Abstract
We conducted a series of experiments examining the effect of polymer stability on FtsZ localization dynamics in Bacillus subtilis. A loss-of-function mutation in ezrA, a putative polymer-destabilizing factor, suppresses the defects in FtsZ polymer stability associated with minCD overexpression. In addition, a mutation that is predicted to stabilize the FtsZ polymer leads to the formation of polar FtsZ rings. These data support the hypothesis that carefully balanced polymer stability is important for the assembly and localization of FtsZ during the bacterial cell cycle.
Cell division is tightly regulated to ensure accurate and efficient segregation of chromosomal material and to maintain cell size and shape. Throughout the bacterial and archaeal kingdoms, the position of the division septum appears to be controlled largely via the localization of the tubulin-like GTPase FtsZ (17). In response to one or more cell cycle signals, FtsZ forms a ring at the nascent division site. This ring then serves as a framework for assembly of the division apparatus (17, 30). Although FtsZ has been shown to be required for cell division in several organisms, the presence of an FtsZ ring alone is not sufficient for cytokinesis (14, 17).
Two factors are known to play important roles in modulating the position of the division septum in the gram-positive bacterium Bacillus subtilis: MinC and MinD, which function as a complex (18), and the 65-kDa protein EzrA (13). MinCD is concentrated at the cell poles, where it inhibits aberrant FtsZ ring formation (24). Null mutations in minC or minD lead to the formation of polar FtsZ rings and polar septa (12, 15, 16, 27). Escherichia coli MinC inhibits FtsZ polymerization in vitro (9), and it is likely that MinCD inhibition of polar FtsZ ring formation in B. subtilis also takes place through direct interactions between FtsZ and MinC. The polar localization of MinCD in B. subtilis is dependent on the 164-residue DivIVA, whereas in E. coli the MinCD complex is kept away from midcell by MinE, an 88-residue protein that shares no apparent homology with DivIVA (7, 24).
Null mutations in ezrA, like those in minCD, lead to the formation of polar FtsZ rings (13). However, in contrast to MinCD, EzrA is distributed throughout the plasma membrane and is concentrated at the nascent septal site in an FtsZ-dependent manner (13). A minCD ezrA double mutant displays a more severe division defect than either single mutant, suggesting that the two factors act independently to inhibit inappropriate FtsZ ring formation. The loss of EzrA apparently results in hyperstabilization of the FtsZ polymer. First, a null mutation in ezrA suppresses the temperature-sensitive phenotype of a conditional allele of ftsZ (13). Also, the loss of ezrA significantly lowers the concentration of FtsZ required to initiate ring formation in vivo (13). These data support a model in which EzrA acts throughout the plasma membrane to destabilize the FtsZ polymer and inhibit inappropriate FtsZ assembly. At the same time, however, a positively acting factor (perhaps a component of the putative FtsZ nucleation site) overcomes EzrA inhibition at midcell, permitting the formation of a medial FtsZ ring even in the presence of EzrA. Although it does not prevent FtsZ assembly at midcell, EzrA presumably contributes to the dynamic nature of the medial FtsZ ring.
Our observation that a loss-of-function mutation in ezrA, a putative destabilizer of the FtsZ polymer, leads to the formation of polar FtsZ rings led us to consider the idea that polymer stability plays an important role in the spatial regulation of FtsZ. In support of this model, increasing the intracellular concentration of FtsZ leads to polar divisions and the formation of anucleate minicells in E. coli (29) and the formation of polar FtsZ rings in B. subtilis (P. A. Levin, unpublished data). A twofold increase in expression of the FtsZ-stabilizing protein ZipA in E. coli also results in the formation of polar FtsZ rings (19). In this report, we examine the effect of factors predicted to stabilize the FtsZ polymer on FtsZ assembly and localization dynamics.
Overexpression of minCD leads to filamentation in B. subtilis
Overexpression of minCD in E. coli leads to extensive filamentation and cell death (3, 11). Similarly, Marston and Errington have shown that overexpression of either GFP-minD alone or GFP-minCD is sufficient to inhibit cell division in otherwise wild-type B. subtilis cells (18). To establish that overexpression of wild-type minCD is sufficient to inhibit cell division in B. subtilis, we constructed a strain in which a second copy of minCD was placed under the control of a modified version of the LacI-repressible, IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter Pspac (J. D. Quisel, W. Burkholder, and A. D. Grossman, submitted for publication) at the amylase locus (PL1138). This promoter (Pspac-hy) is 10- to 20-fold stronger than Pspac (Quisel et al., submitted).
Induction of Pspac-hy-minCD strongly inhibited cell division, resulting in severe filamentation and eventually cell death. Viability assays indicated that the plating efficiency of this strain (PL1138) is 50- to 100-fold lower in the presence of 1 mM IPTG than in the absence of inducer. Cells were sampled from a mid-exponential-phase culture (optical density at 600 nm [OD600] of 0.5) and serially diluted onto solid medium in the presence or absence of IPTG. The CFU per milliliter were 1.7 × 105 in the absence of IPTG and 4.0 × 103 in the presence of IPTG for the Pspac-hy-minCD ezrA::spc strain. In the absence of IPTG, MinD levels in these cells were at most twofold above wild type, as measured by quantitative immunoblotting using antibodies against MinD (data not shown). However, in the presence of IPTG, the intracellular concentration of MinD was increased approximately 15-fold within 90 min of induction (data not shown).
Overexpression of minCD inhibits FtsZ ring formation.
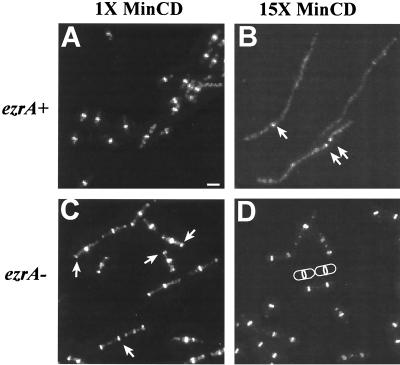
To examine the effect of minCD overexpression on FtsZ ring formation, we used antibodies against FtsZ and immunofluorescence microscopy to localize FtsZ in cells encoding Pspac-hy-minCD grown in the absence and presence of IPTG (Fig. 1A and B). We found that overexpression of minCD severely inhibited FtsZ ring formation (Fig. 1B). Filaments examined 90 min after induction of minCD were 10 to 15 times longer than wild-type cells. Before induction, the frequency of FtsZ ring formation in this strain background was approximately one ring per 1.3 cell lengths. After induction of minCD, the frequency of FtsZ ring formation fell to less than one ring per 6 cell lengths. (Average cell length was determined by measuring wild-type cells grown under conditions identical to those used to induce Pspac-hy-minCD). These data suggest that an increase in the intracellular concentration of MinCD leads to a mislocalization of the complex and prevents FtsZ from forming stable rings at the nascent division site.
FIG. 1.
Stabilization of the FtsZ polymer in the absence of ezrA suppresses the division defect associated with minCD overexpression. The figure shows the immunolocalization of FtsZ in cells in which minCD expression is under the control of a strong IPTG-inducible promoter. Cells were grown to an OD600 of approximately 0.5 in Luria-Bertani medium, after which they were resuspended at a 1:10 dilution in fresh medium in the absence (A and C) or presence (B and D) of 1 mM IPTG. Samples were taken 90 min after resuspension, fixed in methanol, and stained essentially as described elsewhere (13). (A and B) PL1138 (amyE::Pspac-hy-minCD ezrA+); (C and D) PL1152 (amyE::Pspac-hy-minCD ezrA::spc). Arrows point to rings of FtsZ in panel B and examples of polar rings of FtsZ in panel C. A cartoon illustrates a pair of cells with medial rings in panel D. Bar = 2 μm.
The target of MinCD division inhibition in E. coli is somewhat controversial. Biochemical data indicate that E. coli MinC is sufficient to inhibit FtsZ assembly in vitro (9). However, genetic and cell biological data suggest that the primary target of the MinCD division inhibitor in E. coli may not be FtsZ directly but rather the interaction between FtsZ and the putative polymer-stabilizing protein FtsA (11). In contrast to E. coli, FtsA is not essential for viability in B. subtilis (1), and although we cannot rule out the possibility that FtsA is also involved, we believe our data favor the hypothesis that FtsZ is the primary target of MinCD in B. subtilis.
A null mutation in ezrA suppresses the growth defect associated with minCD overexpression.
A null mutation in ezrA leads to the formation of polar FtsZ rings in B. subtilis (13). Although other interpretations are possible (e.g., EzrA binds to and inactivates a membrane-bound FtsZ localization determinant at cell poles), these data suggest that an EzrA deficiency may stabilize FtsZ polymers such that they are resistant to MinCD activity at the cell poles, leading to the formation of polar FtsZ rings. We determined whether the increase in polymer stability observed in the absence of EzrA was sufficient to overcome the inhibition of FtsZ polymerization at midcell resulting from overexpression of minCD.
As expected, in the absence of IPTG, the ezrA null mutant (PL1151 amyE::Pspac-hy-minCD ezrA::spc) exhibits a typical ezrA phenotype with regard to the frequency and position of FtsZ ring formation (Fig. 1C). Cells often had an FtsZ ring at one or both poles in addition to the medial ring, and the frequency of FtsZ ring formation was approximately one ring per 0.8 cell lengths, consistent with the high frequency of FtsZ ring formation.
In contrast to the congenic ezrA+ strain (PL1138), the plating efficiency of the ezrA null mutant is identical in the presence and the absence of IPTG, indicating that the loss of ezrA completely suppresses the growth defect associated with overexpression of minCD. The CFU per milliliter were 4.2 × 108 in the absence of IPTG and 3.9 × 108 in the presence of IPTG for the Pspac-hy-minCD ezrA::spc strain. Consistent with this observation, immunofluorescence microscopy indicates that the loss-of-function mutation in ezrA restores normal FtsZ ring formation to cells in the presence of excess MinCD (Fig. 1D). The frequency of FtsZ ring formation in the ezrA null mutant background in the presence of 1 mM IPTG was one ring per cell length, approximately sixfold higher than the ezrA+ background. Polar FtsZ rings were not visible in the ezrA null cells following induction of minCD, indicating that the loss of one division inhibitor (EzrA) can be compensated for by increasing the level of a second division inhibitor (MinCD). Quantitative immunoblotting demonstrates that there is no difference in MinCD concentration in the presence and the absence of ezrA (data not shown). These results suggest that the loss of EzrA stabilizes the FtsZ polymer to an extent sufficient to overcome the inhibition of ring formation associated with minCD overexpression.
A mutation in the GTP binding site of B. subtilis FtsZ suppresses the minCD overexpression phenotype.
FtsZ polymerizes in vitro in a GTP-dependent manner (5, 17). Work with GTPase-defective alleles of ftsZ suggests that GTP hydrolysis is essential for turnover of FtsZ polymers in vitro and for FtsZ activity in vivo (6, 20, 26). Structural and biochemical data indicate that dimerization of FtsZ creates an active site for the hydrolysis of a single shared GTP molecule (8). GDP-bound multimers of FtsZ are unstable and quickly fall apart (21, 22, 25). Factors that inhibit GTPase activity but do not affect GTP binding are, therefore, expected to stabilize the FtsZ polymer.
Overexpression of minCD in E. coli can be suppressed by four- to fivefold overexpression of ftsZ (4) or by one of several rsa (also called sulB) mutations in ftsZ (10) originally isolated on the basis of their ability to suppress filamentation caused by expression of the division inhibitor SulA (2, 4). Although the resistance of these ftsZ alleles to minCD overexpression could be explained by the loss of residues important for FtsZ-MinCD interaction, we favor the hypothesis that the rsa/sulB mutations in ftsZ stabilize the FtsZ polymer, rendering it resistant to both MinCD and SulA. Consistent with this model, six of the E. coli rsa/sulB mutations have been shown to alter the interaction between FtsZ and GTP and several of them permit GTP binding but specifically inhibit hydrolysis (6, 26).
To determine if a similar mutation in the GTP binding site of B. subtilis ftsZ can also render cells insensitive to minCD overexpression, we constructed an allele of B. subtilis ftsZ (D213G) corresponding to E. coli ftsZ2 (D212G) based on sequence alignment data (8; H. P. Erickson, personal communication). E. coli FtsZ2 binds GTP almost as well as wild-type FtsZ but is at least 200-fold reduced in GTPase activity (6, 26). Although we cannot be certain that the D213G mutation in B. subtilis FtsZ has a phenotype identical to that of E. coli FtsZ2 with regard to GTP binding and hydrolysis, Asp212 is conserved among all known FtsZs and has been implicated in coordinating a metal ion and the nucleotide at the interface of two FtsZ monomers (23). A similar mutation in Caulobacter crescentus ftsZ (DVR216AVA) functions as a dominant lethal mutation when expressed in conjunction with wild-type ftsZ, suggesting that it incorporates into the FtsZ ring at midcell and prevents division altogether (28). Like E. coli ftsZ2, B. subtilis ftsZ2 is not able to support cell division in the absence of wild-type ftsZ (data not shown).
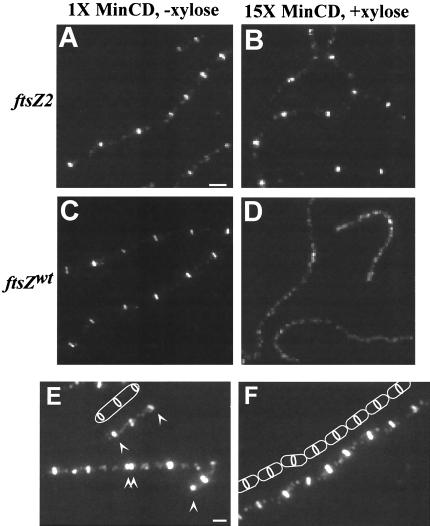
To test the ability of B. subtilis ftsZ2 to suppress minCD overexpression, ftsZ2 was placed under the control of a xylose-inducible promoter at the threonine locus. Using this construct in conjunction with Pspac-hy-minCD (strain PL1190), we found that, like its E. coli counterpart, B. subtilis ftsZ2 is able to suppress the division defect and restore ring formation to cells in the presence of excess MinCD (Fig. 2A and B). In contrast, expression of a second copy of wild-type ftsZ from the same xylose promoter in an identical strain background (PL1192) was not sufficient to overcome the actions of the MinCD division inhibitor (Fig. 2C and D). FtsZ protein levels are identical in the Pxyl-ftsZ and Pxyl-ftsZ2 strains in the presence of xylose (data not shown). Thus, suppression of the lethality associated with minCD overexpression by ftsZ2 is not simply the result of an increase in FtsZ protein levels. Instead, these results support our model that an increase in FtsZ polymer stability in the presence of the GTPase-defective FtsZ protein overcomes the effect of minCD overexpression.
FIG. 2.
The GTPase-defective allele of ftsZ (ftsZ2 D213G) suppresses the division defect associated with minCD overexpression and leads to the formation of aberrant polar FtsZ rings. (A to D) Immunolocalization of FtsZ in cells encoding both a copy of minCD under the control of a strong IPTG-inducible promoter at the amyE locus and a second copy of ftsZ under the control of a xylose-inducible promoter at the thrC locus. Cells were grown to an OD600 of approximately 0.5 in Luria-Bertani medium and diluted 1:10 in fresh medium in the absence (A and C) or presence (B and D) of 1 mM IPTG and 0.5% xylose. Cells were fixed and stained for immunofluorescence microscopy 90 min after resuspension. (A and B) PL1190 (amyE::Pspac-hy-minCD thrC::Pxyl-ftsZ2). (C and D) PL1192 (amyE::Pspac-hy-minCD thrC::Pxyl-ftsZ). Bar = 2 μm. (E and F) Immunolocalization of FtsZ in cells expressing either a copy of the GTPase-defective ftsZ2 allele, Pxyl-ftsZ2 (E), or a second copy of wild-type ftsZ, Pxyl-ftsZ2 (F), under the control of a xylose-inducible promoter at thrC. Cells were grown to an OD600 of approximately 0.5 in Luria-Bertani medium, diluted 1:10 in fresh medium in the presence of 0.5% xylose, and fixed and stained for immunofluorescence microscopy 90 min after resuspension. Arrows point to polar rings of FtsZ in panel E. Cartoons illustrate a cell with medial and polar FtsZ rings in panel E and cell boundaries and FtsZ rings in the chain of cells in panel F. Bar = 1 μm.
The GTPase-defective allele of B. subtilis ftsZ leads to the formation of extra FtsZ rings at polar positions in otherwise wild-type cells.
Based on data from the ezrA null mutant, we predicted that stabilization of the FtsZ polymer by ftsZ2 (and not ftsZwt) would lead to the formation of extra FtsZ rings at polar positions in otherwise wild-type cells. Using a strain (PL1187) encoding the xylose-inducible allele of ftsZ2 at the threonine locus (thrC::Pxyl-ftsZ2), we found that a 90-min induction was sufficient to cause the formation of extra FtsZ rings at cell poles, similar to those observed in ezrA null mutant cells (Fig. 2E). In contrast, a congenic strain (PL1118) encoding a wild-type allele of ftsZ under the same xylose promoter (thrC::Pxyl-ftsZ) had only single medial FtsZ rings after 90 min of growth in the presence of xylose (Fig. 2F). Quantitative immunoblotting showed no difference in FtsZ protein levels between the thrC::Pxyl-ftsZ strain and the thrC::Pxyl-ftsZ2 strain in the presence of xylose (data not shown).
Polymer stability and the assembly and localization of FtsZ.
Our results suggest that the relative stability of the FtsZ polymer plays an important role in modulating the frequency and position of FtsZ ring formation. Both the loss of ezrA, an inhibitor of FtsZ ring formation, and a mutation in ftsZ that is predicted to stabilize the FtsZ polymer, ftsZ2, lead to the formation of aberrant polar FtsZ rings. These aberrant polar FtsZ rings are apparently the result of the hyperstable FtsZ polymer overcoming MinCD inhibition at cell poles. Consistent with this, both an ezrA null mutation and coexpression of ftsZ2 with wild-type ftsZ are able to suppress the growth defect associated with minCD overexpression, restoring normal growth and ring formation in the presence of excess MinCD.
These data support a model in which the localization of FtsZ, and hence the spatial regulation of cell division, is governed by an assortment of factors that modulate FtsZ polymer dynamics throughout the cell and throughout the cell cycle. The activity of some factors, like MinCD, is normally restricted to one region of the cell, while others, like EzrA, function in a more global manner, to alter the concentration of FtsZ required for assembly at the plasma membrane. This model predicts the existence not only of negative-acting factors that inhibit FtsZ ring formation but also of positive-acting factors, such as components of the putative FtsZ nucleation site at midcell, that promote FtsZ ring formation, ensuring that it occurs at both the correct position and the appropriate stage of the cell cycle.
Acknowledgments
We thank Debabrata RayChaudhuri for experimental suggestions and Peter Chivers, Bob Kranz, and Laura Romberg for valuable comments on the manuscript. We also thank Jeffery Errington for the generous gift of antiserum against MinD. Finally, we gratefully acknowledge discussions with M & M participants and members of the Grossman laboratory.
This work was supported by Public Health Services grant GM41934 to A.D.G., by the Merck/MIT Collaborative Program, a Merck/MIT postdoctoral fellowship to P.A.L., and an institutional research grant from the American Cancer Society (IRG-58-010-44) to P.A.L.
REFERENCES
- 1.Beall B, Lutkenhaus J. Impaired cell division and sporulation of a Bacillus subtilis strain with the ftsA gene deleted. J Bacteriol. 1992;174:2398–2403. doi: 10.1128/jb.174.7.2398-2403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi E, Lutkenhaus J. Analysis of ftsZ mutations that confer resistance to the cell division inhibitor SulA (SfiA) J Bacteriol. 1990;172:5602–5609. doi: 10.1128/jb.172.10.5602-5609.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bi E, Lutkenhaus J. Interaction between the min locus and ftsZ. J Bacteriol. 1990;172:5610–5616. doi: 10.1128/jb.172.10.5610-5616.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramhill D, Thompson C M. GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc Natl Acad Sci USA. 1994;91:5813–5817. doi: 10.1073/pnas.91.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai K, Mukherjee A, Xu Y, Lutkenhaus J. Mutations in ftsZ that confer resistance to SulA affect the interaction of FtsZ with GTP. J Bacteriol. 1994;176:130–136. doi: 10.1128/jb.176.1.130-136.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Boer P A J, Crossley R E, Rothfield L I. A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum. Cell. 1989;56:641–649. doi: 10.1016/0092-8674(89)90586-2. [DOI] [PubMed] [Google Scholar]
- 8.Erickson H P. Atomic structures of tubulin and FtsZ. Trends Cell Biol. 1998;8:133–137. doi: 10.1016/s0962-8924(98)01237-9. [DOI] [PubMed] [Google Scholar]
- 9.Hu Z, Mukherjee A, Pichoff S, Lutkenhaus J. The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc Natl Acad Sci USA. 1999;96:14819–14824. doi: 10.1073/pnas.96.26.14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Cao C, Lutkenhaus J. Interaction between FtsZ and inhibitors of cell division. J Bacteriol. 1996;178:5080–5085. doi: 10.1128/jb.178.17.5080-5085.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Justice S S, Garcia-Lara J, Rothfield L I. Cell division inhibitors SulA and MinC/MinD block septum formation at different steps in the assembly of the Escherichia coli division machinery. Mol Microbiol. 2000;37:410–423. doi: 10.1046/j.1365-2958.2000.02007.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee S, Price C W. The minCD locus of Bacillus subtilis lacks the minE determinant that provides topological specificity to cell division. Mol Microbiol. 1993;7:601–610. doi: 10.1111/j.1365-2958.1993.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 13.Levin P A, Kurtser I G, Grossman A D. Identification and characterization of a negative regulator of FtsZ ring formation in Bacillus subtilis. Proc Natl Acad Sci USA. 1999;96:9642–9647. doi: 10.1073/pnas.96.17.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levin P A, Losick R. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 15.Levin P A, Margolis P, Setlow P, Losick R, Sun D. Identification of Bacillus subtilis genes for septum placement and shape determination. J Bacteriol. 1992;174:6717–6728. doi: 10.1128/jb.174.21.6717-6728.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin P A, Shim J J, Grossman A D. Effect of minCD on FtsZ ring position and polar septation in Bacillus subtilis. J Bacteriol. 1998;180:6048–6051. doi: 10.1128/jb.180.22.6048-6051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutkenhaus J, Addinall S G. Bacterial cell division and the Z ring. Annu Rev Biochem. 1997;66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 18.Marston A L, Errington J. Selection of the midcell division site in Bacillus subtilis through MinD-dependent polar localization and activation of MinC. Mol Microbiol. 1999;33:84–96. doi: 10.1046/j.1365-2958.1999.01450.x. [DOI] [PubMed] [Google Scholar]
- 19.RayChaudhuri D. ZipA is a MAP-Tau homolog and is essential for structural integrity of the cytokinetic FtsZ ring during bacterial cell division. EMBO J. 1999;18:2372–2383. doi: 10.1093/emboj/18.9.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.RayChaudhuri D, Park J T. A point mutation converts Escherichia coli FtsZ septation GTPase to an ATPase. J Biol Chem. 1994;269:22941–22944. [PubMed] [Google Scholar]
- 21.Rivas G, Lopez A, Mingorance J, Ferrandiz M J, Zorrilla S, Minton A P, Vicente M, Andreu J M. Magnesium-induced linear self-association of the FtsZ bacterial cell division protein monomer. The primary steps for FtsZ assembly. J Biol Chem. 2000;275:11740–11749. doi: 10.1074/jbc.275.16.11740. [DOI] [PubMed] [Google Scholar]
- 22.Romberg L, Simon M, Erickson H P. Polymerization of FtsZ, a bacterial homolog of tubulin: is assembly cooperative? J Biol Chem. 2001;4:11743–11753. doi: 10.1074/jbc.M009033200. [DOI] [PubMed] [Google Scholar]
- 23.Scheffers D, de Wit J G, den Blaauwen T, Driessen A J. Substitution of a conserved aspartate allows cation-induced polymerization of FtsZ. FEBS Lett. 2001;494:34–37. doi: 10.1016/s0014-5793(01)02310-9. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro L, Losick R. Dynamic spatial regulation in the bacterial cell. Cell. 2000;100:89–98. doi: 10.1016/s0092-8674(00)81686-4. [DOI] [PubMed] [Google Scholar]
- 25.Sossong T M, Jr, Brigham-Burke M R, Hensley P, Pearce K H., Jr Self-activation of guanosine triphosphatase activity by oligomerization of the bacterial cell division protein FtsZ. Biochemistry. 1999;38:14843–14850. doi: 10.1021/bi990917e. [DOI] [PubMed] [Google Scholar]
- 26.Trusca D, Scott S, Thompson C, Bramhill D. Bacterial SOS checkpoint protein SulA inhibits polymerization of purified FtsZ cell division protein. J Bacteriol. 1998;180:3946–3953. doi: 10.1128/jb.180.15.3946-3953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varley A W, Stewart G C. The divIVB region of the Bacillus subtilis chromosome encodes homologues of Escherichia coli septum placement (MinCD) and cell shape (MreBCD) determinants. J Bacteriol. 1992;174:6729–6742. doi: 10.1128/jb.174.21.6729-6742.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Jones B D, Brun Y V. A set of ftsZ mutants blocked at different stages of cell division in Caulobacter. Mol Microbiol. 2001;40:347–350. doi: 10.1046/j.1365-2958.2001.02395.x. [DOI] [PubMed] [Google Scholar]
- 29.Ward J E, Jr, Lutkenhaus J. Overproduction of FtsZ induces minicell formation in E. coli. Cell. 1985;42:941–949. doi: 10.1016/0092-8674(85)90290-9. [DOI] [PubMed] [Google Scholar]
- 30.Weiss D S, Chen J C, Ghigo J M, Boyd D, Beckwith J. Localization of FtsI (PBP3) to the septal ring requires its membrane anchor, the Z ring, FtsA, FtsQ, and FtsL. J Bacteriol. 1999;181:508–520. doi: 10.1128/jb.181.2.508-520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]