Abstract
Objective
There is a widespread use of buffered crystalloid solutions in clinical practice. However, guidelines do not distinguish between specific types of buffered solutions and clinical equipoise exists. We aimed to assess the desirable and undesirable effects of acetate‐ versus lactate‐buffered solutions in hospitalised patients.
Methods
We conducted a systematic review with meta‐analysis and trial sequential analysis of randomised clinical trials assessing the use of acetate‐ versus lactate‐buffered solutions for intravenous administration in hospitalised adults and children. The primary outcome was all‐cause short‐term mortality. We adhered to our published protocol, the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement, the Cochrane Handbook and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) methodology.
Results
We included five RCTs enrolling 390 patients. We found no statistically significant difference in short‐term mortality (random effects, risk ratio [RR] 0.29; 95% confidence interval [CI] 0.06–1.51, p = .14, I 2 = 0%) or hospital length of stay (LOS) (random effects, mean difference [MD]—1.31, 95% CI −3.66 to 1.05, p = .28, I 2 = 0%) between acetate‐ versus lactate‐buffered solutions. The quality of evidence was very low. Data regarding intensive care unit LOS were reported by three trials and duration of vasopressor treatment by one trial; none of these data allowed for pooling in meta‐analyses. No trials reported data on long‐term mortality, health‐related quality of life, adverse events, duration of mechanical ventilation or renal replacement therapy.
Conclusion
In this systematic review, we found very low quantity and quality of evidence on the use of acetate‐ versus lactate‐buffered solutions in hospitalised patients.
Keywords: acetate, crystalloid, fluid therapy, lactate
Editorial Comment
Buffered crystalloid solutions are increasingly used in balanced fluid therapy. This include acetated and lactated solutions. It is uncertain if these solutions differ in adverse events or are harmful for patients. The effects of these solutions could potentially influence a significant number of patients worldwide, particularly those with critical illness who are treated at the ICU. The review points towards a significant need for further and large scale trials investigating benefit and harm of these everyday treatments.
1. BACKGROUND
Intravenous (IV) fluid therapy is widely used in daily clinical practice. 1 Several different types of fluid, intended for various purposes, are available. Among these are isotonic crystalloid solutions comprising normal saline and different buffered solutions.
The use of isotonic crystalloids has increased over the last decade. 2 , 3 , 4 However, clinical practice guidelines increasingly recommend buffered solutions over normal saline as the latter has been associated with unwarranted effects such as hyperchloremic acidosis and possibly increased risk of acute kidney injury (AKI). 5 , 6 , 7 , 8
The buffered solutions (often referred to as “balanced” or “physiologic” solutions) were originally developed to resemble the composition of extracellular fluid closer than normal saline. However, these solutions continue to differ from the composition of plasma. In comparison, they are relatively hypotonic and contain alternative anions, such as acetate or lactate, as a substitute to the naturally occurring buffer bicarbonate. 9 , 10
Harm has been suggested from excessive administration of acetate‐ and lactate‐buffered crystalloid solutions. Acetated solutions might cause cardiotoxicity 11 , 12 , 13 while lactated solutions may result in hyperlactatemia and possibly be inappropriate for patients with impaired liver function. 14 , 15 Importantly, these concerns are based on findings from experimental‐ and observational studies as well as theoretical conceptions. Hence, the clinical implications remain unknown.
The effects of these alternative buffers in clinical practice are poorly described, and the adverse events differ between the solutions. 16 Current clinical practice guidelines do not distinguish between the different types of buffered solutions depending on their buffering anion. 6 , 7 As buffered crystalloid solutions have varying compositions, considering them as a single class of fluid could potentially cause for misinterpretation of different effects between the different solutions thus underlining the importance of this research question. Assessment of interventions used in everyday clinical practice is highly important, as several commonly used interventions have been shown to be at best ineffective, but also harmful for some interventions. 17 This highlights the need to scrutinise routine clinical interventions used by many patients, including the use of buffered crystalloid solutions.
Accordingly, we aimed to summarise and assess the benefits and harms of acetate‐ versus lactate‐buffered crystalloid solutions in hospitalised patients. We hypothesised that the available evidence on the use of acetate‐ versus lactate buffered solutions in hospitalised patients would be sparse.
2. METHODS
We conducted this systematic review according to a prespecified published protocol, 18 and the protocol was registered in the International Prospective Register of Systematic Reviews PROSPERO(CRD42020199743) prior to publication.
We followed the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions; 19 the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement 20 (Electronic supplementary material [ESM], PRISMA checklist); and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. 21
2.1. Types of studies
We included randomised clinical trials (RCTs) assessing the use of acetate‐ versus lactate‐buffered crystalloid solutions in hospitalised patients. We imposed no restrictions regarding language, blinding, publication source or ‐status. We excluded quasi‐randomised trials, individual crossover trials and observational studies. 18
2.2. Types of participants
We included trials conducted in adults or children admitted to hospital for any reason. We excluded trials in animals and healthy subjects. 18
2.3. Types of interventions
We included RCTs assessing IV administration of any primarily acetate‐buffered solution (i.e., Ringer's Acetate, Plasmalyte™, Normosol™, Kabilyte™, Sterofundin™, Ionosteril™ etc.) versus any primarily lactate‐buffered solution (i.e., Ringer's Lactate or Hartmann's solution). Interventions were considered eligible irrespective of timing, dosing, and duration of treatment and multiple intervention‐ and control groups were permitted. Trials in which patients were randomised to other supplemental IV fluids in both the intervention and control group were permitted, if identical solutions were administered in both arms. 18
2.4. Types of outcome measures
2.4.1. Primary outcome measure
The primary outcome was all‐cause short‐term mortality ≤90 days, including in‐Intensive Care Unit (ICU) and in‐hospital mortality.
2.4.2. Secondary outcome measures
Secondary outcomes included;
(1) All‐cause long‐term mortality >90 days, (2) adverse events (as defined by the original trials) at the longest follow‐up, (3) health‐related quality of life (any continuous scale used in the included trials) at the longest follow‐up, (4) hospital length of stay (LOS), (5) ICU LOS, (6) days alive without/duration of mechanical ventilation (if both were available, we report the outcome for which most data exist), (7) days alive without/duration of renal replacement therapy (if both were available, we report the outcome for which most data exist), (8) days alive without/duration of vasopressor/inotropic treatment (if both were available, we report the outcome for which most data exist).
2.4.3. Search methods for identification of studies
We systematically searched MEDLINE (PubMed interface, 1966 onwards), Embase (OVID interface, 1947 onwards), The Cochrane Central Register of Controlled Trials (2018, Issue 10) and Epistemonikos. In addition, we searched databases of ongoing trials from clinical trial registries. We manually searched reference lists of relevant trials and systematic reviews. All searches were conducted from inception to December 6, 2021, when the last search was performed. The full search strategy is provided in the ESM 2.
2.5. Data collection and analysis
2.5.1. Selection of studies
Two authors (K.L.E. and P.S.) independently screened articles for inclusion based on titles and abstracts. Potentially eligible articles were independently evaluated in full text by two authors (K.L.E. and P.S.). Disagreements were resolved by discussion with a third senior author (M.H.M. or A.P.).
2.5.2. Data extraction and management
Two authors independently extracted data using a standardised data extraction form in duplicate (ESM Table 3.1–3.4). Extracted data items included trial characteristics (year of publication, country and number of trial sites), characteristics of trial settings (i.e. medical‐, surgical‐ or emergency department [ED] or intensive care unit) population (inclusion‐ and exclusion criteria), intervention/comparator (type of fluid, indication for use, amount of fluid infused, fluid administration protocol and duration of intervention) and data on the predefined outcome measures.
If prespecified data were not available authors were contacted for further data. Authors of all five included trials were contacted 22 , 23 , 24 , 25 , 26 of which one author of two trials replied and provided additional data. 24 , 25 Disagreements were resolved by discussion with a third senior author (M.H.M. or A.P.).
2.5.3. Risk of bias
Two authors independently assessed the risk of bias of the included trials using the Risk Of Bias tool 2 (ROB2) from the Cochrane Collaboration. 19 , 27 For each included trial, we assessed the following bias domains: (1) bias arising from the randomisation process, (2) bias because of deviations from intended interventions, (3) bias because of missing outcome data, (4) bias in measurement of the outcome and (5) bias in selection of the reported result. 27 Trials were adjudicated as having an either overall low risk of bias, some concerns or high risk of bias for individual outcome measures. The overall risk of bias assessment for each specific outcome was based on the worst risk of bias assessment in any of the prespecified domains, that is, if one or more domains were judged as being high risk of bias, we classified the trial as having overall high risk of bias for the particular outcome, if one or more domains were judged as some concerns of bias we judged the trial as having overall some concerns of bias, and if all trials were judged as low risk of bias we judged the trial as having overall low risk of bias. 27 Disagreements were resolved by discussion with a third senior author (M.H.M. or A.P.).
2.6. Data synthesis
2.6.1. Measures of treatment effect
We calculated relative risks (RRs) with corresponding 95% confidence intervals (CIs) for dichotomous outcomes and mean difference (MD) with corresponding standard deviation (SD) for continuous outcomes. If trials reported alternative measures for continues outcomes, for example, median and inter quartile range (IQR), authors were contacted for additional data. If authors failed to reply, the respective continuous outcome data were not pooled, and we reported median and IQR descriptively. Intention‐to‐treat (ITT) analyses were reported if available. We reported the most conservative treatment effect estimate using either fixed or random effects models. 18
The primary result is based on data from all available trials regardless of the risk of bias evaluation.
2.6.2. Data analysis
We used R version 4.0.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria) with the meta package to conduct the conventional meta‐ and subgroup analyses. We used Trial Sequential Analysis (TSA) version 0.9.5.10 b (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, available from http://www.ctu.dk/tsa) to conduct the TSA.
2.6.3. Assessment of heterogeneity
We assessed statistical heterogeneity by inspecting forest plots and by using the inconsistency (I2) and diversity (D2) statistics 28 , 29 with thresholds as suggested by the Cochrane handbook. 19 We used both random‐effects models (assuming that the true intervention effects in the included trials are not identical but follow normal distribution) and fixed‐effect models (assuming that the true effect of the intervention in both direction and magnitude is fixed across included trials) across outcomes, and we reported the most conservative estimates (highest p‐value) for cautiousness. 30 , 31 We addressed potential statistical heterogeneity in the pre‐planned subgroup analyses. 18
2.6.4. Assessment of small trial bias
Fewer than 10 trials were included, thus we did not assess the risk of small trial bias. 18
2.6.5. Subgroup analyses
We planned to conduct five subgroup analyses; (1) trials with an overall high versus low risk of bias, (2) trials conducted in ICU versus non‐ICU patients, (3) trials conducted in surgical versus non‐surgical settings, (4) trials in children versus adults (as defined by the original trial) and (5) trials assessing administration of higher versus lower volumes of buffered crystalloid solution (defined as < versus > the median of administered fluid volumes across trials). 18 We used the chi‐squared test to assess the statistical heterogeneity across patient subgroups considering a p‐value of .10 as statistically significant.
2.6.6. Sensitivity analysis
We applied empirical continuity correction if zero event trials were included in our meta‐analyses. 32 We did not conduct sensitivity analysis with best‐to‐worst‐case and worst‐to‐best‐case scenarios, as no trials reported loss to follow‐up. 18
2.6.7. Assessment of risk of random errors
We used trial sequential analysis (TSA) to assess the risk of random errors because of sparse data and multiple significance testing. In brief, TSA estimates the required information size needed to detect or reject an a priori pre‐specified realistic intervention effect in a meta‐analysis and widens the CIs (TSA adjusted CI) in cases where data are too sparse to draw conclusions. 29 , 33 , 34 Moreover, we used TSA to test for futility by applying futility boundaries. We applied trial sequential monitoring boundaries according to an a priori 15% RR difference for dichotomous outcomes and an a priori MD of 1 day for continuous outcomes, a family‐wise error rate equal to an alpha of 5% for all outcomes, a beta of 10% (power of 90%), and a control event proportion as per the control arm of the included trials. 30 , 31 , 35 According to our statistical analysis plan, we considered p < .05 as statistically significant for the primary‐ and secondary outcomes, given that we had data available for analysis for only one of eight secondary outcomes. 18 As per our protocol we do not present TSA details or plots if, according to the TSA, <5% of the diversity‐adjusted required information size was accrued. 18
2.6.8. Assessment of the overall quality of evidence
Two authors (K.L.E. and P.S.) independently assessed the quality of evidence for all outcomes using the GRADE methodology. 21 The overall quality of evidence was rated high, moderate, low or very low based on evaluations of risks of bias, inconsistency, indirectness, imprecision and publication bias across studies.
3. RESULTS
We screened 7475 records, assessed 50 trials in full‐text and included five trials (n = 392 randomised patients) in the systematic review. 22 , 23 , 24 , 25 , 26 A total of four trials (n = 288) could be included in the meta‐analyses. 22 , 23 , 24 , 25 In addition, we identified three on‐going trials. 36 , 37 , 38 The main reasons for excluding trials were wrong study design and wrong outcome measures (Figure 1—PRISMA flowchart).
FIGURE 1.
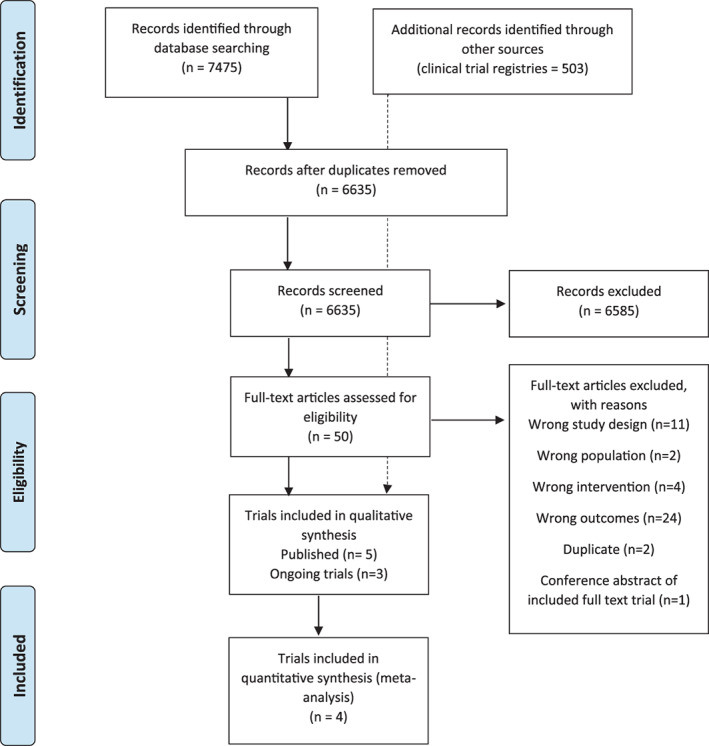
PRISMA flow chart
3.1. Characteristics of trials
The included trials were published from 2011 through 2020. Four were single‐centre trials 22 , 23 , 24 , 26 and one was a multicentre trial; 25 the largest trial included 150 patients. 23 Four trials included patients in a planned surgical setting, 23 , 24 , 25 , 26 and one trial included burn patients in a specialised ICU. 22 All trials were published in English. Further trial characteristics are provided in Table 1.
TABLE 1.
Trial characteristics
| Study/year | Country | Single/multicentre trial (no. of sites) | No. of patients | Clinical setting | Population | Exclusion criteria | Acetate‐buffered solution (no. randomised) | Lactate‐buffered solution (no. randomised) | Patient‐important outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Chaussard et al/2020 22 | France | Single | 28 | Tertiary Burn ICU | Patients >18 years, with burns (>30% TBSA) | Pregnancy, refusal to participate and do not resuscitate orders | Plasmalyte (n = 14) | Ringers Lactate (n = 14) | 28‐day mortality and ICU length of stay |
| Pfortmüller et al/2019 23 | Switzerland | Single | 150 | Surgery and ICU | Patients 18–80 years, undergoing elective open cardiac surgery | <18 years or >80 years. Pre‐existing impaired cardiac or renal function, anaemia, chronic inflammatory‐ or liver disease. Long term steroid medication, active infection, emergency‐ or redo surgery and isolated CABG surgery. Planned use of minimal extracorporeal circuits, early extubation protocols or restrictions to full therapy | Ringers Acetate (n = 75) | Ringers Lactate (n = 75) | In‐hospital mortality, hospital length of stay, ICU length of stay and duration of inotropic /vasopressor treatment |
| Weinberg et al/2018 24 | Australia | Single | 50 | Surgery | Adults undergoing elective primary CABG or valve surgery | Requirement of Custodial® HTK cardioplegia solution, pregnancy, abnormal plasma bicarbonate concentration, hypercapnic respiratory failure, chronic renal‐ or liver disease, diabetes, anaemia or morbid obesity | Plasmalyte‐148 (n = 25) | Hartmann's solution (n = 25) | In‐hospital mortality, hospital length of stay and ICU length of stay |
| Weinberg et al/2015 25 | Australia | Multi (4) | 60 | Surgery | Adult patients, undergoing elective open major liver resection | <18 years, laparoscopic or minimally invasive surgery, hepatic‐ or renal impairment, preoperative coagulopathy, and ASA score 4 or 5 | Plasmalyte‐148 (n = 30) | Hartmann's solution (n = 30) | 30‐day mortality, hospital length of stay and ICU length of stay |
| Shin et al/2011 26 | Korea | Single | 104 | Surgery | Living liver donors undergoing scheduled right hepatectomy | >40 years, emergency donors, left hepatectomy, had >30% fatty change | Plasmalyte (n = 52) | Ringers Lactate (n = 52) | Post‐operative hospital stay |
| Semler MW/Ongoing trial (NCT03537898) | USA | Single | Expected 2093 | Medical ICU | Patients ≥18 years admitted to the Medical ICU | Prisoners | Normosol | Ringers Lactate | 30‐day in‐hospital mortality, renal replacement therapy‐free days, vasopressor‐free days, ventilator‐free days, intensive care unit‐free days |
| Weiss S/Ongoing trial (NCT04102371) | USA | Multi (30+) |
Expected 8800 |
Paediatric dept. and ED's receiving paediatric patients | Patients >6 months to <18 years with suspected sepsis who received <40 ml/kg IV/IO total crystalloid fluid prior to randomisation | Hyperkalaemia, hypercalcemia, hepatic‐ or renal impairment, metabolic disorder, impending brain herniation, pregnancy or known allergy to study fluids. prisoners | Plasmalyte | Ringers Lactate | All‐cause 90‐day mortality |
| Raman S/Ongoing trial 38 | Australia | Single | Expected 480 | Paediatric ICU | Patients age from birth to <16 years admitted to PICU within the last 24 h and received IVFT for ≤4 h in PICU. All fluids must have been administered in PICU. Patients sodium level >130 mmol/L | Age >16 years, received IVFT for >4 h in PICU or admitted for cardiac condition or chronic kidney disease. Patients with Traumatic brain injury, burns, post‐liver transplant, post‐renal transplant, ketoacidosis, major electrolyte abnormalities or oncologic patients in need of rehydration | Plasmalyte | Compound sodium lactate | Organ dysfunction free survival, PICU LOS, hospital LOS, PICU free survival and adverse event |
Abbreviations: ASA, American Society of Anaesthesiology score; CABG, coronary artery bypass grafting; ED, emergency department; ICU, intensive care unit; IO, intraosseous; IV, intravenous; IVFT, intravenous fluid therapy; PICU, paediatric intensive care unit; TBSA, total burn surface area.
3.2. Description of the intervention
The type of intervention/comparator and fluid administration protocols varied between trials. In three trials the administration of trial fluid was limited to the perioperative period, 23 , 25 , 26 one trial assessed the use of crystalloid solutions intraoperatively as CABG priming fluid 24 and one trial assessed the use of buffered crystalloid solutions as resuscitation fluid in critically ill burn patients for 5 days following admission to the ICU. 22 Total volumes of trial fluids infused varied from a minimum of 2.2 L per patient 25 to a maximum of 19.6 L within the study period. 22 The most studied solutions were Plasma‐Lyte versus Ringer's lactate. Full details on fluid consumption within the trials are available in the ESM Table 4.
3.3. Risk of bias
All included trials were adjudicated as having overall ‘low’ or ‘some concerns’ risk of bias for all available outcomes (Figures 2.1–2.4). The main reason for concerns were risk of bias because of deviations from the intended interventions and selection of reported results. Further details on the risk of bias assessment are presented in ESM Table 6.1–6.5.
FIGURE 2.
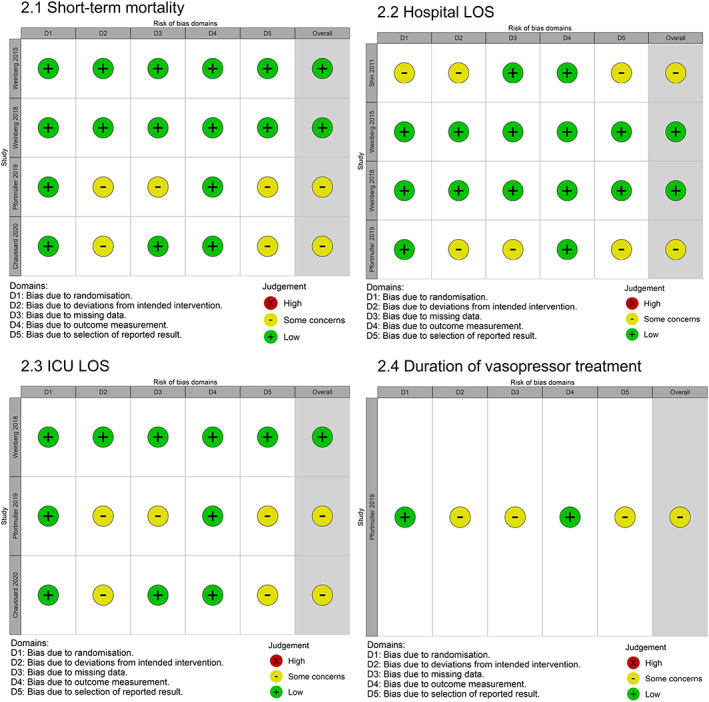
Risk of bias evaluation (ROB2) summary figures. Risk of bias was estimated for the following bias domains: (1) bias arising from the randomisation process, (2) bias because of deviations from intended interventions, (3) bias because of missing outcome data, (4) bias in measurement of the outcome and (5) bias in selection of the reported result
3.4. Primary outcome
3.4.1. All‐cause short‐term mortality ≤90 days
Four trials (n = 286) reported data on short‐term mortality. 22 , 23 , 24 , 25 We found no statistically significant difference in short‐term mortality between patients receiving acetate‐ versus lactate buffered crystalloid solutions (random effects, RR 0.29; 95% CI 0.06–1.51, p = .14, I 2 = 0%) (Figure 3). TSA could not be performed as <1% of the required information size of 39,835 patients had been accrued. Subgroup analyses were consistent with the primary estimate (ESM Table 7.3). The quality of evidence was very low because of inconsistency and imprecision (Table 2).
FIGURE 3.
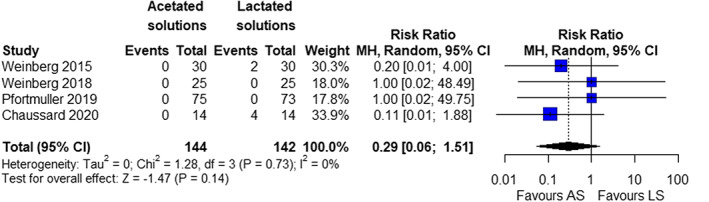
Forrest plot of primary outcome; all cause short‐term mortality (random‐effect model)
TABLE 2.
Summary of findings table. Evaluation of the quality of evidence according to Grading of Recommendations Assessment, Development, and Evaluation (GRADE)
| Certainty assessment | № of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Acetate‐buffered crystalloid solutions | Lactate‐buffered crystalloid solutions | Relative (95% CI) | Absolute (95% CI) | ||
| All‐cause short‐term mortality ≤ 90 days | ||||||||||||
| 4 | Randomised trials | Not serious a | Serious b , c | Not serious | Very serious d | None | 0/144 (0.0%) | 6/142 (4.2%) | RR 0.15 (0.02 to 1.15) | 36 fewer per 1.000 (from 41 fewer to 6 more) |
⨁◯◯◯ Very low |
Critical |
| All‐cause long‐term mortality >90 days—not reported | ||||||||||||
| – | – | – | – | – | – | – | – | – | – | – | – | Critical |
| Adverse events—not reported | ||||||||||||
| – | – | – | – | – | – | – | – | – | – | – | – | Critical |
| Health related quality of life (any scale)—not reported | ||||||||||||
| – | – | – | – | – | – | – | – | – | – | – | – | Critical |
| Hospital length of stay (assessed with: days) | ||||||||||||
| 2 | Randomised trials | Not serious a | Serious c | Not serious | Very serious e | None | 182 | 180 | – | MD 1.31 days lower (3.66 lower to 1.05 higher) |
⨁◯◯◯ Very low |
Important |
| ICU length of stay (assessed with days) | ||||||||||||
| 3 | Randomised trials | Not serious a | Serious b , c | Not serious | Very serious d | None | 114 | 112 | – | Not pooled | ⨁◯◯◯Very low | Important |
| Duration of mechanical ventilation (MV)—not reported | ||||||||||||
| – | – | – | – | – | – | – | – | – | – | – | – | Important |
| Duration of renal replacement therapy (RRT)—not reported | ||||||||||||
| – | – | – | – | – | – | – | – | – | – | – | – | Important |
| Duration of vasopressor/inotropics | ||||||||||||
| 1 | Randomised trials | Not serious a | Not serious | Not serious | Very serious f | None | 75 | 73 | – | 0 (0–0) |
⨁⨁◯◯ Low |
Important |
Abbreviations: CI, confidence interval; MD, mean difference; RR, risk ratio.
Trials were judged as overall ‘low’ risk of bias or ‘some concerns’ (unclear risk of bias). Potential limitations are unlikely to lower confidence in the estimate of effect.
Inconsistency downgraded because of differences in population.
Inconsistency downgraded because of difference in interventions (e.g. one trial applied fluids as pump prime during CABG and one trial used fluids as resuscitation therapy in critically ill burn patients).
TSA could not be performed because <5% of the required information size had been reached.
TSA revealed that only 7% of the required information size had been accrued.
Duration of vasopressor was reported by only 1 trial (n = 150).
3.5. Secondary outcome measures
3.5.1. Hospital LOS
A total of four trials (n = 362) reported data on hospital LOS. 23 , 24 , 25 , 26 Data from two trials (n = 110) allowed for pooling in a meta‐analysis. 24 , 25 We found no statistically significant difference in hospital LOS between the use of acetate versus lactate buffered crystalloid solutions (random effects, MD – 1.31, 95% CI −3.66 to 1.05, p = .28, I 2 = 0%) (Figure 4). TSA found that 7% of the required information size of 1669 patients had been accrued (TSA‐adjusted CI, −10.94 to 8.28) (ESM Figure 8). Data were not available for any subgroup analyses. The quality of evidence was very low because of inconsistency and imprecision (Table 2).
FIGURE 4.
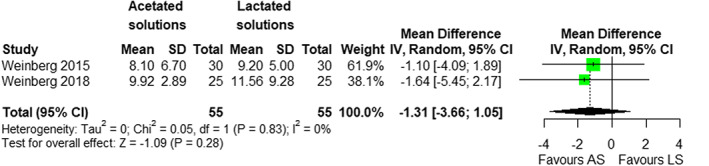
Forrest plot of hospital length of stay (random‐effect model)
3.5.2. ICU LOS
A total of three trials (n = 226) reported data on ICU LOS. 23 , 24 , 26 Data could not be pooled as only one trial provided results as mean (SD) (MD −1.30, 95% CI −3.66 to 1.06, p = .28) 24 while the remaining two trials reported LOS as median (IQR) (ESM Table 5.3). 23 , 26 All trials individually suggested no statistically significant difference in ICU LOS between patients receiving acetate‐ versus lactate‐buffered solutions. The quality of evidence was very low because of inconsistency and imprecision (Table 2).
3.5.3. Vasopressor/inotropic treatment
A total of one trial (n = 150) reported data on duration of vasopressor and inotropic treatment. 23 There was no statistically significant difference in duration of vasopressor/inotropics in between groups (ESM Table 5.4). The quality of evidence was low because of imprecision (Table 2).
3.5.4. Other outcomes
No studies reported data on all‐cause long‐term mortality >90 days, adverse events, health‐related quality of life, duration of/days alive without mechanical ventilation or duration of/days alive without renal replacement therapy.
3.6. Subgroup analyses
We observed no interaction in the subgroup analyses of trials conducted in ICU versus non‐ICU, trials conducted in medical‐ versus. surgical settings and trials administering lower versus higher volumes of trial fluid for the primary outcome short‐term mortality (ESM Table 7.3). The remaining predefined subgroup analyses could not be carried out.
4. DISCUSSION
In this systematic review of RCTs assessing the use of IV acetate‐ versus lactate‐buffered crystalloid solutions in in adults or children admitted to hospital for any reason, we found very low quantity and quality of evidence supporting the decision on the type of buffered crystalloid solution used in hospitalised patients, that is, there was no firm evidence of benefit or harm.
4.1. Summary of evidence
We found no statistically significant difference in all‐cause short‐term mortality, hospital‐ or ICU LOS between patients receiving IV acetate‐ versus lactate‐buffered crystalloid solutions. TSA indicated a high risk of random errors and low quantity of data, and the overall quality of evidence was very low for most outcome measures. Thus, further high‐quality trials are needed to establish firm evidence. Importantly, no trials reported data on “critical” secondary outcome measures, including the outcome adverse effects. Failure to report information on adverse effects hinders interpretation of overall effects. 39 , 40
4.2. Relation to current evidence
This review is the first systematic review of RCTs with meta‐analysis and TSA comparing the use of buffered crystalloid solutions based on their buffering anion, that is, acetate‐ versus lactate‐buffered solutions.
Several RCTs and reviews hereof have compared buffered solutions with normal saline without distinctively assessing the different types of buffered solutions. 41 , 42 , 43 , 44 , 45 , 46 A review by Curran et al. 47 assessed the use of different types of buffered crystalloid solutions based on manufactural origin rather than on their buffering agent. They concluded that data on patient‐important outcomes were too sparse and heterogeneous to allow for pooling, which is in line with our results. In addition, they suggested that, based on surrogate outcomes, Plasmalyte (a primarily acetate‐buffered solution) may result in less metabolic abnormalities as compared with other buffered crystalloids. However, the clinical implications of these metabolic abnormalities are unknown.
The population investigated in our review primarily consisted of planned surgical patients. Hence, the possibility of heterogeneity of treatment effect cannot be dismissed, and the results may not be generalisable to all groups of hospitalised patients. This includes critically ill patients who might be more susceptible to the possible metabolic changes caused by different buffered solutions. 48
Regarding the intervention, the amounts of fluid‐ and the duration of fluid therapy currently investigated, might not adequately represent daily clinical practice. It is likely, that patients in daily clinical practice receive larger amounts of fluid throughout hospitalisation, including inadvertently administered volumes as drug diluents or to preserve catheter patency. 49 , 50 A greater exposure to the intervention could potentially have a greater impact on patient‐important outcomes. The importance of this is illustrated by a secondary analysis of the Isotonic Solutions and Major Adverse Renal Events Trial (SMART). 51 Among patients with sepsis, the effect of buffered crystalloids versus saline on mortality was greater among patients for whom fluid choice was controlled starting in the ED than in those commencing in the ICU. 51
We exclusively evaluated patient‐important outcome measures. Interestingly, a previous scoping review comparing acetate‐ versus lactate‐buffered solutions regardless of study design or type of outcome found that less than 25% of studies reported patient‐centered outcome measures. 16 Importantly, trials reporting surrogate outcome measures are more likely to produce falsely inflated estimates and are at increased risk of false‐positive findings. 52 , 53
Considering the vast use of buffered crystalloid solutions in clinical practice, the lack of data comparing their individual effects on patient‐important outcomes is surprising. Importantly, we identified three on‐going trials 36 , 37 , 38 investigating the effects of acetate‐ versus lactate‐buffered solutions on patient‐important outcome measures, including the large “BASE” pilot trial (Semler et al. expected n = 2093), 36 which will contribute markedly to the existing body of evidence.
4.3. Implications for further research
Buffered crystalloid solutions are widely used in daily clinical practice likely because they are perceived as safe based on assumptions of their similarities to extracellular fluid. However, there is no firm evidence supporting their safety. Importantly, RCTs have previously shown harm from routine interventions in clinical practice, including that of fluid therapy. 17 Administration of colloid solutions have been shown to harm some patients groups as observed for albumin in patients with traumatic brain injury 54 and hydroxyethyl starch in patients with sepsis. 55 Clearly, we cannot treat our patients based on preconceived notions of the effectiveness and safety of a given intervention, as this can have potential serious ramifications for the individual patient.
It is possible that the use of fluids depending on their buffering anion, for example, acetate versus lactate does not significantly affect patient‐centered outcomes. However, considering their vast use in clinical practice, even minor differences in the desirable or undesirable effects will affect many patients.
4.4. Strengths and limitations
We published a protocol and statistical analysis plan prior to conducting the review. The review was planned and reported according to recommendations from the Cochrane collaboration, PRISMA statement and GRADE methodology. Furthermore, we used TSA to test the robustness of the meta‐analysis and to estimate the required information size. 56 Other strengths include the comprehensive systematic search strategy, duplicate independent screening, data extraction and risk of bias assessment and assessment of patient‐important outcome measures only.
Our review also holds limitations. First, the body of evidence consists of a limited number of small trials with overall some concern of bias for most outcomes, increasing the risk of falsely inflated effect estimates. 52 Second, the included trials were clinically heterogenous in terms of patient population, setting intervention/comparator (e.g. types of solution) and fluid administration protocol. Third, many of our predefined patient‐important outcome measures were not reported, and the body of evidence describing these outcomes was limited resulting in clinical equipoise on the balance between benefits and harms. Fourth, we were not able to pool all available data as some trials reported continues outcomes only as median (range/IQR). Authors were contacted for additional data but only one replied. We refrained from calculating mean and SD using the reported median and IQR as suggested by the Cochrane Handbook, 19 , 57 as this approach assumes that the data are normally distributed. Furthermore, data conversion was not prespecified in the study protocol. Finally, we a priori considered a 15% RRD between groups clinically relevant. Consequently, smaller treatment effects cannot be ruled out.
5. CONCLUSION
In this systematic review of RCTs assessing the use of IV acetate‐ versus lactate‐buffered crystalloid solutions in adults and children admitted to hospital for any reason, we found very low quantity and quality of evidence supporting the decision on the type of buffered crystalloid solution, that is, there was no firm evidence for benefit or harm.
AUTHOR CONTRIBUTIONS
KLE: study design, data extraction, data analysis and drafting of manuscript and approval of the final manuscript. AP: study design and critical revision of manuscript for important intellectual content and approval of the final manuscript. PS: data extraction and critical revision of manuscript for important intellectual content, and approval of the final manuscript. MHH: study design and critical revision of manuscript for important intellectual content and approval of the final manuscript.
CONFLICT OF INTEREST
The Department of Intensive Care, Copenhagen University Hospital, Rigshospitalet, receives academic funding from The Novo Nordisk Foundation, Sygeforsikringen ‘danmark’, Fresenius Kabi and Pfizer Denmark. None of the organisations have been involved in the design, conduct, analyses, or reporting of this review. Authors K.L.E., A.P., P.S. and M.H.M. have no individual conflicts of interest to declare.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
None.
Ellekjaer KL, Perner A, Sivapalan P, Møller MH. Acetate‐ versus lactate‐buffered crystalloid solutions: A systematic review with meta‐analysis and trial sequential analysis. Acta Anaesthesiol Scand. 2022;66(7):782‐794. doi: 10.1111/aas.14076
Funding informationThis research project was funded by the Ehrenreich's foundation and Rigshospitalets research foundation. The funding organisations were not involved in the design, conduct, analyses or reporting of the review.
REFERENCES
- 1. Padhi S, Bullock I, Li L, Stroud M. Intravenous fluid therapy for adults in hospital: summary of NICE guidance. BMJ. 2013;347:f7073. [DOI] [PubMed] [Google Scholar]
- 2. Glassford NJ, French CJ, Bailey M, Mârtensson J, Eastwood GM, Bellomo R. Changes in intravenous fluid use patterns in Australia and New Zealand: evidence of research translating into practice. Crit Care Resusc. 2016;18:78‐88. [PubMed] [Google Scholar]
- 3. Miller TE, Bunke M, Nisbet P, Brudney CS. Fluid resuscitation practice patterns in intensive care units of the USA: a cross‐sectional survey of critical care physicians. Perioper Med (Lond). 2016;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hammond NE, Taylor C, Finfer S, et al. Patterns of intravenous fluid resuscitation use in adult intensive care patients between 2007 and 2014: an international cross‐sectional study. PLoS One. 2017;12:e0176292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Semler MW, Kellum JA. Balanced crystalloid solutions. Am J Respir Crit Care Med. 2019;199:952‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soni N. British consensus guidelines on intravenous fluid therapy for adult surgical patients (GIFTASUP): Cassandra's view. Anaesthesia. 2009;64:235‐238. [DOI] [PubMed] [Google Scholar]
- 7. Malbrain M, Langer T, Annane D, et al. Intravenous fluid therapy in the perioperative and critical care setting: executive summary of the international fluid academy (IFA). Ann Intensive Care. 2020;10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans L, Rhodes A, Alhazzani W, et al. Executive summary: surviving sepsis campaign: international guidelines for the Management of Sepsis and Septic Shock 2021. Crit Care Med. 2021;49:1974‐1982. [DOI] [PubMed] [Google Scholar]
- 9. Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Nat Rev Nephrol. 2018;14:541‐557. [DOI] [PubMed] [Google Scholar]
- 10. Morgan TJ. The ideal crystalloid ‐ what is 'balanced'? Curr Opin Crit Care. 2013;19:299‐307. [DOI] [PubMed] [Google Scholar]
- 11. Schrander‐vd Meer AM, ter Wee PM, Kan G, Donker AJ, van Dorp WT. Improved cardiovascular variables during acetate free biofiltration. Clin Nephrol. 1999;51:304‐309. [PubMed] [Google Scholar]
- 12. Kirkendol RL, Pearson JE, Bower JD, Holbert RD. Myocardial depressant effects of sodium acetate. Cardiovasc Res. 1978;12:127‐136. [DOI] [PubMed] [Google Scholar]
- 13. Jacob AD, Elkins N, Reiss OK, Chan L, Shapiro JI. Effects of acetate on energy metabolism and function in the isolated perfused rat heart. Kidney Int. 1997;52:755‐760. [DOI] [PubMed] [Google Scholar]
- 14. Ergin B, Kapucu A, Guerci P, Ince C. The role of bicarbonate precursors in balanced fluids during haemorrhagic shock with and without compromised liver function. Br J Anaesth. 2016;117:521‐528. [DOI] [PubMed] [Google Scholar]
- 15. Kierdorf HP, Leue C, Arns S. Lactate‐ or bicarbonate‐buffered solutions in continuous extracorporeal renal replacement therapies. Kidney Int Suppl. 1999;56:S32‐S36. [PubMed] [Google Scholar]
- 16. Ellekjaer KL, Perner A, Jensen MM, Møller MH. Lactate versus acetate buffered intravenous crystalloid solutions: a scoping review. Br J Anaesth. 2020;125:693‐703. [DOI] [PubMed] [Google Scholar]
- 17. Landoni G, Comis M, Conte M, et al. Mortality in multicenter critical care trials: an analysis of interventions with a significant effect. Crit Care Med. 2015;43:1559‐1568. [DOI] [PubMed] [Google Scholar]
- 18. Ellekjaer KL, Perner A, Sivapalan P, Hylander MM. Acetate‐ vs lactate‐buffered crystalloid solutions: protocol for a systematic review with meta‐analysis and trial sequential analysis. Acta Anaesthesiol Scand. 2021;65:123‐127. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. (accessed June 20th 2020)
- 20. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chaussard M, Dépret F, Saint‐Aubin O, et al. Physiological response to fluid resuscitation with ringer lactate versus Plasmalyte in critically ill burn patients. J Appl Physiol. 1985;2020(128):709‐714. [DOI] [PubMed] [Google Scholar]
- 23. Pfortmueller CA, Faeh L, Müller M, et al. Fluid management in patients undergoing cardiac surgery: effects of an acetate‐ versus lactate‐buffered balanced infusion solution on hemodynamic stability (HEMACETAT). Crit Care. 2019;23:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weinberg L, Chiam E, Hooper J, et al. Plasma‐Lyte 148 vs. Hartmann's solution for cardiopulmonary bypass pump prime: a prospective double‐blind randomized trial. Perfusion. 2018;33:310‐319. [DOI] [PubMed] [Google Scholar]
- 25. Weinberg L, Pearce B, Sullivan R, et al. The effects of plasmalyte‐148 vs. Hartmann's solution during major liver resection: a multicentre, double‐blind, randomized controlled trial. Minerva Anestesiol. 2015;81:1288‐1297. [PubMed] [Google Scholar]
- 26. Shin WJ, Kim YK, Bang JY, Cho SK, Han SM, Hwang GS. Lactate and liver function tests after living donor right hepatectomy: a comparison of solutions with and without lactate. Acta Anaesthesiol Scand. 2011;55:558‐564. [DOI] [PubMed] [Google Scholar]
- 27. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 29. Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Med Res Methodol. 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Med Res Methodol. 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jakobsen JC, Gluud C, Winkel P, Lange T, Wetterslev J. The thresholds for statistical and clinical significance ‐ a five‐step procedure for evaluation of intervention effects in randomised clinical trials. BMC Med Res Methodol. 2014;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Stat Med. 2004;23:1351‐1375. [DOI] [PubMed] [Google Scholar]
- 33. Turner RM, Bird SM, Higgins JP. The impact of study size on meta‐analyses: examination of underpowered studies in Cochrane reviews. PLoS One. 2013;8:e59202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. J Clin Epidemiol. 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
- 35. Jakobsen JC, Wetterslev J, Lange T, Gluud C. Viewpoint: taking into account risks of random errors when analysing multiple outcomes in systematic reviews. Cochrane Database Syst Rev. 2016;3:Ed000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Semler M. Balanced solutions and plasma electrolytes (BASE). Ongoing
- 37. Weiss S. Pragmatic pediatric trial of balanced versus Normal saline fluid in sepsis (PRoMPT BOLUS). Ongoing [DOI] [PMC free article] [PubMed]
- 38. Raman S, Schibler A, Marsney RL, et al. 0.9% sodium chloride solution versus plasma‐Lyte 148 versus compound sodium lacTate solution in children admitted to PICU‐a randomized controlled trial (SPLYT‐P): study protocol for an intravenous fluid therapy trial. Trials. 2021;22:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ioannidis JP, Contopoulos‐Ioannidis DG. Reporting of safety data from randomised trials. Lancet. 1998;352:1752‐1753. [DOI] [PubMed] [Google Scholar]
- 40. Phillips R, Hazell L, Sauzet O, Cornelius V. Analysis and reporting of adverse events in randomised controlled trials: a review. BMJ Open. 2019;9:e024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:1951‐1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378:819‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zampieri FG, Machado FR, Biondi RS, et al. Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: the BaSICS randomized clinical trial. JAMA. 2021;326(9):818‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Young P, Bailey M, Beasley R, et al. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701‐1710. [DOI] [PubMed] [Google Scholar]
- 45. Odor PM, Bampoe S, Dushianthan A, et al. Perioperative administration of buffered versus non‐buffered crystalloid intravenous fluid to improve outcomes following adult surgical procedures: a Cochrane systematic review. Perioper Med (Lond). 2018;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Antequera Martín AM, Barea Mendoza JA, Muriel A, et al. Buffered solutions versus 0.9% saline for resuscitation in critically ill adults and children. Cochrane Database Syst Rev. 2019;7:CD012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Curran JD, Major P, Tang K, et al. Comparison of balanced crystalloid solutions: a systematic review and meta‐analysis of randomized controlled trials. Crit Care Explor. 2021;3:e0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192:1045‐1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44:409‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Glassford NJ, Bellomo R. The complexities of intravenous fluid research: questions of scale, volume, and accumulation. Korean J Crit Care Med. 2016;31:276‐299. [Google Scholar]
- 51. Jackson KE, Wang L, Casey JD, et al. Effect of early balanced crystalloids before ICU admission on sepsis outcomes. Chest. 2021;159:585‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ciani O, Buyse M, Garside R, et al. Comparison of treatment effect sizes associated with surrogate and final patient relevant outcomes in randomised controlled trials: meta‐epidemiological study. BMJ. 2013;346:f457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Savovic J, Turner RM, Mawdsley D, et al. Association between risk‐of‐bias assessments and results of randomized trials in Cochrane reviews: the ROBES meta‐epidemiologic study. Am J Epidemiol. 2018;187:1113‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Myburgh J, Cooper DJ, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357:874‐884. [DOI] [PubMed] [Google Scholar]
- 55. Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med. 2012;367:124‐134. [DOI] [PubMed] [Google Scholar]
- 56. Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta‐analysis. BMC Med Res Methodol. 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


