Abstract
Uncorrected refractive astigmatism degrades visual acuity. Spherical intraocular lenses (IOLs) leave astigmatic errors resident in the cornea manifest in refractive astigmatism. Toric IOLs, correcting for this corneal astigmatism, contribute to spectacle‐free vision in the pseudophakic eye. This review provides information to assist surgeons in a rational choice of eyes suitable for toric IOL implantation, methods of IOL cylinder power calculation, surgical techniques for toric IOLs and management of complications. With appropriate application of this information, correction of visually detrimental astigmatism can be achieved routinely.
Keywords: biometry, cataract, intraocular lens (IOL), IOL optics, Toric
1. INTRODUCTION
Full refractive correction of the aphakic eye requires not only correction of the profound spherical error, but also the astigmatic element of the aphakic refractive state. Correction of the spherical error with an intraocular lens (IOL) allows comfortable spectacle correction of remaining astigmatic refractive error, but the goal of spectacle‐free vision can only be achieved if the astigmatic element is also corrected.
The simple principle behind the use of toric intralocular lenses is that aphakic astigmatism mainly derives from corneal astigmatism. This latter entity must, however, be predictable from pre‐operative measurement to be useful in calculating the cylinder power of an IOL. Surgical incisions in or near the cornea alter corneal astigmatism. 1 Advances over the last 30 years of cataract surgery have enabled these predictions to be made with increasing accuracy. Extracapsular cataract extraction with 11 mm incisions did not allow good prediction of postoperative corneal astigmatism whereas modern phacoemulsification through incisions under 2.75 mm do. 2 , 3 These incisions when very small (under 2 mm) are astigmatically neutral, meaning the change in corneal shape they induce is below our ability to measure it, and predictable enough to allow use of toric intraocular lenses and to produce good refractive correction in the pseudophakic eye. 4 Slightly larger incisions, though astigmatically active, are predictable in their astigmatic effect. 5 This predictable effect can be incorporated in the calculation of toric IOL cylinder power and meridian of placement.
Predictable and stable post‐operative corneal astigmatism along with rotational stability are the sine qua non of toric IOL use. With their use comes the promise of spectacle‐free vision in the pseudophakic eye.
2. HISTORY
Some forms of toric IOL have been in existence for over 30 years. Initial PMMA IOLs met with limited success because of the large incision size and scleral incision approach required for their insertion. 6 A silicone version could be folded for insertion, but encountered problems with rotational stability and still required a sizable incision. 7 With highly compressible materials with strong structural memory such as hydrophobic and hydrophilic acrylics, the astigmatic benefits of smaller and smaller phacoemulsification incisions have been utilised. 7 With low or no astigmatic power and axis change brought on by the act of crystalline lens extraction, an IOL could be chosen of appropriate power and accurate axis of placement.
3. THE EFFECT ON UNAIDED ACUITY OF ASTIGMATISM AND THE INDICATIONS FOR INSERTION OF TORIC IOLS
The loss of unaided acuity with increasing astigmatic error is seen in Figure 1. 8 , 9 The loss is influenced not only by the power of the astigmatism, but also its orientation.
FIGURE 1.
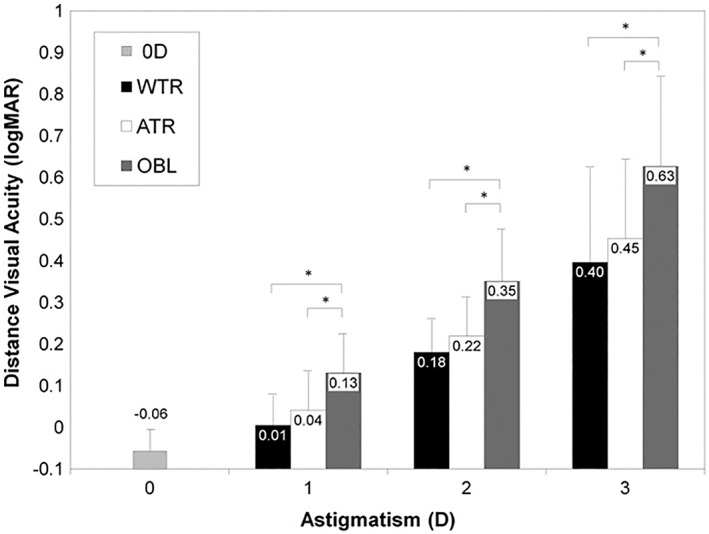
Distance visual acuity with uncorrected astigmatism was significantly influenced by axis orientation at each diopter of astigmatism (P!0.001 for 1.0D, 2.0D, and 3.0D, ANOVA). The asterisks represent a significant difference (Fisher LSD post hoc test). ATR, against‐the‐rule astigmatism; OBL Z, oblique astigmatism; WTR, with‐the‐rule astigmatism. Source: Reproduced from Reference [8]
Toric IOLs come in refractive steps starting with the lowest available power of 1D. 10 , 11 This lower end of the power range is capable of correction of 0.5D–0.6D of corneal astigmatism. The steps of increasing power vary between manufacturers but typically 0.5D–0.75D with upper ranges of between 6D and 9D, typically, depending on the manufacturer. 11 , 12 These steps and ranges provide correction for most physiological astigmatism and a wide range of pathological astigmatic error.
These ranges of fixed powers mirror the range and steps of spherical power available in commercially available IOLs. Surgeons are familiar with the concept of a non‐zero target that the use of these devices with step‐wise progressions of power impose. Our routine calculation of sphere power is resident on our biometers, and a table of possible IOL sphere powers is presented to us with a prediction of the likely sphere equivalent refractive outcome for each choice. Seldom is the predicted outcome emmetropic even if that is desired. It is a small non‐zero compromise on that ideal post‐operative refraction dictated by the available steps in IOL sphere power. The same non‐zero astigmatic target is necessary when calculating the appropriate IOL cylinder power, even if perfect axis alignment is achieved.
For the insertion of a toric IOL to be appropriate, the minimum requirement is that the predicted remaining astigmatism is less than the predicted post‐operative corneal astigmatism and the patient receives a refractive benefit. At what point above this minimum a toric IOL is indicated, in terms of unaided visual acuity, is disputed, particularly in terms of cost‐effectiveness since toric IOLs cost more than spherical IOLs. Surgeons set various thresholds of corneal astigmatism based on these considerations. 12 , 13
One limitation on toric IOL step selection seems to be the commonly held, but erroneous, view that the corneal astigmatism should not be overcorrected. The advice given is that the ‘axis should not be flipped’. 14 This advice seems to emanate from optometric practice where overcorrection and ‘flipping the axis’ with spectacle correction leads to visual discomfort from meridional magnification and unusual distortion of the visual environment. This does not apply with IOLs. They are not prone to these aberrations, and overcorrection is indicated if it leads to less remaining refractive astigmatism than under‐correction. 15
A further layer of decision‐making when choosing to use a toric IOL is imposed by the intended refractive effect of the IOL. Specifically, if the IOL is being chosen to minimise or eliminate the use of spectacles, then astigmatic correction is quite obviously mandatory. There is little point in inserting a multifocal IOL if remaining refractive astigmatism is predicted. In such cases, no remaining astigmatism is useful.
Toric IOLs cost more than spherical IOLs. 16 Limited access therefore occurs in less well funded health care systems. Typically, optometric services to the post‐operative patient in such systems would also be limited. In those circumstances, it could be argued the need for toric IOLs is greater than in a well‐funded system, because the patient in a well‐funded system can access spectacles for remaining astigmatism whereas the less well‐funded patient cannot.
4. CALCULATION OF TORIC IOL CYLINDER POWER
There is a universally held, but perhaps oversimplified, assumption that all refractive astigmatism in an aphakic eye is resident in the cornea. 17 Corneal astigmatism must therefore be measured accurately, and its axis and power in the postoperative eye accurately predicted to achieve refractive success using toric IOLs.
4.1. Accurate corneal power and axis measurement
Today's cataract surgeons are presented with a wide range of devices that measure corneal curvature and convert those data into useful and accurate power and axis information that can be inserted into readily available toric calculators. There is a large body of published data on these devices demonstrating their comparability and the accuracy of the refractive outcome they provide both for spherical and toric IOLs. 18 , 19 , 20 , 21 Once the surgeon has decided on the preferred device a few steps can be taken to ensure success. It is evident, even with minimal experience of these devices, that there is a test‐to‐test variation in measurements with all of them. 22 To avoid inaccurate outcome from this phenomenon, most surgeons will compare the data from at least two independent measurements to examine for consistency. Barrett has provided a method of combination of information from several devices into one set of composite data used in toric IOL power calculation and designation of axis. 23
4.2. Accurate corneal power and axis prediction
4.2.1. Surgically induced astigmatism
Because of the propensity of the surgical incision to reduce corneal curvature along the incised meridian and increase curvature on the orthogonal meridian, it is necessary to predict what the corneal shape will be once the cataract is extracted and the IOL has been inserted. The simplest approach to this is to reduce incision size to a point where no measurable change occurs. This is probably around 2 mm incision size in the clear cornea. 4 Most routine phacoemulsification devices require an incision size in the region of 2.4 mm or slightly larger. This is astigmatically active. 5 A surgically induced astigmatism can be calculated by examining the vector difference between the preoperative corneal astigmatism and the postoperative corneal astigmatism. This value, averaged vectorially or arithmetically over a population of eyes, can then be included in the power and axis requirement of the IOL. This method tends to over‐estimate the change that occurs due to the test‐to‐test variability of corneal power and axis measurement. 24 An alternative is to derive the flattening that occurs on average on the incised meridian by vector analysis. 25 Once again that value depends on possibly inaccurate corneal measurement. A third option is to apply a theoretical value, 0.1D for instance, of induced flattening on the incised meridian. Whatever method is used, a new set of corneal curvature data is derived that predicts the altered corneal astigmatism power and axis that is then used to calculate the cylinder power of the toric IOL and its ideal axis.
4.2.2. Posterior corneal astigmatism
The cornea has two refracting surfaces, anterior and posterior. 26 The latter, because the difference between the refractive indices of cornea and aqueous humour is less than between cornea and air, the refractive effect of the posterior cornea is greatly less than the anterior corneal surface. It is more highly curved than the anterior surface so has a concave or negative lens effect of the order of 5 or 6 dioptres. It also has an astigmatic component. This astigmatism contributes to a total corneal astigmatism. 18 Growing numbers of devices can measure this astigmatism which appears to have its steep component near the vertical corneal meridian in the majority of but not all eyes. 27 For accurate prediction of corneal astigmatism, this posterior corneal astigmatism effect needs to be either measured or estimated and included in toric IOL calculations. 28 , 29
There is a difference between the measured and estimated effect of posterior corneal astigmatism. These estimates of non‐anterior corneal astigmatism are from refraction of eyes with intraocular lenses. 32 Such eyes have known lenticular astigmatism, making calculation of the non‐anterior corneal and non‐lenticular astigmatism possible, and the measured posterior astigmatism is smaller than the values derived from such a calculation. This implies that the measurement of posterior corneal astigmatism is inaccurate or that there is another ‘left‐over’ astigmatism present derived from other sources—lens tilt or decentration, for instance. 30 This entity is poorly understood as yet.
For the present, the use of measured posterior corneal astigmatism values to derive a total corneal astigmatism for inclusion in toric IOL calculation is less successful in predicting outcome than the application of estimated or outcome‐derived values. 31 , 32 The later methods automatically include the effect of this ‘left‐over astigmatism’. This may explain their higher success rates. These estimated or theoretical methods include the Baylor Nomogram, 33 the Goggin Nomogram 34 and the Barrett/Abulaffia/Koch method. 35
The effect of the posterior corneal astigmatism on total corneal astigmatism varies with the difference between the anterior and posterior corneal astigmatism axes. The posterior corneal curvature has a negative lens effect. If the posterior corneal steep axis is predominantly at or near the vertical meridian 18 and the anterior steep axis is near vertical (a with‐the‐rule cornea), the anterior astigmatic power effect at that steep vertical meridian will be significantly reduced. Conversely, the reduction of effect on the flat axis power, at or near the horizontal in a with‐the‐rule eye, will be less. The overall effect is to reduce the total corneal astigmatism power in with‐the‐rule eyes by comparison with the anterior corneal astigmatism power. With consistent near vertical orientation of the posterior corneal steep axis, the opposite effect will occur in against‐the‐rule corneas. There is an augmentation of the total corneal cylinder power versus the anterior power. There is evidence that these effects are maximal in eyes with lower astigmatism power and may not to occur in eyes with oblique corneal astigmatism. 36 , 37
Several online resources exist for the calculation of toric IOL powers from pre‐operative data, provided by IOL manufacturers. 38 , 39 , 40 All require keratometric data. Some will accept combined anterior and posterior corneal data (commonly called ‘total cornea’ data), anterior chamber depth and axial length. Some will provide automatic application of theoretical or population‐based adjustment for the effect of the posterior corneal astigmatism and will incorporate an estimate of corneal surgically induced astigmatism. Surgeons are well advised to be familiar with what the calculator of their preferred manufacturer actually does. Some third‐party calculators and nomograms also exist (Kane, 41 ASSORT, 42 Barrett, 24 Goggin 43 ) for assistance in all or part of the calculation. Surgeons should be aware that the sphere power of the IOL influences the required cylinder power for a constant corneal astigmatic power as does the anterior chamber depth and its correlate, the effective lens position. This is a consequence of the effect on power of these parameters for all IOLs not only toric IOLs. 44 , 45
4.2.3. Post‐keratorefractive corneas
Keratorefractive procedures alter corneal shape. It is by this effect that these procedures alter the refractive error of the eye. Use of routine keratometers to measure such corneas yields erroneous corneal power values. 46 First, the corneal power is measured by standard keratometers at a ring that is roughly 3.2 mm to 3.4 mm in diameter. In post‐keratorefractive corneas, this may lie outside the visually active more central zone of the cornea that has been altered by the procedure from its normal shape. Some devices measure at a more central ring that does reflect the true power of the visually important part of the cornea. The IOLMaster (Carl Zeiss Meditec Gmb, Oberkochen, Germany), for instance, measures nearer to a 2 mm ring and is more accurate in these eyes as a result. Second, the corneal refractive index used by almost all keratometers includes the effect of the anterior and posterior cornea in one value. Keratorefractive corneas have an altered relationship of anterior and posterior corneal curvature rendering this combined value invalid. The conversion of radius of curvature to refractive power therefore becomes invalid. Third, the keratometric values influence the prediction of the effective lens position in some IOL power calculation formulae not specifically designed for use in post‐keratorefractive eyes. These differences versus normal corneas affect the IOL sphere power calculation and consequently the toric component in a similar way. Resources for use in such eyes to calculate toric IOLs exist online and in some biometers. They have long been available for IOL sphere calculation and now for toric IOLs specifically. The Barrett True‐K toric calculator 47 or the Haigis‐L 48 are examples. The significant errors in outcome from IOL power calculations are avoided by use of such methods. 49
5. SURGICAL TECHNIQUES FOR TORIC IOLS
Surgery for toric IOls must include a method of identification of the intended meridian of placement of the IOL, use of an incision with predictable corneal astigmatic effect and accurate rotational placement of the IOL at that meridian.
5.1. Axis identification
The eye will tort with movement of the patient from an upright position to a recumbent position for surgery, so identification of appropriate corneal meridia must take place in the upright position. 50 Local anaesthetic can alter the rotational position of the eye by variable motor block effect, and identification of the required meridian must take place before any anaesthetic procedure. This can be achieved in a number of ways.
5.1.1. Pre‐operative manual meridional marking
Immediately prior to surgery a mark can be placed to identify a typical meridian, the horizontal or vertical meridian, for instance. 51 This is used per‐operatively to identify the meridian for IOL axis placement. All toric IOLs have the least powered axis of the IOL marked on the optic of the IOL. This is common to all manufacturers. This axis is, therefore, placed on the most powered corneal meridian identified, as described above. The marking is carried using the surgeons naked eye or one of several instruments or other tools such as a smart phone. 52
5.1.2. Pre‐operative identification using an automated system
A number of automated image guidance systems exist that use preoperative measured or programmed data to identify the appropriate corneal meridian per‐operatively. They rely on identification of conjunctival vessels. Examples are the Alcon Inc. (Geneva, Switzerland) Varion™ and Carl Zeiss Meditec Gmb (Oberkochen, Germany) Callisto eye™ systems. Automation holds the promise of increased IOL placement accuracy. 53 It remains to be shown whether they improve toric refractive outcome in a clinically useful manner. Even if accuracy of meridional placement of a toric IOL can be improved, consequent improvement in refractive outcome may or may not occur given test‐to‐test variability in steep axis identification combined with the very small effect on refraction of very small variations in astigmatic alignment. In fact, since most corneas show some form of topographic variation from exact orthogonal astigmatism, there is probably a range of axes of toric IOL placement that will yield the same refractive result in most eyes. Intraoperative aberrometry may assist in assessing the IOL power and axis requirement of the aphakic eye but with limited clinical benefit. 54 , 55
5.2. Incision placement and width
The surgically induced astigmatism of any incision in or near the cornea is proportional to its arc length and inversely proportional to its distance from the centre of the cornea. 56 , 57 Clear corneal incisions placed on the horizontal meridian (the widest corneal diameter in most eyes) are less astigmatically active than those on a more vertical meridian, given the limbus is an oval structure. 58 Scleral incisions are less active and longer incision at all sites are more active. Astigmatic neutrality is achieved at about 2–2.2 mm in the periphery of the clear cornea. 4 Above this threshold the effect may be variable but is largely predictable and can be used to augment the astigmatic effect of the toric implant. The alternative of increased toric IOL power is likely to be more predictable. 59 A power value and axis value of the incision‐induced astigmatism can be included in the toric IOL power calculation, as outlined above. The astigmatic effect of sutured incisions is unpredictable.
6. POST‐OPERATIVE ASSESSMENT AND MANAGEMENT OF TORIC IOLS
If accurate power calculation and meridional placement has taken place, rotational stability of the IOL must continue in order to achieve appropriate refractive results. Malposition of a toric IOL in the postoperative period can arise from erroneous per‐operative placement or post‐operative rotation. The former can be avoided by good pre‐operative measurement and good surgical technique. Eyes at risk of the latter are large and often highly myopic. 60 , 61 It is assumed that the capsular bag is sufficiently large that poor contact of the IOL haptic with the equator of the capsular bag occurs and allows rotation. It can occur in smaller eyes but less commonly.
The phenomenon of toric IOL rotation or malpositioning can be recognised by examination with a dilated pupil. It can also be inferred from a failure to achieve the expected vision as it recovers post‐operatively. If an astigmatic refractive surprise is identified, there are three causes: the IOL is off axis; the IOL cylinder power is incorrect; or a combination of these two problems. Analysis of the problem should be undertaken whenever a reasonable manifest refraction can be obtained, usually from about week 2 post‐operatively. The manifest refraction should be accompanied by a post‐operative keratometry in case the corneal astigmatic axis has changed, a measurement of the exact IOL axis and ideally a measured anterior chamber depth. A check should be made that the correct IOL power was inserted and that the correct axis was chosen. A transcription error might, for instance, have led to the IOL being placed on the flat corneal meridian rather than the steep meridian. With these data, specifically a knowledge of the IOL sphere and cylinder power and its axis and the refraction, the ideal axis of placement and the ideal IOL cylinder power can be vector calculated. This kind of analysis can be done using a number of online resources that vector analyse the problem and provide a solution. 62 , 63 , 64 If the power is at fault, a lens exchange, a secondary IOL or an excimer ablation might be considered, but an isolated axis error can be rectified by a rotation of the IOL. The severity of a power error can be assessed by calculating if the ideal IOL cylinder power is more than half way to the next available cylinder power step, up or down. If so, consideration should be given to an IOL exchange. Post‐operative keratometry and anterior chamber depth measurement provide corroborating information. The calculated ideal IOL meridian should, for instance, coincide with the postoperative steep axis which may have changed from its preoperative position having been altered by the incision.
The best timing of an IOL rotation is not established by trials, but is logically best carried out when some capsular shrinkage has occurred to avoid a further rotation. This may be between 2 and 4 weeks post‐operatively. Later intervention may be complicated by inability to free the IOL in a tightly adherent capsular bag. Consideration should be given to placement of a capsular tension ring, expanding the capsular equator and thus approximating the anterior and posterior capsular leaves to each other and to the IOL.
6.1. Deliberate off‐axis placement of toric IOLs
The advice to place a toric IOL deliberately off‐axis to ‘depower’ its astigmatism correcting effect is occasionally seen in non‐peer‐reviewed publications. It has not appeared in the peer‐reviewed literature. The idea is that if one does not have access to a lower powered IOL, reducing the astigmatic correction by deliberately placing it off‐axis may be a way of providing a good outcome. An example would be if the corneal astigmatism was 1D, but the only IOL available had a 1.5D cylinder, as is the cases in some health care systems; placing the IOL off‐axis might reduce its correcting power to a suitable level. This is a misunderstanding of toric IOL optics. Reducing the correcting effect of the cylinder in the IOL leads to increasing, not decreasing, remaining astigmatism. 65 An eye receiving such a misaligned IOL will be worse off refractively than if the IOL were perfectly placed. The best correction any toric IOL will achieve, with the least possible remaining astigmatism, is if it is perfectly aligned. This applies to under‐ and over‐correcting IOL cylinders equally.
7. TORIC IOL REFRACTIVE OUTCOME
With good pre‐operative measurement, good IOL toric power calculation and good surgical technique, excellent refractive results can be achieved with toric IOLs, providing spectacle‐free vision. Of course, to the patient, successful use of a toric IOL means least refractive astigmatism post‐operatively. To achieve that the two components of success are adequate alignment of the IOL and adequate power selection, and each of these can be analysed as measures of success. There are many studies of possible outcome of application of newer techniques by calculation of the probable refractive result of the new technique if applied to the results from a previous set of eyes treated using an older technique. 66 , 67 , 68 This applies particularly to IOL toric power calculation methods. This methodology assumes the eyes studied would behave the same with a differently chosen IOL. In experimental terms, this assumption may not be safe. There is significant test‐to‐test variation in almost all the parameters used to calculate IOL toric power (keratometry, axial length and anterior chamber depth) and in the post‐operative assessment of outcome (manifest refraction). This method gives information, but would be enhanced by actual results of application of the techniques in prospective case series. Below are data collected, where possible from reported case series, where the implanted IOL has been calculated by the method being examined.
7.1. Axis placement accuracy
Routine accuracy of axis placement is reported as within 50 of the intended axis. 69 , 70 Average of misalignment where the alignment axis is signed (positive for counter‐clockwise from the intended axis and negative for clockwise) is close to zero with a mean absolute value of less than 50. 71
7.2. Power calculation accuracy
The success of power calculation can only be assessed by vector analysis, since the effect of off‐axis placement on remaining refractive astigmatism must be removed. The basic measure of success is a vector value called the ‘magnitude of error’. It is the difference between the planned power effect of the IOL cylinder and the actual power effect achieved, calculated from the post‐operative refraction. This can be calculated even if the IOL is misaligned. The median magnitude of error is reported as 0.2D or less, particularly when corneal surgically induced astigmatism is included in the cylinder power choice and allowance is made for the effect of the posterior cornel astigmatism. 24 , 72 , 73
7.3. Remaining refractive astigmatism
Because toric IOLs are manufactured in steps (0.5D or 0.75D depending on the manufacturer), there is seldom a situation where exactly zero remaining refractive astigmatism can be targeted. Instead, a non‐zero target of minimal but acceptable power is set as close to zero as possible. This is similar to the setting of a spherical equivalent target for the sphere power of IOLs. Average achieved remaining refractive astigmatism is around 0.4D. 24 This is acceptable to most patients if the sphere equivalent remaining error is similarly on target. Achieving astigmatic refractive outcome of close to 100% of eyes within 1D or less from the target and 90% within 0.5D is possible. 32
8. CONCLUSION
In combination with appropriate sphere power choice, accurate use of toric IOLs enhances the patient's visual experience post‐operatively. In fact, it will commonly improve on the patient's pre‐morbid state. Ophthalmic surgeons are privileged in those terms. With accurate pre‐operative measurement, good calculation of IOL cylinder power, including allowance for corneal surgically induced astigmatism and the effects of astigmatism arising from sources other than the anterior cornea (chiefly the posterior cornea) and good surgical technique, excellent refractive results can commonly be achieved. Market share data suggests that toric IOL are implanted in 10%–15% in most of the developed world. In some regions this rises to closer to 30% (Australia, for instance). The latter rate reflects the prevalence of visually significant corneal astigmatism in any human population. 74 The limiting factor is probably cost. We as ophthalmologists should advocate that this anomaly be addressed.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ACKNOWLEDGEMENTS
Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
Goggin M. Toric intraocular lenses: Evidence‐based use. Clin Experiment Ophthalmol. 2022;50(5):481‐489. doi: 10.1111/ceo.14106
REFERENCES
- 1. Pfleger T, Skorpik C, Menapace R, Scholz U, Weghaupt H, Zehetmayer M. Long‐term course of induced astigmatism after clear corneal incision cataract surgery. J Cataract Refract Surg. 1996;1(22):72‐77. [DOI] [PubMed] [Google Scholar]
- 2. Swinger CA. Postoperative astigmatism. Surv Ophthalmol. 1987;31(4):219‐248. [DOI] [PubMed] [Google Scholar]
- 3. Kaufmann C, Thiel MA, Esterman A, Dougherty PJ, Goggin M. Astigmatic change in biaxial microincisional cataract surgery with enlargement of one incision: a prospective controlled study. Clin Experiment Ophthalmol. 2009;37:254‐261. [DOI] [PubMed] [Google Scholar]
- 4. Kaufmann C, Krishnan A, Landers J, Esterman A, Thiel MA, Goggin M. Astigmatic neutrality in biaxial microincision cataract surgery. J Cataract Refract Surg. 2009;35:1555‐1562. [DOI] [PubMed] [Google Scholar]
- 5. Ferreira TB, Ribeiro FJ, Pinheiro J, Ribeiro P, O'Neill JG. Comparison of surgically induced astigmatism and morphologic features resulting from femtosecond laser and manual clear corneal incisions for cataract surgery. J Refract Surg. 2018. May;1(34):322‐329. [DOI] [PubMed] [Google Scholar]
- 6. Frohn A, Dick HB, Thiel HJ. Implantation of a toric poly(methyl methacrylate) intraocular lens to correct high astigmatism. J Cataract Refract Surg. 1999;25:1675‐1678. [DOI] [PubMed] [Google Scholar]
- 7. Chang DF. Comparative rotational stability of single‐piece open‐loop acrylic and plate‐haptic silicone toric intraocular lenses. J Cataract Refract Surg. 2008;34:1842‐1847. [DOI] [PubMed] [Google Scholar]
- 8. Kobashi H, Kamiya K, Shimizu K, Kawamorita T, Uozato H. Effect of axis orientation on visual performance in astigmatic eyes. J Cataract Refract Surg. 2012;38:1352‐1359. [DOI] [PubMed] [Google Scholar]
- 9. Berdahl JP, Hardten DR, Kramer BA, Potvin R. Effect of astigmatism on visual acuity after multifocal versus monofocal intraocular lens implantation. J Cataract Refract Surg. 2018;44:1192‐1197. [DOI] [PubMed] [Google Scholar]
- 10. https://www.zeiss.com/meditec/int/product-portfolio/iols/toric-iols.html#cluster. Accessed May 2022.
- 11. https://www.alcon.com/eye-care-products. Accessed May 22.
- 12. Statham M, Apel A, Stephensen D. Comparison of the AcrySof SA60 spherical intraocular lens and the AcrySof Toric SN60T3 intraocular lens outcomes in patients with low amounts of corneal astigmatism. Clin Experiment Ophthalmol. 2009;37(8):775‐779. [DOI] [PubMed] [Google Scholar]
- 13. Aujla JS, Vincent SJ, White S, Panchapakesan J. Cataract surgery in eyes with low corneal astigmatism: implantation of the Acrysof IQ Toric SN6AT2 intraocular lens. J Ophthalmic Vis Res. 2014;324‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. https://www.reviewofophthalmology.com/article/37-ways-to-get-great-outcomes-with-torics. Accessed May 2022.
- 15. Beheregaray S, Goggin M, LaHood B. Astigmatic overcorrection and axis flip for targeting minimal remaining refractive astigmatism with toric intraocular lenses. J Cataract Refract Surg. 2018;44:109‐110. [DOI] [PubMed] [Google Scholar]
- 16. Simons R, Visser N, van den Biggelaar F, et al. Trial‐based cost‐effectiveness analysis of toric versus monofocal intraocular lenses in cataract patients with bilateral corneal astigmatism in The Netherlands. J Cataract Refract Surg. 2019;45(2):146‐152. [DOI] [PubMed] [Google Scholar]
- 17. Koch DD, Ali SF, Weikert MP, Shirayama M, Jenkins R, Wang L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J Cataract Refract Surg. 2012;38(12):2080‐2087. [DOI] [PubMed] [Google Scholar]
- 18. Franzco CM, Niyazmand H, Seo S, Franzco GB, Nilagiri VK, Franzco JM. Agreement between two swept‐source ocular coherence tomography biometry devices. J Cataract Refract Surg. 2022;10‐97. doi: 10.1097/j.jcrs.0000000000000942 [DOI] [Google Scholar]
- 19. Dong J, Yao J, Chang S, Kanclerz P, Khoramnia R, Wang X. Comparison study of the two biometers based on swept‐source optical coherence tomography technology. Diagnostics. 2022;12:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ashena Z, Gallagher S, Naveed H, Spalton DJ, Nanavaty MA. Comparison of anterior corneal Aberrometry, Keratometry and pupil size with Scheimpflug tomography and ray tracing Aberrometer. Vision. 2022;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lupardi E, Taroni L, Hoffer KJ, Schiano‐Lomoriello D, Savini G. Comparison of corneal power calculation by standard Keratometry and Total Keratometry in eyes with previous myopic FS‐LASIK. J Refract Surg. 2021;37:848‐852. [DOI] [PubMed] [Google Scholar]
- 22. Goggin M, Patel I, Billing K, Esterman A. Variation in surgically induced astigmatism estimation due to test‐to‐test variations in keratometry. J Cataract Refract Surg. 2010;36:1792‐1793. [DOI] [PubMed] [Google Scholar]
- 23. https://calc.apacrs.org/TrueKToricTK_preview/TrueKToricTK.aspx. Accessed April 2022.
- 24. Kamiya K, Iijima K, Ando W, Shoji N. Comparison of mean and centroid of surgically induced astigmatism after standard cataract surgery. Front Med. 2021;8:822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernández‐Rosés J, Julien À, Lamarca J, Barraquer RI. Flattening effect is the key parameter for toric calculator. J Cataract Refract Surg. 2019;45:1210. [DOI] [PubMed] [Google Scholar]
- 26. Javal E. Memoires d'Ophtalmometrie: Annotes et Precedes d'une Introduction. G. Masson; 1890:131. [Google Scholar]
- 27. LaHood BR, Goggin M. Measurement of posterior corneal astigmatism by the IOLMaster 700. J Refract Surg. 2018;34:331‐336. [DOI] [PubMed] [Google Scholar]
- 28. Goggin M, Zamora‐Alejo K, Esterman A, van Zyl L. Adjustment of anterior corneal astigmatism values to incorporate the likely effect of posterior corneal curvature for toric intraocular lens calculation. J Refract Surg. 2015;31:98‐102. [DOI] [PubMed] [Google Scholar]
- 29. Goggin M, van Zyl L, Caputo S, Esterman A. Outcome of adjustment for posterior corneal curvature in toric intraocular lens calculation and selection. J Cataract Refract Surg. 2016;42(10):1441‐1448. [DOI] [PubMed] [Google Scholar]
- 30. Goggin M, LaHood BR, Roggia M, Beheregaray S. Leftover astigmatism: the missing link between measured and calculated posterior corneal astigmatism. J Refract Surg. (in press). [DOI] [PubMed] [Google Scholar]
- 31. Saini P, Choudhry S, Rajput M, Sen S. To compare the refractive outcomes after cataract surgery using conventional keratometry (K) with Barrett universal II formula and total keratometry (TK) with Barrett TK universal II formula for intraocular lens power calculation. Investig Ophthalmol Vis Sci. 2020;61:609. [Google Scholar]
- 32. Abulafia A, Barrett GD, Porat‐Rein A, et al. Measured corneal astigmatism versus pseudophakic predicted refractive astigmatism in cataract surgery candidates [published online ahead of print, 2022 mar 11]. Am J Ophthalmol. 2022;240:225‐231. [DOI] [PubMed] [Google Scholar]
- 33. Koch DD, Jenkins RB, Weikert MP, Yeu E, Wang L. Correcting astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J Cataract Refract Surg. 2013;39:1803‐1809. [DOI] [PubMed] [Google Scholar]
- 34. Goggin M, Zamora‐Alejo K, Esterman A, van Zyl L. Adjustment of anterior corneal astigmatism values to incorporate the likely effect of posterior corneal curvature for toric intraocular lens calculation. J Refract Surg. 2015;31:98‐102. [DOI] [PubMed] [Google Scholar]
- 35. Abulafia A, Koch DD, Wang L, et al. New regression formula for toric intraocular lens calculations. J Cataract Refract Surg. 2016;42:663‐671. [DOI] [PubMed] [Google Scholar]
- 36. LaHood BR, Goggin M, Esterman A. Assessing the likely effect of posterior corneal curvature on Toric IOL calculation for IOLs of 2.50 D or greater cylinder power. J Refract Surg. 2017;33:730‐734. [DOI] [PubMed] [Google Scholar]
- 37. Sheen Ophir S, LaHood B, Goggin M. Refractive outcome of toric intraocular lens calculation in cases of oblique anterior corneal astigmatism. J Cataract Refract Surg. 2020;46:688‐693. [DOI] [PubMed] [Google Scholar]
- 38. https://zcalc.meditec.zeiss.com/. Accessed May 2022.
- 39. https://www.myalcon-toriccalc.com/. Accessed May 2022.
- 40. https://www.bausch-lomb.be/nl/professionals/voor-uw-praktijk/toric-calculator/. Accessed May 2022.
- 41. https://www.iolformula.com/. Accessed April 2022.
- 42. https://assort.com/assort-toric-iol-calculator-0. Accessed April 2022.
- 43. http://goggintoric.com/. Accessed April 2022.
- 44. Goggin M, Moore S, Esterman A. Outcome of toric intraocular lens implantation after adjusting for anterior chamber depth and intraocular lens sphere equivalent power effects [published correction appears in arch Ophthalmol. 2011;129:1494]. Arch Ophthalmol. 2011;129:998‐1003. [DOI] [PubMed] [Google Scholar]
- 45. Goggin M, Moore S, Esterman A. Toric intraocular lens outcome using the manufacturer's prediction of corneal plane equivalent intraocular lens cylinder power. Arch Ophthalmol. 2011;129(8):1004‐1008. [DOI] [PubMed] [Google Scholar]
- 46. Pantanelli SM, Lin CC, Al‐Mohtaseb Z, et al. Intraocular lens power calculation in eyes with previous excimer laser surgery for myopia: a report by the American Academy of ophthalmology. Ophthalmology. 2021;128(5):781‐792. [DOI] [PubMed] [Google Scholar]
- 47. https://calc.apacrs.org/TrueKToricTK_preview/TrueKToricTK.aspx. Accessed April 2022.
- 48. Haigis W. Intraocular lens calculation after refractive surgery for myopia: Haigis‐L formula. J Cataract Refract Surg. 2008;34(10):1658‐1663. [DOI] [PubMed] [Google Scholar]
- 49. Cao D, Wang L, Koch DD. Outcome of toric intraocular lenses implanted in eyes with previous corneal refractive surgery. J Cataract Refract Surg. 2020;46:534‐539. [DOI] [PubMed] [Google Scholar]
- 50. Chang J. Cyclotorsion during laser in situ keratomileusis. J Cataract Refract Surg. 2008;34:1720‐1726. [DOI] [PubMed] [Google Scholar]
- 51. Durkin SR, Goggin M. A one‐point technique for per‐operative corneal meridian identification: corneal marking technique. Graefes Arch Clin Exp Ophthalmol. 2013;251:2481‐2482. [DOI] [PubMed] [Google Scholar]
- 52. Teichman JC, Baig K, Ahmed II. Simple technique to measure toric intraocular lens alignment and stability using a smartphone. J Cataract Refract Surg. 2014;40:1949‐1952. [DOI] [PubMed] [Google Scholar]
- 53. Khatib ZI, Haldipurkar SS, Shetty V. Verion digital marking versus smartphone‐assisted manual marking and isolated manual marking in toric intraocular lens implantation. Indian J Ophthalmol. 2020;68:455‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kane JX, Chang DF. A review. Ophthalmology. 2021;128:e94‐e114. [DOI] [PubMed] [Google Scholar]
- 55. Solomon KD, Sandoval HP, Potvin R. Evaluating the relative value of intraoperative aberrometry versus current formulas for toric IOL sphere, cylinder, and orientation planning. J Cataract Refract Surg. 2019;45:1430‐1435. [DOI] [PubMed] [Google Scholar]
- 56. Anders N, Pham DT, Linke C, Wollensak J. Bogenförmige lamellierende Keratotomie zur Astigmatismuskorrektur. Experimentelle und erste klinische Ergebnisse [curved lamellar keratotomy for correction of astigmatism. Experimental and initial clinical results]. Ophthalmologe. 1996;93(3):279‐283. [PubMed] [Google Scholar]
- 57. Ho HC, Chen KH, Hsu WM, Lee SM, Chiang CC, Li YS. Linear‐long incisions with a small optical zone for the correction of astigmatism in older patients. Ophthalmology. 2004;111(1):28‐33. [DOI] [PubMed] [Google Scholar]
- 58. Baharozian CJ, Song C, Hatch KM, Talamo JH. A novel nomogram for the treatment of astigmatism with femtosecond‐laser arcuate incisions at the time of cataract surgery. Clin Ophthalmol. 2017;11:1841‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kaufmann C, Peter J, Ooi K, Phipps S, Cooper P, Goggin M. Queen Elizabeth astigmatism study group. Limbal relaxing incisions versus on‐axis incisions to reduce corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2005;31:2261‐2265. [DOI] [PubMed] [Google Scholar]
- 60. Zhu X, He W, Zhang K, Lu Y. Factors influencing 1‐year rotational stability of AcrySof Toric intraocular lenses. Br J Ophthalmol. 2016;100:263‐268. [DOI] [PubMed] [Google Scholar]
- 61. Yao Y, Meng J, He W, et al. Associations between anterior segment parameters and rotational stability of a plate‐haptic toric intraocular lens. J Cataract Refract Surg. 2021;47(11):1436‐1440. [DOI] [PubMed] [Google Scholar]
- 62. https://www.astigmatismfix.com/. Accessed April 2022.
- 63. http://goggintoric.com/. Accessed April 2022.
- 64. https://toricpro.com/. Accessed April 2022.
- 65. LaHood B, Goggin M. Debunking the myth of depowering a toric intraocular lens. J Cataract Refract Surg. 2022;48:370‐371. [DOI] [PubMed] [Google Scholar]
- 66. Kane JX, Connell B. A comparison of the accuracy of 6 modern Toric intraocular lens formulas. Ophthalmology. 2020;127(11):1472‐1486. [DOI] [PubMed] [Google Scholar]
- 67. Yang S, Byun YS, Kim HS, Chung SH. Comparative accuracy of Barrett Toric calculator with and without posterior corneal astigmatism measurements and the Kane Toric formula. Am J Ophthalmol. 2021;231:48‐57. [DOI] [PubMed] [Google Scholar]
- 68. Solomon KD, Sandoval HP, Potvin R. Evaluating the relative value of intraoperative aberrometry versus current formulas for toric IOL sphere, cylinder, and orientation planning. J Cataract Refract Surg. 2019;45(10):1430‐1435. [DOI] [PubMed] [Google Scholar]
- 69. Vukich JA, Ang RE, Straker BJK, et al. Evaluation of intraocular lens rotational stability in a multicenter clinical trial. Clin Ophthalmol. 2021;15:3001‐3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee BS, Onishi AC, Chang DF. Comparison of rotational stability and repositioning rates of 2 presbyopia‐correcting and 2 monofocal toric intraocular lenses. J Cataract Refract Surg. 2021;47:622‐626. [DOI] [PubMed] [Google Scholar]
- 71. Savini G, Alessio G, Perone G, Rossi S, Schiano‐Lomoriello D. Rotational stability and refractive outcomes of a single‐piece aspheric toric intraocular lens with 4 fenestrated haptics. J Cataract Refract Surg. 2019;45:1275‐1279. [DOI] [PubMed] [Google Scholar]
- 72. Orts P, Piñero DP, Aguilar S, Tañá P. Efficacy of astigmatic correction after femtosecond laser‐guided cataract surgery using intraoperative aberrometry in eyes with low‐to‐moderate levels of corneal astigmatism. Int Ophthalmol. 2020;40:1181‐1189. [DOI] [PubMed] [Google Scholar]
- 73. Piovella M, Colonval S, Kapp A, Reiter J, Van Cauwenberge F, Alfonso J. Patient outcomes following implantation with a trifocal toric IOL: twelve‐month prospective multicentre study. Eye (Lond). 2019;33(1):144‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hoffmann PC, Hütz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. J Cataract Refract Surg. 2010;36(9):1479‐1485. [DOI] [PubMed] [Google Scholar]


