Abstract
Background
The risk of cutaneous squamous cell carcinoma (cSCC) is significantly increased in organ transplant recipients (OTRs). Clearance of actinic keratoses (AKs) is generally regarded as a surrogate biomarker for cSCC prevention. OTR‐cSCC chemoprevention with topical AK treatments has not been investigated in randomized controlled trials (RCTs), although there is evidence that 5% 5‐fluorouracil (5‐FU) may be chemoprotective in immunocompetent patients.
Objectives
To assess the feasibility, activity and evaluation outcomes relevant to the design of a future phase III RCT of topical cSCC chemoprevention in OTRs.
Methods
OTRs with 10 or more AKs in predefined areas were randomized 1 : 1 : 1 to topical 5‐FU, 5% imiquimod (IMIQ) or sunscreen (sun‐protective factor 30+) in a phase II, open‐label RCT over 15 months. Feasibility outcomes included proportions of eligible OTRs randomized, completing treatment and willing to be re‐treated. AK activity [AK clearance, new AK development, patient‐centred outcomes (toxicity, health‐related quality of life, HRQoL)] and evaluation methodology (clinical vs. photographic) were assessed.
Results
Forty OTRs with 903 AKs were randomized. All feasibility outcomes were met (56% of eligible OTRs were randomized; 89% completed treatment; 81% were willing to be re‐treated). AK activity analyses found 5‐FU and IMIQ were superior to sunscreen for AK clearance and prevention of new AKs. 5‐FU was more effective than IMIQ in AK clearance and prevention in exploratory analyses. Although toxicity was greater with 5‐FU, HRQoL outcomes were similar.
Conclusions
Trials of topical AK treatments in OTRs for cSCC chemoprevention are feasible and AK activity results support further investigation of 5‐FU‐based treatments in future phase III trials.
What is already known about this topic?
Cutaneous squamous cell carcinoma (cSCC) is significantly more common in immunocompromised individuals including organ transplant recipients (OTRs) compared with immunocompetent populations.
cSCC chemoprevention activity of sunscreen and 5‐fluorouracil‐based (5‐FU) actinic keratosis (AK) treatments has been demonstrated in randomized controlled trials (RCTs) in immunocompetent populations but not in OTRs.
AKs are cSCC precursors and their clearance and prevention are generally regarded as surrogate endpoint biomarkers for potential cSCC chemoprevention activity.
What does this study add?
SPOT (SCC Prevention in OTRs using Topical treatments) has confirmed that RCTs of OTR‐cSCC chemoprevention with topical AK treatments are feasible.
It also suggests that topical 5‐FU may be superior to 5% imiquimod and sunscreen in AK clearance and prevention.
Together with recent evidence from several RCTs in the general population, these data provide a compelling rationale for further studies of intervention with 5‐FU‐based topical chemoprevention approaches in OTR‐cSCC prevention.
Clearance of actinic keratoses (AK) is generally regarded as a surrogate biomarker for CSCC prevention. Our aim is to assess feasibility, activity and evaluation outcomes relevant to design of a future phase III RCT of topical CSCC chemoprevention in OTRs. We have concluded that trials of topical AK treatments in OTRs for CSCC chemoprevention are feasible and AK activity results support further investigation of 5‐FU‐based treatments in future phase III trials.

Linked Comment: M. Samimi. Br J Dermatol 2022; 187:281–282.
Cutaneous squamous cell carcinoma (cSCC) is the second most common skin cancer, with more than 50 000 cases annually in the UK and a rising incidence. 1 Immunosuppressed individuals, including organ transplant recipients (OTRs), have a significantly increased cSCC risk, tumour burden and metastatic risk. 2 , 3
Actinic keratoses (AKs) are intraepidermal cSCC precursors and harbour many molecular features in common with cSCC. 4 An estimated 0·075–0·53% of AKs progress to cSCC each year and this is accelerated in immunosuppressed individuals. 2 , 5 In OTRs, AKs frequently colocalize as confluent areas of ‘field cancerization’ from which cSCCs arise. 2 , 5 Topical field‐directed treatments are therefore a rational approach to cSCC prevention. 6 A randomized controlled trial (RCT) in the 1990s confirmed a modest effect of sunscreen on AK/cSCC prevention and sunscreen is therefore regarded as the standard of care. 7 , 8 , 9 Evidence for activity of topical field‐directed chemoprevention emerged only in 2017–18 with two 5% 5‐fluorouracil (5‐FU)‐based interventional studies. 10 , 11 OTRs were excluded from both these trials and from an RCT demonstrating superior efficacy of 5‐FU vs. 5% imiquimod cream (IMIQ), methyl aminolevulinate photodynamic therapy (MAL‐PDT) or 0·015% ingenol mebutate gel in AK clearance. 12 In OTRs, few RCTs have compared AK clearance and prevention with topical field therapies and none have confirmed cSCC chemoprevention activity, 13 , 14 although AK activity is commonly regarded as a surrogate biomarker for cSCC prevention.
SPOT (Squamous cell carcinoma Prevention in Organ transplant recipients with Topical treatments) is a prospective, multicentre, open‐label, phase II RCT comparing AK treatment with 5‐FU and IMIQ vs. sunscreen alone in OTRs. It was designed as a feasibility study to inform a future phase III chemoprevention RCT. 5‐FU and IMIQ are the two AK topical treatments used most often in specialist OTR clinics in the UK and sun‐protective factor (SPF) 30+ sunscreen is the standard of care.
Methods
Patient population, eligibility, screening, randomization and allocation concealment
SPOT was conducted in three UK specialist OTR dermatology clinics (the SPOT protocol is available in File S1; see Supporting Information). Eligibility criteria included those aged 18 years or above; 10 or more AKs in one to two contiguous, predefined treatment zones and matched clinically equivalent zones (CEZs); stable graft function and immunosuppression regimen in the previous 6 months; no topical AK treatment in the 4 weeks prior to randomization. Acitretin was permitted if the dose was stable for the preceding 6 months.
Ten potential treatment zones were defined as either head and neck (HN: right/left cheek and nose; right/left forehead; scalp; upper chest) or upper limb (UL: right/left hand; right/left forearm) (Figure S1a; see Supporting Information). A 4‐mm punch biopsy from a representative AK was taken for confirmation of clinical diagnosis. Participants were randomized 1 : 1 : 1 to 5‐FU, IMIQ or sunscreen using a bespoke computer algorithm with a block stratification method developed by Cancer Research UK Clinical Trials Unit. Randomization was stratified by HN vs. UL location. This was an open‐label trial and neither participants nor clinicians assessing clinical response were blinded to treatment allocation. However, all investigators subsequently evaluating photographic images were blinded to treatment allocation.
Treatment protocols
5‐FU and IMIQ dosing regimens were those used in routine clinical practice (see File S1). 15 Participants received detailed advice on application and adverse effects. To maximize the chances of 100% AK clearance, dose escalation was permitted, with repeat treatment after 4 weeks (Figure 1). SPF 30+ sunscreen was applied a minimum of once a day in the sunscreen arm and discretionary use was recommended for all study zones (5‐FU, IMIQ and CEZ) according to standard clinical advice (File S1). 16 Use of topical corticosteroids and emollients for local skin reactions (LSRs) was recorded (see Table S1). Cryotherapy was not permitted within study zones. All patients were followed up for a total of 12 months after the 3‐month treatment period ended.
Figure 1.
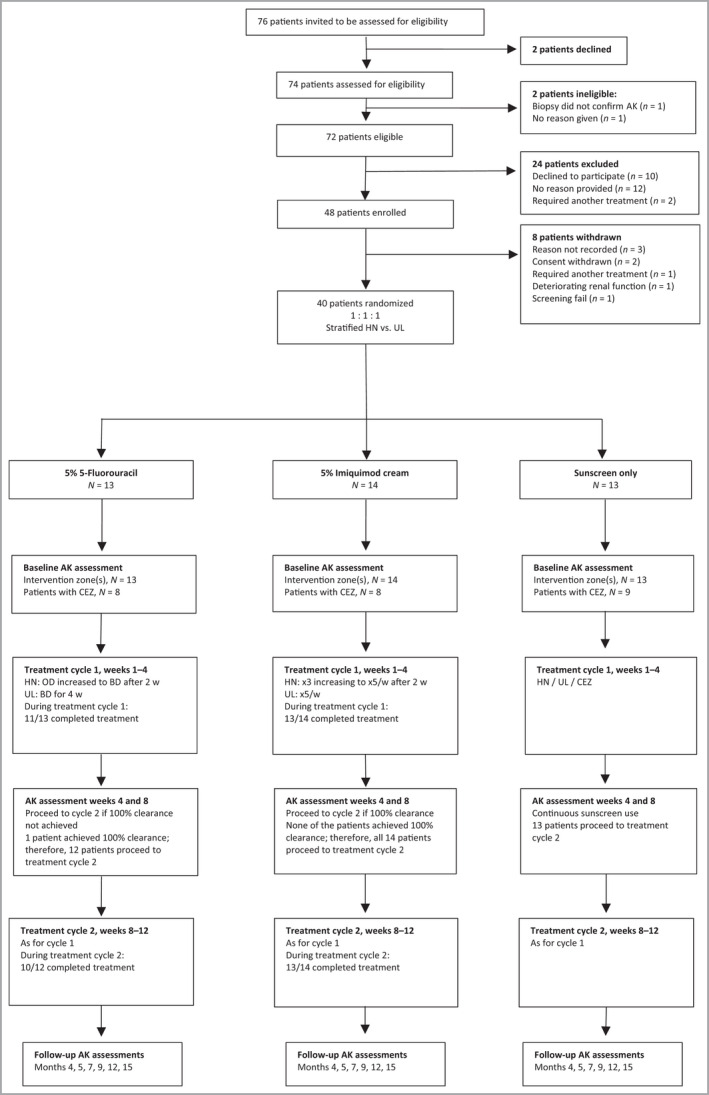
Trial design and patient flow through the study including feasibility outcomes. Of 72 eligible patients, 48 (67%, 95% CI 55–77%) agreed to participate and 40 (56%) were randomized, meeting the feasibility criterion of at least 30%. The second feasibility criterion was at least 70% of patients should complete treatment cycle 1 for the active treatment arms: this was achieved: 5‐FU 11 of 13, 85% (95% CI 55–98%); IMIQ 13 of 14, 93% (95% CI 66–100%). The third feasibility criterion was that at least 70% of patients requiring treatment should complete cycle 2 for the active treatment arms: this was achieved: [5‐FU 10 of 12, 83% (95% CI 52–98%); IMIQ 13 of 14, 93% (95% CI 66–100%)]. AK, actinic keratosis; BD, twice daily; CI, confidence interval; CEZ, clinically equivalent zone; 5‐ FU, 5% 5‐fluorouracil; HN, head and neck; IMIQ, 5% imiquimod; OD, once daily; w, week; UL, upper limbsActinic keratosis clinical assessment
Actinic Keratosis clinical asessment
Four pretrial consensus exercises were undertaken to optimize inter‐ and intra‐observer concordance. 17 AK area was assessed by tracing AK margins on 1‐cm squared transparent acetate sheets (Figure S1b); keratosis was assessed by palpation (1, barely palpable; 2, moderate keratosis; 3, marked keratosis); erythema was graded as 1, mild or 2, marked. Clinical diagnosis of AK required a keratosis score ≥ 1 and erythema score ≥ 1. The total AK burden was derived as an exploratory score (product of total AK count, median keratosis grade and median area). Treatment zones and CEZs were assessed at baseline and subsequently at 10 and three timepoints, respectively. Dose escalation/reduction was supervised in telephone interviews at six timepoints and monitored by treatment diary entries for weeks 1–16 (data not shown).
Local skin reactions, toxicity and safety
Redness, swelling, ulceration and discomfort were assessed using a four‐point scale at five timepoints (see Appendix S2 of File S1). Adverse events were reported using the Common Terminology Criteria for Adverse Events (CTCAE, v4·0). 18 25‐hydroxyvitamin D3 levels were measured at baseline and month 15. Renal function was monitored as per transplant surveillance protocols.
Health‐related quality of life
Two validated health‐related quality of life (HRQoL) tools were used: the EuroQol™ 5D‐3L questionnaire and the Dermatology Life Quality Index. 19 When SPOT was designed there were no validated AK‐specific HRQoL tools. The AK Index questionnaire – an exploratory questionnaire based on the Skin Cancer Index 20 – was piloted. All HRQoL tools were completed at baseline, weeks 4 and 12, months 5 and 15.
High‐resolution digital photography actinic keratosis assessment
Digital photographs of treatment and CEZs at eight and four timepoints, respectively, were taken using a standardized protocol (File S1). Photography and clinical AK assessments were compared in a three‐stage exploratory analysis by two investigators not involved in the RCT. In stage 1, 10 randomly selected study images were annotated using an electronic image capture programme (GIMP, v. 2·10·14; https://www.gimp.org/) and scores compared with clinical assessment recorded on mapping acetates. This information was used for consensus modification of scoring criteria. In stage 2, 10 further images were then scored by two separate investigators (C.A.H. and Z.H.) who compared these results with matched clinical assessments, informing further modification of scoring. In stage 3, 10 images were scored and concordance between observers and with clinical assessments were calculated [Table S2 (see Supporting Information); Figure 2a,b].
Figure 2.
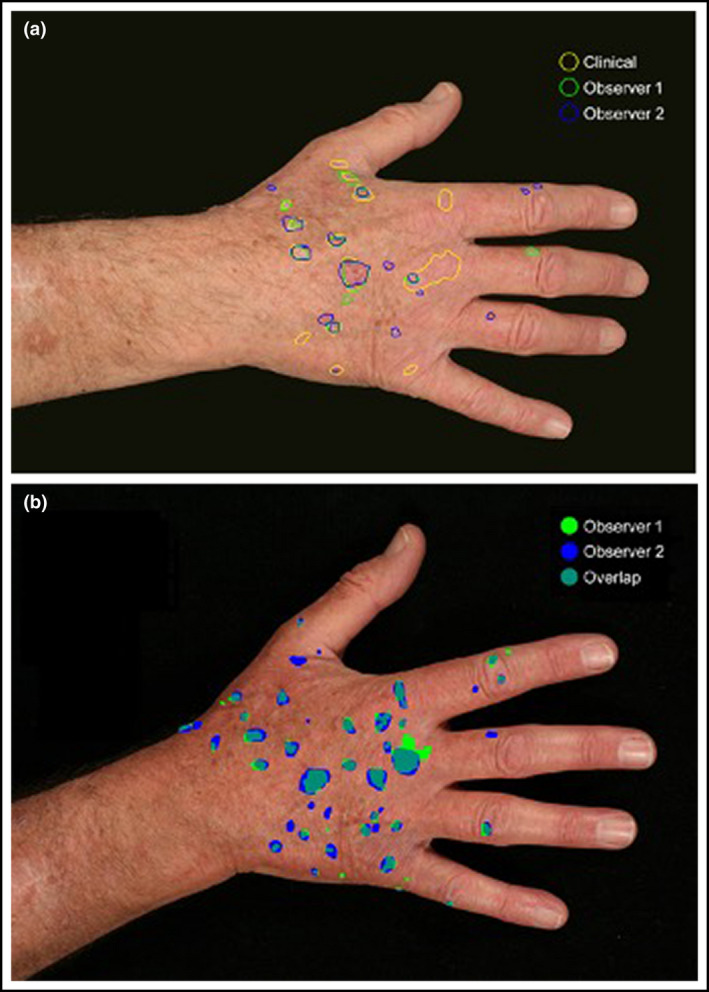
Digital photographs corresponding with clinical actinic keratosis (AK) assessments were taken at all timepoints. An electronic image capture programme was used by two independent observers (observer 1 and 2) to annotate images. (a) These annotated images were matched with the original clinical assessment (clinical) by two separate observers. These results were used to calculate the sensitivity and false discovery rates for photography vs. clinical assessment of individual AKs. (b) The annotated images were also used to derive a Dice coefficient for interobserver concordance (overlap) in the photographic assessment of AKs annotated by each observer. [Colour figure can be viewed at wileyonlinelibrary.com]
Outcomes
Feasibility outcomes included the proportion of eligible participants willing to be randomized, completing 5‐FU or IMIQ treatment (assessed at the end of cycles 1 and 2) and willing to use the treatment again. Activity outcomes included lesion‐specific clearance of baseline AKs; proportion of patients achieving either 100% or 75% baseline AK clearance (AK100 and AK75, respectively – the latter exploratory and unplanned); prevention of new AKs; total number of AKs; durability of AK clearance (proportion of AKs cleared by months 4 and 5 that remained clear at month 15); tolerability; cSCC event rate. Unless specified elsewhere, activity outcomes were assessed at weeks 4, 8 and 12 (cycles 1 and 2), and months 4, 5, 7, 9, 12 and 15 post‐trial entry. Information on adverse events and treatment tolerance was assessed at weeks 2, 4, 8 and 12 and month 4. Willingness to use the treatment again was assessed at the end of the study. Evaluation and patient‐centred outcomes included AK scoring concordance by clinical assessment and photography and sensitivity of HRQoL tools, respectively.
Statistical analyses
As a conventional sample size calculation is not applicable to feasibility, this was based on criteria for AK100, predicted to be ≥50% for 5‐FU and IMIQ and 0% for sunscreen (a factor of 0·05% was added to facilitate calculation). The study was powered to compare each treatment with sunscreen, giving 17 patients per arm to detect a difference between 0·05% and 50% with 90% power using a two‐sided α of 0·025. The total sample size was 60 allowing for loss to follow‐up of 10%.
Risk difference and 95% confidence intervals (CIs) for activity outcomes in each arm vs. sunscreen were calculated and compared using Fisher’s exact test. AK clearance, new AKs and HRQoL were analysed using hierarchical models. Overall worst LSR was compared using Fisher’s exact test. cSCC development was reported using the Kaplan–Meier method. CIs for proportions were calculated using Wilson’s method. For single timepoint comparisons of both 5‐FU and IMIQ to sunscreen for the persistence outcome Dunnett’s test was used. Unplanned sensitivity analyses for activity AK outcomes are given in Appendix S1 (see Supporting Information). Statistical analyses used in the photography assessments are summarized in Table S2 (see Supporting Information).
Ethical approval
The trial was performed according to the principles of the Declaration of Helsinki and approved by the London‐Chelsea Research Ethics Committee (REC 13/LO/1579). Informed written consent was obtained from all participants.
Results
Trial population and centres
Participants were randomized from March 2015 to September 2016, with the last follow‐up in January 2018. Seventy‐two OTRs were approached, 48 (67%) agreed to participate and 40 were randomized. Recruitment of 20 participants was prevented by the unexpected closure of one planned centre after trial opening. Thirteen participants were randomized to 5‐FU, 14 to IMIQ and 13 to sunscreen (Figure 1). CEZs were monitored in 25 of 40 (62·5%) participants. Stratification was equivalent for HN and UL sites (Table 1). No participants were lost to follow‐up.
Table 1.
Baseline actinic keratosis (AK) characteristics in treatment zones and clinically equivalent zones (CEZs)
| Characteristics | 5% 5‐Fluorouracil | 5% Imiquimod | Sunscreen | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention, all sites | HN | UL | CEZ | Intervention, all sites | HN | UL | CEZ | Intervention, all sites | HN | UL | CEZ | Intervention, all sites | CEZ | |
| Participants, n | 13 | 7 | 6 | 8 | 14 | 7 | 7 | 8 | 13 | 6 | 7 | 9 | 40 | 25 |
| AKs, total n | 178 | 103 | 75 | 104 | 214 | 98 | 116 | 106 | 178 | 74 | 104 | 123 | 570 | 333 |
| Median AKs, n (range) | 12 (10–25) | 12 (10–25) | 12 (10–25) | 11·5 (10–21) | 13 (10–29) | 13 (10–25) | 15 (10–29) | 12·5 (6–26) | 13 (5–21) | 11·5 (10–16) | 17 (5–21) | 14 (5–17) | 12·5 (5–29) | 13 (5–26) |
| Median AK area (range), cm2 | 0·4 (0.1–5·5) | 0·5 (0·2–5·5) | 0·4 (0·1–4) | 0·4 (0·1–5·5) | 0·4 (0·1–3) | 0·4 (0·2–3) | 0·4 (0·1–2) | 0·55 (0·1–6·5) | 0·38 (0·1–9) | 0·3 (0·2–3) | 0·4 (0·1–9) | 0·4 (0·1–5) | 0·4 (0·1–9) | 0·4 (0·1–6·5) |
| KSS; AKs, n (%) | ||||||||||||||
| 1 | 112 (63) | 59 (57) | 53 (71) | 62 (60) | 121 (57) | 59 (60) | 62 (53) | 55 (52) | 113 (63) | 48 (65) | 65 (62) | 80 (65) | 346 (61) | 197 (59) |
| 2 | 59 (33) | 38 (37) | 21 (28) | 33 (32) | 76 (36) | 30 (31) | 46 (40) | 46 (43) | 57 (32) | 26 (35) | 31 (30) | 36 (29) | 192 (34) | 115 (35) |
| 3 | 7 (4) | 6 (6) | 1 (1) | 9 (9) | 17 (8) | 9 (9) | 8 (7) | 5 (5) | 8 (4) | 0 (0) | 8 (8) | 7 (6) | 32 (6) | 21 (6) |
| Mean; median | 1·41; 1·0 | 1·49; 1·0 | 1·31; 1·0 | 1·49; 1·0 | 1·51; 1·0 | 1·49; 1·0 | 1·53; 1·0 | 1·53; 1 | 1·41; 1·0 | 1·35; 1·0 | 1·43; 1 | 1·41; 1·0 | 1·45; 1·0 | 1·47; 1·0 |
| ESS; AKs, n (%) | ||||||||||||||
| 1 | 122 (69) | 77 (75) | 45 (60) | 54 (52) | 154 (72) | 72 (73) | 82 (71) | 53 (50) | 127 (71) | 60 (81) | 67 (64) | 75 (61) | 403 (71) | 182 (55) |
| 2 | 56 (31) | 26 (25) | 30 (40) | 50 (48) | 60 (28) | 26 (27) | 34 (29) | 53 (50) | 51 (29) | 14 (19) | 37 (36) | 48 (39) | 167 (29) | 151 (45) |
| TAB, median (range)a | 6·6 (2–30) | 8·1 (2–30) | 6·1 (2·4–9) | 6·3 (2–21) | 6·6 (2·2–23·2) | 6·0 (3·3–10·4) | 7·5 (2·2–23·2) | 6·35 (4·8–31·2) | 5·6 (2·2–22·5) | 6·0 (2·2–5·6) | 6·8 (2·2–22·5) | 6·3 (2–17) | 6·15 (2–30) | 6·3 (2–31·2) |
aTotal AK burden (TAB) score for each patient was derived as a product of the total AK count, median keratosis grade and median area.
ESS, Erythema Scale Score; HN, right/left cheek and nose, right/left forehead, scalp, upper chest; KSS, Keratosis Scale Score; UL, upper limbs
Baseline demographics and actinic keratosis characteristics
The 40 OTRs randomized had a total of 903 AKs (treatment zones, n = 570; CEZs, n = 333). Patient demographics were similar in all three arms including sex (31 of 40, 77·5% male), age [median 65 (range 45–79) years], transplant type, immunosuppressive drug regimen, duration of transplantation [median 26 (range 2–41) years], skin cancer history and AK treatment history with the exception of previous acitretin use (Table 2). AK clinical characteristics were also similar (Table 1).
Table 2.
Baseline characteristics of study participants
| Characteristics | 5‐FU (n = 13) | IMIQ (n = 14) | SS (n = 13) | Total (n = 40) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 11 (85) | 10 (71) | 10 (77) | 31 (78) |
| Female | 2 (15) | 4 (29) | 3 (23) | 9 (23) |
| Median age (range), years | 65 (45–77) | 66 (51–79) | 66 (50–72) | 65 (45–79) |
| Organ transplant type, n (%) | ||||
| Kidney | 12 (92) | 14 (100) | 13 (100) | 39 (98) |
| Hearta | 1 (8) | 0 | 1 (8) | 2 (5) |
| Liverb | 0 | 0 | 1 (8) | 1 (2·5) |
| Median duration of transplantation (range), years | 26 (3–41) | 28 (2–37) | 22 (2–37) | 26 (2–41) |
| Immunosuppressive drugs, n (%)c,d | ||||
| Azathioprine | 3 (25) | 7 (50) | 4 (31) | 14 (36) |
| Mycophenolate | 3 (25) | 5 (36) | 3 (23) | 11 (28) |
| Sirolimus | 1 (8) | 0 (0) | 0 (0) | 1 (3) |
| Ciclosporin | 5 (42) | 4 (29) | 4 (31) | 13 (33) |
| Prednisolone | 11 (92) | 11 (79) | 10 (77) | 32 (82) |
| Tacrolimus | 2 (17) | 4 (29) | 3 (23) | 9 (23) |
| History of skin cancer | ||||
| Participants, n (%) | 8 (62) | 7 (50) | 9 (69) | 24 (60) |
| Unknown, n (%) | 0 | 1 (7) | 0 | 1 (2·5) |
| Median (range) per patient | 7·5 (1–30) | 9 (2–25) | 5 (1–18) | 7 (1–30) |
| BCC / (cSCC + CIS) | 3 | 4·5 | 1·6 | 2·9 |
| Acitretin use, n (%) | 6 (46) | 2 (14) | 1 (8) | 9 (23) |
| History of AK treatment, n (%) | ||||
| Yes | 12 (92) | 12 (86) | 11 (85) | 35 (88) |
| Unknown | 1 (8) | 0 | 0 | 1 (2·5) |
| AK treatments used, n (%) | ||||
| Surgery | 3 (23) | 6 (43) | 3 (23) | 12 (30) |
| Cryotherapy | 11 (85) | 9 (64) | 9 (69) | 29 (73) |
| 5‐FU | 10 (77) | 10 (71) | 9 (69) | 29 (73) |
| IMIQ | 4 (31) | 6 (43) | 3 (23) | 13 (33) |
| Diclofenac gel | 1 (8) | 2 (14) | 3 (23) | 6 (15) |
| Ingenol mebutate gel | 1 (8) | 1 (7) | 1 (8) | 3 (8) |
| PDT | 1 (8) | 1 (7) | 0 (0) | 2 (5) |
aOne patient had dual cardiac and renal transplants; bone renal transplant recipient received a liver transplant during the follow‐up period and ciclosporin was switched to tacrolimus; cin the IMIQ arm, two of 14 participants had a reduction in immunosuppression during the trial period; done patient from the 5‐FU study arm did not have a full immunosuppressive medication history available (for this section, 5‐FU, n = 12; total, n = 39).
AK, actinic keratosis; BCC, basal cell carcinoma; CIS, carcinoma in situ; cSCC, cutaneous squamous cell carcinoma; 5‐FU, 5% 5‐fluorouracil cream; IMIQ, 5% imiquimod; PDT, photodynamic therapy; SS, sunscreen
Feasibility outcomes
Of 72 eligible participants, 40 (56%, 95% CI 43–67%) were randomized, meeting the feasibility criterion of 30% (details are shown in Figure 1). At least 70% of participants were required to have completed treatment cycle 1. In total, 89% of patients completed cycle 1 (11 of 13 and 13 of 14 in the 5‐FU and IMIQ, respectively). One patient on 5‐FU stopped treatment in cycle 1 after 3 weeks because of a severe LSR but achieved 100% AK clearance and did not proceed to treatment cycle 2. All other participants required a second cycle and 10 of 12 (83%) and 13 of 14 (93%) in the 5‐FU and IMIQ arms, respectively, completed this. Overall, 89% of patients across the 5‐FU and IMIQ arms completed their treatment cycles, as per the study protocol. The feasibility criterion that 70% of participants would be willing to be re‐treated was met for 81% across all arms (data not shown).
Activity outcomes
Lesion‐specific actinic keratosis clearance rates
Hierarchical modelling showed that clearance rates were consistently higher for 5‐FU: 85%, 60% and 28%, respectively, for 5‐FU, IMIQ and sunscreen at 4 weeks post‐treatment (month 4); 83%, 57% and 19% by 8 weeks post‐treatment (month 5); 73%, 59% and 39% by 12 months post‐treatment (month 15). Clearance was lower on UL vs. HN sites with IMIQ, but no site‐related differences were seen with 5‐FU and sunscreen. Figure 3 shows the output for the model described in Appendix S2 (see Supporting Information) and considers all randomized patients for assessment of new AKs. Note, it shows a smoothed function of how the percentage of AKs cleared changes with time rather than the observed data.
Figure 3.
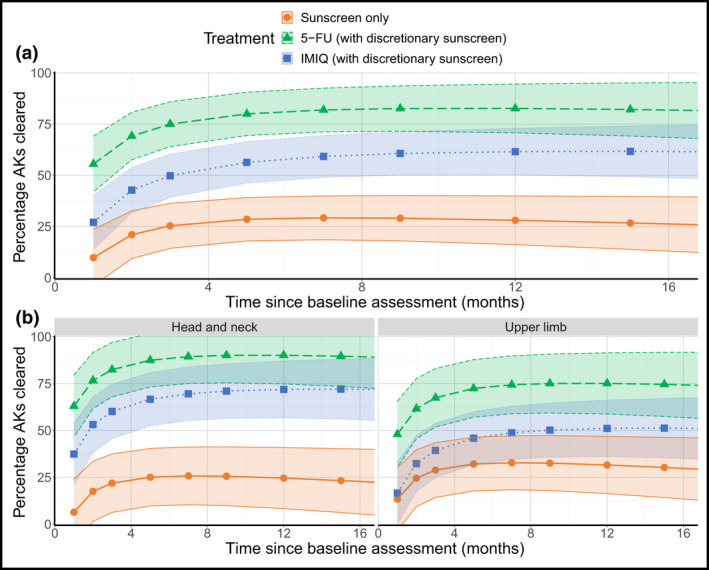
Fixed‐effects from complete AK clearance model. Points indicate the mean estimate with 95% confidence intervals shown by the shaded region, shaded according to treatment, in the pooled population (a) and by stratification variables (b). Note: this figure shows the output for the model described in Appendix S2 and considers all randomized patients for assessment on new AKs. It is a smoothed function of how the percentage of AKs cleared changes with time, rather than the observed data. AK, actinic keratosis; 5‐FU, 5% 5‐fluorouracil; IMIQ, 5% imiquimod. [Colour figure can be viewed at wileyonlinelibrary.com]
Actinic keratosis clearance (75% and 100% of baseline)
AK75 and AK100 clearance values are shown in Figure S2 (see Supporting Information). At 4 weeks post‐treatment (month 4) the proportions of participants achieving AK75 for 5‐FU, IMIQ and sunscreen were 92%, 43% and 9%, respectively, and at 8 weeks post‐treatment (month 5), the proportions were 75%, 43% and 8%. At 12 months post‐treatment (month 15), AK75 was significantly higher for 5‐FU compared with sunscreen (58% vs. 15%, P = 0·041), but the difference for IMIQ compared with sunscreen (29% vs. 15%, P = 0·648) was nonsignificant. AK100 at 4 weeks post‐treatment (month 4) was achieved in 23%, 14% and 9% and at 8 weeks post‐treatment (month 5) in 42%, 14% and 0% for 5‐FU, IMIQ and sunscreen, respectively, but by 12 months post‐treatment (month 15) neither 5‐FU nor IMIQ were significantly more effective than sunscreen (17% and 7% for 5‐FU and IMIQ, respectively, vs. 8% for sunscreen, P = 0·593 and P = 1·00).
Persistence of actinic keratosis clearance
The proportion of AKs cleared at weeks 4 and 8 post‐treatment (months 4 and 5, Figure 4) that remained undetectable at 12 months post‐treatment was superior with 5‐FU vs. sunscreen (mean values: 4 weeks: 75% vs. 51%, 95% CI –4 to 52; P = 0·10; 8 weeks: 77% vs. 53%, 95% CI 4–51; P = 0·10). IMIQ may also be superior to sunscreen (4 weeks: 73% vs. 51%, 95% CI –7 to 50, P = 0·15; 8 weeks: 74% vs. 53%, 95% CI –6 to 49; P = 0·14, respectively). This outcome was sensitive to timing of the month 15 assessment (Appendix S1; see Supporting Information).
Figure 4.
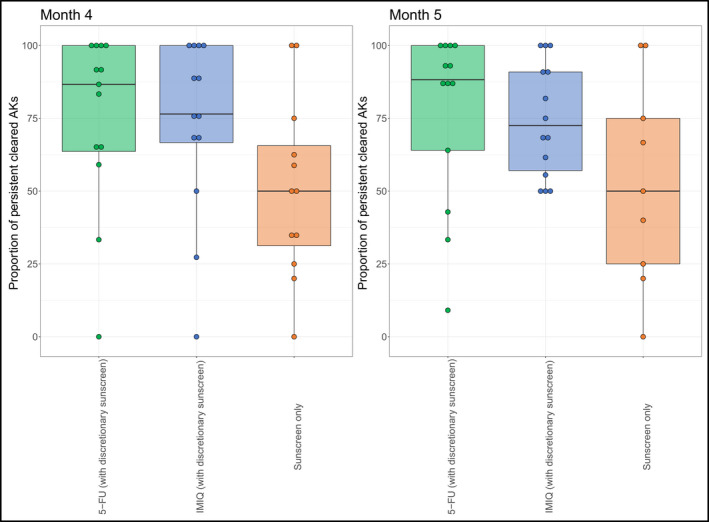
Boxplots indicating the proportion of AKs cleared at months 4 and 5 which remained clear by the final follow‐up appointment (month 15, 12 months post‐treatment) by treatment. The horizontal line within each box represents the median, the extremities of the box the 25th and 75th quantiles, the lines then extend to ~1·5*IQR (as a crude measure of where 95% of the data is anticipated to fall), with the remaining points corresponding to outliers. Due to the small sample size, there may be some discrepancy between the mean (as presented in the text) and the median (as shown in the boxplots).AK, actinic keratosis; 5‐FU, 5% 5‐fluorouracil; IMIQ, 5% imiquimod; IQR, interquartile range [Colour figure can be viewed at wileyonlinelibrary.com]
Prevention of new actinic keratoses
See Appendix S3 and Figure S3 in the Supporting Information and Figure 5. New AK rates were lower with 5‐FU vs. sunscreen whereas the rate was comparable for IMIQ and sunscreen [0·08, 1·36 and 0·73 for 5‐FU, IMIQ and sunscreen, respectively, at 4 weeks post‐treatment (month 4); 0·5, 1·14 and 1·0 at 8 weeks post‐treatment (month 5); 0·08, 0·5 and 1·0 at 12 months post‐treatment (month 15)]. Despite initial variability in the number of new AKs in the CEZs, by 12 months post‐treatment (month 15), the generation of new AKs compared with the previous visit was similar across all treatments: at 12 months post‐treatment, new AK rates in treatment vs. CEZ were a mean of 0·14 (SD 0·38) vs. 2·43 (3·04) for the 5‐FU arm, 0·88 (1·13) vs. 2·14 (4·06) for IMIQ and 1·44 (1·74) vs. 1·57 (0·98) for sunscreen.
Figure 5.
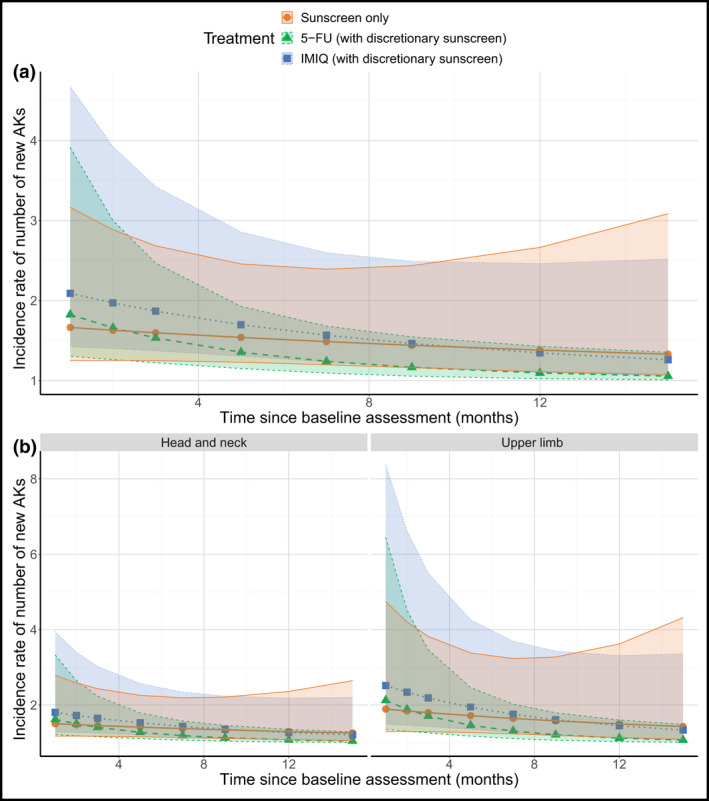
Fixed effects from new AKs in the treatment zone model. Points indicate the mean estimate with 95% confidence intervals indicated by the shaded region, shaded according to treatment, in the pooled population (a) and by stratification variables (b). Note: this figure shows the output for the model described in Appendix S3 and considers all randomized patients for assessment of new AKs. It shows a smoothed function of how the incidence rate of new AKs changes with time rather than the observed data. AK, actinic keratosis; 5‐FU, 5% 5‐fluorouracil; IMIQ, 5% imiquimod. [Colour figure can be viewed at wileyonlinelibrary.com]
Total number of actinic keratoses
See Appendix S4, Figure S4 (see Supporting Information) and Figure 6. Exploratory analysis of mean treatment effect which combined AK clearance and new AK formation rates confirmed the mean number of AKs at each timepoint were lowest with 5‐FU (2, 9 and 12 at 4 weeks post‐treatment (month 4); 3, 10 and 13 at 8 weeks post‐treatment (month 5); 5, 9 and 11 at 12 months post‐treatment (month 15) for 5‐FU, IMIQ and sunscreen, respectively).
Figure 6.
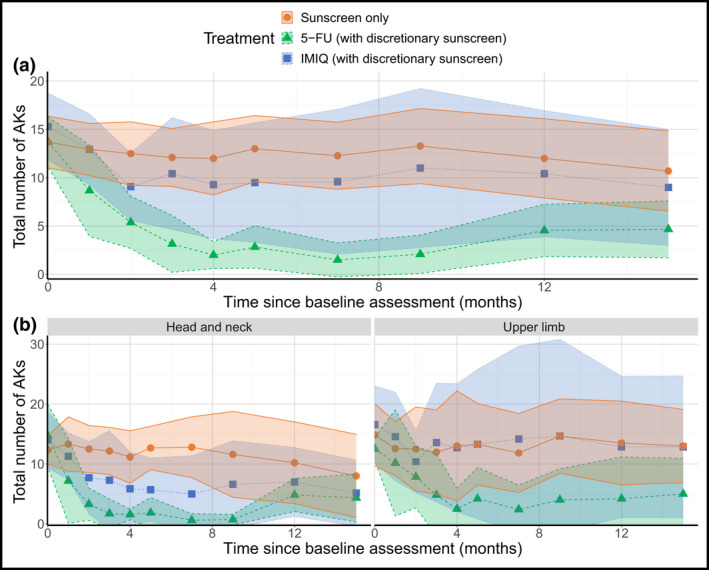
Mean estimates of the total number of AKs with 95% confidence intervals indicated by the shaded region in the pooled population (a) and by stratification variables (b). AK, actinic keratosis; 5‐FU, 5% 5‐fluorouracil; IMIQ, 5% imiquimod. [Colour figure can be viewed at wileyonlinelibrary.com]
Cutaneous squamous cell carcinoma development
Fifty‐three cSCCs developed in 17 participants by month 15 and all except one arose outside trial zones. The month‐15 (12 months post‐treatment) event rate was 47% for 5‐FU (95% CI, 25–77%; 15 cSCCs in six participants), 50% for IMIQ (CI 28–77%; 23 cSCCs in seven participants) and 31% for sunscreen (CI 13–63%; 15 cSCCs in four participants). See Figure S5 in the Supporting Information.
Safety and toxicity
LSRs occurred in 100%, 93% and 8% of participants in 5‐FU, IMIQ and sunscreen arms, respectively (see Tables S1 and S3; see Supporting Information). LSRs were worse for 5‐FU vs. IMIQ (exploratory analysis P = 0·024) and both were worse than sunscreen (P < 0·001). LSR intensity was greatest at week 4 (cycle 1) and milder for cycle 2. Adverse events occurred in 62%, 79% and 23% in the 5‐FU, IMIQ and sunscreen arms, respectively, with pruritus and fatigue most common. Serious adverse events were reported in three participants (5‐FU, n = 1; IMIQ, n = 2) and were unrelated to treatment. Vitamin D levels were similar across arms for baseline and month 15. Use of topical corticosteroids or cryotherapy was similar across arms.
Health‐related quality of life outcomes
No significant changes were seen during and after treatment for any of the treatment arms and there were no significant HRQoL differences between each of the three HRQoL tools (Table S4; see Supporting Information).
High‐resolution digital photography
Details are available in Table S2. After modifying scoring in stage 1 (the training set), 10 images with 93 clinically identified AKs were assessed in stage 2. Observers scored 223 and 195 AKs, respectively, of which 79 and 72 were correctly matched with clinical assessment (sensitivity 85% and 77%; false discovery rates 65% and 63%). Erythema was identified as the main source of low specificity in consensus discussion and excluded. In stage 3, 10 images with 98 clinically identified AKs were assessed: 51 and 74 AKs were scored of which 43 and 48 were correctly matched with clinical assessment (sensitivity 44% and 49%; false discovery rates 16% and 35%). Intraclass correlation coefficient for total AKs improved (0·2–0·76 for observer 1; 0·08–0·66 for observer 2) as did Cohen’s κ for interobserver concordance for AKs correctly matched to clinical assessment (0·34–0·57). DICE coefficient analysis of interobserver concordance improved from 0·51 to 0·54 overall and from 0·65 to 0·72 for matched AKs. Kendall’s coefficient of concordance for keratosis did not improve.
Discussion
SPOT is the first RCT to compare standard topical AK field treatments against sunscreen in OTRs. It provides important information on feasibility, activity and evaluation outcomes for the design of a future phase III OTR‐cSCC topical chemoprevention trial, with a signal that 5‐FU is more effective than IMIQ or sunscreen in OTR AK treatment and prevention. Key strengths were the detailed characterization and tracking of individual AKs at multiple timepoints, inclusion of a sunscreen arm and matched CEZs, and inclusion of UL sites which have generally been excluded in previous studies.
In terms of feasibility outcomes, acceptability and tolerability of treatment and patient preferences may be barriers to the use of topical AK treatments in cSCC chemoprevention. However, SPOT provided encouraging evidence for these outcomes. In particular, predetermined criteria for recruitment, adherence to treatment and willingness to be re‐treated were all met across all intervention arms, despite differences in the intensity of LSRs. We have previously reported data generated by the SPOT discrete choice experiment on patient AK preferences. 21
In terms of activity outcomes, when SPOT was designed, head‐to‐head RCT comparisons of AK field treatments in the general population were few and conflicting 22 , 23 and information in OTRs sparse. Despite the limitations of a feasibility trial design, there was a signal that 5‐FU was superior to sunscreen in AK clearance, proportion of patients achieving at least 75% AK clearance and AK prevention, with effects persisting to 12 months post‐treatment. Comparisons between IMIQ and sunscreen showed lower efficacy of IMIQ; this may reflect its mechanism of action as a toll‐like receptor agonist and inherent dependence on an intact immune system. It may also reflect inclusion of UL sites in which IMIQ appears relatively less effective, possibly due to its lower solubility in the thicker stratum corneum on UL skin. 24 , 25 It is notable that approximately one‐third of AKs cleared in the sunscreen arm, underscoring the importance of including routine sunscreen in OTR AK treatment and prevention strategies in clinical practice and future phase III trials.
In comparison with previous studies of topical AK treatments in OTRs, although since the inception of SPOT there has been progress in addressing the evidence gap in AK treatment trials in the general population, 12 , 26 data in OTRs remain limited, 14 with only eight, largely low‐quality, RCTs identified in a 2019 systematic review. 13 PDT was investigated in six of eight RCTs, with 5‐FU and IMIQ included in only one of eight and two of eight RCTs, respectively (Table S5; see Supporting Information). The 5‐FU study was an intrapatient comparison of MAL‐PDT and 5‐FU in eight OTRs: 5‐FU clearance rates at 6 months were similar to those observed in SPOT, but AK100 rates were lower, possibly reflecting both the inclusion of Bowen disease and the use of 5‐FU as a single 3‐week cycle in this trial. 27 In the first RCT investigating IMIQ, 15 , 26 AK clearance rates at 8 weeks when used on HN sites for 16 weeks were similar to those seen in SPOT. 15 A second RCT that compared IMIQ and MAL‐PDT and AK clearance at 3 months with IMIQ was also similar. 28 PDT outcomes are broadly equivalent or inferior to those seen with 5‐FU in SPOT, but superior to IMIQ. 13 , 27 In the general population superiority and cost‐effectiveness of 5‐FU has recently been confirmed in an RCT randomizing 624 non‐OTRs to 5‐FU, IMIQ, MAL‐PDT or 0·015% ingenol mebutate. 12 , 29 Despite differences in the treatment regimens and exclusion of UL sites in this study, it is notable that AK75 for both IMIQ and 5‐FU in OTRs in SPOT were equivalent at 3 or 4 months post‐treatment, but lower by 12 months post‐treatment.
In comparison with previous cSCC prevention studies, until recently, few AK intervention studies included cSCC prevention as a primary endpoint. An exception was the landmark population‐based RCT conducted in Nambour, Australia, which showed significant reduction in AKs and 40% reduction in cSCCs with intensive sunscreen use in an immunocompetent population. 7 , 30 , 31 In OTRs, a nonrandomized study in Germany provided a signal of activity for intensive sunscreen use in cSCC prevention. 32 With the exception of PDT, which has not shown significant OTR‐cSCC prevention activity, 33 , 34 , 35 previous RCTs have investigated systemic chemoprevention in OTRs. In particular, cSCC chemoprevention activity with oral retinoids has been confirmed in several RCTs. 36 , 37 , 38 , 39 , 40 Modification of immunosuppression by switching to mTOR inhibitors also significantly reduced new cSCCs, but only after the first cSCC. 41 , 42 , 43
Since SPOT was initiated, two additional cSCC chemoprevention approaches have been evaluated in RCTs in the general population. 10 , 11 , 44 Oral nicotinamide compared with placebo reduced AKs by 13% and cSCCs by 30% over 12 months in Australian high‐risk immunocompetent individuals 44 and a study in OTRs is ongoing. 45 The Veterans Affairs Keratinocyte Carcinoma Chemoprevention (VAKCC) study compared 2–4 weeks of 5‐FU on HN vs. placebo in 932 non‐OTRs with two or more previous keratinocyte cancers: incident cSCCs were reduced by 75% in the first year, although this was nonsignificant by 4 years. 10 Similar to SPOT, VAKCC reported 74% reduction in AKs treated with 5‐FU at 6 months and 50% fewer new AKs at 12 months. 46 , 47 In a separate RCT, 4 days’ treatment with 5‐FU combined with 0·005% calcipotriol ointment was superior to 5‐FU alone in clearing AKs in 130 non‐OTRs and cSCC incidence in a subgroup of participants was reduced at 3 years, although this was significant only for HN sites. 48 The mechanism of calcipotriol action is believed to be through enhancement of T‐cell activation, and whether effectiveness is equivalent in OTRs is currently uncertain.
In terms of AK evaluation methodology outcomes, an important consideration for future trials is optimal AK assessment methodology. Before SPOT started, there were few validated methods for reliable clinical AK assessment. 49 , 50 Most studies quantify AKs using ‘total count’ and although consensus discussion improves interobserver reliability, this does not specifically capture new and regressing AKs. 49 , 50 , 51 , 52 , 53 To address these challenges, SPOT was preceded by a series of consensus studies to improve inter‐ and intra‐observer AK assessment. 17 Subsequently, AKASI (Actinic Keratosis Area and Severity Index) and photograph‐based AK‐FAS (AK field assessment scale) have been reported, but neither accurately tracks individual AKs as was required in SPOT. 54 , 55 However, the AK scoring methodology used in SPOT was time consuming. Photography is potentially more practical for larger, multicentre studies, but concordance in SPOT between clinical AK assessment and digital photography was limited. Photography currently appears to be insufficiently reliable to replace clinical AK assessment in the context of OTRs who often have a high burden of AKs and non‐AK keratotic lesions. Machine‐learning approaches for analysing photographic images may overcome these limitations in the future. 56 , 57
There are limitations in our study. Due to the unexpected closure of one of the trial sites after initiation of the study we were unable to meet our target sample size. Furthermore, the use of AKs as a surrogate endpoint biomarker for cSCC development is limited by the low and uncertain progression rate of AK to cSCC in both the general population and in OTRs. Nonetheless, in the context of a feasibility study, it was considered to be a justifiable surrogate outcome measure.
In conclusion, SPOT has provided information on important feasibility issues relevant to the development of phase III clinical trials for OTRs with AKs. It also provides a signal in OTRs that 5‐FU is consistently superior to sunscreen with IMIQ demonstrating some but less consistent superiority to sunscreen. Combined with recent evidence from the immunocompetent population, these data provide a compelling rationale for further investigation of 5‐FU‐based topical chemoprevention approaches in OTR‐cSCC prevention.
Author contributions
Zeeshaan Hasan: Data curation (equal); formal analysis (equal); investigation (equal); project administration (equal); writing – review and editing (equal). Ikhlaaq Ahmed: Formal analysis (equal); methodology (supporting); software (equal); validation (supporting); writing – review and editing (supporting). Rubeta N Matin: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); project administration (equal); supervision (equal); writing – review and editing (equal). Victoria s Homer: Data curation (equal); formal analysis (equal); methodology (equal); software (equal); writing – review and editing (equal). John Lear: Conceptualization (equal); funding acquisition (lead); investigation (equal); methodology (equal); supervision (equal); writing – review and editing (equal). Ferina Ismail: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (lead); investigation (equal); methodology (equal); project administration (equal); supervision (equal); writing – review and editing (equal). Tristan Whitmarsh: Data curation (equal); investigation (equal); software (equal); writing – review and editing (equal). Adele Green: Conceptualization (equal); funding acquisition (lead); methodology (equal); writing – review and editing (equal). Jason Thomson: Investigation (equal); writing – review and editing (equal). Alan Milligan: Investigation (equal); writing – review and editing (equal). Sarah Hogan: Investigation (equal); writing – review and editing (equal). Vanessa Van‐de‐Velde: Investigation (equal); writing – review and editing (equal). Liza Mitchell‐Worsford: Investigation (equal); writing – review and editing (equal). Jonathan Kentley: Investigation (equal); writing – review and editing (equal). Sarah J Bowden: Data curation (equal); formal analysis (equal); methodology (equal); project administration (equal); resources (equal); software (equal); supervision (equal); writing – review and editing (equal). Piers Gaunt: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); software (equal); validation (equal); writing – review and editing (equal). Keith Wheatley: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); methodology (equal); project administration (equal); software (equal); supervision (equal); validation (equal); writing – review and editing (equal). Charlotte M Proby: Conceptualization (equal); data curation (equal); formal analysis (equal); funding acquisition (lead); investigation (equal); methodology (equal); project administration (equal); supervision (equal); writing – review and editing (equal). Catherine Harwood: Conceptualization (lead); data curation (lead); formal analysis (supporting); funding acquisition (lead); investigation (lead); methodology (lead); project administration (lead); resources (lead); supervision (lead); writing – review and editing (lead).
Funding sources
This was an investigator‐initiated and investigator‐led trial funded by the National Institute for Health Research via the Research for Patient Benefit programme (PB‐PG‐0110‐21244). The study was coordinated by and developed in collaboration with the Cancer Research UK Clinical Trials Unit (CRUKCTU), University of Birmingham. Staff at the CRUKCTU are supported by a core funding grant from Cancer Research UK (C22436/A25354). The study was also developed with support from the UK Dermatology Clinical Trials Network (UK DCTN), which is financially supported by the British Association of Dermatologists and the University of Nottingham. The National Cancer Research Institute Skin Cancer Clinical Studies Group were also involved in trial development. Commercial support was provided by MEDA pharmaceuticals who supplied the 5% 5‐fluorouracil and 5% imiquimod creams. No pharmaceutical company had any role in the design of the trial, collection or analysis of the data, or writing of the manuscript. The sponsor was Queen Mary University of London. The EudraCT number is 2013‐000893‐32.
Conflicts of interest
The authors declare they have no conflicts of interest.
Data availability
Data are available in the accompanying online Supporting Information.
Ethics statement
The trial was approved by the London‐Chelsea Research Ethics Committee (REC 13/LO/1579).
Supporting information
File S1 Squamous cell carcinoma Prevention in Organ transplant recipients using Topical treatments: a feasibility study (SPOT protocol).
Table S1 Local skin reactions.
Table S2 High‐resolution digital photography assessment vs. clinical assessment.
Table S3 Adverse events and serious adverse events.
Table S4 Health‐related quality of life assessments.
Table S5 Comparison of results from SPOT study with selected RCTs.
Figure S1 Clinical assessments of actinic keratoses.
Figure S2 Mean estimate of the proportion of patients achieving AK100 and AK75.
Figure S3 Mean number of new actinic keratoses per trial visit.
Figure S4 Number of new actinic keratoses in the treatment and clinically equivalent zones.
Figure S5 Cumulative incidence of cutaneous squamous cell carcinoma development by treatment arm.
Appendix S1 Sensitivity analyses.
Appendix S2 Hierarchical modelling of percentage actinic keratosis clearance.
Appendix S3 Hierarchical modelling of new actinic keratoses.
Appendix S4 Hierarchical modelling of total actinic keratoses.
I.A. and R.N.M. contributed equally.
Plain language summary available online
References
- 1. Venables ZC, Nijsten T, Wong KF et al. Epidemiology of basal and cutaneous squamous cell carcinoma in the U.K. 2013–15: a cohort study. Br J Dermatol 2019; 181:474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harwood CA, Toland AE, Proby CM et al. The pathogenesis of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol 2017; 177:1217–24. [DOI] [PubMed] [Google Scholar]
- 3. Venables ZC, Autier P, Nijsten T et al. Nationwide incidence of metastatic cutaneous squamous cell carcinoma in England. JAMA Dermatol 2019; 155:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomson J, Bewicke‐Copley F, Anene CA et al. The genomic landscape of actinic keratosis. J Invest Dermatol 2021; 141:1664–74.e7. 10.1016/j.jid.2020.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallingford SC, Russell SA, Vail A et al. Actinic keratoses, actinic field change and associations with squamous cell carcinoma in renal transplant recipients in Manchester, UK. Acta Derm Venereol 2015; 95:830–4. [DOI] [PubMed] [Google Scholar]
- 6. Christensen GB, Ingvar C, Hartman LW et al. Sunbed use increases cutaneous squamous cell carcinoma risk in women: a large‐scale, prospective study in Sweden. Acta Derm Venereol 2019; 99:878–83. [DOI] [PubMed] [Google Scholar]
- 7. Green A, Williams G, Neale R et al. Daily sunscreen application and betacarotene supplementation in prevention of basal‐cell and squamous‐cell carcinomas of the skin: a randomised controlled trial. Lancet 1999; 354:723–9. [DOI] [PubMed] [Google Scholar]
- 8. Marks R, Rennie G, Selwood TS. Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet 1988; 1:795–7. [DOI] [PubMed] [Google Scholar]
- 9. Darlington S, Williams G, Neale R et al. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol 2003; 139:451–5. [DOI] [PubMed] [Google Scholar]
- 10. Weinstock MA, Thwin SS, Siegel JA et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol 2018; 154:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cunningham TJ, Tabacchi M, Eliane JP et al. Randomized trial of calcipotriol combined with 5‐fluorouracil for skin cancer precursor immunotherapy. J Clin Invest 2017; 127:106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jansen MHE, Kessels JPHM, Nelemans PJ et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med 2019; 380:935–46. [DOI] [PubMed] [Google Scholar]
- 13. Heppt MV, Steeb T, Niesert AC et al. Local interventions for actinic keratosis in organ transplant recipients: a systematic review. Br J Dermatol 2019; 180:43–50. [DOI] [PubMed] [Google Scholar]
- 14. Werner RN, Nast A. ‘Surprisingly little evidence’ on how best to treat actinic keratosis in organ transplant recipients. Br J Dermatol 2019; 180:11–12. [DOI] [PubMed] [Google Scholar]
- 15. Ulrich C, Bichel J, Euvrard S et al. Topical immunomodulation under systemic immunosuppression: results of a multicentre, randomized, placebo‐controlled safety and efficacy study of imiquimod 5% cream for the treatment of actinic keratoses in kidney, heart, and liver transplant patients. Br J Dermatol 2007; 157 (Suppl. 2):25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ismail F, Mitchell L, Casabonne D et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol 2006; 155:916–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matin RN, Gaunt P, McCartney et al. Developing a standardized actinic keratosis assessment protocol. Br J Dermatol 2013; 169:99–112. [Google Scholar]
- 18. US Department of Health and Human Services . Common Terminology Criteria for Adverse Events (CTCAE), v4.0: 28 May 2009; v4.03: 14 June 2010. Available at: https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010‐06‐14_quickreference_5x7.pdf
- 19. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19:210–16. [DOI] [PubMed] [Google Scholar]
- 20. Rhee JS, Matthews BA, Neuburg M et al. The Skin Cancer Index: clinical responsiveness and predictors of quality of life. Laryngoscope 2007; 117:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kopasker D, Kwiatkowski A, Matin RN et al. Patient preferences for topical treatment of actinic keratoses: a discrete‐choice experiment. Br J Dermatol 2019; 180:902–9. [DOI] [PubMed] [Google Scholar]
- 22. Gupta AK, Paquet M, Villanueva E, Brintnell W. Interventions for actinic keratoses. Cochrane Database Syst Rev 2012; 12:CD004415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vegter S, Tolley K. A network meta‐analysis of the relative efficacy of treatments for actinic keratosis of the face or scalp in Europe. PLoS One 2014; 9:e96829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Telò I, Pescina S, Padula C et al. Mechanisms of imiquimod skin penetration. Int J Pharm 2016; 511:516–23. [DOI] [PubMed] [Google Scholar]
- 25. Monnier J, Tognetti L, Miyamoto M et al. In vivo characterization of healthy human skin with a novel, non‐invasive imaging technique: line‐field confocal optical coherence tomography. J Eur Acad Dermatol Venereol 2020; 34:2914–21. [DOI] [PubMed] [Google Scholar]
- 26. Cornejo CM, Jambusaria‐Pahlajani A, Willenbrink TJ et al. Field cancerization: treatment. J Am Acad Dermatol 2020; 83:719–30. [DOI] [PubMed] [Google Scholar]
- 27. Perrett CM, McGregor JM, Warwick J et al. Treatment of post‐transplant premalignant skin disease: a randomized intrapatient comparative study of 5‐fluorouracil cream and topical photodynamic therapy. Br J Dermatol 2007; 156:320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Togsverd‐Bo K, Halldin C, Sandberg C et al. Photodynamic therapy is more effective than imiquimod for actinic keratosis in organ transplant recipients: a randomized intraindividual controlled trial. Br J Dermatol 2018; 178:903–9. [DOI] [PubMed] [Google Scholar]
- 29. Jansen MHE, Kessels JPHM, Merks I et al. A trial‐based cost‐effectiveness analysis of topical 5‐fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br J Dermatol 2020; 183:738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van der Pols JC, Williams GM, Pandeya N et al. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol Biomarkers Prev 2006; 15:2546–8. [DOI] [PubMed] [Google Scholar]
- 31. Gordon LG, Scuffham PA, van der Pols JC et al. Regular sunscreen use is a cost‐effective approach to skin cancer prevention in subtropical settings. J Invest Dermatol 2009; 129:2766–71. [DOI] [PubMed] [Google Scholar]
- 32. Ulrich C, Jürgensen JS, Degen A et al. Prevention of non‐melanoma skin cancer in organ transplant patients by regular use of a sunscreen: a 24 months, prospective, case–control study. Br J Dermatol 2009; 161 (Suppl. 3):78–84. [DOI] [PubMed] [Google Scholar]
- 33. de Graaf YG, Kennedy C, Wolterbeek R et al. Photodynamic therapy does not prevent cutaneous squamous‐cell carcinoma in organ‐transplant recipients: results of a randomized‐controlled trial. J Invest Dermatol 2006; 126:569–74. [DOI] [PubMed] [Google Scholar]
- 34. Wulf HC, Pavel S, Stender I et al. Topical photodynamic therapy for prevention of new skin lesions in renal transplant recipients. Acta Derm Venereol 2006; 86:25–8. [DOI] [PubMed] [Google Scholar]
- 35. Wennberg AM, Stenquist B, Stockfleth E et al. Photodynamic therapy with methyl aminolevulinate for prevention of new skin lesions in transplant recipients: a randomized study. Transplantation 2008; 86:423–9. [DOI] [PubMed] [Google Scholar]
- 36. George R, Weightman W, Russ GR et al. Acitretin for chemoprevention of non‐melanoma skin cancers in renal transplant recipients. Australas J Dermatol 2002; 43:269–73. [DOI] [PubMed] [Google Scholar]
- 37. de Sévaux RG, Smit JV, de Jong EM et al. Acitretin treatment of premalignant and malignant skin disorders in renal transplant recipients: clinical effects of a randomized trial comparing two doses of acitretin. J Am Acad Dermatol 2003; 49:407–12. [DOI] [PubMed] [Google Scholar]
- 38. Badri O, Schmults CD, Karia PS, Ruiz ES. Efficacy and cost analysis for acitretin for basal and squamous cell carcinoma prophylaxis in renal transplant recipients. Dermatol Surg 2021; 47:125–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tee LY, Sultana R, Tam SYC, Oh CC. Chemoprevention of keratinocyte carcinoma and actinic keratosis in solid‐organ transplant recipients: systematic review and meta‐analyses. J Am Acad Dermatol 2021; 84:528–30. [DOI] [PubMed] [Google Scholar]
- 40. Hoegler KM, Khachemoune A. Is the first‐line systemic chemoprevention of nonmelanoma skin cancer nicotinamide or acitretin? Int J Dermatol 2021; 60:749–50. [DOI] [PubMed] [Google Scholar]
- 41. Euvrard S, Morelon E, Rostaing L et al. Sirolimus and secondary skin‐cancer prevention in kidney transplantation. N Engl J Med 2012; 367:329–39. [DOI] [PubMed] [Google Scholar]
- 42. Hoogendijk‐van den Akker JM, Harden PN, Hoitsma AJ et al. Two‐year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol 2013; 31:1317–23. [DOI] [PubMed] [Google Scholar]
- 43. Alter M, Satzger I, Schrem H et al. Non‐melanoma skin cancer is reduced after switch of immunosuppression to mTOR‐inhibitors in organ transplant recipients. J Dtsch Dermatol Ges 2014; 12:480–8. [DOI] [PubMed] [Google Scholar]
- 44. Chen AC, Martin AJ, Choy B et al. A phase 3 randomized trial of nicotinamide for skin‐cancer chemoprevention. N Engl J Med 2015; 373:1618–26. [DOI] [PubMed] [Google Scholar]
- 45. Damain D. Effect of oral nicotinamide on non‐melanoma skin cancer incidence and actinic keratosis in renal, hepatic, heart and lung transplant recipients: a randomised controlled trial. Trial ACTRN12617000599370, 2017, registered at: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372709 (accessed 21 February 2021).
- 46. Pomerantz H, Hogan D, Eilers D et al. Long‐term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol 2015; 151:952–60. [DOI] [PubMed] [Google Scholar]
- 47. Walker JL, Siegel JA, Sachar M et al. 5‐Fluorouracil for actinic keratosis treatment and chemoprevention: a randomized controlled trial. J Invest Dermatol 2017; 137:1367–70. [DOI] [PubMed] [Google Scholar]
- 48. Rosenberg AR, Tabacchi M, Ngo KH et al. Skin cancer precursor immunotherapy for squamous cell carcinoma prevention. JCI Insight 2019; 4:e125476. 10.1172/jci.insight.125476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Weinstock MA, Bingham SF, Cole GW et al. Reliability of counting actinic keratoses before and after brief consensus discussion: the VA Topical Tretinoin Chemoprevention (VATTC) trial. Arch Dermatol 2001; 137:1055–8. [PubMed] [Google Scholar]
- 50. Epstein E. Quantifying actinic keratosis: assessing the evidence. Am J Clin Dermatol 2004; 5:141–4. [DOI] [PubMed] [Google Scholar]
- 51. Chen SC, Hill ND, Veledar E et al. Reliability of quantification measures of actinic keratosis. Br J Dermatol 2013; 169:1219–22. [DOI] [PubMed] [Google Scholar]
- 52. Lee KC, Lew R, Weinstock MA. Improvement in precision of counting actinic keratoses. Br J Dermatol 2014; 170:188–91. [DOI] [PubMed] [Google Scholar]
- 53. Jiyad Z, Marquart L, O’Rourke P, Green AC. Incidence and regression of actinic keratoses in organ transplant recipients. Acta Derm Venereol 2018; 98:77–81. [DOI] [PubMed] [Google Scholar]
- 54. Dirschka T, Pellacani G, Micali G et al. A proposed scoring system for assessing the severity of actinic keratosis on the head: actinic keratosis area and severity index. J Eur Acad Dermatol Venereol 2017; 31:1295–302. [DOI] [PubMed] [Google Scholar]
- 55. Dréno B, Cerio R, Dirschka T et al. A novel actinic keratosis field assessment scale for grading actinic keratosis disease severity. Acta Derm Venereol 2017; 97:1108–13. [DOI] [PubMed] [Google Scholar]
- 56. Sinnya S, O’Rourke P, Ballard E et al. Counting actinic keratosis – is photographic assessment a reliable alternative to physical examination in clinical trials? Acta Derm Venereol 2015; 95:604–5. [DOI] [PubMed] [Google Scholar]
- 57. Jiyad Z, O’Rourke P, Soyer HP, Green AC. Assessing the concordance of actinic keratosis counts on digital photographs with clinical examination in organ transplant recipients. Acta Derm Venereol 2017; 97:351–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File S1 Squamous cell carcinoma Prevention in Organ transplant recipients using Topical treatments: a feasibility study (SPOT protocol).
Table S1 Local skin reactions.
Table S2 High‐resolution digital photography assessment vs. clinical assessment.
Table S3 Adverse events and serious adverse events.
Table S4 Health‐related quality of life assessments.
Table S5 Comparison of results from SPOT study with selected RCTs.
Figure S1 Clinical assessments of actinic keratoses.
Figure S2 Mean estimate of the proportion of patients achieving AK100 and AK75.
Figure S3 Mean number of new actinic keratoses per trial visit.
Figure S4 Number of new actinic keratoses in the treatment and clinically equivalent zones.
Figure S5 Cumulative incidence of cutaneous squamous cell carcinoma development by treatment arm.
Appendix S1 Sensitivity analyses.
Appendix S2 Hierarchical modelling of percentage actinic keratosis clearance.
Appendix S3 Hierarchical modelling of new actinic keratoses.
Appendix S4 Hierarchical modelling of total actinic keratoses.
Data Availability Statement
Data are available in the accompanying online Supporting Information.


