Abstract
Background and purpose
Venous thromboembolic events (VTEs) are a major complication in cancer patients, and therefore, also in brain cancer patients, anticoagulants are considered appropriate in the treatment of VTEs.
Methods
Frequency, risk factors, and treatment of VTEs, as well as associated complications, were assessed in a population‐based cohort of glioblastoma patients in the Canton of Zurich, Switzerland. Correlations between clinical data and survival were retrospectively analyzed using the log‐rank test and Cox regression models.
Results
Four hundred fourteen glioblastoma patients with isocitrate dehydrogenase wild‐type status were identified. VTEs were documented in 65 patients (15.7%). Median time from tumor diagnosis to the occurrence of a VTE was 1.8 months, and 27 patients were diagnosed with VTEs postoperatively (within 35 days; 42.2%). History of a prior VTE was more common in patients who developed VTEs than in those who did not (p = 0.004). Bevacizumab treatment at any time during the disease course was not associated with occurrence of VTEs (p = 0.593). Most patients with VTEs (n = 61, 93.8%) were treated with therapeutic anticoagulation. Complications occurred in 14 patients (23.0%), mainly intracranial hemorrhages (n = 7, 11.5%). Overall survival did not differ between patients diagnosed with VTEs and those who had no VTE (p = 0.139). Tumor progression was the major cause of death (n = 283, 90.7%), and only three patients (1.0%) died in association with acute VTEs.
Conclusions
Venous thromboembolic events occurred early in the disease course, suggesting that the implementation of primary venous thromboembolism prophylaxis during first‐line chemoradiotherapy could be explored in a randomized setting.
Keywords: anticoagulation, bleeding, epidemiology, glioblastoma, VTE
Venous thromboembolic events (VTEs) are a major complication in cancer patients. Frequency, risk factors, and treatment of VTEs, as well as associated complications, were assessed in a population‐based cohort of 414 glioblastoma patients in the Canton of Zurich, Switzerland. VTEs occurred early in the disease course, suggesting that the implementation of primary venous thromboembolism prophylaxis during first‐line chemoradiotherapy could be explored in a randomized setting.

INTRODUCTION
Patients diagnosed with primary brain tumors have a high risk for venous thromboembolic events (VTEs), reported in a range of 14%–32% [1, 2, 3, 4, 5, 6]. The incidence of VTEs is high throughout the course of disease [2], but seems to peak in the first 6 months after diagnosis [7]. Recurrent VTEs may be seen in one third of patients diagnosed with glioblastoma [3].
Risk factors for VTEs in brain tumor patients include immobility, higher age, obesity, comorbidities, history of VTEs, subtotal tumor resection, glioblastoma histology, and recurrent disease [8, 9]. Bevacizumab appears not to increase the risk of VTEs in glioblastoma patients [8, 10, 11, 12]. The association of blood group with VTEs in glioma patients has been discussed controversially. Whereas B blood group was found to be predictive for VTEs in a cohort of 139 glioblastoma patients [13], this was not confirmed in a prospective study of patients diagnosed with glioma, including glioblastoma [7]. Elevated factor VIII levels [7] and low platelets or high leukocytes [14] have been reported to increase the risk of VTEs in glioblastoma patients. Moreover, podoplanin expression by primary brain tumors induces platelet aggregation and is associated with increased risk of VTEs [15].
Recommendations for prophylaxis and treatment of VTEs in patients diagnosed with primary brain tumors are based largely on extrapolation from patients with other tumors and consensus [8, 16]. Primary venous thromboembolism prophylaxis in the ambulatory setting is not recommended but should be considered for hospitalized patients [17]. Low‐molecular‐weight heparin (LMWH) should be chosen as the first‐line venous thromboembolism prophylaxis and initiated within 24 h after brain surgery [8, 18]. A single prospective randomized study (PRODIGE trial) on primary prophylaxis with LMWH for VTEs in patients with primary brain tumors, including glioblastoma, noticed a trend toward reduced VTEs, but the trial was stopped prematurely [19]. Prophylactic anticoagulant use is associated with increased risk of bleeding in glioblastoma patients [19, 20, 21].
Therapeutic doses of LMWH are recommended for the treatment of VTEs. Direct oral anticoagulants (DOACs) should not be routinely used in glioblastoma patients, given the lack of data from clinical trials in this population. The duration of therapeutic anticoagulation for treatment of VTEs should be at least 6 months.
In an analysis of three randomized trials including patients with newly diagnosed glioblastoma, therapeutic anticoagulation initiated between the start of concomitant temozolomide with radiotherapy and the start of maintenance temozolomide therapy was associated with inferior survival [22]. It is unclear whether VTEs in these patients contributed to inferior survival. No association with survival was observed in patients treated with prophylactic doses of anticoagulants or with antiplatelet agents [22].
VTEs are associated with inferior survival in cancer patients [23]. It has remained unclear whether there is an association between VTEs and survival in glioblastoma patients. Anticoagulants have been speculated to improve survival in cancer patients, not only by preventing VTEs, but also because of direct anticancer effects [24, 25, 26, 27, 28]. Data from two Cochrane reviews on cancer patients do not suggest a mortality benefit from oral or parenteral anticoagulation, respectively, and the risk for bleeding is likely increased [29, 30].
The association of VTEs, anticoagulant use, and its complications with survival in glioblastoma patients on a population‐based level remains unclear. Here, we performed a retrospective study of VTEs in 414 patients with isocitrate dehydrogenase (IDH) wild‐type glioblastoma, who were diagnosed over a 10‐year time frame in the Canton of Zurich, Switzerland.
PATIENTS AND METHODS
Patient identification
All patients, 18 years or older, who resided in the Canton of Zurich, Switzerland, and were diagnosed with glioblastoma between 2005 and 2014, were included in a glioblastoma cancer registry in the Canton of Zurich. Patient identification data were provided by the Cancer Registry of the Cantons of Zurich and Zug. Epidemiological data on this patient cohort were published previously [31, 32]. For the present analysis, we excluded all patients who lacked molecular data on IDH mutation status based on the 2021 World Health Organization (WHO) classification [33].
Disease characteristics
All tumors in the glioblastoma cancer registry had been classified according to the WHO 2007 criteria [34] in the local pathology departments, and in a second step were classified by IDH mutation status based on the WHO 2021 classification [33]. The O6‐methylguanine DNA methyltransferase (MGMT) promoter methylation status was determined by methylation‐specific polymerase chain reaction. IDH mutation status was assessed using IDH1‐R132H immunohistochemistry. According to WHO 2021 criteria, IDH1‐R132H‐negative glioblastomas in patients 55 years or older at diagnosis (n = 296) could be regarded as IDH wild‐type tumors without any additional sequencing [33]. In cases with available tissue (n = 66), IDH1‐R132H‐negative tumors in patients younger than 55 years were subjected to IDH1 and IDH2 sequence analyses. In 52 patients younger than 55 years, the IDH mutation status was obtained by immunohistochemistry only. Extent of resection was determined by early postoperative magnetic resonance imaging (MRI) or, if no MRI was available, by cranial computed tomography (CT). Macroscopic (gross) total resection was defined by the absence of contrast enhancement. Clinical and treatment data were extracted from medical records. History of cancer was defined by any cancer diagnosis, independent of histology or WHO grade. A history of intracranial bleeding or stroke was defined by any listed event in a medical report prior to the diagnosis of glioblastoma.
Statistical analyses
Demographic, clinical, molecular tumor marker and comedication data were analyzed by descriptive statistics. The chi‐squared test was performed for analysis of nominal variables, and the Mann–Whitney U test was used for the comparison of quantitative variables between groups. Overall survival (OS) was calculated from primary surgery for glioblastoma to death or last follow‐up. Patients were censored at last follow‐up. Kaplan–Meier curves were used to estimate OS, and differences were analyzed using the log‐rank test. Cox proportional hazards regression models were used for multivariate analyses to test the association of clinical and molecular markers, including the occurrence of VTEs, with survival. A time‐dependent term of VTEs considering the time from tumor diagnosis to VTE was included where indicated. All statistical analyses were performed using SPSS version 27 (IBM), and a p‐value of 0.05 was set as statistically significant.
Ethics
This study was approved by the ethics committee of the Canton of Zurich (KEK‐ZH‐Nr. 2009–0135/1; KEK‐ZH‐Nr. 2015–0437).
RESULTS
Patient characteristics
A total of 414 patients diagnosed with IDH wild‐type glioblastoma in the Canton of Zurich, Switzerland, were included in this study. Median follow‐up of surviving patients was 10.1 months (95% confidence interval [CI] = 5.2–13.2), assessed from the time of diagnosis. Patient characteristics are summarized in Table 1. Venous thromboembolic events were documented in 65 patients (15.7%) during the course of disease. In the cohort of patients diagnosed with VTEs (VTE cohort), 45 patients (69.2%) were male, compared to 216 male patients (61.9%) in the cohort of glioblastoma patients who were not diagnosed with VTEs (p = 0.260). Karnofsky performance status (KPS), extent of resection, MGMT promoter methylation status, and first‐line treatment did not differ between these two cohorts. Exposure to bevacizumab at any time was not associated with an increased risk of VTEs. History of intracranial hemorrhage or cerebrovascular stroke was similar in both groups, whereas history of VTEs was seen more often in the VTE cohort (n = 6, 9.2%) than in the control cohort (n = 8, 2.3%, p = 0.004; Table 1).
TABLE 1.
Patient characteristics at baseline
| Characteristic | Non‐VTE cohort, n = 349, 84.3% | VTE cohort, n = 65, 15.7% | p |
|---|---|---|---|
| Age, years a | |||
| Median | 63.0 | 59.7 | 0.065 |
| Range | 18–90 | 37–83 | |
| Sex, n | |||
| Male | 216 (61.9%) | 45 (69.2%) | 0.260 |
| Female | 133 (38.1%) | 20 (30.8%) | |
| KPS, n a | |||
| 90%–100% | 54 (15.6%) | 6 (9.2%) | 0.299 |
| 70%–80% | 203 (58.7%) | 44 (67.7%) | |
| <70% | 89 (25.7%) | 15 (23.1%) | |
| No data | 3 (−) | – | |
| Extent of surgical resection, n a | |||
| Gross total resection, ≥99% | 114 (32.8%) | 31 (47.7%) | 0.125 |
| Incomplete resection, <99% | 155 (44.5%) | 21 (32.3%) | |
| Biopsy | 78 (22.4%) | 13 (20.0%) | |
| Autopsy | 1 (0.3%) | – | |
| No data | 1 (−) | – | |
| MGMT promoter methylation status, n | |||
| Methylated | 111 (45.7%) | 16 (34.8%) | 0.172 |
| Unmethylated | 132 (54.3%) | 30 (65.2%) | |
| No data | 106 (−) | 19 (−) | |
| First‐line therapy, n | |||
| Radiotherapy plus TMZ | 170 (49.7%) | 38 (58.5%) | 0.336 |
| Radiotherapy alone | 68 (19.9%) | 13 (20.0%) | |
| Chemotherapy alone | 26 (7.6%) | 1 (1.5%) | |
| Others b | 22 (6.4%) | 5 (7.7%) | |
| No therapy | 56 (16.4%) | 8 (12.3%) | |
| No data | 7 (−) | – | |
| Bevacizumab at any time during the disease, n | |||
| Yes | 123 (36.5%) | 26 (40.0%) | 0.593 |
| No | 214 (63.5%) | 39 (60.0%) | |
| No data | 12 (−) | – | |
| History of cancer, n | |||
| Yes | 52 (15.0%) | 8 (12.3%) | 0.574 |
| No | 295 (85.0%) | 57 (87.7%) | |
| No data | 2 (−) | – | |
| History of intracranial bleeding, n | |||
| Yes | 6 (1.7%) | 0 (0%) | 0.287 |
| No | 343 (98.3%) | 65 (100%) | |
| History of cerebrovascular stroke, n | |||
| Yes | 9 (2.6%) | 2 (3.1%) | 0.819 |
| No | 340 (97.4%) | 63 (96.9%) | |
| History of VTE, n | |||
| Yes | 8 (2.3%) | 6 (9.2%) | 0.004 |
| No | 341 (97.7%) | 59 (90.8%) | |
| Analyzed time frame | |||
| 2005–2009 | 141 (40.4%) | 30 (46.2%) | 0.387 |
| 2010–2014 | 208 (59.6%) | 35 (53.8%) | |
Abbreviations: KPS, Karnofsky performance status; MGMT, O6‐methylguanine DNA methyltransferase; n, number of patients; TMZ, temozolomide; VTE, venous thromboembolic event.
At time of diagnosis.
Mainly experimental drugs in clinical trials or bevacizumab.
Characterization of the VTE cohort
Patient characteristics at time of VTE diagnosis are summarized in Table 2. Median time from tumor diagnosis to a VTE was 1.8 months. At the time of VTE diagnosis, patients commonly had reduced performance status. Most patients were on steroids (n = 39, 60.9%) and were overweight, with a body mass index (BMI) > 25 kg/m2 (n = 28, 63.6%). The majority of VTEs were observed within 35 days after initial surgery (n = 27, 42.2%). During first‐line treatment, an additional 18 patients (28.1%) were diagnosed with VTEs. A swimmer plot depicting the occurrence of VTEs for each patient of the VTE cohort over the time of the course of disease is shown in Figure 1. In patients experiencing VTEs later in the course of disease, essentially all occurred during phases of tumor‐specific treatment rather than in treatment‐free intervals.
TABLE 2.
Patient characteristics at time of VTE
| VTE cohort, n = 65 | n (%) |
|---|---|
| Type of VTE | |
| Deep vein thrombosis | 26 (40.6%) |
| Pulmonary embolism | 29 (45.3%) |
| Sinus vein thrombosis | 2 (3.1%) |
| Deep vein thrombosis plus pulmonary embolism | 7 (10.9%) |
| No data | 1 (−) |
| KPS | |
| 90%–100% | 10 (16.1%) |
| 70%–80% | 17 (27.4%) |
| <70% | 35 (56.5%) |
| No data | 3 (−) |
| Steroids | |
| Yes | 39 (60.9%) |
| No | 25 (39.1%) |
| No data | 1 (−) |
| BMI | |
| Underweight, <18.5 kg/m2 | 1 (2.3%) |
| Normal weight, ≥18.5–24.9 kg/m2 | 15 (34.1%) |
| Overweight, ≥25–29.9 kg/m2 | 11 (25.0%) |
| Obese, ≥30 kg/m2 | 17 (38.6%) |
| No data | 21 (−) |
| Platelets | |
| Normal level | 47 (78.3%) |
| Decreased level, CTCAE Grade ≥ 1 | 13 (21.7%) |
| No data | 5 (−) |
| Tumor status | |
| Prior to first recurrence | 48 (75.0%) |
| After first recurrence | 16 (25.0%) |
| No data | 1 (−) |
| Disease phase | |
| Postoperatively, ≤35 days | 27 (42.2%) |
| First‐line treatment | 18 (28.1%) |
| Second‐line treatment | 7 (10.9%) |
| Third‐line treatment | 5 (7.8%) |
| No tumor‐specific treatment, >35 days | 7 (10.9%) |
| No data | 1 (−) |
| Cotreatments | |
| Radiotherapy, concomitant TMZ | 9 (14.1%) |
| Maintenance TMZ | 5 (7.8%) |
| Chemotherapy alone | 2 (3.1%) |
| Radiotherapy alone | 3 (4.7%) |
| Immune checkpoint inhibitor alone | 1 (1.6%) |
| Bevacizumab alone | 7 (10.9%) |
| Radiotherapy plus bevacizumab | 2 (3.1%) |
| Chemotherapy plus bevacizumab | 1 (1.6%) |
| No treatment | 34 (53.1%) |
| No data | 1 (−) |
| Antiplatelet treatment/anticoagulation at the time of VTE | |
| Antiplatelet treatment | 3 (4.9%) |
| Prophylactic anticoagulation | 10 (16.4%) |
| Therapeutic anticoagulation | 0 (0.0%) |
| Antiplatelet treatment plus prophylactic anticoagulation | 2 (3.3%) |
| No treatment | 46 (75.4%) |
| No data | 4 (−) |
| Treatment of VTE | |
| Prophylactic anticoagulation | 2 (3.1%) |
| LMWH | 2 (3.1%) |
| Therapeutic anticoagulation | 61 (93.8%) |
| LMWH | 29 (47.5%) a |
| DOAC | 4 (6.6%) a |
| Vitamin K antagonist | 21 (34.4%) a |
| Unfractionated heparin | 2 (3.3%) a |
| Vena cava filter/vitamin K antagonist | 5 (8.2%) a |
| No treatment | 2 (3.1%) [died immediately of VTE] |
| Complications from therapeutic anticoagulation for VTE | |
| Intracranial hemorrhage | 7 (11.5%) |
| CTCAE Grade 2 | 3 (4.9%) |
| CTCAE Grade 3 | 4 (6.6%) |
| Subdural hematoma, CTCAE Grade 2 | 2 (3.3%) |
| Oral hemorrhage, CTCAE Grade 1 | 1 (1.6%) |
| Anal bleeding | 2 (3.3%) |
| CTCAE Grade 2 | 1 (1.6%) |
| CTCAE Grade 3 | 1 (1.6%) |
| Epistaxis, CTCAE Grade 2 | 1 (1.6%) |
| Gastrointestinal hemorrhage, CTCAE Grade 3 | 1 (1.6%) |
| No complication | 47 (77.0%) |
| Complications from what kind of therapeutic anticoagulation for VTE (percentage of n = 14 patients with complications, or percentage of the number of patients under the respective treatment) | |
| LMWH, n = 19 | 6 (42.9%, 20.7%) |
| DOAC, n = 4 | 2 (14.3%, 50.0%) |
| Vitamin K antagonist, n = 21 | 4 (28.6%, 19.0%) |
| Unfractionated heparin, n = 2 | 1 (7.1%, 50.0%) |
| Vena cava filter/vitamin K antagonist, n = 5 | 1 (7.1%, 20.0%) |
| Time from tumor diagnosis to VTE | |
| Months, median (SD; range) | 1.8 (13.3; 0.0–78.0) |
| Duration of anticoagulation after VTE | |
| Months, median (SD; range) | |
| All VTE patients with anticoagulation, n = 60 | 4.6 (8.4; 0.1–58.4) |
| Patients who had anticoagulation until death, n = 29 | 2.1 (4.0; 0.1–16.9) |
| Patients who stopped anticoagulation for other reasons, n = 31 | 6.4 (10.6; 0.7–58.4) |
| Reason for stopping anticoagulation after VTE (percentage of n = 61 patients with anticoagulation) | |
| Death | 29 (47.5%) |
| Complication | 10 (16.4%) |
| Decision of the physician | 14 (22.9%) |
| Unknown | 8 (13.1%) |
| Recurrent VTE under anticoagulation (percentage of n = 61 patients with anticoagulation) | |
| Failure rate | 2 (3.3%) |
| Median time to treatment failure, months (95% CI) | 12.4 (12.3–12.6) |
Abbreviations: BMI, body mass index; CI, confidence interval; CTCAE, Common Terminology Criteria for Adverse Events; DOAC, direct oral anticoagulant; KPE, Karnofsky performance status; LMWH, low‐molecular‐weight heparin; n, number of patients; TMZ, temozolomide; VTE, venous thromboembolic event.
Percentages calculated for the group of patients who received therapeutic anticoagulation (n = 61).
FIGURE 1.
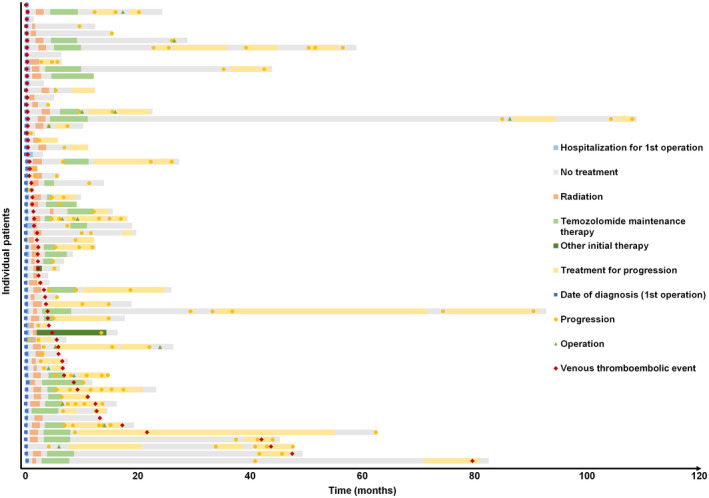
Swimmer plot depicting the occurrence of venous thromboembolic events for each patient. Each bar of the y‐axis represents one individual patient of the venous thromboembolic events (VTEs) cohort (n = 65). The x‐axis represents time in months since hospitalization for initial surgery. Red diamonds represent the VTEs. Blue squares represent the date of first operation. Yellow dots represent dates of tumor progression. Green triangles represent any further operation other than the initial surgery. Bars represent the duration of hospitalization for the first operation (blue), treatment‐free periods (gray), duration of initial radiotherapy plus/minus concomitant temozolomide (orange), duration of maintenance temozolomide (bright green), duration of initial therapy other than temozolomide (dark green), and duration of any other treatment at time of progression (yellow)
Perioperative prophylaxis for VTEs could be confirmed in 32 patients (82.1%), whereas seven patients (17.9%) had no documented prophylaxis in the medical records; data were missing for 26 patients. Bevacizumab comedication at time of VTE diagnosis was noted in 10 patients (15.6%). Approximately half of the patients diagnosed with VTEs had pulmonary embolism (n = 36, 56.3%). Of the patients with pulmonary embolism, seven patients (10.9%) were diagnosed with concomitant deep vein thrombosis. At the time of VTE diagnosis, 53 patients (82.8%) were symptomatic, and 45 patients (70.3%) reported their symptoms to the clinician. In 11 patients (17.2%), the clinician first noted the symptoms leading to the VTE diagnosis, whereas the patients themselves were not aware of them (Table S1). All seven patients diagnosed with both pulmonary embolism and deep vein thrombosis had a CT scan of the chest, as well as an ultrasound examination or a CT venography of the legs. Two patients were diagnosed with pulmonary embolism based exclusively on their clinical symptoms and died immediately. Patients with isolated deep vein thrombosis, who had no desaturation or chest pain, did not undergo a chest CT scan (Table S1).
At the time of VTE diagnosis, 12 patients (19.7%) were on prophylactic anticoagulation (Table 2). Almost all patients (n = 61, 93.8%) received therapeutic anticoagulation for the treatment of VTEs. Only two patients were given prophylactic anticoagulation (3.1%). Another two patients died immediately from VTEs (3.1%) before anticoagulation could be initiated. Therapeutic anticoagulation was achieved mostly with LMWH (n = 29, 47.5%), followed by vitamin K antagonists (n = 26, 42.6%). The latter were combined with inferior vena cava filters in five patients (8.2%), mostly to postpone full anticoagulation for a few days in freshly operated patients. Median time of therapeutic anticoagulation was 4.6 months for all patients with implemented anticoagulation after VTEs (n = 60). Twenty‐nine patients underwent therapeutic anticoagulation until death, with a median duration of therapeutic anticoagulation of 2.1 months. The remaining 31 patients (n = 1 patient with missing data on duration of anticoagulation) received therapeutic anticoagulation for a median of 6.4 months (Table 2).
Fourteen of 61 patients (23.0%) who received therapeutic anticoagulation suffered from complications (Table 2), including intracranial hemorrhage (n = 7, 11.5%; n = 5 symptomatic), subdural hematoma (n = 2, 3.3%), anal bleeding (n = 2, 3.3%), and oral hemorrhage (n = 1.6%). One of these 14 patients died due to intracranial bleeding. Therapeutic anticoagulation had to be stopped in 10 patients, whereas three patients were able to continue the treatment. Of the 14 patients with complications under therapeutic anticoagulation, six patients (42.9%) were on LMWH, five patients (35.7%) were on vitamin K antagonists (combined with a vena cava filter in one patient), two patients were on a DOAC, and one patient was on unfractionated heparin (Table 2). In most patients (n = 60), the VTEs had no impact on the treatment schedule (92.3%), whereas in five patients (76.9%), chemotherapy or bevacizumab, respectively, were delayed or stopped.
Four patients in the VTE cohort had a second VTE. Two patients were on therapeutic anticoagulation at the time of the second VTE, with a median time to the occurrence of the second VTE of 6.4 months. Two other patients were not anticoagulated (median time between the VTEs was 12.4 months).
Survival data
Median OS for the whole cohort was 12.1 months (95% CI = 11.0–13.3). Median OS for patients diagnosed with VTEs was 14.2 months (95% CI = 10.8–17.5) compared to 11.0 months (95% CI = 10.7–13.1) for patients without VTEs (Table S2). Kaplan–Meier survival curves stratified according to the diagnosis of VTEs are shown in Figure 2. Survival in patients diagnosed with VTEs compared to those patients who were not was similar (p = 0.139; Table S2, Figure 2).
FIGURE 2.

Venous thromboembolic events and survival. Kaplan–Meier survival curves are shown for glioblastoma patients diagnosed with (blue line) or without (red line) venous thromboembolic events (VTE). The log‐rank test was used for comparison
Most patients died of tumor progression (90.7%; Table 3). In patients in an end‐of‐life setting with a main focus on palliative and supportive care, death as a result of neurological decline over time was assumed to be because of tumor progression. Sudden and unexpected death was observed in five patients (1.6%). Of these five patients, one patient had a VTE approximately 2 months before his death and was treated with a vena cava filter. Another four patients (1.3%) died of intracranial hemorrhage. One of these patients was on prophylactic anticoagulation at the time of death, and none was on therapeutic anticoagulation. One patient, who was on prophylactic anticoagulation, died of extensive bleeding in the gluteal muscle. Sixteen patients (5.1%) died from other causes, mainly systemic infections (n = 8, 50%), and there were two lethal accidents (12.5%). In patients who had VTEs during the course of disease, three patients (6.4%) died in association with acute VTEs, presumably cardiopulmonary failure, based on the clinical presentation (Table 3).
TABLE 3.
Cause of death
| Cause of death | All patients, n = 414 | Non‐VTE cohort, n = 349 | VTE cohort, n = 65 |
|---|---|---|---|
| Tumor progression | 283 (90.7%) | 246 (92.8%) | 37 (78.7%) |
| Bleeding | 5 (1.6%) | 4 (1.5%) | 1 (2.1%) |
| Intracranial | 4 (1.3%) | 3 (1.1%) | 1 (2.1%) |
| Gluteal muscle | 1 (0.3%) | 1 (0.4%) | 0 |
| Thromboembolic event, assumed cardiopulmonary failure | 3 (1.0%) | 0 | 3 (6.4%) |
| Sudden unexpected death of unknown cause | 5 (1.6%) | 4 (1.5%) | 1 (2.1%) |
| Other a | 16 (5.1%) | 11 (6.7%) | 5 (10.6%) |
| Alive/lost to follow‐up | 51 (−) | 41 (−) | 10 (−) |
| No data | 51 (−) | 43 (−) | 8 (−) |
Abbreviation: VTE, venous thromboembolic event.
Accident (n = 2), suicide (n = 1), gastrointestinal disease (n = 1), hyperglycemia (n = 1), infection (n = 8), intraoperative complication (n = 1), liver disease (n = 1), myocardial infarction (n = 1).
VTEs were not associated with risk of death in univariable Cox regression analysis (p = 0.14, hazard ratio [HR] = 0.80, 95% CI = 0.60–1.07). Multivariable Cox regression analysis confirmed known prognostic or predictive markers in glioblastoma, including age, KPS, extent of resection, MGMT promoter methylation status, and first‐line treatment. In contrast, the diagnosis of a VTE at any time during the course of disease again was not associated with increased risk for death (HR = 0.86, 95% CI = 0.60–1.25, p = 0.435; Table 4). In addition, we included a time‐dependent covariable for the time of tumor diagnosis to time of VTE in this Cox regression for risk of death (p = 0.007, HR = 16.4, 95% CI = 2.15–124.81); however, it was confirmed that VTEs were not associated with risk of death in this adjusted model (p = 0.12, HR = 0.79, 95% CI = 0.59–1.06).
TABLE 4.
Multivariate analysis with regard to death (Cox regression)
| Characteristic | n | HR (95% CI) | p |
|---|---|---|---|
| Age a | |||
| >65 years | 120 | 1 | Ref |
| ≤65 years | 163 | 0.64 (0.47–0.88) | 0.006 |
| Sex | |||
| Male | 185 | 1 | Ref |
| Female | 98 | 1.16 (0.88–1.53) | 0.296 |
| KPS a | |||
| <70% | 68 | 2.00 (1.44–2.78) | <0.001 |
| 70%–80% | 171 | 1 | Ref |
| 90%–100% | 44 | 0.64 (0.43–0.94) | 0.022 |
| Extent of resection a | |||
| Biopsy | 49 | 1 | Ref |
| Incomplete | 123 | 0.43 (0.29–0.62) | <0.001 |
| Gross total, ≥99% | 111 | 0.27 (0.18–0.40) | <0.001 |
| MGMT promoter methylation status | |||
| Unmethylated | 161 | 1 | Ref |
| Methylated | 122 | 0.57 (0.43–0.75) | <0.001 |
| Postsurgical therapy | |||
| No therapy | 34 | 1 | Ref |
| RT alone | 52 | 0.40 (0.24–0.64) | <0.001 |
| CT alone | 23 | 0.30 (0.16–0.55) | <0.001 |
| RT plus TMZ | 156 | 0.19 (0.12–0.30) | <0.001 |
| Others b | 18 | 0.16 (0.85–0.31) | <0.001 |
| VTE during the course of disease | |||
| Yes | 46 | 0.86 (0.60–1.25) | 0.435 |
| No | 237 | 1 | Ref |
Abbreviations: CI, confidence interval; CT, chemotherapy; HR, hazard ratio; KPS, Karnofsky performance status; MGMT, O6‐methylguanine DNA methyltransferase; Ref, reference; RT, radiotherapy; TMZ, temozolomide; VTE, venous thromboembolic event.
At time of diagnosis.
Mainly experimental drugs in clinical trials, or bevacizumab.
DISCUSSION
This study was performed to explore the incidence of VTEs, a major complication in cancer patients, and the association with survival in glioblastoma patients on a population level. In the present IDH wild‐type glioblastoma cohort, 65 patients (15.7%) were diagnosed with VTEs during the course of disease (Table 1). Several reports, mainly single‐center studies, describe an incidence of VTEs between 14.5% and 32.3% in glioma cohorts [3, 4, 35, 36] (Table 5). Differences in the incidence of VTE in these studies can probably be explained by the retrospective nature and the lack of standardized screening for VTEs, especially in asymptomatic patients. Higher VTE incidences, compared to glioblastoma patients, were reported in other cohorts, including patients with WHO Grade 2 and 3 astrocytoma [35, 36]. The concept of an increased risk for VTE in IDH‐mutant gliomas remains controversial. Whereas Diaz and colleagues postulated an increased risk for VTEs in patients with IDH‐mutant tumors [35], this finding was not confirmed in a subsequent study [36]. Unruh and colleagues described a higher frequency of intratumoral microthrombi in IDH1/2 wild‐type tumors, independent of histological grade [6]. The authors suggested that mutant IDH1/2 has potent antithrombotic activity within gliomas as well as in the peripheral circulation, probably because mutant IDH1/2 initiates F3 promoter hypermethylation, resulting in a relative lack of tissue factor‐containing microparticles [37]. In addition, the IDH1/2 metabolite D‐2‐hydroxyglutarate has been discussed as having antiplatelet activity based on calcium‐dependent inhibition of human platelet aggregation and clotting.
TABLE 5.
Review of the literature (selected papers)
| Study | Prospective/retrospective study | Single‐/multicenter study | Included patients (n) | Screening | Follow‐up, months | Patients diagnosed with VTE, n (%) | Treatment, n (%) |
|---|---|---|---|---|---|---|---|
| Our study, 2022 | Retrospective | Multicenter (Canton of Zurich, Switzerland) | IDH wild‐type glioblastoma (414) | VTE during the course of disease | 10.1 | 65 (15.7%) |
Prophylactic AC, LMWH, 2 (3.1%) Therapeutic AC LMWH, 29 (47.5%) DOAC, 4 (6.6%) Vitamin K antagonist, 21 (34.4%) Unfractionated heparin, 2 (3.3%) IVC filter/vitamin K antagonist, 5 (8.2%) None, 2 (3.1%) |
| Mandel et al., 2021 | Retrospective | Single center (MD Anderson, Texas) | Astrocytoma [IDH mutant or IDH wild‐type status] (282) | VTE during the course of disease | – |
All tumors: 52 (18.4%) All grades, IDH wild‐type: 45 (19.3%) All grades, IDH mutant: 7 (14.3%) |
Vitamin K antagonist, 4 (7.7%) IVC filter/vitamin K antagonist, 2 (3.8%) IVC filter alone, 9 (17.3%) IVC filter/DOAC, 1 (1.9%) LMWH, 29 (55.8%) IVC filter/LMWH, 3 (5.8%) DOAC, 2 (3.8%) Other, 2 (3.8%) |
| Diaz et al., 2020 | Prospectively collected data; retrospectively analyzed | Single center (University of Virginia) |
Glioma WHO Grade 2 (147) WHO Grade 3 (109) WHO Grade 4 (334) |
VTE during the course of disease | 17.9 |
WHO Grade 2: 12 (8.2%) WHO Grade 3: 10 (9.2%) WHO Grade 4: 103 (30.8%) All grades, IDH wild‐type: 102 (26.5%) All grades IDH mutant: 18 (8.7%) |
– |
| Le Rhun et al., 2018 | Prospective studies; retrospectively analyzed | Multicenter | Glioblastoma [IDH mutant or IDH wild‐type status] (1273) | Anticoagulant exposure at time from (i) randomization to start combined radiochemotherapy or (ii) from combined radiochemotherapy to start of temozolomide maintenance | – |
(i) 1 of 1273 (0.1%) (ii) 22 of 1017 (2.2%) |
New VTEs except 1 were treated with therapeutic AC |
| Edwin et al., 2016 | Retrospective | Single center (Cleveland) | Glioblastoma (450) | VTE during the course of disease | Minimum of 6 | 145 (32.2%) |
IVC filter alone, 39 (26.9%) IVC filter with AC, 21 (14.5%) AC alone, 54 (37.2%) LMWH, 36 (24.8%) Warfarin, 15 (10.3%) Heparin, 2 (1.4%) DOAC, 1 (0.7%) None, 31 (21.4%) |
| Yust‐Katz et al., 2015 | Retrospective | Single center (MD Anderson) | Glioblastoma (440) | VTE after starting adjuvant chemotherapy | Minimum of 6 | 64 (14.5%) |
AC alone, 36 Coumadin, 8 LMWH, 28 IVC filter, 2 IVC/AC, 21 Unknown, 3 None, 2 |
Abbreviations: AC, anticoagulation; DOAC, direct oral anticoagulants; IDH, isocitrate dehydrogenase; IVC, inferior vena cava; LMWH, low‐molecular‐weight heparin; VTE, venous thromboembolic event; WHO, World Health Organization.
Our data indicate that the risk of VTEs is already high early in the disease course, with a median time to VTE of 1.8 months (Table 2). In our cohort, many patients were diagnosed with VTEs in the first 35 days after neurosurgical intervention. This is in line with the recommendation that patients with cancer, including patients diagnosed with brain tumor and brain metastasis, should receive perioperative VTE prophylaxis [16]. In addition, a large retrospective analysis confirmed craniotomy for brain tumors to be an independent risk factor for VTEs compared to craniotomy for other reasons, including ischemic stroke or bleedings [38]. Among brain tumor patients, the risk of VTEs in patients diagnosed with glioblastoma is reportedly higher than the risk in those diagnosed with meningioma [39]. As a mechanism, a systemic procoagulative state has been reported more frequently in glioblastoma patients compared to meningioma patients [40]. Additionally, in our dataset, the phase of initial treatment appears to place patients at risk, suggesting that prophylactic anticoagulation might be useful specifically during the early disease trajectory.
Conversely, it seems counterintuitive to assume that the risk of VTEs decreases during the course of disease, because several risk factors such as steroid use, immobility, possible increase of BMI, and tumor burden should be more prevalent in later disease stages. Thus, it is also conceivable that the apparently lower rate of VTEs in later disease stages reflects less stringent follow‐up or follow‐up at less experienced sites.
In our study, history of a VTE was significantly associated with the incidence of VTEs after diagnosis of glioblastoma (Table 1). Patients who developed VTEs were characterized by increased BMI (≥25 kg/m2, 63.6%), reduced KPS (<70%, 56.5%), and comedication with steroids (60.9%; Table 2), which are known risk factors for VTEs in brain tumor patients [8, 9]. Our study does not allow for drawing conclusions regarding causality of risk factors for VTEs. Although we found decreased platelet levels at the time of VTE diagnosis in 21.7% of our patients, it remains unclear whether this represents an independent risk factor for VTEs in glioblastoma patients. A negative association of VTEs and platelet count at the time of diagnosis in primary brain tumors has been described [14].
Bevacizumab appears not to be associated with an increased risk of VTEs in randomized clinical trials on glioblastoma patients [8, 10, 11, 12]. On a population‐based level, we confirm that bevacizumab treatment at any time during the course of disease was not associated with VTEs (Table 1).
Although VTEs have been linked to inferior survival in cancer patients in general [23], such a negative association between VTEs in glioblastoma patients and survival was not seen in our cohort (Table S2, Table 3, Figure 2). We even noticed a trend toward superior survival in glioblastoma patients with VTEs, almost all of which received full anticoagulation. As patients in our study were diagnosed between 2005 and 2014 and the first DOAC (rivaroxaban) for the treatment of VTEs was approved in Switzerland in 2012, these anticoagulants are underrepresented for VTE treatment in our cohort. A recent study showed a satisfactory safety profile of DOACs compared to LMWH in glioblastoma patients with pulmonary embolism, and the advantages of avoiding injections, less need of monitoring, and fewer interactions nowadays might favor their use in clinical practice [41]. The complication rate of all patients receiving DOACs for VTE treatment was high in our study (50%; Table 2), but due to the very limited number of patients in this cohort, comparisons with the complication rate under LMWH or vitamin K antagonists cannot be made. The complication rate among patients receiving LMWH (20.7%) or vitamin K antagonists (19.0%) were similar.
These population‐based data appear to be at odds with observations on the significance of VTEs in clinical trial populations. No association with survival was observed in patients treated with prophylactic doses of anticoagulants or with antiplatelet agents in a retrospective analysis of three randomized clinical trials in glioblastoma patients [22]. In this dataset, therapeutic anticoagulation initiated between the start of concomitant temozolomide with radiotherapy and maintenance temozolomide was associated with inferior survival. Although three patients (6.4%) with VTEs in our study died in association with an acute thromboembolic event, VTEs were not a major cause of death (Table 3).
The main limitations of this study include the retrospective nature of data collection, including missing data on steroid administration over time and treatment intensity, as well as the lack of data on IDH mutation status by sequencing in some patients younger than 55 years, and on MGMT promoter methylation status. Based on the retrospective nature of this study, the prevalence of VTEs, notably in later disease stages, as well as the prevalence of prescription or intensity of VTE prophylaxis, may be underestimated.
Although VTEs were identified in 15.7% of glioblastoma patients on this population‐based level, VTEs were not associated with death. Therefore, our data do not support the implementation of primary venous thromboembolism prophylaxis in glioblastoma patients without further data from controlled studies. However, VTEs occurred early in the course of disease, suggesting that the implementation of primary venous thromboembolism prophylaxis using a safe and well‐tolerated agent could be explored during first‐line chemoradiotherapy in a randomized setting.
AUTHOR CONTRIBUTIONS
Amanda Eisele: Conceptualization (equal); data curation (lead); formal analysis (lead); investigation (lead); project administration (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead). Katharina Seystahl: Data curation (equal); formal analysis (equal); funding acquisition (lead); investigation (equal); methodology (lead); validation (equal); writing – review and editing (equal). Elisabeth Rushing: Data curation (equal); investigation (equal); project administration (equal); validation (equal); writing – review and editing (equal). Patrick Roth: Data curation (equal); investigation (equal); project administration (equal); validation (equal); writing – review and editing (equal). Emilie Le Rhun: Conceptualization (lead); data curation (equal); investigation (equal); project administration (equal); validation (equal); writing – review and editing (equal). Michael Weller: Conceptualization (lead); funding acquisition (lead); investigation (lead); project administration (lead); supervision (lead); writing – original draft (lead); writing – review and editing (lead). Dorothee Gramatzki: Conceptualization (lead); data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); validation (lead); visualization (lead); writing – original draft (lead); writing – review and editing (lead).
CONFLICT OF INTEREST
K.S. has received honoraria for board participation from Roche. E.L.R. has received personal fees from AbbVie, Adastra, Bayer, LEO Pharma, Seagen, and Tocagen. P.R. has received honoraria for lectures or advisory board participation from Bristol Myers Squibb, Covagen, Debiopharm, Merck Sharp & Dohme, Novocure, QED, Roche, and Virometix and research support from Merck Sharp & Dohme and Novocure. M.W. has received research grants from Abbvie, Adastra, Apogenix, Merck Sharp & Dohme, Merck (EMD), Novocure, and Quercis, and honoraria for lectures, advisory board participation, or consulting from Abbvie, Adastra, Bristol Myers Squibb, Celgene, Medac, Merck Sharp & Dohme, Merck (EMD), Nerviano Medical Sciences, Novartis, Orbus, Philogen, Roche, Tocagen, and yMabs. All remaining authors declare that they have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
The authors would like to thank the patients and their families for making this study possible and acknowledge the contributions of the multidisciplinary teams taking care of glioblastoma patients in the Canton of Zurich, Switzerland. Open access funding provided by Universitat Zurich.
Eisele A, Seystahl K, Rushing EJ, et al.. Venous thromboembolic events in glioblastoma patients: An epidemiological study. Eur J Neurol. 2022;29:2386‐2397. doi: 10.1111/ene.15404
Funding information
This work was supported by a grant from Krebsliga Zurich to M.W. and D.G. (KLS‐3110‐02‐2013); a grant from the Betty and David Koetser Foundation for Brain Research of the University of Zurich, Switzerland (F‐86001‐60‐01); a personal grant (“Filling the Gap”) of the University of Zurich, Switzerland to D.G.; and a personal grant (“Filling the Gap”) of the University of Zurich, Switzerland and of the Walter und Gertrud Siegenthaler Foundation to K.S
DATA AVAILABILITY STATEMENT
Coded data not provided in the article will be made available upon request of other qualified investigators for purposes of replicating results.
REFERENCES
- 1. Muster V, Gary T. Incidence, Therapy, and bleeding risk‐cancer‐ associated thrombosis in patients with glioblastoma. Cancers (Basel). 2020;12:1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma: an evidence‐based review. Cancer. 2000;89:640‐646. [DOI] [PubMed] [Google Scholar]
- 3. Edwin NC, Khoury MN, Sohal D, McCrae KR, Ahluwalia MS, Khorana AA. Recurrent venous thromboembolism in glioblastoma. Thromb Res. 2016;137:184‐188. [DOI] [PubMed] [Google Scholar]
- 4. Yust‐Katz S, Mandel JJ, Wu J, et al. Venous thromboembolism (VTE) and glioblastoma. J Neurooncol. 2015;124:87‐94. [DOI] [PubMed] [Google Scholar]
- 5. Taillibert S, Taillandier L, Le Rhun E. Venous thrombosis in patients with high‐grade glioma. Curr Opin Oncol. 2015;27:516‐521. [DOI] [PubMed] [Google Scholar]
- 6. Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016;132:917‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Streiff MB, Ye X, Kickler TS, et al. A prospective multicenter study of venous thromboembolism in patients with newly‐diagnosed high‐grade glioma: hazard rate and risk factors. J Neurooncol. 2015;124:299‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roth P, Pace A, Le Rhun E, et al. Neurological and vascular complications of primary and secondary brain tumours: EANO‐ESMO clinical practice guidelines for prophylaxis, diagnosis, treatment and follow‐up. Ann Oncol. 2021;32:171‐182. [DOI] [PubMed] [Google Scholar]
- 9. Jo JT, Schiff D, Perry JR. Thrombosis in brain tumors. Semin Thromb Hemost. 2014;40:325‐331. [DOI] [PubMed] [Google Scholar]
- 10. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy‐temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709‐722. [DOI] [PubMed] [Google Scholar]
- 11. Gilbert MR, Sulman EP, Mehta MP. Bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:2048‐2049. [DOI] [PubMed] [Google Scholar]
- 12. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377:1954‐1963. [DOI] [PubMed] [Google Scholar]
- 13. Heenkenda MK, Malmstrom A, Lysiak M, et al. Assessment of genetic and non‐genetic risk factors for venous thromboembolism in glioblastoma ‐ the predictive significance of B blood group. Thromb Res. 2019;183:136‐142. [DOI] [PubMed] [Google Scholar]
- 14. Thaler J, Ay C, Kaider A, et al. Biomarkers predictive of venous thromboembolism in patients with newly diagnosed high‐grade gliomas. Neuro Oncol. 2014;16:1645‐1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riedl J, Preusser M, Nazari PM, et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129:1831‐1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38:496‐520. [DOI] [PubMed] [Google Scholar]
- 17. Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: american society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agnelli G, Piovella F, Buoncristiani P, et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med. 1998;339:80‐85. [DOI] [PubMed] [Google Scholar]
- 19. Perry JR, Julian JA, Laperriere NJ, et al. PRODIGE: a randomized placebo‐controlled trial of dalteparin low‐molecular‐weight heparin thromboprophylaxis in patients with newly diagnosed malignant glioma. J Thromb Haemost. 2010;8:1959‐1965. [DOI] [PubMed] [Google Scholar]
- 20. Perry SL, Bohlin C, Reardon DA, et al. Tinzaparin prophylaxis against venous thromboembolic complications in brain tumor patients. J Neurooncol. 2009;95:129‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robins HI, O'Neill A, Gilbert M, et al. Effect of dalteparin and radiation on survival and thromboembolic events in glioblastoma multiforme: a phase II ECOG trial. Cancer Chemother Pharmacol. 2008;62:227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Rhun E, Genbrugge E, Stupp R, et al. Associations of anticoagulant use with outcome in newly diagnosed glioblastoma. Eur J Cancer. 2018;101:95‐104. [DOI] [PubMed] [Google Scholar]
- 23. Kuderer NM, Ortel TL, Francis CW. Impact of venous thromboembolism and anticoagulation on cancer and cancer survival. J Clin Oncol. 2009;27:4902‐4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mousa SA, Petersen LJ. Anti‐cancer properties of low‐molecular‐weight heparin: preclinical evidence. Thromb Haemost. 2009;102:258‐267. [DOI] [PubMed] [Google Scholar]
- 25. Mousa SA, Mohamed S. Inhibition of endothelial cell tube formation by the low molecular weight heparin, tinzaparin, is mediated by tissue factor pathway inhibitor. Thromb Haemost. 2004;92:627‐633. [DOI] [PubMed] [Google Scholar]
- 26. Collen A, Smorenburg SM, Peters E, et al. Unfractionated and low molecular weight heparin affect fibrin structure and angiogenesis in vitro. Cancer Res. 2000;60:6196‐6200. [PubMed] [Google Scholar]
- 27. Takeuchi A, Yamamoto Y, Munesue S, et al. Low molecular weight heparin suppresses receptor for advanced glycation end products‐mediated expression of malignant phenotype in human fibrosarcoma cells. Cancer Sci. 2013;104:740‐749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balzarotti M, Fontana F, Marras C, et al. In vitro study of low molecular weight heparin effect on cell growth and cell invasion in primary cell cultures of high‐grade gliomas. Oncol Res. 2006;16:245‐250. [DOI] [PubMed] [Google Scholar]
- 29. Kahale LA, Hakoum MB, Tsolakian IG, et al. Oral anticoagulation in people with cancer who have no therapeutic or prophylactic indication for anticoagulation. Cochrane Database Syst Rev. 2017;12:CD006466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Akl EA, Kahale LA, Hakoum MB, et al. Parenteral anticoagulation in ambulatory patients with cancer. Cochrane Database Syst Rev. 2017;9:Cd006652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gramatzki D, Roth P, Rushing E, et al. Bevacizumab may improve quality of life, but not overall survival in glioblastoma: an epidemiological study. Ann Oncol. 2018;29:1431‐1436. [DOI] [PubMed] [Google Scholar]
- 32. Gramatzki D, Dehler S, Rushing EJ, et al. Glioblastoma in the Canton of Zurich, Switzerland revisited: 2005 to 2009. Cancer. 2016;122:2206‐2215. [DOI] [PubMed] [Google Scholar]
- 33. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diaz M, Jo J, Smolkin M, Ratcliffe SJ, Schiff D. Risk of venous thromboembolism in grade II‐IV gliomas as a function of molecular subtype. Neurology. 2021;96:e1063‐e1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mandel JJ, Youssef M, Yust‐Katz S, et al. IDH mutation status and the development of venous thromboembolism in astrocytoma patients. J Neurol Sci. 2021;427:117538. [DOI] [PubMed] [Google Scholar]
- 37. Turcan S, Rohle D, Goenka A, et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kimmell KT, Jahromi BS. Clinical factors associated with venous thromboembolism risk in patients undergoing craniotomy. J Neurosurg. 2015;122:1004‐1011. [DOI] [PubMed] [Google Scholar]
- 39. Fluss R, Kobets AJ, Inocencio JF, et al. The incidence of venous thromboembolism following surgical resection of intracranial and intraspinal meningioma. A systematic review and retrospective study. Clin Neurol Neurosurg. 2021;201:106460. [DOI] [PubMed] [Google Scholar]
- 40. Yerrabothala S, Gourley BL, Ford JC, et al. Systemic coagulation is activated in patients with meningioma and glioblastoma. J Neurooncol. 2021;155:173‐180. [DOI] [PubMed] [Google Scholar]
- 41. Dubinski D, Won SY, Voss M, et al. Direct oral anticoagulants vs. low‐molecular‐weight heparin for pulmonary embolism in patients with glioblastoma. Neurosurg Rev. 2022;45:451‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Coded data not provided in the article will be made available upon request of other qualified investigators for purposes of replicating results.


