Abstract
Background
This qualitative study assessed the experience of patients with chronic rhinosinusitis with nasal polyposis (NP) to inform the development of a novel symptom diary for clinical study use.
Methods
Concept elicitation and cognitive interviews were conducted with patients who had a physician‐verified diagnosis of NP and a history of intranasal corticosteroid use. Concepts were identified via open‐ended and follow‐up questions. Relative symptom/impact disturbance level was assessed using a scale of 0 (not at all disturbing) to 10 (extremely disturbing).
Results
Patients (n = 30) attributed numerous symptoms and impacts to NP; the most prevalent and disturbing were nasal congestion (identified by 100% of patients; average disturbance rating = 7.9), nasal blockage/obstruction (97%; 8.2), difficulty with sense of smell (97%; 7.6), facial pressure (90%; 6.2), postnasal drip (87%; 6.5), runny nose (87%; 6.2), facial pain (80%; 6.3), and headache (77%; 6.5). These symptoms, along with the impact of NP on sleep and daily activities, were included in the Nasal Polyposis Symptom Diary (NPSD). Cognitive interviews confirmed that patients understood the NPSD items and could select a response reflective of their experience at its worst over the past 24 hours using a four‐point scale (none, mild, moderate, or severe).
Conclusion
The most relevant and disturbing symptoms, according to patients with NP, were included in the NPSD. Interviews confirmed the suitability of NPSD in capturing the daily experience of patients. These findings support the content validity of the NPSD as a suitable tool for capturing NP symptoms and impacts.
Keywords: chronic rhinosinusitis with nasal polyposis, content validity, instrument development, patient‐reported outcomes, qualitative research
1. INTRODUCTION
Chronic rhinosinusitis with nasal polyposis (hereafter NP) is a chronic inflammatory disease of the nasal mucosa characterized by persistent sinonasal symptoms and endoscopic signs of nasal polyps, mucopurulent discharge, or edema. 1 , 2 , 3 Patients with NP experience nasal blockage, obstruction, and congestion as well as other symptoms such as nasal discharge, facial pain/pressure, and impaired sense of smell that have a profound impact on patient functioning and health‐related quality of life (HRQoL). 4 , 5 , 6 , 7 Improving symptoms is an important treatment goal and consequently a key objective in clinical studies intended to evaluate new therapies for NP. 1 , 8 Well‐developed, fit‐for‐purpose, patient‐reported outcome (PRO) assessments are essential for capturing patient‐perceived symptom severity in clinical studies. 9 Selection of meaningful study endpoints and fit‐for‐purpose assessments are contingent on having a comprehensive understanding of the condition as perceived by patients. 10 Qualitative studies identifying relevant concepts of interest and defining aspects of those concepts to be measured are critical in PRO development. Furthermore, patient interviews serve as an important tool for evaluating PRO assessments to ensure that the content is comprehendible and capable of capturing patient experience with the concepts of interest. 11 , 12 , 13 , 14 These data are necessary to evaluate the content validity of the PRO assessment and are prerequisites to evaluations of its measurement properties. 11 , 12 , 13 , 14 , 15
The aim of this qualitative study was to understand the symptoms and impacts experienced by patients with NP to inform the development of a novel PRO instrument, the Nasal Polyposis Symptom Diary (NPSD).
2. MATERIALS AND METHODS
2.1. Literature review
A literature review was conducted to gather information on the most common symptoms and impacts experienced by patients with NP as well as to assess existing instruments used to evaluate the NP patient experience. The symptoms and impacts review was conducted through a PubMed search for articles published from January 1, 2007 to December 31, 2017. Prespecified search terms to identify NP‐specific concepts included “chronic rhinosinusitis with nasal polyps,” “nasal polyposis,” “signs,” and “symptoms.” The existing instrument review was conducted via searches of PubMed, Cochrane, and PsycINFO for articles published within the same time frame. Prespecified search terms to identify PRO instruments used for patients with NP included “chronic rhinosinusitis with nasal polyps,” “nasal polyposis,” “patient‐reported outcomes,” and “quality of life.” ClinicalTrials.gov and the Patient‐Reported Outcomes and Quality of Life Instruments Database (PROQOLID) 16 were also reviewed for further information on PROs previously used in NP clinical studies. The goal of the literature review was to inform the design of a preliminary conceptual model that organized and prioritized the most relevant symptoms and impacts experienced by patients with NP to support the development of a fit‐for‐purpose PRO assessment.
2.2. Patients
Patient recruitment was conducted in the United States (US) and the United Kingdom (UK). Patients were eligible for the study if they were aged ≥18 years; had a health care provider‐confirmed diagnosis of severe NP, severe NP and comorbid asthma, or NP with surgery to remove polyps but still experienced mild to moderate symptoms post‐surgery; and had experienced NP symptoms within the last 12 months. Severe NP was defined as bilateral sinonasal polyposis that, despite treatment with intranasal corticosteroids and a history of treatment with systemic corticosteroids (oral or parenteral) or prior surgery for NP, had severity consistent with a need for surgery as described by: (1) a minimum bilateral nasal polyp score (NPS) of 5 out of a maximum score of 8 (with a unilateral score of at least 2 for each nostril); (2) ongoing symptoms for at least 12 weeks; and (3) patient‐reported moderate to severe nasal blockage (score 2 or 3) on a scale of 0 = none; 1 = mild; 2 = moderate; and 3 = severe. Patients enrolled in a clinical study or with a diagnosis of antrochoanal polyps, nasal septal deviation that occludes at least one nostril, Churg‐Strauss syndrome, Young syndrome, Kartagener syndrome, rhinitis medicamentosa, or allergic fungal rhinosinusitis were excluded. All patients provided written informed consent prior to participation in the study. Additional details on the patient recruitment methodology are available in the Supplemental Material.
2.3. Patient interviews
Following the development of the preliminary conceptual model, combined concept elicitation (CE) and cognitive interviews were conducted to understand patient experience with NP and to evaluate the content of the NPSD. Interviews were conducted in accordance with the recommendations of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Good Research Practices Task Force. 13 , 14 Approval was obtained from Quorum Review Institutional Review Board for individual qualitative CE and cognitive interviews with adult patients with NP.
This study involved three interviewers and one interviewing supervisor, all of whom are experienced qualitative researchers with training in CE and cognitive interviewing techniques. Interviews were conducted via teleconference and followed a semistructured interview guide that was evaluated and standardized via mock interviewing sessions, during which the interviewers identified areas for improvement and standardized practices. Each interview started with open‐ended questions designed to capture descriptions of the patient experience in their own words and then moved to probes of key concepts of interest. CE topics in the interview guide included symptom characteristics (spontaneous and probed) and whether the patient felt the symptoms were related to NP or treatment; the level of disturbance of these symptoms on their lives; how symptoms had evolved over time; how symptoms impacted or affected their lives; and level of disturbance of these impacts on their lives. During each interview, patients rated the level of disturbance for each NP symptom or impact on a 0–10 numeric rating scale, ranging from 0 = “not disturbing at all” to 10 = “extremely disturbing.”
The cognitive interviews portion of the interviews captured patients’ comprehension of the NPSD content, including the instructions, instrument items, and response scales. For each item, patients were asked to describe the concept of interest (specific symptom or impact) in their own words, describe the meaning of the response options and their thought process while selecting a response for each item, and elaborate on any aspect of the NPSD that they found to be problematic or confusing.
2.4. Qualitative analyses interviews
Thematic analyses were used to identify concepts that were subsequently categorized into two broad categories: symptoms and impacts. Impacts were further categorized as either proximal or distal. Proximal impacts are hypothesized to be directly associated with symptoms, whereas distal impacts are considered to have a less direct causal relationship to symptoms and may be influenced by other external factors. 17
For all concepts, the number of patients who mentioned the concept (including total, spontaneous, and probed mentions) and average disturbance ratings were tabulated. Concepts that were mentioned by ≥50% of patients and had an average disturbance rating of ≥5 were considered salient. Concept saturation—the point at which successive interviews yield no new meaningful information—was conducted after each wave of interviews (out of six waves) and evaluated at the end of the study to determine when it was reached. Additional details on the methodology used for concept coding and concept saturation are available in the Supplemental Material.
3. RESULTS
3.1. Literature review
The literature review was conducted in January 2018. A total of 20 articles met the search inclusion criteria for the symptoms and impacts review (Table S1). The 20 articles covering symptoms and impacts of NP were examined to prioritize concepts that are potentially most salient for patients with NP. Literature describing important concepts from the patients’ perspectives through qualitative patient interview studies were prioritized during the search; however, no such articles were identified in the search results. Ten commonly reported symptoms and seven common impacts relevant to patients with NP were prioritized based on the results of the literature review (Table 1).
TABLE 1.
Results of literature review of commonly reported symptoms and impacts of NP
| Common symptoms | Common impacts |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Abbreviation: NP, chronic rhinosinusitis with nasal polyposis.
Articles including PRO instruments that could appropriately assess the prioritized concepts in the NP patient population and searches of ClinicalTrials.gov and PROQOLID databases generated a list of PRO instruments that included both generic instruments and those developed for various nasal conditions. No instruments were specifically developed for patients with NP. Therefore, a total of 12 PRO instruments developed for nasal conditions were selected for further analysis and mapped to the most relevant symptoms identified in the literature review (Figure 1).
FIGURE 1.
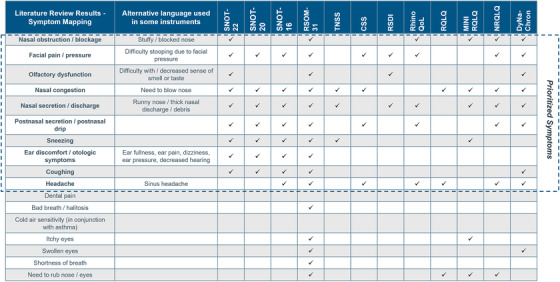
Symptom mapping of PROs from instruments identified in literature reviewa.
aConcepts with high reported prevalence and frequent mentions in the literature were prioritized for patients with NP.
Abbreviations: CSS, Chronic Sinusitis Survey; MINIRQLQ, Mini Rhinoconjunctivitis QoL Questionnaire; NP, chronic rhinosinusitis with nasal polyposis; NRQLQ, Nocturnal Rhinoconjunctivitis QoL Questionnaire; RhinoQoL, Rhinosinusitis QoL survey; RQLQ, Rhinoconjunctivitis QoL Questionnaire; PRO, patient‐reported outcome; QoL, quality of life; RSDI, Rhinosinusitis Disability Index; RSOM‐31, Rhinosinusitis Outcome Measure 31; SNOT‐16, 16‐item Sino‐Nasal Outcome Test; SNOT‐20, 20‐item Sino‐Nasal Outcome Test; SNOT‐22, 22‐item Sino‐Nasal Outcome Test; TNSS, Total Nasal Symptom Score.
3.2. Patients
A total of 30 patients (20 patients in the US and 10 patients in the UK) who had a variety of experiences with severe NP, severe NP with comorbid asthma, or surgery to remove nasal polyps followed by mild/moderate symptoms postsurgery participated in combined CE and cognitive interviews. Demographics and baseline characteristics were similar between patients in the US and the UK (Table 2). The 30 interviews conducted were split into six waves of five patients each to assess saturation. Group assignments for each wave were determined by the chronological order in which each interview was conducted. The number of new concepts appearing in each wave of interviews was used to assess saturation, with concept saturation calculated based on concepts identified in coded transcripts.
TABLE 2.
Baseline demographics and clinical features of patients in CE/cognitive interview
| Demographic or clinical variable | Patients (n = 30) |
|---|---|
| Age (years), mean (range) | 52 (28–72) |
| Female, n (%) | 18 (60) |
| Past NP surgery, n (%) | 20 (67) |
| Asthma, n (%) | 20 (67) |
| Employment status, n (%) | |
| Full‐time | 10 (33) |
| Part‐time | 9 (30) |
| Retired | 4 (13) |
| Unemployed, not seeking employment | 6 (20) |
| Unemployed, seeking employment | 1 (3) |
| Education, n (%) | |
| High school | 8 (27) |
| Some college | 8 (27) |
| Bachelor's degree | 7 (23) |
| Graduate degree | 7 (23) |
Abbreviations: CE, concept elicitation; NP, chronic rhinosinusitis with nasal polyposis.
3.3. Concept elicitation interviews
A total of 43 symptoms were identified across 30 patient interviews, including nasal congestion (mentioned by 100% of patients), nasal blockage/obstruction and difficulty with sense of smell (97% of patients for both), facial pressure (90% of patients), postnasal drip and runny nose (87% of patients for both), facial pain (80% of patients), and headache (77% of patients).
Of the 43 total symptoms identified, 53% were from the first wave of interviews, and 19%, 9%, 9%, 7%, and 2% were identified in waves 2, 3, 4, 5, and 6, respectively. One new symptom (sensitivity to light) emerged in wave 6. This symptom, reported by one patient, was not a direct symptom of NP but rather resulted exclusively from headaches caused by nasal polyps. As this concept was not a direct symptom of NP, the research team concluded that symptom saturation was achieved in wave 5.
Salient symptoms were identified by mapping the number of patients mentioning each symptom against the average disturbance rating of the symptom (Figure S1). Fifteen symptoms from patient interviews were identified as salient: nasal congestion, nasal blockage/obstruction, difficulty with sense of smell, facial pressure, postnasal drip, runny nose, facial pain, headache, difficulty with sense of taste, coughing, sneezing, discolored mucus, ear discomfort, dry mouth, and shortness of breath.
Patient interviews elicited 28 impacts, with 12 impacts identified as salient: impaired sleep, frustration, impact on work/productivity, fatigue, irritability, impact on daily activities/hobbies, impact on social life, embarrassment, reduced concentration, impact on voice, financial impact, and impact on family life (Figure S2). Impacts on sleep and on daily activities/hobbies were selected for inclusion in the NPSD as these were the impacts with the most frequent spontaneous mentions by patients, with 90% of them mentioning impaired sleep and 73% mentioning impact on daily activities/hobbies. Saturation was achieved for proximal impacts determined to be a result of NP symptoms.
3.4. Conceptual model and NPSD item selection
The key symptoms and impacts determined through CE interviews were used to finalize the conceptual model for NP. The final conceptual model included 26 symptoms and 19 impacts. Of these concepts, 15 symptoms and 12 impacts were identified as salient to the NP patient experience. Salient symptoms are shown in Figure 2.
FIGURE 2.
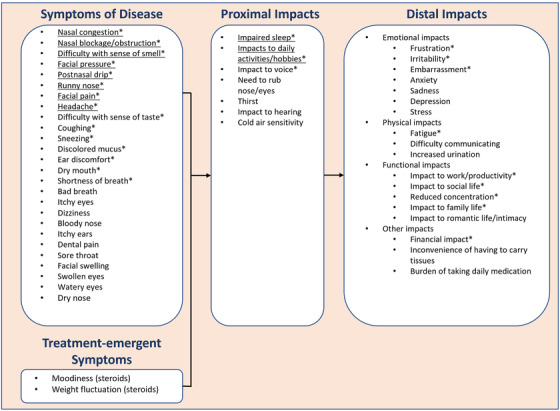
Final conceptual model for chronic rhinosinusitis with nasal polyposisa,b.
aUnderlined concepts were selected for inclusion in the final NPSD. Symptoms that were mentioned by ≤4 patients and impacts that were mentioned by ≤3 patients (of 30 total patients) were not included in the revised conceptual model. Due to the low rate of reporting by patients, these concepts were judged to be less significant and/or more distal to the typical NP patient experience.
bConcepts with an asterisk were identified as a salient concept (mentioned by ≥50% of patients and a total average disturbance rating of ≥5) from patient interviews. Not all salient concepts were included in the final NPSD to ensure the focus remained on only those concepts considered by patients to be the most important and to maintain its brevity.
Abbreviations: NP, chronic rhinosinusitis with nasal polyposis; NPSD, Nasal Polyposis Symptom Diary.
The research team intended for the NPSD to be a brief assessment to capture the severity and impact of key symptoms of NP. The most frequently reported and disturbing symptoms directly attributable to NP were selected for inclusion in the final NPSD instrument: nasal congestion, nasal blockage/obstruction, difficulty with sense of smell, facial pressure, postnasal drip, runny nose, facial pain, and headache. Symptom impact on sleep and daily activities was evident in the qualitative data; as a result, items related to these concepts were added to the NPSD as independent assessments of NP‐related impairment.
3.5. Cognitive interviews
Cognitive interviews of all 30 patients followed CE to debrief the draft NPSD. Approximately half of the patients (53%) expressed that the NPSD was not missing any key concepts that were meaningful to their experiences, and the remaining patients (47%) suggested adding one or more additional concepts to the NPSD. Three concepts were suggested by more than one patient: difficulty with sense of taste (mentioned by four patients), coughing (mentioned by three patients), and dry mouth (mentioned by three patients). Evaluation of the data showed that patients related these concepts to other concepts that are already captured in the NPSD (i.e., difficulty with sense of taste was a result of difficulty with sense of smell and coughing and dry mouth were considered to be attributable to other factors such as postnasal drip). To limit patient burden and target concepts that are relevant across the entire target NP patient population, a decision was made to exclude these three additional concepts in the NPSD.
Patients reported that the instrument was easy to understand, relevant to their experience, and appropriate for use in a daily diary. Most patients (77%) indicated that the NPSD items and instructions were clear and had no suggestions to improve the clarity of the items and instructions. The same percentage of patients indicated that the NPSD response options (none, mild, moderate, and severe) were appropriate and easy to understand. The majority of patients (93%) indicated that they could easily recall their NP symptoms over the past 24 hours. When asked about their willingness to complete the NPSD, 73% of patients indicated that they would be willing to complete the NPSD every day for up to a year in a clinical trial setting.
Evaluation of item redundancy focused on nasal blockage versus nasal congestion, headache versus facial pain versus facial pressure, and runny nose versus postnasal drip. Two‐thirds of patients (67%) indicated that nasal blockage and nasal congestion are distinct concepts, thus necessitating separate items to evaluate the concepts independently. Many patients described nasal blockage as physical obstruction of the nasal cavity caused by nasal polyps compared with nasal congestion, which was more frequently associated with mucus and inflammation of the nasal lining. Some patients indicated that blowing their nose or taking medication could provide relief for nasal congestion but not nasal blockage. However, 33% of patients suggested combining nasal blockage with nasal congestion in the NPSD. These individuals either had difficulty differentiating between nasal blockage and nasal congestion or indicated that any difference between the two concepts was not meaningful. The research team ultimately determined that blockage and congestion would be included as two items to capture more evidence of the NP patient experience, as per the opinion of most patients.
Half of the patients indicated that headache, facial pain, and facial pressure are distinct but often concurring symptoms and recommended maintaining separate items to capture these concepts. Of those reporting these as distinct concepts, patients indicated that facial pain differs from headache in regard to localization of pain—headache typically occurs in the forehead and head, whereas facial pain is on the face below the forehead. Of the patients interviewed, 30% recommended combining the facial pain with facial pressure items in the NPSD but indicated that headache should remain a distinct item because a headache can occur without facial pain or facial pressure. Only 7% of patients recommended combining headache, facial pain, and facial pressure into one item in the NPSD because they had trouble differentiating between the concepts or they did not commonly experience these symptoms. Given the proportion of patients who viewed these symptoms as distinct concepts, the research team decided to keep headache, facial pain, and facial pressure as separate items to capture these experiences in greater detail.
Patients viewed runny nose and postnasal drip as distinct concepts, but interview results indicated that some patients may benefit from clarifying text highlighting that postnasal drip pertains to “mucus drainage down the throat.” Additional clarifying text was added to this item to ensure that postnasal drip was not confused with a runny nose. All patients who were asked about this item indicated that the clarifying text was clear, easy to understand, and an accurate description based on their experience.
Overall, cognitive interview results indicated that patients understood the NPSD items and could select a meaningful response when asked to rate symptom or impact severity at its worst in the past 24 hours using a four‐point response scale (none, mild, moderate, and severe). No revisions to the NPSD were necessary following cognitive interviews, and the final NPSD (Figure 3) was considered suitable for clinical study use and psychometric evaluation.
FIGURE 3.
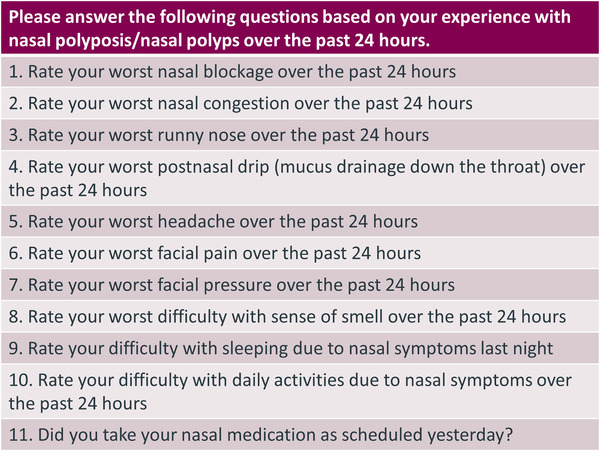
Nasal Polyposis Symptom Diarya.
aChoice for response to items 1–10 (4‐point scale): none, mild, moderate, severe. Choice for response to item 11 (a binary item asking about compliance with nasal medication not to be included in NPSD scoring): no, yes.
Abbreviation: NPSD, Nasal Polyposis Symptom Diary.
4. DISCUSSION
Capturing the experience of patients in clinical studies using PROs is increasingly important for drug development. With an expanding focus on patient‐centered drug development, health regulatory authorities and decision makers need direct evidence of patient benefit as captured by a PRO to complement treatment benefits captured using clinical assessments. As such, PRO measures are increasingly used as primary or key secondary endpoints in clinical studies as patient‐centric and clinically relevant assessments of the condition and treatment. 9
A challenge for clinical studies in NP is that the available PRO instruments were developed as comprehensive measures of HRQoL and were not designed specifically for NP, nor do their longer recall periods capture day‐to‐day variability of NP symptoms and impacts. For example, our review of the literature found that the Rhinosinusitis Outcome Measure 31 (RSOM‐31), originally developed as an HRQoL assessment in rhinosinusitis, 18 includes all of the prioritized symptoms for patients with NP; however, the instrument assesses items beyond the symptoms of NP that may not be relevant to the NP patient experience. In an NP clinical study setting, these additional items would not contribute meaningful data, but would increase the burden on patients participating in the study. The Total Nasal Symptom Score (TNSS) 19 questionnaire has versions with shorter recall periods; however, this tool was originally developed for allergic rhinitis and does not capture all relevant aspects of the NP patient experience (e.g., impaired sense of smell). PROs developed specifically for chronic rhinosinusitis have been used in studies of patients both with and without NP due to the similarity of symptoms experienced; however, these instruments appear to be more responsive in patients without NP compared with those with NP. 20 Patients with NP have a higher overall disease burden compared with those with chronic rhinosinusitis and are more affected by certain symptoms (e.g., difficulty with sense of smell). 20 The evaluation of the utility of existing instruments based on the published literature and previous clinical study use indicated a gap to be filled with a novel, brief, diary PRO tool for use with patients who have NP to track changes in symptoms and impacts during clinical studies.
This qualitative study was conducted in an effort to address the need for a brief daily symptom assessment, developed with patient input, that is suitable for use in NP clinical studies. The NPSD captures the eight most common and disturbing NP symptoms on a daily basis to limit recall bias and capture the variability of these symptoms, thus allowing clinical study researchers to pinpoint the onset of treatment effect with greater precision than less frequently administered assessments. In addition to the core symptom items, the NPSD also captures the patient‐perceived impact of symptoms on sleep and daily activities as well as patient‐reported use of nasal medication. These items were included to generate data that may be used to contextualize symptom severity and monitor adherence to NP maintenance medication use in a clinical study.
The NPSD is intended to evaluate individual and total symptom experiences of patients with NP participating in clinical studies. Within this context, use of the NPSD complements other measures of patient health status or HRQoL that capture symptom impact in a more comprehensive way than is possible with a daily diary. Although the brevity of the assessment and daily administration format are beneficial in a clinical study setting, the NPSD may be impractical for use in clinical practice due to its daily, electronic symptom reporting. Other comprehensive, fit‐for‐purpose HRQoL tools such as the 22‐item Sino‐Nasal Outcome Test (SNOT‐22) 21 or the recently developed Chronic Rhinosinusitis Patient‐Reported Outcome (CRS‐PRO) 20 with a longer recall period may be better suited to assess HRQoL in clinical practice.
A strength of the current qualitative study is the CE and cognitive interviews conducted that provided a deeper understanding of the NP patient experience. Building on the identification of symptoms relevant to the NP population, patients in a subsequent qualitative study conducted after the NPSD development discussed symptom frequency, duration, and severity, providing an in‐depth understanding of the symptomatology and complexity of the patient experience with links between the primary symptom associated with NP and other symptoms occurring as a consequence of the primary symptom. 10 As in the current qualitative study, further discussion during the probing interviews revealed links between symptoms and sleep difficulty, 10 further supporting the symptoms identified in the literature and currently assessed in clinical studies.
Limitations, including potential selection bias and limited generalizability to broader populations, may be present in this body of work, but these are frequently inherent in qualitative research. Another potential limitation of this study is the relatively small convenience sample, although concept saturation was determined to be adequate. Additionally, independent confirmation of patients’ NP diagnoses via endoscopy was not conducted specifically for this study. This is typical of qualitative studies in which physician chart review and attestation are common approaches for identifying eligible participants; however, it leaves open the possibility of misdiagnosis of current NP status. Although this is a limitation of the present work, it is notable that patients must have had a prior endoscopy to meet the NPS eligibility criteria and, for surgical patients, assessment prior to NP surgery. These requirements provide assurance that patients participating in the study had a verified NP diagnosis.
Quantitative data to evaluate item performance characteristics, confirm scoring, and evaluate overall measurement properties of the NPSD will be derived from a phase III confirmatory study (OSTRO; NCT03401229) evaluating the use of benralizumab for treatment of more than 400 patients with severe bilateral NP who are symptomatic despite receiving standard‐of‐care therapy. 22
5. CONCLUSION
Existing PRO instruments in published literature and/or used in clinical studies were not developed specifically for patients with NP and do not assess critical day‐to‐day variability in their symptom experience. The NPSD is a novel daily PRO tool that captures the most relevant and disturbing symptoms and impacts as identified by patients with NP. Patient interviews confirmed the suitability of the NPSD in capturing the daily experiences of patients without increased burden. These qualitative findings support further quantitative assessment of the NPSD as a suitable tool for measuring NP symptoms and impacts in clinical studies.
CONFLICT OF INTERESTS
Sean O'Quinn, Vivian H. Shih, and Ubaldo J. Martin are employees and shareholders of AstraZeneca. Oren Meyers, Patrick Crooks, and Julie Bailey are employees of IQVIA Consulting Services, which received funding from AstraZeneca to complete the study. Ashley F. Slagle is an employee of Aspen Consulting, LLC, which received funding from AstraZeneca to complete the study.
Supporting information
Table S1. Results of NP concept literature review
Figure S1. NP symptom salience (frequency and average disturbance rating)
Figure S2. NP impact salience (frequency and average disturbance rating)
ACKNOWLEDGMENTS
We thank the patients who participated in the qualitative study. Writing and editing assistance, including preparation of a draft manuscript under the direction and guidance of the authors, incorporation of author feedback, and manuscript submission, was provided by Emily Ruzich (IQVIA Consulting Services, Cambridge, MA, USA) and Wynne Dillon, MS (Kay Square Scientific, Newtown Square, PA, USA). This support was funded by AstraZeneca.
O'Quinn S, Shih VH, Martin UJ, et al. Measuring the patient experience of chronic rhinosinusitis with nasal polyposis: qualitative development of a novel symptom diary. Int Forum Allergy Rhinol. 2022;12:996–1005. 10.1002/alr.22952
REFERENCES
- 1. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1‐464. [DOI] [PubMed] [Google Scholar]
- 2. Hull BP, Chandra RK. Refractory chronic rhinosinusitis with nasal polyposis. Otolaryngol Clin North Am. 2017;50(1):61‐81. [DOI] [PubMed] [Google Scholar]
- 3. Mygind N, Dahl R, Bachert C. Nasal polyposis, eosinophil dominated inflammation, and allergy. Thorax. 2000;55(Suppl 2):S79‐S83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bequignon E, Mangin D, Bécaud J, et al. Pathogenesis of chronic rhinosinusitis with nasal polyps: role of IL‐6 in airway epithelial cell dysfunction. J Transl Med. 2020;18(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Franzese CB. The role of biologics in the treatment of nasal polyps. Immunol Allergy Clin North Am. 2020;40(2):295‐302. [DOI] [PubMed] [Google Scholar]
- 6. Roufosse F. Targeting the interleukin‐5 pathway for treatment of eosinophilic conditions other than asthma. Front Med (Lausanne). 2018;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alobid I, Benitez P, Bernal‐Sprekelsen M, et al. Nasal polyposis and its impact on quality of life: comparison between the effects of medical and surgical treatments. Allergy. 2005;60(4):452‐458. [DOI] [PubMed] [Google Scholar]
- 8. Bachert C, Bhattacharyya N, Desrosiers M, Khan AH. Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy. 2021;14:127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mercieca‐Bebber R, King MT, Calvert MJ, et al. The importance of patient‐reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall R, Trennery C, Chan R, et al. Understanding the patient experience of severe, recurrent, bilateral nasal polyps: a qualitative interview study in the United States and Germany. Value Health. 2020;23(5):632‐641. [DOI] [PubMed] [Google Scholar]
- 11. U.S. Food and Drug Administration, Center for Drug Evaluation and Research, Center for Devices and Radiological Health, Center for Biologics Evaluation and Research . Guidance for Industry Patient‐Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims . Accessed December 24, 2021. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/patient‐reported‐outcome‐measures‐use‐medical‐product‐development‐support‐labeling‐claims
- 12. U.S. Food and Drug Administration . FDA Patient‐Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient's Voice in Medical Product Development and Regulatory Decision Making . Accessed December 24, 2021. https://www.fda.gov/drugs/development‐approval‐process‐drugs/fda‐patient‐focused‐drug‐development‐guidance‐series‐enhancing‐incorporation‐patients‐voice‐medical
- 13. Patrick DL, Burke LB, Gwaltney CJ. Content validity—establishing and reporting the evidence in newly developed patient‐reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force Report: Part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967‐977. 10.1016/j.jval.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 14. Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient‐reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force Report: Part 2—assessing respondent understanding. Value Health. 2011;14(8):978‐988. 10.1016/j.jval.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 15. Rothman M, Burke L, Erickson P, et al. Use of existing patient‐reported outcome (PRO) instruments and their modification: the ISPOR Good Research Practices for evaluating and documenting content validity for the use of existing instruments and their modification PRO task force report. Value Health. 2009;12(8):1075‐1083. [DOI] [PubMed] [Google Scholar]
- 16. Patient‐Reported Outcomes and Quality of Life Database (PROQOLID) (Online). Mapi Research Institute . Accessed December 24, 2021. https://eprovide.mapi‐trust.org/about/about‐proqolid.
- 17. Wilson IB, Cleary PD. Linking clinical variables with health‐related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59‐65. [PubMed] [Google Scholar]
- 18. Piccirillo JF, Edwards D, Haiduk A, Yonan C, Thawley SE. Psychometric and clinimetric validity of the 31‐item Rhinosinusitis Outcome Measure (RSOM‐31). Am J Rhinol. 1995;9(6):297‐308. [Google Scholar]
- 19. Downie SR, Andersson M, Rimmer J, et al. Symptoms of persistent allergic rhinitis during a full calendar year in house dust mite‐sensitive subjects. Allergy. 2004;59(4):406‐414. [DOI] [PubMed] [Google Scholar]
- 20. Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22‐item sinonasal outcome test. Clin Otolaryngol. 2009;34(5):447‐454. [DOI] [PubMed] [Google Scholar]
- 21. Ghadersohi S, Price CPE, Beaumont JL, et al. Responsiveness and convergent validity of a new patient‐reported outcome measure for chronic rhinosinusitis (CRS‐PRO). J Allergy Clin Immunol Pract. 2020;8(7):2351‐2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bachert C, Han JK, Desrosiers MY, et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: a randomized, placebo‐controlled trial. J Allergy Clin Immunol. Published online September 29, 2021. 10.1016/j.jaci.2021.08.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of NP concept literature review
Figure S1. NP symptom salience (frequency and average disturbance rating)
Figure S2. NP impact salience (frequency and average disturbance rating)


