is able to access SNDS data upon authorization from the French Data Protection Office (CNIL Commission Nationale de l Informatique et des Libertes) to carry out a study, research, or an evaluation of public interest (https://www.snds.gouv.fr/SNDS/Processus‐d‐acces‐aux‐donnees and https://www.snds.gouv.fr/).


