Abstract
One of the most successful achievements of fetal intervention is the pharmacologic management of fetal arrhythmias. This management usually takes place during the second or third trimester. While most arrhythmias in the fetus are benign, both tachy‐ and bradyarrhythmias can lead to fetal hydrops or cardiac dysfunction and require treatment under certain conditions. This review will highlight precise diagnosis by fetal echocardiography and magnetocardiography, the 2 primary means of diagnosing fetuses with arrhythmia. Additionally, transient or hidden arrhythmias such as bundle branch block, QT prolongation, and torsades de pointes, which can lead to cardiomyopathy and sudden unexplained death in the fetus, may also need pharmacologic treatment. The review will address the types of drug therapies; current knowledge of drug usage, efficacy, and precautions; and the transition to neonatal treatments when indicated. Finally, we will highlight new assessments, including the role of the nurse in the care of fetal arrhythmias. The prognosis for the human fetus with arrhythmias continues to improve as we expand our ability to provide intensive care unit–like monitoring, to better understand drug treatments, to optimize subsequent pregnancy monitoring, to effectively predict timing for delivery, and to follow up these conditions into the neonatal period and into childhood. Coordinated initiatives that facilitate clinical fetal research are needed to address gaps in knowledge and to facilitate fetal drug and device development.
Keywords: antiarrhythmic drugs, arrhythmia, congenital heart block, fetal arrhythmia, fetal magnetocardiography, fetal echocardiography, fetal pharmacology, fetal tachycardia, long QT syndrome, torsades de pointes
Collaborations between pediatric cardiologists and obstetricians to implement drug treatments for tachy‐ and bradyarrhythmias have led, in some of the cases, to dramatically successful outcomes for rhythm disturbances that had previously resulted in premature birth, hydrops, and death. Fetal echocardiography became a mainstay of both anatomic and rhythm diagnosis and improved over the decades, implementing advanced 2‐dimensional, M‐mode, color Doppler, and pulsed Doppler techniques.
Diagnostic Approach of Fetal Arrhythmia
Electrocardiographic monitoring never developed in the fetus, due mainly to technical reasons such as the near‐complete insulating effect of the fetal vernix and maternal tissues that made it impossible to consistently record a fetal cardiac signal from the maternal abdominal surface. Fetal electrocardiography was complicated further by fetal movement. Cardiotocography, a Doppler‐based fetal heart rate monitoring technique, remains largely restricted to normal fetal rhythm and heart rate (HR) assessments in the third trimester and during labor, leaving a major gap in arrhythmia assessment of the high‐ risk fetus.
During the early parts of the 21st century, a small number of medical physics laboratories from around the world were recognizing that fetal electrocardiographic monitoring would likely never offer the capabilities that electrocardiography offers postnatally. They began evaluating super‐conducting quantum interference devices, that capture the electrocardiogram‐analogous magnetocardiogram. Rather than catching on, with implementation in every fetal care center, super‐conducting quantum interference devices have been largely restricted to a few labs due to the expense of helium and magnetically shielded rooms.
Figure 1 shows typical fetal magnetocardiogram (fMCG) tracings from a case of fetal supraventricular tachycardia (SVT) evaluated using fetal magnetocardiography.
Figure 1.
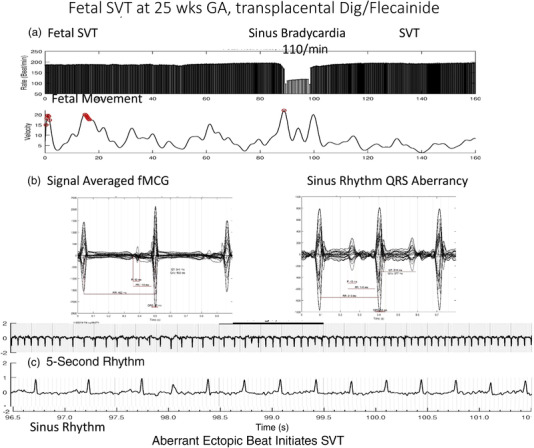
Fetal SVT. (a) Heart rate trend over 5 minutes. In this case, the fetus is in SVT at about 194/min for nearly the entire tracing. Sinus bradycardia at 110/min is likely due to the transplacental effects of digoxin and flecainide. (b) cardiac time intervals in both sinus rhythm and SVT with 1:1 VA conduction. Note that the QRS is wider during SVT, likely related to flecainide effect. (c) Aberrantly conducted PAC that initiates SVT. Note that T‐wave inversion is present. This could be due to digoxin. Dig, digoxin; fMCG, fetal magnetocardiogram; GA, gestational age; PAC, premature atrial contraction; SVT, supraventricular tachycardia.
A recent breakthrough in the field of biomagnetism has led to the introduction of optically pumped magnetometers, which are compact, inexpensive, and can be used within a person‐sized noise‐reducing shield rather than requiring a large shielded room. 1 , 2 These may be ready for Food and Drug Administration (FDA) approval within 2 years. Meanwhile, some centers continue to try to develop fetal electrocardiography, but to date no commercial models are approved for precise arrhythmia diagnosis.
Unique Aspects of Pharmacologic Treatment of the Fetus
The treatments for various arrhythmias are rhythm specific and, to a large extent, still based on limited actual pharmacologic drug data due to the invasive nature of sampling the fetus.
Table 1 shows the unique aspects of treatment of the fetus as compared to treatment of the infant.
Table 1.
Differences in Pharmacological Treatment Between the Fetus and Neonate/Infant
| Fetus | Neonate/Infant | |
|---|---|---|
| Knowledge of treatment of arrhythmias | Limited published experience (<20% of the infant studies), mainly from fetal care centers, within the past 4 decades, even less published for direct fetal therapies | Small to medium case series have been published for most major cardiac drugs used in infants |
| Pharmacologic assessment |
Requires cordocentesis for direct drug measurement; not routine Maternal serum levels and corresponding umbilical cord concentrations can be obtained at delivery Amniotic fluid drug concentration may be higher than maternal serum for some drugs |
Serum levels available for digoxin, propranolol, procainamide, flecainide, amiodarone, lidocaine, mexiletine, +/– sotalol Not routinely available for sotalol and beta blockers other than propranolol ECG and heart rate changes used as surrogate to assess drug effect |
| Electrophysiologic assessment | Maternal home hand‐held Doppler and/or frequent obstetric monitoring critical to detecting SVT recurrence, or bradycardia; if available, fMCG can provide QRS and QTc, HR variability, and STT changes; maternal ECG helpful | Outpatient: Cardiac monitor or Transtelephonic transmitter, Holter monitor, periodic ECGs; transesophageal pacing before discharge may identify fetuses that will never have SVT again and thus do not require treatment or follow‐up |
| Drug administration |
Physiologic changes in later pregnancy (higher cardiac output and volume of distribution), play an important role in transplacental drug delivery; single transplacental drug therapy, followed by combination drug therapy or direct fetal IM or IC therapy No clearly superior first‐line drug for SVT or atrial flutter; currently under study Upward dose adjustment may be needed in the third trimester. |
Single drug therapy, followed by dual or combination drug therapy for refractory cases Reduced Compliance with medication administration can be seen if twice‐daily or 4‐times‐daily dosing of drug Drug levels can be used to assess longer‐term compliance; with rapid neonatal/infant growth, drug adjustment every other week is recommended on a mg/kg/day basis. Important to teach neonatal resuscitation to caregivers, and also poison prevention measures for siblings and infant. Maternal postpartum depression can impact medication compliance for infant |
| Drug withdrawal | Once treatment is initiated, it is usually continued to delivery | Infants after fetal tachycardia should be weaned from treatment at birth unless recent SVT. Most recurrences are within 72 hours. TEP may be useful. If postnatal antiarrhythmics are used, wean off at 6‐12 months of age. Long‐term, if SVT persists, radiofrequency ablation is usually done after 4‐7 years of age |
| Long‐term prognosis | 87% with fetal SVT will wean from drug therapy by 1 year; 13% will still require treatment; higher for those with long‐RP tachycardia | About 90% will wean from therapy; however, late recurrences can be seen in up to one‐third. RF ablation is generally performed over 4‐7 years of age. Younger age risks coronary injury with RF ablation |
ECG, electrocardiogram; fMCG, fetal magnetocardiogram; HR, heart rate; IC, intracordal; IM, intramuscular; RF, radiofrequency; SVT, supraventricular tachycardia; TEP, transesophageal pacing.
For references, see text.
Treatment of the fetus is quite different from treatment of the infant or child. The most common approach is transplacental therapy (TPT), where the pregnant patient is given escalating and sometimes top‐therapeutic doses of antiarrhythmic or other medication alone or in combination in order to reach the fetus. Most drugs are of small molecules that readily cross the placenta. Another means is direct administration to the fetus via intracordal, intramuscular, or intraperitoneal instillation. Fetal vascular access has significant risk, which will be addressed.
During the latter part of pregnancy, the physiologic changes in pregnancy that result in a higher cardiac output and volume of distribution, are an important factor in TPT. This is because increased cardiac output and decreased vascular afterload result in higher peripheral tissue and organ perfusion. A larger volume of distribution provides for higher net distribution for the drug. This often “outstrips” the gains in free drug in the circulating volume (which is increased due to lower albumin levels). 3 Recurrences of well‐controlled tachyarrhythmias can occur when maternal drug levels fall (higher distribution and lower patient compliance) or when hydrops develops (impaired transplacental flow). Digoxin has poor transplacental transfer during hydrops, and when hydrops resolves, the impact of high maternal digoxin dosing may manifest as fetal bradycardia. 4 Fetal drug effects may rise during long‐term therapy with sotalol or flecainide due to accumulation of certain drugs in the amniotic space. 5 , 6 The placenta was once felt to be the only means of transfer of drug into or out of the amniotic fluid space, but Menon 7 has suggested that fetal membranes also contain the same enzymes as the placenta and may be important in drug transfer (both ingress and egress).
Different Types of Fetal Arrhythmias
The most common arrhythmias are isolated ectopic atrial contractions, which occur in about 1% to 2% of pregnancies. It is important to distinguish these from premature ventricular contractions. Premature atrial contractions (PACs) are commonly caused by redundancy of the septum primum or flap of the foramen ovale. They do not require antiarrhythmic treatment. They can produce bradycardia (heart rate <110/min) when PAC's alternately block at the atrioventricular (AV) node (blocked atrial bigeminy). PACs are associated with the SVT in about 1% of ectopy cases; however, this risk climbs to 10% to 14% risk for blocked atrial bigeminy. Thus, when ectopy develops, close weekly observation or hand‐held home monitors should be employed to detect SVT before the onset of hydrops, which can develop in within several days. Isolated premature ventricular contractions also do not require treatment; however, care should be taken to exclude commonly associated conditions such as ventricular aneurysms or diverticuli, tumors, congenital heart disease or cardiomyopathy, or familial long QT syndrome and other inherited arrhythmia syndromes.
Treatment of Fetal Tachyarrhythmia
Pharmacologic Treatment of Fetal Tachyarrhythmia—A Historical Perspective
Tachyarrhythmias of severity to require intrauterine treatment are seen in about 1 in 2500 pregnancies. The most serious complication is the development of fetal congestive heart failure or hydrops fetalis. Digoxin was the first drug used to treat fetal tachyarrhythmia and fetal heart failure. 8 , 9 , 10 , 11 , 12 This was followed shortly by the use of verapamil, 13 , 14 , 15 beta blockers, 15 procainamide, 15 , 16 quinidine, 9 , 15 and amiodarone. 15 , 17 During the 1990s, new drugs such as flecainide and sotalol came along with good transplacental transfer and effectiveness for severe life‐threatening tachyarrhythmia, and these were used, alone and in combination, in fetuses with drug‐refractory tachycardia. 5 , 6 , 11 , 12 , 18 , 19 , 20 , 21 , 22 , 23 While digoxin was about 60% effective in the absence of hydrops, it had very poor transplacental transfer in fetuses with hydrops (<20%), and beta blockers also appeared to be minimally effective. Verapamil, 13 , 14 , 15 which was quite efficacious for reentrant arrhythmias, had a side effect profile that included sudden demise and renal failure. Procainamide, while also effective, increased uterine irritability, and with intravenous maternal administration, could actually trigger preterm labor. Quinidine caused gastrointestinal side effects. Amiodarone, while exceedingly effective even in drug‐refractory fetal tachyarrhythmia, also had many side effects, including thyroid dysfunction in both mother and fetus, and potential fetal neurodevelopmental concerns, limiting its broader application, though it is still used for the most life‐threatening maternal and fetal arrhythmias. 23 , 24 , 25 , 26 , 27
While direct fetal therapy with intracordal adenosine for rapid conversion or instilling other potent drugs into the fetal cord seemed enticing, the risk of direct cord access had to be weighed against the risk of fetal death from the arrhythmia. Several studies have shown higher mortality (25%‐50%) in fetuses treated directly via the cord, than in those treated only with maternal medication or by intramuscular injection. 25 Intracordal adenosine has been effective in converting SVT; however, premature beats lead to reinitiations, rendering adenosine overall ineffective.
One approach, however, has proven useful for fetuses with hydrops. Intramuscular digoxin has been reported to both shorten time to conversion and improve the rate of conversion, but it has been associated anecdotally with sciatic nerve injury in about 1 in 20 cases where injection was given into the thigh or buttocks. 4
Pharmacologic Treatment of Fetal Tachyarrhythmia—Current Perspective
The advent of the 21st century has seen more systematic evaluation of fetal tachyarrhythmia through multicenter approaches. 28 Miyoshi et al 2 published a protocol for the fetus without hydrops, starting with digoxin and adding other drugs (digoxin/sotalol then digoxin/flecainide). Overall survival was 96%, and loss was only in those with hydrops. Miyoshi et al showed that oral digoxin loading was comparable to intravenous digoxin dosing, making administration in the hospital setting safer. SVT conversion to sinus rhythm in utero was 89% without hydrops and 75% with hydrops. Those with long‐RP tachycardia were responsive to sotalol. Jaeggi 29 and colleagues are currently conducting a multicenter randomized clinical trial, called the FAST trial, of 600 fetuses with SVT or atrial flutter (AFl) from at least 70 centers (and also a registry for those unable to be randomized). This study is evaluating digoxin, sotalol, and flecainide monotherapy for fetuses without hydrops with SVT; digoxin or sotalol monotherapy for fetuses with AFl (digoxin+sotalol for AFl with hydrops); and digoxin+flecainide vs digoxin+sotalol for SVT with hydrops. The study is blinded, and results will not be released until total enrollment is reached.
Impact of Fetal Tachycardia Treatment on Maternal and Child Health
Studies have shown relatively high rates of maternal side effects and premature delivery related to fetal transplacental treatment. Maternal nausea and anorexia can lead to poor weight gain. In hydrops, once sinus rhythm is restored, preterm labor can be triggered due to rapid fetal diuresis into the confined intra‐amniotic space, leading to polyhydramnios. In Miyoshi's 2 series, 20% of fetuses delivered prematurely. Rarely, amniotic fluid reduction procedures have been necessary. To our knowledge, maternal death from fetal tachyarrhythmia drug treatment has not been reported, but side effects are common and usually well tolerated. 30 These are often specific to the drug and are more common when drugs are given concurrently, in quick succession, or rapidly escalated. Daily inpatient electrocardiograms and heart monitoring or telemetry are indicated during initiation of drug therapy. For the fetus, third‐trimester biophysical profiles or non‐stress‐testing assessments are indicated. Generally, if good control of the rhythm has been achieved, as is the case in about 75% to 90% of cases, vaginal delivery is possible. 31 The infant should be monitored in a neonatal intensive care unit (ICU) setting during the withdrawal phase from medication, and if SVT or AFl has not recurred during the first 72 hours, home heart rate monitoring or transesophageal electrophysiologic study can be considered. 31
Mechanisms of Fetal Tachycardia and the Drugs That Are Used to Treat These
Reentrant SVT
The vast majority of fetal SVT is reentrant SVT. In this case, the fetal heart rate (FHR) is usually 220 to 320/min and regular, with a 1:1 relationship of atrium and ventricle. SVT is usually initiated by PACs. 32 The average time to conversion/control is 6 to 7 days. The fetus with sustained durations of tachycardia lasting >12 hours and with rates in excess of 200/min, with a gestation <36 weeks should receive TPT. 33 For the fetus >36 weeks, delivery can be considered, but given the need for cesarean section when delivering a baby in SVT, several major centers have now begun treating transplacentally even at this mature gestation, in the hopes that tachycardia control might afford a vaginal delivery and avert the developmental risks associated with late‐preterm births. Likewise, fetuses with hydrops are sometimes treated transplacentally at gestations >36 weeks, since resolution of hydrops can improve respiratory resuscitation (E Jaeggi and BF Cuneo, personal communication, June 2022). Argument for delivery after SVT presentation >36 weeks’ gestation includes the potential for directly administering drugs to the infant, as well as averting stillbirth potential and not exposing the mother to the side effects of transplacental antiarrhythmic drugs. Shared decision making with the care team and patient is optimal.
Transplacental dosing does not always lead to adequate fetal efficacy, which may necessitate higher maternal doses or even the addition of multiple concurrent drugs in order to control the tachycardia. Three drugs are generally used in the first‐line treatment of SVT: digoxin, flecainide, and sotalol. Currently, none have been found to be superior for reentrant SVT. Sotalol, flecainide, and other drugs can concentrate in the amniotic fluid to levels up to 28 times the maternal serum level. 6 Maternal clearance through the placenta and possibly through fetal membranes is often sufficient to prevent fetal reabsorption of significant quantities. 34
Atrial Flutter
Atrial flutter, which accompanies SVT in up to 30% of cases and is associated with a much higher atrial rate than SVT (atrial rates of 375‐550 bpm, with ventricular rates of 190‐230/min) is treated in the same way as SVT. Digoxin and Sotalol are drugs of choice for atrial flutter. Amiodarone, despite its high side effects profile, is >90% effective for controlling drug‐refractory SVT; it is less so for AFl and often simply slows the flutter rate. 25
Table 2 shows the mechanism, side effects profiles, and transplacental maternal‐fetal drug concentration ratios for the commonly used drugs to treat SVT and AFl.
Table 2.
Drugs to Treat Fetal SVT and Atrial Flutter a
| Drug 2 , 6 , 12 , 25 , 27 , 28 , 31 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 | Type of Arrhythmia | F:M Drug RatioRoute | Efficacy Acute and Chronic | Elimination | Intra‐amniotic | Side Effects |
|---|---|---|---|---|---|---|
|
Digoxin Na,K‐ATPase inhibitor Class C |
SVT, AFl |
0.8:1, ↓ if hydrops to 0.2:1 PO, IV, fetal IM/IC |
50%‐60%, combined with other AA 80% | Renal | Higher, not reflected in fetal | N/V, arrhythmias, anorexia, poor weight gain |
|
Flecainide Calcium channel inhibitor Class C |
SVT, AFl, AET, PJRT, VT (non‐LQTS) |
1:1(+) PO |
60% | Renal | Up to 27× maternal serum level | CNS, bradycardia, ↑QRS, ↑QTc |
|
Sotalol Potassium channel inhibitor/beta blocker Class B |
SVT, AFl, AET, PJRT |
0.9:1(+) PO |
50%‐60% for SVT, up to 80% for AFl | Renal | 1.6‐28× maternal serum level | CNS, bradycardia, ↑QTc |
|
Amiodarone Multichannel inhibitor Class D |
SVT, AFl (±), AET, PJRT, JET, VT not if ↑QTc |
0.4:1, long half‐life after PO loading Rare IC or peritoneal administration |
90+% | Hepatic | Lipophilic, all tissues |
Bradycardia, M/F hypothyroidism, ↑QTc, breastfeeding CI |
| Adenosine | 0 | Not recommended, Direct IC administration | Low | Erythrocytes | 0 | Short‐acting |
AA, antiarrhythmic agent; AET, atrial ectopic tachycardia; AFl, atrial flutter; CI, contraindicated; CNS, central nervous system; F:M, fetal:maternal; IC, intracordal; IM, intramuscular; IV, intravenous; JET, junctional ectopic tachycardia; LQTS, long QT syndrome; M/F, maternal/fetal; N/V, nausea/vomiting; PJRT, permanent junctional reciprocating tachycardia; PO, per os (orally); QTc, corrected QT interval; SVT, supraventricular tachycardia; VT, ventricular tachycardia.
These doses are based on often small case series. Even when treatment goes well, these drugs have been reported to have a stillbirth rate of 4% and prematurity rate of almost 20%. 2 Much of the prematurity is due to inadequate time for transplacental conversion (which can take 3‐7 days per each drug administered), and there is no question that the fetal and neonatal morbidity of delivering a very premature infant in SVT or AFl far surpasses the morbidity of transplacental treatment. There is a tendency to underestimate the time to conversion of SVT. By delivering the infant prematurely, the effective drug processing power of the placenta and maternal circulation is exchanged for the immature drug processing of the premature infant's liver and kidneys. 35 At least 40% to 60% of infants will require neonatal drug treatment 31 ; therefore, having a mature neonate delivered in sinus rhythm is a marked advantage. The need for transfusions for anemia from bone marrow suppression, exchange transfusions for hyperbilirubinemia due to drug displacement of bilirubin‐binding sites, and prolonged neonatal ICU care for regulating drug therapy are much higher for the premature infant. In addition, premature infants often have markedly different metabolic rates. For example, digoxin half‐life can be as long as 30 hours. 35 This is an area for research.
Tachycardia
Rare forms of slower fetal tachycardia have a long VA interval (atrial ectopic tachycardia and permanent junctional reciprocating tachycardia) with a predominantly 1:1 AV relationship and can respond to sotalol, flecainide, or amiodarone. These also have a greater tendency to persist beyond 1 year of age and may require radio frequency ablation.
Fetal ventricular tachycardia (VT) or junctional ectopic tachycardia are much rarer than SVT and AFl. These are treated differently depending on the etiology. fMCG is extremely helpful in assessing these types of rare arrhythmias and in risk stratifying the cases. Many of the drugs cause corrected QT (QTc) prolongation and are contraindicated in torsades de pointes (TdP).
Table 3 lists drugs for TdP as well as for VT not associated with QTc prolongation.
Table 3.
Antiarrhythmic Agents for VT a
| Drug 71 , 74 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 | F:M Drug Ratio | Efficacy Acute and Chronic | Elimination | Intra‐amniotic Accumulation | Side Effects |
|---|---|---|---|---|---|
| Drugs to treat all VT, including torsades de pointes | |||||
|
Magnesium sulfate Class D after 48 h |
1:1(+) IV, PO coadministered with Vit D if 25‐OH Vit D level is low |
80+% | Renal | Baseline high | CNS |
|
Propranolol, other beta blockers Class C |
0.25:1 IV, PO |
Partial, ↓QTc, lowers ventricular fibrillation risk; metoprolol less effective in LQTS | Hepatic | 2‐4× | Bradycardia; nadolol concentrates in breast milk |
|
Lidocaine/Mexiletine Calcium channel inhibitor |
0.5:1 IV/PO |
50+% | Hepatic, renal | 0.5‐1.0 | CNS, Paradox ↑QTc |
|
Drugs to treat VT with normal QTc, not torsades de pointes | |||||
|
Flecainide Calcium inhibitor Class C |
1:1 | 60% | Hepatic, renal | 1.6‐27× serum level | CNS, brady, ↑QTc |
|
Sotalol Potassium inhibitor/beta blocker class B |
0.9:1(+) | 50%‐60% | Renal | 28× serum level | CNS, bradycardia, ↑QTc |
|
Amiodarone Class D |
0.4:1, long‐term after loading | 90+% | Hepatic, biliary excretion, long half‐life | All tissues | Bradycardia, no breastfeeding |
Neonatal Antiarrhythmic Therapy for Tachyarrhythmias
The need for postnatal antiarrhythmic therapy varies, but conservatively, only 50% to 70% of fetal tachycardia cases will need treatment for SVT once born, since the natural history is for accessory AV connections to regress with time, and because blood flow dynamics change at birth. AFl that has been direct‐current cardioverted or converted using transesophageal pacing is even less likely to recur and require neonatal antiarrhythmic agents. 36 , 37 , 38 , 39 , 40 Both VT and junctional ectopic tachycardia require treatment. When fetal SVT recurrences occur, about 80% will recur in the first 3 days. For this reason, a drug‐free period is usually instituted shortly after birth. For those that recur, treatment is usually instituted with a beta blocker or sometimes digoxin or flecainide. Recurrence is more common with long‐RP types of tachycardias. Sometimes fetal bundle branch block is noted immediately after delivery. 41 , 42 , 43 This is most common with flecainide, especially when its treatments are overlapped with sotalol or amiodarone. Drug levels should be sent and the drug withheld if possible, until conduction normalizes.
Recognition and Treatment of Fetal Bradyarrhythmias
Sinus Bradycardia
Bradyarrhythmias such as persistent sinus bradycardia can be underrecognized. They can be markers for fetal long QT syndrome (LQTS), inherited bradycardia syndromes, congenital heart disease (heterotaxy with low atrial rhythm), isoimmune disease, and other life‐threatening conditions. Many of the fetal arrhythmias that contribute to fetal demise are hidden from existing types of clinical assessment. 44 , 45 , 46 , 47 , 48 , 49 Obstetricians have generally used American College of Obstetricians and Gynecologists guidelines to establish FHR ranges, with normal FHRs being between 110 and 180 bpm. 50 But this largely ignores the gestation effect on FHR, which is significant. Several gestation‐based HR norms exist 51 , 52 , 53 , 54 , 55 and can and should be applied to better recognize those fetuses with HRs outside the normal range for gestation. In general, bradycardia does not require treatment; however, recognition of underlying cause is important, as many of these diseases do require treatment in the fetus or neonate.
Atrioventricular Block
Sustained second‐ or third‐degree AV block is seen most commonly in Sjogren's Antibody A isoimmunization from maternal collagen vascular disease. Currently, the StopBloQ study 56 is enrolling women at risk for development of congenital atrioventricular block (CAVB). CAVB is seen in about 1% to 2% of pregnant lupus patients, 5% with active disease, and after an affected pregnancy, this risk increases to 16%.
Figure 2 shows an example of a fetus with isoimmune CAVB.
Figure 2.
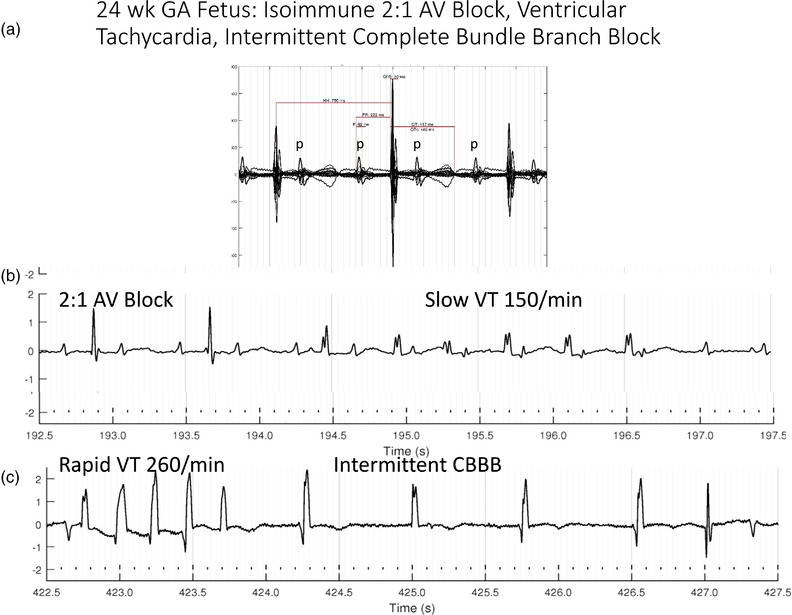
24‐week GA fetus with complex 2:1 isoimmune AV block, HR 75/min. (a) The signal averaged wave form, which documents 2 P waves for each QRS and a stable but prolonged PR interval. (b) Slow ventricular tachycardia is shown, which is common during the first several weeks of AV block. (c) Image shows that this fetus also had periods of rapid ventricular tachycardia and complete AV block with wide QRS escape rhythm (complete bundle branch block). These unstable findings are most common during the early adaptive phase, when the fetus is adjusting to the sudden drop in heart rate associated with AV block. AV, atrioventricular; CBBB, complete bundle branch block; GA, gestational age; VT, ventricular tachycardia.
It is important to realize that maternal autoimmune disease is a risk factor for stillbirth, growth restriction, and other complications of pregnancy even without fetal AV block (AVB). AVB onset is commonly between 18 and 24 weeks’ gestational age, and rarely develops after 28 weeks’ gestational age. 57 , 58 , 59 Based on what we have learned from fMCG and echocardiography, the early stages of AVB are associated with frequent ectopy, junctional and ventricular tachycardia, STT wave changes, and QT prolongation. After several weeks of adapting to the lower FHR, which is often in the 50 to 70/min range, the fetus will show stabilization for the majority of the remainder of pregnancy. The HR may continue to fall and lose variability and for rates <50 bpm with low variability, and most of these infants will require pacemaker implantation in the newborn period. If the HR is < 50 bpm, augmentation with terbutaline may raise ventricular rate, though it has not been linked to better survival. The use of dexamethasone both in partial (second‐degree AVB) and complete (third‐degree AVB) has been shown in some cases to prevent progression to a higher‐grade AVB, as well as to reverse partial block in up to 25%. Dexamethasone's use in third‐degree AVB is controversial still, with some centers finding reduced long‐term cardiomyopathy and others not finding this. 60 Additionally, intravenous immunoglobulin has been shown to prevent mortality in most fetuses with hydrops due to CAVB. Many centers are using intravenous immunoglobulin to reduce inflammation in high‐risk cases where extensive endocardial fibroelastosis or ventricular dysfunction is present. Hopefully, the StopBloq trial will provide more insight into its role.
Table 4 lists the common medications used to treat fetal AV block.
Table 4.
Drugs for Treatment of Isoimmune AV block a
| Drug 84 , 93 , 94 , 95 , 96 , 97 | Indication/Duration Route | F:M Drug Ratio | Efficacy Acute and Chronic | Elimination | Side Effects |
|---|---|---|---|---|---|
| Dexamethasone Fluorinated glucocorticoid |
PR on echo >170 ms or AV block onset PO |
0.5 F:M, ↓ Mab levels | 20%‐40% reversal of 2:1 block, may ↓ postnatal cardiomyopathy | Hepatic and renal | Maternal HTN, ↑ glucose, Cushing syndrome, CNS, osteoporosis, etc; transfer to breast milk |
|
IVIG Anti‐inflammatory, blocks F2/FAB receptors in placenta |
Hydrops IV |
0.5‐1.0:1 |
In HF, ↓ mortality from 80%‐25% $$$, preapproval needed |
Depends on target, mostly renal | Allergic Rxn, vaccines |
|
Hydroxychloroquine TLR blocker, ↓ endosomal pH |
Prior infant with NLE PO |
1.04:1 | ↓ heart block risk from 16% to 7% in subsequent pregnancy | Half‐life, 40‐50 days; mostly renal, some retained long‐term | ↑QTc |
|
Terbutaline Beta agonist (isoimmune and nonisoimmune AV block) |
FHR <50/min, if CHD <55/min or with hydrops PO |
1‐1.5:1 | ↑ FHR by 5‐10 beats/min, not proven to ↑ survival | Renal | ↑ maternal HR, arrhythmias, CNS |
AV, atrioventricular; CHD, congenital heart disease; CNS, central nervous system; FAB, fragment antigen‐binding; FHR, fetal heart rate; F:M, fetal:maternal; HF, hydrops fetalis; HR, heart rate; HTN, hypertension; MAb, monoclonal antibody; NLE, neonatal lupus erythematosus; Rxn, reactions; TLR, toll‐like receptor.
Recognition and Treatment of Fetal Arrhythmias That Are Not Picked Up by Echocardiography
Recently, we and others have gained a much better understanding of the impact of silent arrhythmias and conduction defects on fetal morbidity and mortality. Genetic conditions are known to be associated with high rates of stillbirth and sudden infant death syndrome. Ion channelopathies may play a role. Vitamin D and magnesium or calcium deficiency and maternal QT prolonging or HR‐augmenting medications have the potential to increase risk in susceptible populations. 61 , 62 , 63 , 64 , 65 , 66 One of the most important impacts of newer cardiac monitoring such as fMCG will be in uncovering the predisposition for fetal QT prolongation. We recently reported that 16 of 33 fetuses with congenital heart disease had either QTc >500 milliseconds (10/33), complete bundle branch block (4/33), or both (2/33) (Wacker‐Gussmann et al 67 ). Unlike manifest fetal arrhythmias, many of the most serious rhythm disorders occur when the FHR is within the normal range, and rhythm may be entirely normal, making these arrhythmias nearly impossible to detect using standard obstetrical monitoring techniques alone. It is within this group of rhythm disturbances that the majority of fetal deaths occur. Even postnatally, this group represents a challenge to detect, though electrocardiography will detect these if performed. Some countries have adopted universal electrocardiographic screening techniques (Japan, Italy) to recognize these infant conditions that account for 10% to 15% of sudden infant death syndrome and up to 30% of sudden deaths in older children. 68 , 69 In the fetus, 5% to 10% of all unexplained stillbirths have been linked in several studies to these inherited repolarization abnormalities, and in families with LQTS the risk of miscarriage in doubled and stillbirth is 8 times higher than in the general population. 49 The mechanism of death in the inherited channelopathy group is likely due to TdP VT, and has been associated with markedly prolonged fetal QTc >600 milliseconds. T‐wave and systolic mechanical alternans (echocardiographically), and late‐coupled premature ventricular contractions discovered with fMCG can be warning signs preceding TdP or lethal events (B. Hughes, personal communication, August 2022). While TdP is usually associated with rapid development of heart failure and hydrops, it is better tolerated in the fetus than in the neonate due to the placental perfusion pressure that maintains fetal circulation. Unlike other forms of VT, TdP is very difficult to recognize echocardiographically because the semilunar valves (aortic and pulmonary) do not open consistently and the rate appears slower than what it actually is (Wacker‐Gussmann et al 67 ; Bolin et al 70 ).
Figure 3 shows a case of a fetus with familial LQTS type 2 and 2:1 AVB, where TdP was discovered only at the time of fMCG. Serial follow‐up in this fetus demonstrated that transplacental mexiletine, added to oral propranolol, to stabilize the fetal rhythm was actually proarrhythmic, making the TdP worse. The fetus was taken off mexiletine, left on high‐dose propranolol, and adequately managed despite some ongoing fetal torsades until birth, when nadolol and pacemaker therapy were instituted. She is now 2 years old. Her mother, interestingly, had also presented with 2:1 block, and required neonatal pacemaker.
Figure 3.
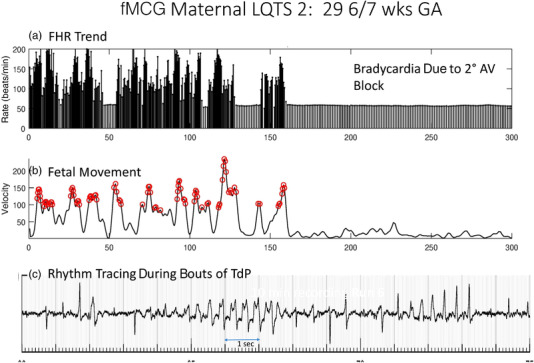
29‐week GA fetus with familial LQTS type 2. The top tracing shows low fetal heart rates of 52/min due to functional 2:1 AV block. Irregular tachycardia is seen (left half) with rates of 100‐200/min. The bottom panel shows the rhythm associated with the higher rates, which is torsades de pointes and not sinus rhythm with ectopy as suspected by referral echocardiogram. AV, atrioventricular; FHR, fetal heart rate; fMCG, fetal magnetocardiogram; GA, gestational age; LQTS, long QT syndrome; TdP, torsades de pointes; VT, ventricular tachycardia.
The mechanisms of fetal demise in cardiomyopathies (noncompaction, idiopathic) and inherited metabolic syndromes are different than for LQTS, and many of these are not associated with repolarization abnormalities, but rather depolarization abnormalities. Complete right or left bundle branch block is common. Unfortunately, unlike inherited arrhythmias that can often be effectively treated once recognized, many cardiomyopathies with such profoundly wide QRS complexes will go on to experience severe decelerations, ST‐T abnormalities, hypertrophic changes, and fetal demise. A widely divergent group of additional conditions and arrhythmias, some of which are acquired, such as myocarditis, cardiac rhabdomyomas from tuberous sclerosis, cardiac fibromas, congenital heart defects (heterotaxy syndrome, Ebstein malformation, etc), and ventricular aneurysms, can manifest both overt and hidden rhythm and conduction abnormalities.
With the use of fMCG, we and others have begun to recognize electrophysiological signs of fetal risk in such conditions as fetal growth restriction, hydrops, mono‐twins, gastroschisis, pregnancies subsequent to a stillborn infant, and others. These are often the same types of findings that one might notice on a cardiac monitor in an ICU setting, such as ST depression, T‐wave inversion, QT prolongation, pathologic FHR decelerations, J waves, intermittent preexcitation, bundle branch block, arrhythmias, and more. Although not a part of regular practice at this time, with advances in newer types of inexpensive magnetometers, it is likely that in the future, obstetric care will marry the diagnostic benefits of the ultrasound/echocardiogram with the advanced monitoring capabilities of fMCG. These will be most helpful in serial follow‐up of certain high‐risk maternal/fetal conditions.
Another underrecognized problem in electrophysiology of the fetus is the complexity of rhythm disturbances. With those LQTS fetuses who were stillborn, novel fetal rhythms were often found, including AVB with 3:1 conduction ratio, QRS alternans in 2:1 AVB, long‐cycle‐length TdP, and slow monomorphic VT, that would not have been recognized without fMCG. 71 It is apparent that a subset of these extremely lethal arrhythmias will not be seen postnatally due to their complete lethality.
The best antiarrhythmic treatments are based on a precise electrophysiologic assessment of rhythm. This is true at all ages. Personalized pharmacologic treatment strategies may need to be developed in the future, especially for the rarer genetic conditions. 72
Nurse's Role in the Care of Patients With Fetal Arrhythmia
The fetal nurse in the United States provides an array of care for patients with fetal cardiology and rhythm diagnoses. These roles can include support for complex diagnoses, including obstetric issues, structural anomalies, and/or arrhythmias. Thus, the nursing background necessary overlaps medical disciplines. Critical roles in the care of these patients, include care coordination, patient education, and collaboration with the various inpatient and outpatient team members, including fetal cardiology, perinatology, obstetric care teams and pharmacy, and the patient and family members. It is critical in the care of these complex patients that all are aware of the medications including over‐the‐counter vitamins and supplements as well as prescription antiarrhythmic agents. Often, these medications are titrated to anthropometric measures that change throughout the progression of pregnancy.
The current standard of practice for the nurse includes regular medication reconciliation at each visit (in‐person, phone, or virtual visits) verbally with the patient as well as documentation in the electronic medical record for accuracy. It may be necessary to retrieve send‐out drug levels. Patients receive extensive education at each visit regarding the administration, dosing, and effects of the medications (over‐the‐counter medications, prescriptions) including side effects, interactions, and potential dietary recommendations. It is advisable that written supportive documents such as after‐visit summaries are provided. The nurse is required to remain up to date with these multiple effects of the medication and required monitoring guidelines using multiple online resources. For example, some drugs may be newly listed to the long QT list, or have new indication based on emerging scientific statements. In addition, new FDA warnings can be posted. The fetal nurse will frequently collaborate with pharmacies to ensure drug availability, especially at initial discharge from the hospital; ensure accurate formulations; and verify any drug‐drug interactions.
Often in the care of fetal cardiology patients, monitoring the maternal and fetal HR is necessary to optimize pharmacologic treatment. The fetal nurse will order the equipment necessary to monitor the maternal and fetal HRs, provide patient and family instruction, and regularly check on the patient to record results. The fetal nurse is responsible for contacting the corresponding care providers when the results are concerning or if any questions arise from the patient. Often, the fetal nurse will arrange for evaluation with the provider when abnormal data arises. Anticipation is key to the nursing role.
Areas for Future Research
More research in pharmacokinetic modeling for drug effect as well as for ion channel effects are needed. Randomized controlled clinical trials like the FAST trial, and StopBloq trial are important for defining proper treatment. In addition, drug usage registries should be actively funded by the National Institutes of Health and the FDA to accurately assess the clinical effects of antiarrhythmic drug treatment on both mother and fetus, and should include data during the breastfeeding period.
Electrophysiologic modeling of the rhythm and conduction has reached an advanced state for adults; however, such modeling during pregnancy, and also in the fetus, where different proteins and enzymes exist, is badly needed. The fetus exists in a buoyant environment, which can impact QTc, as well as a hypoxemic environment, which can trigger arrhythmias more readily. Fetuses are exposed to drugs through transplacental transfer and through intra‐amniotic accumulation and reabsorption that alter cardiac time intervals. 73 Having better pharmacologic and electrophysiologic modeling to predict what effects these may have can benefit the clinician.
Finally, as outlined by Kogutt and Satin 41 in a recent article, research innovations in obstetrics lag far behind those of other medical disciplines with regard to funding, industry investment, patents, and companies producing products. This could be addressed by establishing a fetal drug and device consortium, funded by the National Institutes of Health or FDA to promote research and the translation of promising device technologies and drugs, including future gene therapies, for the human fetus in need of treatment. This coordination effort could substantially reduce the delays in innovative fetal treatments, as has already been experienced for pediatric patients through current FDA drug and device initiatives.
In summary, the needs of the fetus should be identified, and a multidisciplinary effort should be made to address these needs as quickly as possible, through policy changes that facilitate fetal research, technological advances, and more careful monitoring of drug and device treatments for the fetus.
Conflicts of Interest
J.S. receives salary support from National Institutes of Health RO1HL143485 and RO1HL063174. P.N. is employed by Baxter International, Inc. The remaining authors declare no conflicts of interest.
Funding Information
This manuscript contains data supported by research grants from the National Institutes of Health National Heart Lung and Blood Institute, RO1 HL143485 and RO1 HL063174, and from the Dr. Scholl Foundation, Chicago, Il.
Acknowledgments
The authors thank Ronald T. Wakai, director of the Biomagnetism Laboratory, University of Wisconsin–Madison for his long‐term collaboration, including the use of his laboratory.
Data Availability Statement
Data for this manuscript involves vulnerable groups (fetuses, infants), and as such is not openly shared on public databases. Requests for data will be reviewed individually. Research teams can addressed requests to Janette Strasburger, MD (Children's Wisconsin, Herma Heart Institute, MS 713, 9000 W. Wisconsin Ave, Milwaukee, WI 53226).
References
- 1. Strand S, Lutter W, Strasburger JF, Shah V, Baffa O, Wakai RT. Low‐cost fetal magnetocardiography: a comparison of SQUID and optically‐pumped magnetometers. J Am Heart. 2019;8(16):e013436. 10.1161/JAHA.119.013436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miyoshi T, Maeno Y, Hamasaki T, et al. Antenatal therapy for fetal supraventricular tachyarrhythmias: multicenter trial. J Am Coll Cardiol. 2019;74:874‐885. [DOI] [PubMed] [Google Scholar]
- 3. Kazma JM, van den Anker J, Allegaert K, Dallmann A, Ahmadzia HK. Anatomical and physiological alterations of pregnancy. J Pharmacokinet Pharmacodyn. 2020;47:271‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Parilla B, Strasburger J, Socol M. Fetal supraventricular tachycardia complicated by hydrops fetalis: a role for direct fetal intramuscular therapy. Am J Perinatol. 1996;13:483‐486. [DOI] [PubMed] [Google Scholar]
- 5. Bourget P, Pons JC, Delouis C, Fermont L, Frydman R. Flecainide distribution, transplacental passage, and accumulation in the amniotic fluid during the third trimester of pregnancy. Ann Pharmacother. 1994;28:1031‐1034. [DOI] [PubMed] [Google Scholar]
- 6. Oudijk MA, Ruskamp JM, Ververs FF, et al. Treatment of fetal tachycardia with sotalol: transplacental pharmacokinetics and pharmacodynamics. J Am Coll Cardiol. 2003;42:765‐770. [DOI] [PubMed] [Google Scholar]
- 7. Menon R. Human fetal membranes at term: dead tissue or signalers of parturition? Placenta. 2016;44:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lingman G, S O, P O. Intrauterine digoxin treatment of fetal paroxysmal tachycardia. Br J Obstet Gynecol. 1980;1980:340‐342. [DOI] [PubMed] [Google Scholar]
- 9. Spinnato J, Shaver D, Flinn GS, Sibai BM, Watson D, Marin‐Garcia J. Fetal supraventricular tachycardia: in utero therapy with digoxin and quinidine. Obstet Gynecol. 1984;64:730‐735. [PubMed] [Google Scholar]
- 10. Weiner C, Thompson M. Direct treatment of fetal supraventricular tachycardia after failed transplacental therapy. Am J Obstet Gynecol. 1988;158:570‐573. [DOI] [PubMed] [Google Scholar]
- 11. Meijboom E, van Engelen A, van de Beek E, Weijtens O, Lautenschutz J, Benatar A. Fetal arrhythmias. Curr Opin Cardiol. 1994;9:97‐102. [DOI] [PubMed] [Google Scholar]
- 12. Van Engelen A, Weijtens O, Brenner J, et al. Management outcome and follow‐up of fetal tachycardia. J Am Coll Cardiol. 1994;24:1371‐1375. [DOI] [PubMed] [Google Scholar]
- 13. Gembruch U, Hansmann M, Redel DA, Bald R. Intrauterine therapy of fetal tachyarrhythmias: intraperitoneal administration of antiarrhythmic drugs to the fetus in fetal tachyarrhythmias with severe hydrops fetalis. J Perinat Med. 1988;16:39‐44. [DOI] [PubMed] [Google Scholar]
- 14. Murad SH, Tabsh KM, Conklin KA, et al. Verapamil: placental transfer and effects on maternal and fetal hemodynamics and atrioventricular conduction in the pregnant ewe. Anesthesiology. 1985;62:49‐53. [PubMed] [Google Scholar]
- 15. Wladimiroff J, Stewart P. Treatment of fetal cardiac arrhythmias. Br J Hosp Med. 1985;34:134‐140. [PubMed] [Google Scholar]
- 16. Hallak M, Neerhof MG, Perry R, Nazir M, Huhta JC. Fetal supraventricular tachycardia and hydrops fetalis: combined intensive, direct, and transplacental therapy. Obstet Gynecol. 1991;78:523‐525. [PubMed] [Google Scholar]
- 17. Arnoux P, Seyral P, Llurens M, et al. Amiodarone and digoxin for refractory fetal tachycardia. Am J Cardiol. 1987;59:166‐167. [DOI] [PubMed] [Google Scholar]
- 18. Wren C, Hunter S. Maternal administration of flecainide to terminate and suppress fetal tachycardia. Br Med J Clin Res Ed. 1988;296:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Allan L, Chita S, Sharland G, Maxwell D, Priestley K. Flecainide in the treatment of fetal tachycardias. Br Heart J. 1991;65:46‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaeggi E, Fouron JC, Drblik SP. Fetal atrial flutter: diagnosis, clinical features, treatment, and outcome. J Pediatr. 1998;132:335‐339. [DOI] [PubMed] [Google Scholar]
- 21. Sonesson SE, Fouron JC, Wesslen‐Eriksson E, Jaeggi E, Winberg P. Foetal supraventricular tachycardia treated with sotalol. Acta Paediatr. 1998;87:584‐587. [DOI] [PubMed] [Google Scholar]
- 22. Oudijk MA, Michon MM, Kleinman CS, et al. Sotalol in the treatment of fetal dysrhythmias. Circulation. 2000;101:2721‐2726. [DOI] [PubMed] [Google Scholar]
- 23. Oudijk MA, Ruskamp JM, Ambachtsheer BE, et al. Drug treatment of fetal tachycardias. Paediatr Drugs. 2002;4:49‐63. [DOI] [PubMed] [Google Scholar]
- 24. Jouannic JM, Delahaye S, Fermont L et al. Fetal supraventricular tachycardia: a role for amiodarone as second‐line therapy? Prenat Diagn. 2003;23:152‐156. [DOI] [PubMed] [Google Scholar]
- 25. Strasburger JF, Cuneo BF, Michon MM, et al. Amiodarone therapy for drug‐refractory fetal tachycardia. Circulation. 2004;109:375‐379. [DOI] [PubMed] [Google Scholar]
- 26. Cuneo BF. Treatment of fetal tachycardia. Heart Rhythm. 2008;5:1216‐1218. [DOI] [PubMed] [Google Scholar]
- 27. Maeno Y, Hirose A, Kanbe T, Hori D. Fetal arrhythmia: prenatal diagnosis and perinatal management. J Obstet Gynaecol Res. 2009;35:623‐629. [DOI] [PubMed] [Google Scholar]
- 28. Strasburger JF. Predictability in fetal supraventricular tachycardia management. J Am Coll Cardiol. 2019;74:886‐888. [DOI] [PubMed] [Google Scholar]
- 29. Jaeggi E. Prospective Randomized Clinical Trial of Fetal Atrial Flutter & Supraventricular Tachycardia Therapy (FAST RCT). 2016‐2023.
- 30. Purkayastha S, Weinreich M, Fontes JD, Lau JF, Wolfe DS, Bortnick AE. Fetal supraventricular tachycardia: what the adult cardiologist needs to know. Cardiol Rev. 2022;30:31‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michel M, Renaud C, Chiu‐Man C, Gross G, Jaeggi E. Postnatal recurrence and transesophageal inducibility of prenatally treated fetal supraventricular tachycardia. Heart Rhythm J. 2022;19(8):1343‐1349. 10.1016/j.hrthm.2022.04.013 [DOI] [PubMed] [Google Scholar]
- 32. Wakai RT, Strasburger JF, Li Z, Deal BJ, Gotteiner NL. Magnetocardiographic rhythm patterns at initiation and termination of fetal supraventricular tachycardia. Circulation. 2003;107:307‐312. [DOI] [PubMed] [Google Scholar]
- 33. Donofrio MT, Moon‐Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183‐2242. [DOI] [PubMed] [Google Scholar]
- 34. Trotter A, Kaestner M, Pohlandt F, Lang D. Unusual electrocardiogram findings in a preterm infant after fetal tachycardia with hydrops fetalis treated with flecainide. Pediatr Cardiol. 2000;21:259‐262. [DOI] [PubMed] [Google Scholar]
- 35. Pinsky WW, Jacobsen JR, Gillette PC, Adams J, Monroe L, McNamara DG. Dosage of digoxin in premature infants. J Pediatr. 1979;94:639‐642. [DOI] [PubMed] [Google Scholar]
- 36. Vari D, Tadeo D, Kurek N, et al. Transesophageal pacing studies reduce readmission but prolong initial admission in infants with supraventricular tachycardia. Heart Rhythm J. 2022;19:PO‐674‐02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strasburger JF. The transition from fetal to neonatal supraventricular tachycardia: What is the role of transesophageal atrial pacing? Heart Rhythm. 2022;19(8):1350‐1351. 10.1016/j.hrthm.2022.05.017 [DOI] [PubMed] [Google Scholar]
- 38. Bonney WJ, Killen S, Johns JA, Fish FA, Kannankeril PJ. Postnatal transesophageal pacing for infants with fetal supraventricular tachycardia. Heart Rhythm J. 2010;7:PO4‐157. [Google Scholar]
- 39. Kannenkeril PJ, Gotteiner NL, Deal BJ, Johnsrude CL, Strasburger JF. Location of accessory connection in infants presenting with supraventriculartachycardia in utero: clinical correlations. Am J Perinatol. 2003;20(3):115‐9. 10.1055/s-2003-40014 [DOI] [PubMed] [Google Scholar]
- 40. Ko JK, Deal BJ, Strasburger JF, Benson DWJ. Supraventricular tachycardia mechanisms and their age distribution in pediatric patients. Am J Cardiol. 1992;69:1028‐1032. [DOI] [PubMed] [Google Scholar]
- 41. Kogutt BK, Satin AJ. Obstetric innovation. Am J Obstet Gynecol. 2020;223:592‐595e1. [DOI] [PubMed] [Google Scholar]
- 42. Hall CM, Ward Platt MP. Neonatal flecainide toxicity following supraventricular tachycardia treatment. Ann Pharmacother. 2003;37:1343‐1344. [DOI] [PubMed] [Google Scholar]
- 43. Rasheed A, Simpson J, Rosenthal E. Neonatal ECG changes caused by supratherapeutic flecainide following treatment for fetal supraventricular tachycardia. Heart. 2003;89:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Crotti L, Tester DJ, White WM, et al. Long QT syndrome‐associated mutations in intrauterine fetal death. JAMA. 2013;309:1473‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Norstrand DW, Tester DJ, Ackerman MJ. Overrepresentation of the proarrhythmic, sudden death predisposing sodium channel polymorphism S1103Y in a population‐based cohort of African‐American sudden infant death syndrome. Heart Rhythm. 2008;5:712‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tester DJ, Ackerman MJ. The molecular autopsy: should the evaluation continue after the funeral? Pediatr Cardiol. 2012;33:461‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cuneo BF, Etheridge SP, Horigome H, et al. Arrhythmia phenotype during fetal life suggests LQTS genotype: risk stratification of perinatal long QT syndrome. Circ Arrhythm Electrophysiol. 2013;6(5):946‐51. 10.1161/CIRCEP.113.000618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tester DJ, Wong LCH, Chanana P, et al. Cardiac genetic predisposition in sudden infant death syndrome. J Am Coll Cardiol. 2018;71:1217‐1227. [DOI] [PubMed] [Google Scholar]
- 49. Cuneo BF, Kaizer AM, Ann Clur S, et al. Mothers with long QT syndrome are at increased risk for fetal death: Findings from a multicenter international study. Am J Obstet Gynecol. 2020;222(3):263.e1‐263.e11. 10.1016/j.ajog.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 50. ACOG ACoOaG . ACOG Practice Bulletin No. 106: Intrapartum fetal heart rate monitoring: nomenclature, interpretation, and general management principles. Obstet Gynecol. 2009;114:192‐202. [DOI] [PubMed] [Google Scholar]
- 51. Wacker‐Gussmann A, Plankl C, Sewald M, Schneider KM, Oberhoffer R, Lobmaier SM. Fetal cardiac time intervals in healthy pregnancies ‐ an observational study by fetal ECG (Monica Healthcare System). J Perinat Med. 2018;46:587‐592. [DOI] [PubMed] [Google Scholar]
- 52. van Leeuwen P, Schiermeier S, Lange S, et al. Gender‐related changes in magnetocardiographically determined fetal cardiac time intervals in intrauterine growth retardation. Pediatr Res. 2006;59:820‐824. [DOI] [PubMed] [Google Scholar]
- 53. Stinstra J, Golbach E, van Leeuwen P, et al. Multicentre study of fetal cardiac time intervals using magnetocardiography. BJOG: Int J Obstet Gynaecol. 2002;109:1235‐1243. [DOI] [PubMed] [Google Scholar]
- 54. Serra V, Bellver J, Moulden M, Redman CW. Computerized analysis of normal fetal heart rate pattern throughout gestation. Ultrasound Obstet Gynecol. 2009;34:74‐79. [DOI] [PubMed] [Google Scholar]
- 55. Mitchell JL, Cuneo BF, Etheridge SP, Horigome H, Weng HY, Benson DW. Fetal heart rate predictors of long QT syndrome. Circulation. 2012;126:2688‐2695. [DOI] [PubMed] [Google Scholar]
- 56. Cuneo B, Buyon JP. Surveillance and treatment to prevent fetal atrioventricular block likely to occur quickly (STOP BLOQ). https://stopbloq.org/. Accessed July 2022
- 57. Zhao H, Cuneo BF, Strasburger JF, Huhta JC, Gotteiner NL, Wakai RT. Electrophysiological characteristics of fetal atrioventricular block. J Am Coll Cardiol. 2008;51:77‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strasburger JF. Prenatal diagnosis of fetal arrhythmias. Clin Perinatol. 2005;32:891‐912, viiii. [DOI] [PubMed] [Google Scholar]
- 59. Strasburger JF, Cheulkar B, Wichman HJ. Perinatal arrhythmias: diagnosis and management. Clin Perinatol, 2007;34:627‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saito M, Silverman E, Golding F, et al. Effects of transplacental dexamethasone therapy on fetal immune‐mediated complete heart block. Fetal Diagn Ther. 2021;48:183‐188. [DOI] [PubMed] [Google Scholar]
- 61. Wacker‐Gussmann A, Strasburger JF, Cuneo BF, Wakai RT. Diagnosis and treatment of fetal arrhythmia. Am J Perinatol. 2014;31(7):617‐28. 10.1055/s-0034-1372430. Epub 2014 May 23.PMID: 24858320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wierzejska R, Jarosz M, Kleminska‐Nowak M, et al. Maternal and cord blood vitamin D status and anthropometric measurements in term newborns at Birth. Front Endocrinol (Lausanne). 2018;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ambrosi A, Salomonsson S, Eliasson H, et al. Development of heart block in children of SSA/SSB‐autoantibody‐positive women is associated with maternal age and displays a season‐of‐birth pattern. Ann Rheum Dis. 2012;71:334‐340. [DOI] [PubMed] [Google Scholar]
- 64. Cox JT, Phelan ST. Prenatal nutrition: special considerations. Minerva Ginecol. 2009;61:373‐400. [PubMed] [Google Scholar]
- 65. Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf). 2009;70(5):685‐90. 10.1111/j.1365-2265.2008.03403.x [DOI] [PubMed] [Google Scholar]
- 66. Cutolo M, Otsa K. Review: vitamin D, immunity and lupus. Lupus. 2008;17:6‐10. [DOI] [PubMed] [Google Scholar]
- 67. Wacker‐Gussmann A, Strasburger JF, Wakai RT. Contribution of fetal magnetocardiography to diagnosis, risk assessment, and treatment of fetal arrhythmia. J Am Heart Assoc. 2022;11(15):e025224. 10.1161/JAHA.121.025224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schwartz PJ, Stramba‐Badiale M, Segantini A, et al. Prolongation of the QT interval and the sudden infant death syndrome. N Engl J Med. 1998;338:1709‐1714. [DOI] [PubMed] [Google Scholar]
- 69. Schwartz PJ. Stillbirths, sudden infant deaths, and long‐QT syndrome: puzzle or mosaic, the pieces of the Jigsaw are being fitted together. Circulation. 2004;109:2930‐2932. [DOI] [PubMed] [Google Scholar]
- 70. Bolin EH, Whittington JR, Mehl ST, Escalona-Vargas D, Eswaran H. Fetal magnetocardiography for the diagnosis of fetal dysrhythmias: single-center experience over 8 years [published online ahead of print July 19, 2022]. JACC Clin Electrophysiol. 10.1016/j.jacep.2022.05.011 [DOI] [PubMed] [Google Scholar]
- 71. Strand S, Strasburger JF, Cuneo BF, Wakai RT. Complex and novel arrhythmias precede stillbirth in fetuses with de novo long QT syndrome. Circ Arrhythm Electrophysiol. 2020;13:e008082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sinnecker D, Laugwitz KL, Moretti A. Induced pluripotent stem cell‐derived cardiomyocytes for drug development and toxicity testing. Pharmacol Ther. 2014;143:246‐252. [DOI] [PubMed] [Google Scholar]
- 73. Trotter A, Kaestner M, Pohlandt F, Lang D. Unusual electrocardiogram findings in a preterm infant after fetal tachycardia with hydrops fetalis treated with flecanide. Pediatr Cardiol. 2000;21:259‐262. [DOI] [PubMed] [Google Scholar]
- 74. Gozar L, Gabor‐Miklosi D, Toganel R, et al. Fetal tachyarrhythmia management from digoxin to amiodarone–a review. J Clin Med. 2022;11(3):804. 10.3390/jcm11030804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van der Heijden LB, Oudijk MA, Manten GT, ter Heide H, Pistorius L, Freund MW. Sotalol as first‐line treatment for fetal tachycardia and neonatal follow‐up. Ultrasound Obstet Gynecol. 2013;42:285‐293. [DOI] [PubMed] [Google Scholar]
- 76. Shah A, Moon‐Grady A, Bhogal N, et al. Effectiveness of sotalol as first‐line therapy for fetal supraventricular tachyarrhythmias. Am J Cardiol. 2012;109:1614‐1618. [DOI] [PubMed] [Google Scholar]
- 77. Sanatani S, Potts JE, Reed JH, et al. The study of antiarrhythmic medications in infancy (SAMIS): a multicenter, randomized controlled trial comparing the efficacy and safety of digoxin versus propranolol for prophylaxis of supraventricular tachycardia in infants. Circ Arrhythm Electrophysiol. 2012;5:984‐991. [DOI] [PubMed] [Google Scholar]
- 78. Rasiah SV, Ewer AK, Miller P, Kilby MD. Prenatal diagnosis, management and outcome of fetal dysrhythmia: a tertiary fetal medicine centre experience over an eight‐year period. Fetal Diagn Ther. 2011;30:122‐127. [DOI] [PubMed] [Google Scholar]
- 79. Jaeggi ET, Carvalho JS, De Groot E, et al. Comparison of transplacental treatment of fetal supraventricular tachyarrhythmias with digoxin, flecainide, and sotalol: results of a nonrandomized multicenter study. Circulation. 2011;124:1747‐1754. [DOI] [PubMed] [Google Scholar]
- 80. Jaeggi ET, Nii M. Fetal brady‐ and tachyarrhythmias: new and accepted diagnostic and treatment methods. Semin Fetal Neonatal Med. 2005;10:504‐514. [DOI] [PubMed] [Google Scholar]
- 81. Krapp M, Kohl T, Simpson JM, Sharland GK, Katalinic A, Gembruch U. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart. 2003;89:913‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Merson N. Adenosine treatment of supraventricular tachycardia following epidural test dose: a case study. AANA J. 1993;61:521‐523. [PubMed] [Google Scholar]
- 83. Moore JP, Gallotti RG, Shannon KM, et al. Genotype Predicts outcomes in fetuses and neonates with severe congenital long QT syndrome. JACC Clin Electrophysiol. 2020;6:1561‐1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Trucco SM, Jaeggi E, Cuneo B, et al. Use of intravenous gamma globulin and corticosteroids in the treatment of maternal autoantibody‐mediated cardiomyopathy. J Am Coll Cardiol. 2011;57:715‐723. [DOI] [PubMed] [Google Scholar]
- 85. Vaksmann G, Lucidarme S, Henriet E. Fetal ventricular tachycardia: betablockers should be the first line treatment. J Gynecol Obstet Hum Reprod. 2021;50:101946. [DOI] [PubMed] [Google Scholar]
- 86. Takatsuka H, Wakabayashi K, Yamazaki S, et al. Transition of maternal serum concentration of digoxin and flecainide in the third trimester‐A case report of fetal supraventricular tachycardia with hydrops. Clin Case Rep. 2021;9:e03992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cuneo BF, Strasburger JF, Yu S, et al. In utero diagnosis of long QT syndrome by magnetocardiography. Circulation. 2013;128:2183‐2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Horigome H, Nagashima M, Sumitomo N, et al. Clinical characteristics and genetic background of congenital long‐QT syndrome diagnosed in fetal, neonatal, and infantile life: a nationwide questionnaire survey in Japan. Circ Arrhythm Electrophysiol. 2010;3:10‐17. [DOI] [PubMed] [Google Scholar]
- 89. Simpson JM, Maxwell D, Rosenthal E, Gill H. Fetal ventricular tachycardia secondary to long QT syndrome treated with maternal intravenous magnesium: case report and review of the literature. Ultrasound Obstet Gynecol. 2009;34:475‐480. [DOI] [PubMed] [Google Scholar]
- 90. Oudijk MA, Gooskens RH, Stoutenbeek P, De Vries LS, Visser GH, Meijboom EJ. Neurological outcome of children who were treated for fetal tachycardia complicated by hydrops. Ultrasound Obstet Gynecol. 2004;24:154‐158. [DOI] [PubMed] [Google Scholar]
- 91. Perry JC. Ventricular tachycardia in neonates. Pacing Clin Electrophysiol. 1997;20:2061‐2064. [DOI] [PubMed] [Google Scholar]
- 92. Strasburger JF, Wakai RT. Fetal cardiac arrhythmia detection and in utero therapy. Nat Rev Cardiol. 2010;7:277‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti‐Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody‐exposed fetuses and infants. J Am Coll Cardiol. 2010;55:2778‐2784. [DOI] [PubMed] [Google Scholar]
- 94. Mawad W, Hornberger L, Cuneo B, et al. Outcome of antibody‐mediated fetal heart disease with standardized anti‐inflammatory transplacental treatment. J Am Heart Assoc. 2022;11(3):e023000. 10.1161/JAHA.121.023000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Strasburger JF, Wacker‐Gussmann A. Congenital heart block in subsequent pregnancies of SSA/Ro‐positive mothers: cutting recurrence in half. J Am Coll Cardiol. 2020;76:303‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cuneo BF, Strasburger JF, Zhao H, Huhta JC, Gotteiner NL, Wakai RT. Electrophysiologic patterns of fetal heart rate augmentation with terbutaline in complete AV block. Heart Rhythm J. 2005;2:S45. [Google Scholar]
- 97. Eliasson H, Sonesson S‐E, Sharland G, et al. Isolated atrioventricular block in the fetus /clinical perspective. Circulation. 2011;124:1919‐1926. [DOI] [PubMed] [Google Scholar]
- 98. Joglar JA, Kapa S, Saarel EV. et al. HRS Expert Consensus statement on the management of arrhythmias during pregnancy. Heart Rhythm. 2022. In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this manuscript involves vulnerable groups (fetuses, infants), and as such is not openly shared on public databases. Requests for data will be reviewed individually. Research teams can addressed requests to Janette Strasburger, MD (Children's Wisconsin, Herma Heart Institute, MS 713, 9000 W. Wisconsin Ave, Milwaukee, WI 53226).


