Abstract
Purpose
Age‐related macular degeneration (AMD) is a leading cause of vision impairment. This randomised placebo‐controlled trial investigated whether point‐of‐care tools can improve optometrists' AMD knowledge and/or care provision.
Methods
Australian optometrists (n = 31) completed a demographics survey and theoretical AMD case study multiple‐choice questions (MCQs) to assess their confidence in AMD care provision and AMD knowledge. Participants were then randomly assigned to one of three point‐of‐care tools (online ‘Classification of Age‐related macular degeneration and Risk Assessment Tool’ (CARAT), paper CARAT, or ‘placebo’) to use when providing care to their subsequent 5–10 AMD patients. Participants self‐audited the compliance of their AMD care to best practice for these patients, and a similar number of consecutive patients seen prior to enrolment. Post‐intervention, participants retook the AMD knowledge MCQs and confidence survey.
Results
A total of 29 participants completed the study. At the study endpoint, clinical confidence relative to baseline improved with the paper CARAT, relative to placebo, for knowledge of AMD risk factors, asking patients about these factors and referring for medical retinal sub‐specialist care. There were no between‐group differences for the change in AMD knowledge scores. Considering record documentation for patients with any AMD severity, there were no significant between‐group differences for documenting patient risk factors, AMD severity, clinical examination techniques or management. In a sub‐analysis, the change from baseline in compliance for documenting discussions about patient smoking behaviours for early AMD patients was higher with use of the online CARAT relative to placebo (p = 0.04). For patients with intermediate AMD, the change from baseline in documenting the risk of progression to late AMD was greater among practitioners who used the paper CARAT, relative to placebo (p = 0.04).
Conclusions
This study demonstrates that point‐of‐care clinical tools can improve practitioner confidence and aspects of the documentation of AMD clinical care by optometrists as assessed by self‐audit.
Keywords: age‐related macular degeneration, clinical audit, clinical tool, optometry
Key points.
The Classification of Age‐related macular degeneration and Risk Assessment Tool is a novel in‐office clinical tool designed to support optometric care provision to people with age‐related macular degeneration.
Use of a paper‐based, point‐of‐care tool improved optometrists' self‐reported confidence in their knowledge of age‐related macular degeneration and how to manage the condition.
Implementation of age‐related macular degeneration point‐of‐care tools by optometrists to inform care provision improved clinical record documentation of aspects of management for patients with earlier stages of the disease.
INTRODUCTION
Evidence‐based practice (EBP) is commonly defined as the “conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients.” 1 It involves integrating the highest‐quality research evidence, with a practitioner's clinical expertise and a patient's preferences when making healthcare decisions. 2 Clinical practice guidelines (CPGs), based upon a systematic review of relevant research evidence, intend to support EBP and reduce potential variability in care. 3 , 4 However, a range of barriers exists to clinicians' use of, and adherence, to CPGs, 5 , 6 including lack of awareness and insufficient confidence in their implementation. 6 Potentially, some barriers can be addressed by clinical decision support tools that aim to provide evidence‐based information to guide patient assessment and/or management at the point of care. 7 Such tools may also facilitate communication with patients, to assist with incorporating patients' preferences in the decision‐making process.
Clinical decision support tools exist for a range of eye conditions. 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Despite the availability of such tools for optometrists in Australia, including for sight‐threatening conditions such as age‐related macular degeneration (AMD), 16 , 17 the efficacy of clinical decision support tools for supporting EBP in eye care practice has not been studied. AMD, affecting people over 55 years of age, is a degenerative condition of the macula that can lead to progressive central vision loss 18 , 19 and reduced quality of life. 20 Due to the current absence of validated treatments for reversing the pathophysiology of the disease, targeting modifiable risk factors at earlier stages of the disease is crucial for reducing the risk of progression to vision threatening, late‐stage AMD. 21 , 22
As major providers of primary eye care, Australian optometrists have a pivotal role in AMD care provision, including identifying and monitoring signs and symptoms of the disease, instituting appropriate management and referring patients for ophthalmological care when required. 23 , 24 To assist optometrists in managing AMD, several resources exist, including Optometry Australia's 2019 AMD Clinical Practice Guide and the Royal Australian and New Zealand College of Ophthalmologists (RANZCO) Referral Pathway for AMD Screening and Management by Optometrists (2018). 16 , 17 Whilst these resources provide information about recommended clinical examination procedures, AMD severity classification, suggested review periods for different disease severities and the criteria for referral to a medical retinal sub‐specialist, they are not specifically designed as point‐of‐care tools and do not enable calculation of a patients' risk of progression to late‐stage AMD. There is an opportunity to develop AMD clinical decision support tools that are easy for clinicians to understand, time efficient for use in‐office and facilitate communication with patients regarding risk factor modification for shared clinical decision‐making.
The primary aim of this randomised controlled trial (RCT) was to investigate the efficacy of a novel in‐office AMD clinical tool (the Classification of Age‐related macular degeneration and Risk Assessment Tool, CARAT), provided to optometrists in either online or paper formats, relative to a ‘placebo’ tool to support standard clinical care, for improving clinical record documentation and knowledge of AMD care. The main study outcomes were the accuracy of documenting patient risk factors, AMD severity, the calculated risk of progression to late‐stage AMD, advice regarding modifiable risk factors and an appropriate review period, as measured through clinical self‐audit using an online platform, the Macular Degeneration Clinical Care Audit Tool (MaD‐CCAT). 25 Changes to participants' clinical confidence and AMD knowledge (based on responses to theoretical case studies) were also evaluated.
METHODS
This study was conducted in accordance with the 2018 National Health and Medical Research Council (NHMRC) National Statement on Ethical Conduct in Human Research and approved by the University of Melbourne Human Research Ethics Committee (ID: 1851607). The trial was prospectively registered on the Australian and New Zealand Clinical Trials Registry (ACTRN12619001747112). All participants provided written informed consent to participate.
Participant recruitment and eligibility
Advertisements for participants were distributed to the Australian eye care community using professional magazines and e‐newsletter correspondence from September 2019 to September 2020. Colleagues of the researchers were also invited. To be eligible, optometrists needed to be currently practicing in Australia, have access to their own patient records, and be naïve to the MaD‐CCAT audit tool by not having participated in our prior study. 25 Data were collected between December 2019 and December 2020.
Study design
This study involved a participant‐masked RCT. Participants' involvement is summarised in Figure 1. Following provision of written informed consent, participants were invited to attend a one‐on‐one videoconference with a researcher (SAG). During this session, participants completed a baseline online survey (Qualtrics Survey Software, qualtrics.com) to capture their demographics, AMD practice patterns and confidence in AMD care; they also answered multiple choice questions (MCQs) related to theoretical AMD case studies (described below).
FIGURE 1.

Overview of participants' involvement in the study. AMD, Age‐related macular degeneration; MaD‐CCAT, Macular Degeneration Clinical Care Audit Tool; MCQs, Multiple‐choice questions
The information captured in the survey included:
Participant demographics: gender, country of completing their optometry degree, year of graduation, therapeutic endorsement to prescribe scheduled medicines (yes/no), average clinical hours worked per week, principal practice setting (e.g., academic, corporate practice, hospital clinic, public health clinic, private practice etc.) and practice location (postcode).
AMD practice patterns: estimated average number of AMD patients seen per week, AMD grading scale used (if any), AMD clinical guidelines used (if any) and retinal imaging devices available at their practice.
Confidence in AMD practice, on a five‐step Likert scale ranging from “not at all confident” to “very confident”, for: knowledge of risk factors for developing AMD; knowledge of risk factors for AMD progression; asking patients about modifiable risk factors for developing AMD; providing advice to patients about modifiable risk factors for developing AMD; diagnosing earlier stages of AMD; managing earlier stages of AMD and, ability to appropriately refer AMD patients for medical retinal sub‐specialist care.
Theoretical case studies were designed to assess participants' knowledge of AMD diagnosis and management; the cases considered a range of risk factors and disease severities. Participants were randomly presented with each case study, and were required to answer a total of 26 MCQs covering four clinical themes (i.e., patient risk factors, clinical examination, AMD severity diagnosis and management).
Participants were then randomly assigned (1:1:1) to one of three clinical tools (described under subheading ‘Study interventions: AMD clinical tools’) during the videoconference. A researcher not involved in the videoconference and who did not interact with participants (LED), had prospectively prepared concealed, consecutively numbered opaque envelopes for participant randomisation, to ensure allocation concealment. The randomisation sequence was generated using a random number generator (Microsoft Excel, Version 16.39, microsoft.com), with a block size of 15. The masked researcher (SAG) opened the envelope to reveal the participant's assigned clinical tool during the videoconference, and then provided standardised training on how to use the intervention using pre‐recorded presentations (Microsoft PowerPoint, Version 16.39, microsoft.com). The ‘placebo’ tool was used as an attention‐control measure designed for participants to maintain their current practices and AMD knowledge, and to control for any external influences that may have influenced practice behaviours over the study duration (see description below). Participants were unaware of the existence of an inactive intervention in the trial, thus creating a concealed component to the study that was not stated in the plain language statement or in the public clinical trials registry.
Participants were asked to use their assigned AMD clinical tool for the next 5–10 AMD patients to whom they provided care. 25 Participants audited these patient records (post‐intervention) and an equivalent number of AMD patients seen prior to enrolment (pre‐intervention), for comparison. Self‐audits were performed using the Macular Degeneration Clinical Care Audit Tool (MaD‐CCAT), described below.
Participants then attended a second videoconference for a post‐study online survey, to again capture their confidence in their AMD knowledge and care, and to answer the same theoretical AMD case study MCQs. Participants were also asked to indicate their level of agreement, using a five‐step Likert scale that ranged from “strongly disagree” to “strongly agree”, for aspects relating to their perceived value and utility of both their assigned AMD clinical tool and the MaD‐CCAT.
Upon completion of all data collection, participants were invited to attend a debriefing session hosted via Zoom (Zoom Video Communications, zoom.us). During this session, researchers revealed information about the concealment of the ‘placebo’ tool and presented the main findings of the study to participants. This session was recorded and sent to participants unable to attend the scheduled live Zoom session.
Study interventions: AMD clinical tools
Three AMD clinical tools were created for this study, comprising two active and one inactive (placebo). The active interventions provided evidence‐based, patient‐specific information to guide AMD care. The placebo intervention, described under subheading ‘Placebo intervention: Google form’, intended to support standard care.
Active intervention #1: Online ‘Classification of Age‐related macular degeneration and Risk Assessment Tool’ (CARAT)
The online CARAT was hosted on a website. Using individual participant log in details, participants could enter details about their patient's AMD risk factors and AMD clinical signs, in the worst eye based on the macula phenotype in the context of AMD (Figure 2). Based on this information, the CARAT provided a text‐based output to participants that included: (i) AMD classification based on the Beckman Initiative for Macular Research Classification (2013) 26 ; (ii) risk of progression to late‐stage AMD in the next five years (as a percentage, based upon the ‘Advanced AMD Risk Calculator’ obtained from caseyamdcalc.ohsu.edu, last accessed 20 July, 2020) using the risk assessment method detailed by Klein et al. (2011) 27 ; (iii) Age‐Related Eye Disease Study (AREDS) simple severity scale score, so participants could learn more about manual risk calculation using the CARAT supporting manual; (iv) recommended management and (v) recommendations for appropriate review periods and/or if referral to sub‐specialty ophthalmology care was indicated, according to the RANZCO AMD referral pathway 17 .
FIGURE 2.
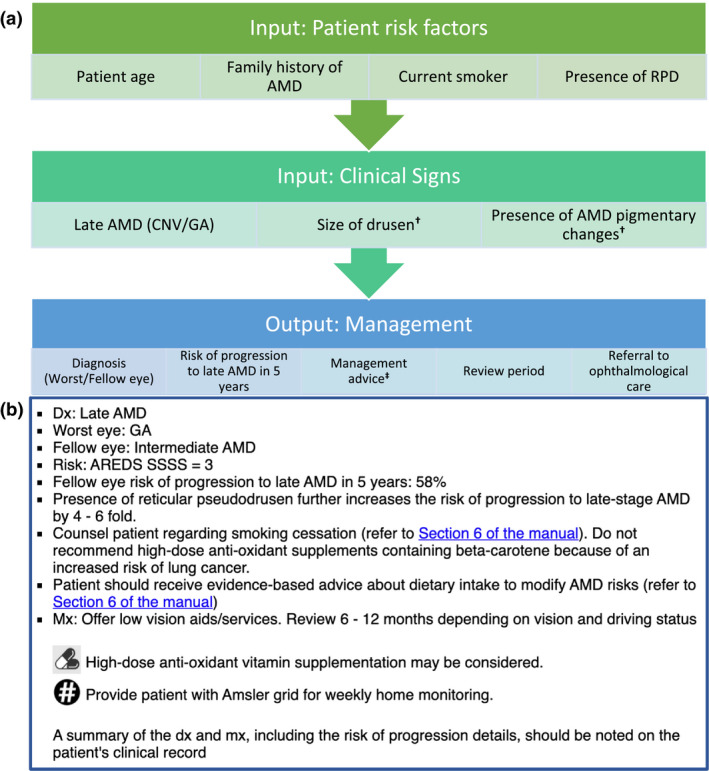
Classification of Age‐related Macular Degeneration and Risk Assessment Tool (CARAT). (a) input, and (b) example of text‐based output of the online CARAT. † Worst eye based on the macula phenotype in the context of AMD. ‡ Management advice includes Amsler grid provision for home monitoring, nutritional supplementation, counselling regarding smoking cessation (when indicated) and dietary intake advice. ‘Section 6 of the manual’ is a hyperlink for downloading the resources section of the CARAT supporting manual to assist with management advice provided to patients with AMD. AMD, age‐related macular degeneration; AREDS, Age‐Related Eye Disease Study; CNV, choroidal neovascularisation; dx, diagnosis; GA, geographic atrophy; mx, management; RPD, reticular pseudodrusen; SSSS, simple severity scale score
A CARAT supporting manual was provided to participants assigned an active intervention, which included general information about AMD definitions, risk factors, clinical examination techniques and a risk of progression manual calculator (Supplementary Material S1).
Active intervention #2: Paper‐based CARAT
The paper CARAT comprised a flowchart with the same information as the online tool. Participants were instructed to use the flowchart for their AMD patient's risk factors and clinical signs to obtain a patient diagnosis and management output summary (Supplementary Material S2). Participants had to manually calculate their patient's risk of progression to late‐stage AMD in five years by referring to the CARAT supporting manual (Supplementary Material S1).
Placebo intervention: Google form
The placebo intervention comprised a Google form with a free‐form textbox for participants to enter information that summarised each patient consultation. Participants were advised to enter a summary of their patient's demographics, clinical signs and management, including review period, using the information in their clinical records. Once five responses were submitted, participants received a summary of their responses from the researcher (SAG), with no additional information.
Macular Degeneration Clinical Care Audit Tool (MaD‐CCAT)
The MaD‐CCAT, described in detail elsewhere, 25 was developed using iAuditor by SafetyCulture (accessible online at https://safetyculture.com/iauditor/). It is a clinical audit tool that enables evaluation of the clinical care provided by optometrists to patients with, or at risk of, AMD, relative to best practice standards.
The MaD‐CCAT assesses: documentation of AMD risk factors; the use of diagnostic tests for comprehensive assessment of disease severity; documentation of patient management and recommended review periods. Most questions in the MaD‐CCAT require yes/no/not applicable responses. Participants were directed to respond using information documented on their patient records. For each participant, the average compliance value from multiple patient records was calculated to represent overall performance.
Statistics
The study involved a convenience sample of 31 participants. Due to the exploratory nature of this study, no formal a priori power calculation was performed. Statistical analyses were undertaken using GraphPad Prism, version 8.0 (GraphPad, graphpad.com). Descriptive statistics were used to summarise participant survey responses for demographics, practice patterns and confidence in AMD care. A Kruskal‐Wallis test was used to compare change from baseline scores between interventions for each outcome. Dunn's multiple comparison was used to evaluate between‐group comparisons, with adjusted p‐values reported. Sub‐group analyses were performed for the analysis of AMD management, stratified by clinical severity (i.e., normal ageing changes, early AMD and intermediate AMD), using the same statistical approaches. An alpha value of 0.05 was used to define statistical significance.
RESULTS
Participant characteristics
Thirty‐one optometrists provided informed consent to participate and were randomly assigned to one of three interventions (AMD clinical tools): placebo (n = 10), paper CARAT (n = 11) and online CARAT (n = 9). Two participants were lost to follow‐up at the self‐audit stage (placebo tool, n = 1; online CARAT, n = 1). In total, 29 participants completed the study; their demographics and practice patterns are summarised in Table 1. Most participants were in metropolitan practice, had completed their optometry training in Australia, had less than five years of practice experience and had therapeutic endorsement to prescribe scheduled topical medications.
TABLE 1.
Baseline participant demographics and self‐reported age‐related macular degeneration practice patterns
| Placebo (n = 9) | Paper CARAT (n = 11) | Online CARAT (n = 9) | |
|---|---|---|---|
| Gender, male: n (%) | 3 (33) | 4 (36) | 5 (56) |
| Degree completed in Australia: n (%) | 9 (100) | 11 (100) | 9 (100) |
| Years since graduation: median; IQR [range] | 4; 2 [2–21] | 3; 5.5 [1–16] | 3; 2 [2–13] |
| Therapeutically endorsed: n (%) | 9 (100) | 11 (100) | 11 (100) |
| Average clinical hours worked per week: median; IQR [range] | 38; 4 [32 – 40] | 38; 2 [12–42] | 40; 2 [38–50] |
| Principal place of practice: n (%) | |||
| Corporate | 5 (56) | 4 (36) | 3 (33) |
| Private | 4 (44) | 5 (45) | 4 (44) |
| Public health centre | – | 2 (18) | 1 (11) |
| Academic | – | – | 1 (11) |
| Practice setting: n (%) | |||
| Metropolitan | 7 (78) | 8 (73) | 7 (78) |
| Regional | 2 (22) | 3 (27) | 2 (22) |
| Average number of AMD patients seen per week: median; IQR [range] | 2.5; 2.5 [0.5–15] | 2; 3 [1–10] | 2; 3 [0.5–7] |
| Use of an AMD grading scale: n (%) | 8 (89) | 7 (64) | 9 (100) |
| Beckman classification | 3 | 1 | 6 |
| AREDS | 3 | 2 | 2 |
| Other a | 2 | 2 | 1 |
| Unspecified | – | 2 | – |
| Use of an AMD guideline: n (%) | 5 (56) b | 7 (64) | 6 (67) b |
| RANZCO AMD referral pathway | – | – | 1 |
| AREDS c | 1 | 1 | 2 |
| Macular Disease Foundation c | 2 | – | – |
| Optometry Australia 2019 AMD clinical practice guide c | 2 | 1 | 2 |
| Other d | 1 | 3 | 2 |
| Unspecified | – | 2 | – |
| Imaging devices available at practice: n (%) | |||
| Retinal fundus photography | 7 | 11 | 8 |
| Optical coherence tomography | 8 | 8 | 8 |
| Retinal fundus autofluorescence | 4 | 9 | 6 |
| Retinal infrared imaging | – | 1 | 2 |
Abbreviations: AMD, age‐related macular degeneration; AREDS, Age‐Related Eye Disease Study; CARAT, Classification of Age‐related Macular Degeneration and Risk Assessment Tool; IQR, interquartile range; n, number of participants; RANZCO, The Royal Australian and New Zealand College of Ophthalmologists.
Other self‐reported AMD grading scales used were “clinical classification of AMD” and “Wisconsin ARM grading”, “Optometry Australia 2019 AMD clinical practice guide”, “Macular Disease Foundation” and “university lecture notes”
Some participants reported using two AMD guidelines for management
These categories reflect the responses of participants who were asked whether they currently use an AMD guideline; we note that AREDS, the Optometry Australia 2019 AMD clinical practice guide and the Macular Disease Foundation documents do not actually constitute clinical guidelines, by definition.
Other AMD guidelines reported to be used by participants were “university lecture notes”, “CPD events”, “NHMRC”, “Centre for Eye Research Australia” and “Beckman Classification”.
Participants' confidence in their AMD knowledge and care provision
Participants indicated their confidence in their AMD knowledge and care provision on a five‐step scale (Figure 3). At baseline (pre‐intervention), there were no inter‐group differences (p < 0.05 for all comparisons). There was an improvement for the change in confidence post‐intervention for the paper CARAT, relative to placebo, for knowledge of risk factors for AMD development (p = 0.01), asking patients about AMD risk factors (p = 0.03) and referring AMD patients to a medical retinal sub‐specialist (p = 0.04). Detailed statistical analyses are provided in Supplementary Material S3.
FIGURE 3.

Self‐reported confidence in age‐related macular degeneration (AMD) clinical care, pre‐ and post‐intervention among participants. Participants (placebo: n = 9, paper CARAT: n = 11, online CARAT: n = 9) indicated their level of confidence with each statement (on the right), using a five‐step Likert scale comprising: not at all confident, barely confident, reasonably confident, confident and very confident. *Refer AMD patients for medical retinal sub‐specialist care. AMD, age‐related macular degeneration; CARAT, classification of age‐related macular degeneration and risk assessment tool; M‐RF, modifiable risk factors; RF, risk factors
Participants' knowledge of AMD care
Participants completed 26 theoretical AMD case study MCQs that assessed knowledge in four key clinical areas: AMD risk factors, clinical examination, AMD severity classification and AMD management. For each clinical area, there were no significant differences in MCQ scores between study groups at baseline (p > 0.05). Comparing change from baseline data (see Supplementary Material S4 for detailed analyses) across the three AMD clinical tools at the end of the study, there were no significant differences in performance in any of the clinical areas (p > 0.05 for all comparisons).
AMD clinical audits
Each participant completed 6 ± 2 (mean ± SD) audits of unique patient records at both the pre‐ and post‐intervention time points. At baseline, there were no differences in compliance between intervention groups across any of the clinical audit domains (p > 0.05 for all comparisons). For reference, pre‐ versus post‐intervention summary data, for each intervention group, are provided for each audit domain in Supplementary Material S5. Over the course of the study, the placebo group showed no changes to their AMD care, except for an improvement in documenting smoking cessation counselling. The efficacy of the two active interventions (paper CARAT and online CARAT) was evaluated by considering the change from baseline, relative to the placebo tool.
AMD patient risk factors
For the change from baseline in compliance for clinical record documentation of AMD risk factors, there were no significant between‐group differences in any domain (i.e., family history of AMD, current smoking status, current dietary behaviours, recording the presence/absence of RPD or current nutritional supplement intake) (see Supplementary Material S6).
Classification of AMD severity
The MaD‐CCAT assessed whether optometrists accurately documented AMD severity based on the Beckman Initiative for Macular Disease Classification (2013), 26 informed by the clinical signs noted on the patient's record. At the study end point, there were no significant between‐group differences for changes to the accuracy in documenting AMD severity (see Figure S7).
Clinical examination
For the change from baseline in documenting clinical examination techniques, there were no significant between‐group differences for in‐office Amsler grid test (for patients with at least early stage AMD), dilated retinal fundus examination (DFE), retinal imaging or multimodal imaging (see Figure S8). These findings should be viewed in the context of relatively high baseline performance in most of these clinical areas (see Supplementary Material S5 for a summary of the pre‐ versus post‐intervention summary data).
AMD management
The assessment of patient management was based on AMD severity in the worst eye, as specified by participants in the MaD‐CCAT. Considering the appropriateness of management advice provided to all patients, there were no significant between‐group differences in any domain (i.e., documentation of counselling about smoking behaviours for current and/or prior smokers, discussing nutritional supplement intake, recording the risk of progression to late‐stage AMD in patients with early or intermediate AMD, provision of an Amsler grid for home monitoring for patients with early or intermediate AMD, or nominating an appropriate review period) (see Supplementary Material S9).
A sub‐analysis was performed to consider patient management based on the clinical severity of the macular findings (i.e., normal ageing changes, early AMD or intermediate AMD). The change from baseline in compliance for documenting discussions about patient smoking behaviours for those with early AMD was significantly greater among practitioners assigned the online CARAT relative to the placebo tool (Figure 4a; p = 0.04). In addition, for patients with intermediate AMD, the change from baseline in documenting the risk of progression to late AMD was significantly higher among practitioners who used the paper CARAT, relative to placebo (Figure 4b; p = 0.04).
FIGURE 4.

(a) Change from baseline in compliance (%) for documentation of discussing patients' smoking behaviours for patients with early AMD. (b) Change from baseline in compliance (%) for documenting patient's risk of progression to late stage AMD, for those with intermediate AMD at the time of examination. *p < 0.05. AMD, age‐related macular degeneration; CARAT, classification of age‐related macular degeneration
AMD clinical tool experience
Participants (n = 29) completed a post‐study survey to examine their experience with their intervention (Figure 5). Most participants agreed or strongly agreed that the AMD clinical tools were easy to use (placebo: 89%, paper CARAT: 100%, online CARAT: 89%) and time efficient (placebo: 56%, paper CARAT: 64%, online CARAT: 78%). Relative to placebo, more participants agreed that the paper CARAT helped AMD patients understand their individualised risk of progression to late‐stage AMD (placebo: 22%, paper CARAT: 100%, online CARAT: 56%) and facilitated patient discussions regarding the risk of progression to late‐stage AMD (placebo: 56%, paper CARAT: 100%, online CARAT: 44%).
FIGURE 5.

Participants' level of agreement with statements for various aspects of their assigned age‐related macular degeneration (AMD) clinical tool. Stacked bar chart showing participants' (placebo, n = 9; paper CARAT, n = 11; online CARAT, n = 9), level of agreement, with each statement for various aspects of their assigned AMD clinical tool, at the end of the study, using the following five‐step Likert scale: strongly disagree, disagree, neither agree nor disagree, agree and strongly agree. AMD, age‐related macular degeneration; CARAT, classification of age‐related macular degeneration and risk assessment tool
DISCUSSION
This randomised controlled trial (RCT) investigated the utility of point‐of‐care tools for enhancing the optometric care provided to AMD patients, as measured using clinical self‐audit. The study compared two active interventions (clinical tools), delivered in paper‐ and online‐based formats, with a placebo tool designed to support standard care. Relative to those who received the placebo, practitioners assigned the paper CARAT showed greater improvements in confidence in relation to their knowledge of risk factors for AMD development, asking patients about such risk factors and referring patients for medical retinal sub‐specialist care. Data from the clinical audit showed that changes, from baseline, for documenting AMD risk factors, disease severity and use of clinical examination techniques did not differ across any of the intervention groups at the study endpoint. Compared with the placebo tool, practitioners who used the online CARAT showed greater improvement in documenting discussions around smoking behaviours for patients with early AMD. Practitioners assigned the paper CARAT showed higher levels of compliance with recording a patient's risk of progression to late AMD, compared to those allocated the placebo tool. The placebo group showed no changes to their AMD care, except for an improvement in documenting smoking cessation counselling; this finding suggests that the placebo tool successfully supported standard care, as a relevant control to compare the effect of the active tools on AMD care.
Efficacy of the clinical tools
Systematic reviews have synthesised evidence from multiple studies to consider the value of point‐of‐care clinical tools for improving compliance to CPGs. 28 , 29 , 30 Sutton et al. summarised the potential advantages of using clinical tools to include reducing the incidence of patient adverse events, promoting clinician adherence to CPGs, reducing costs associated with patient management of chronic conditions, including the patient in the shared decision‐making process and improving clinical record documentation. 30 However, to our knowledge, no studies have assessed the use of point‐of‐care tools in optometric practice. The present study sought to address this evidence gap, by investigating whether implementing in‐office AMD clinical tools alters the optometric care provided to patients with AMD, as measured by clinical self‐audit.
As a key modifiable risk factor, tobacco smoking imparts an at least two‐to‐three‐fold increase in the risk of progression to late‐stage AMD. 31 , 32 , 33 Therefore, asking AMD patients about their smoking status, and providing advice regarding smoking cessation, is crucial for AMD risk reduction. Use of the online CARAT improved clinicians' documentation of counselling patients with early AMD about their smoking behaviours. Although no improvement in AMD knowledge was quantified with any intervention, as assessed using the MCQ scores for the case studies, most participants felt that the paper CARAT improved their knowledge of AMD risk factors and severity staging. Possible reasons for no significant changes to the MCQ scores include that the sample size was not sufficient to detect a change in this outcome and most participants had less than five years of practice experience (suggestive of relatively current AMD knowledge, as supported by participants' generally high baseline knowledge scores).
Features of point‐of‐care tools that enhance clinical practice
The features of clinical tools that successfully promote clinician adherence to evidence‐based recommendations have been thoroughly investigated. A systematic review by Kawamoto et al. identified features of an ‘ideal’ clinical decision support tool as: (i) automated support, as part of clinicians' standard workflow; (ii) actionable recommendations; (iii) support at the time of decision‐making and (iv) computer‐based support. 34 The active AMD clinical tools (CARAT) developed for the present study aimed to provide actionable recommendations that could be implemented at the time of decision‐making. The online CARAT was designed to provide semi‐automated, computer‐based clinical care support based on individual patient data entered into pre‐specified fields, whereas the hard copy (paper) CARAT used a flowchart format that the clinician manually worked through to derive relevant clinical information. The present study did not find a particular implementation mode (i.e., paper versus online versions of the CARAT) to be consistently preferable for improving clinical record documentation.
For the online CARAT, the requirement for clinicians to input patient data manually into a form on a website could potentially have disrupted their clinical workflow, which is known to be an implementation barrier. 35 , 36 Bonner et al. developed an online clinical tool summarising general practitioner (GP) cardiovascular disease prevention guidelines that included a patient risk calculator. 35 The tool improved identification of patients at high risk of developing cardiovascular disease, and supported the prescribing of medications for high‐risk, but not low‐risk, patients. 35 This cardiovascular tool had similar content domains to the online CARAT, to assist with risk factor identification using a risk of progression calculator. 35 However, unlike the present study, the intended users were involved in the development of the tool, which may have led to a high degree of familiarity with its content. The post‐study survey found that GPs suggested the clinical tool should be incorporated into their medical software to improve workflow efficiency. 35 Similar suggestions to improve uptake of clinical tools have been described in the literature. 29 , 37 , 38 , 39
Placebo tool (standard care)
Inconsistency and alterations to standard clinical practice patterns from participation in research studies creates challenges in implementation RCTs, potentially limiting the value of a comparator or control arm. 40 Active control groups are widely used to represent standard care in RCTs, 41 , 42 , 43 , 44 , 45 , 46 particularly for the investigation of behavioural changes. An ‘attention control’ intervention mimics the active intervention group(s) in activities and contact time in the study; however the intervention content or ‘active ingredient’ is absent. 47 The placebo tool for the current study intended to act as an ‘attention control’ to support the participant's typical AMD care provision. Palmer et al. investigated the efficacy of a self‐managed computerised therapy for patients with post‐stroke aphasia compared to standard care and an attention control group. 48 The researchers reported no significant differences between usual care and the attention control groups, supporting the use of attention control groups to represent standard care. 48 The placebo group in the current study controlled for any behavioural changes as a result of participation and/or the anticipated auditing component of the study, as well as any potential external factors. The presence of a placebo tool was concealed to promote equal engagement by all participants. Use of concealment may cause stress in participants in certain contexts; however these negative effects can be minimised by debriefing participants post‐study about the reasons for the concealment. 49 The debriefing session in the present study was designed to reduce misconceptions about the study and provide the placebo group with the CARAT.
The placebo tool was successful in supporting standard clinical care as there were generally no changes to AMD care documentation post‐intervention; documenting counselling regarding smoking behaviours was the only domain to show a significant improvement. This finding may be explained by the Hawthorne effect, relating to improvements in practice behaviours resulting from awareness of performance evaluation using clinical audit for the study. 50 , 51 In addition, during the study period, Quit Victoria® collaborated with Optometry Victoria and South Australia to develop a campaign to raise optometrists' awareness about the importance of counselling regarding smoking cessation. 52 This initiative was extensively publicised, which may have contributed to the behavioural changes observed in the placebo group. Therefore, external factors need to be considered to disambiguate the potential effect(s) of interventions in promoting behavioural changes in implementation studies.
Clinicians' attitudes towards the clinical tools
High practitioner satisfaction has been reported in previous RCTs assessing the use of clinical tools in preventative care. 28 , 53 , 54 Mertz et al. investigated the attitudes of dental clinicians, pre‐ and post‐implementation, of a tool built into an electronic health record system. 55 The researchers reported high (98%) adherence to clinician use of the tool. 55 Clinicians also agreed that the clinical tools supported their commitment to EBP and improving patients' care, health and experience. 55 Similarly, most participants assigned to the paper CARAT (91%) and online CARAT (78%) in the present study agreed that they would continue to use the tool when managing their AMD patients. In contrast, only about half of participants (55%) assigned the placebo tool expressed a willingness to continue using the tool. While this is less than for the active interventions, the reported willingness to continue using the placebo tool may be attributed to reporting bias, where participants respond in a manner that seeks to please the researchers by reporting favourable outcomes. 56
Clinical record documentation and clinical self‐audit barriers
The process of self‐audit using the MaD‐CCAT was recently found to improve documentation of optometric care provided to patients with, or at risk of, AMD. 25 In the current study, self‐audit was used to measure the effects of the interventions on AMD clinical record documentation. Participants were instructed to base their audits only on information documented in their patient records. Therefore, potential discussions with patients that were not recorded were not captured. A study by Shah et al. compared the content of patient records with audio recordings by unannounced actors acting as standardised patients in optometric practices in the United Kingdom. 57 The researchers reported that optometrists provide more verbal advice than was suggested by the documentation in their patient records. 57 Therefore, the compliance scores presented in the current study may underestimate the optometric care provided. Conversely, self‐audit may have led to inadvertent and/or deliberate biases in the entered data, which could overestimate compliance. Independent re‐audit may be considered in future studies to evaluate the validity of self‐audits performed using the MaD‐CCAT.
Considerations relevant to the interpretation of this study
Participants in the current study were mostly recently graduated optometrists. Regarding the potential impact of experience on clinical practice, Downie et al. considered the influence of practitioner age on the self‐reported practices of optometrists in relation to smoking cessation counselling and dietary intake advice. 58 This study found that younger optometrists, aged 20‐29 years, were less likely to enquire about their patients' smoking behaviours, compared to more experienced practitioners. 58 It is thus unclear whether findings in the present study are representative of the effect of the studied interventions on the general optometric profession. This consideration could be addressed in future studies that engage a greater breadth of the profession, including more experienced practitioners.
Data collection for the present study was conducted between December 2019 and December 2020. Over this period there were extended COVID‐19 restrictions and Government‐mandated lockdowns that included optometric care being restricted to urgent and critical cases only in parts of Australia. These factors affected participant recruitment and reduced the number of relevant patients available for the self‐audits. We acknowledge that the study involved a modest sample size (n = 31) and thus had limited statistical power. A post hoc consideration of the potential sample required for an average effect size of d = 0.5, using a mixed‐model analysis of variance (ANOVA), indicates a need for 15 optometrists per intervention for 80% statistical power. In view of this, non‐significant outcomes should not be viewed definitively, but rather as hypothesis‐generating findings that can be used to inform subsequent adequately powered trials.
Further limitations include the duration of clinical tool implementation and audit, based on the number of AMD patients seen following provision of clinical tools. Future studies may consider enrolling more participants and re‐auditing clinical records over a longer period or regular intervals, to better represent the optometry community and assess whether improvements are sustained.
In conclusion, clinical decision support tools are designed to support healthcare provision by providing evidence‐based information in an accessible format, for use at the point‐of‐care. A novel in‐office AMD clinical tool, the CARAT, was developed and tested to assess whether it supports evidence‐based optometric care. The study found that the use of point‐of‐care tools improved some aspects of documenting AMD management advice, as assessed by self‐audit using the MaD‐CCAT. Incorporating online tools into electronic medical record systems to reduce disruptions to clinical workflow and/or individualised clinician‐targeted training may support further improvements in documentation and knowledge of AMD care.
CONFLICT OF INTEREST
All authors declare no conflicts of interest in relation to this work.
AUTHOR CONTRIBUTIONS
Sena Ayse Gocuk: Conceptualization (equal); Data curation (lead); Formal analysis (equal); Investigation (equal); Methodology (equal); Project administration (lead); Writing – original draft (lead); Writing – review & editing (supporting). Allison M McKendrick: Conceptualization (equal); Data curation (supporting); Formal analysis (equal); Investigation (supporting); Methodology (equal); Project administration (supporting); Resources (supporting); Supervision (equal); Writing – original draft (supporting); Writing – review & editing (equal). Laura Elizabeth Downie: Conceptualization (equal); Data curation (supporting); Formal analysis (equal); Funding acquisition (lead); Investigation (supporting); Methodology (equal); Project administration (supporting); Resources (lead); Supervision (lead); Writing – original draft (supporting); Writing – review & editing (equal).
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We thank Prof. Robyn Guymer and A/Prof. Lauren Ayton for their feedback for the development of the point‐of‐care tools (CARAT). We also thank Ziying Yang for her contribution to the programming of the online CARAT. This work was supported by the Australian Government Research Training Program Scholarship awarded to Sena Ayse Gocuk, a 2015 Macular Disease Foundation Australia ‐ Blackmores Grant awarded to Laura Elizabeth Downie, and a NHMRC Translating Research Into Practice (TRIP) Fellowship (APP1091833) awarded to Laura Elizabeth Downie. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians. Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians. [Correction added on 14‐May‐2022, after first online publication: CAUL funding statement has been added.]
Gocuk SA, McKendrick AM, Downie LE. Point‐of‐care tools to support optometric care provision to people with age‐related macular degeneration: A randomised, placebo‐controlled trial. Ophthalmic Physiol Opt 2022;42:814–827. 10.1111/opo.12970
Funding information
This project was partially funded by a 2015 Macular Disease Foundation Australia Blackmores research grant (Principal Investigator: Downie), and a NHMRC Translating Research Into Practice (TRIP) Fellowship (APP1091833 Downie).
REFERENCES
- 1. Sackett DL, Rosenberg WM, Gray JM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn't. BMJ. 1996;312:71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dawes M, Summerskill W, Glasziou P, et al. Sicily statement on evidence‐based practice. BMC Med Educ. 2005;5:1. 10.1186/1472-6920-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henshall C, Marzano L, Smith K, et al. A web‐based clinical decision tool to support treatment decision‐making in psychiatry: a pilot focus group study with clinicians, patients and carers. BMC Psychiatry. 2017;17:265. 10.1186/s12888-017-1406-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ten Teije A, Miksch S, Lucas P. Computer‐based medical guidelines and protocols: a primer and current trends. Amsterdam: IOS Press; 2008. [Google Scholar]
- 5. Farquhar CM, Kofa EW, Slutsky JR. Clinicians’ attitudes to clinical practice guidelines: a systematic review. Med J Aust. 2002;177:502–6. [DOI] [PubMed] [Google Scholar]
- 6. Ryan MA. Adherence to clinical practice guidelines. Otolaryngol Head Neck Surg. 2017;157:548–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hudson P, Collins A, Bostanci A, et al. Toward a systematic approach to assessment and care planning in palliative care: a practical review of clinical tools. Palliat Support Care. 2016;14:161–73. [DOI] [PubMed] [Google Scholar]
- 8. Efron N. Efron grading scales for contact lens complications. Oxford: Butterworth‐Heinemann; 2010. [Google Scholar]
- 9. Mitchell P, Foran S, Wong T, et al. Guidelines for the management of diabetic retinopathy. Barton, ACT: National Health and Medical Research Council; 2008. [Google Scholar]
- 10. National Health Medical Research Council . NHMRC additional levels of evidence and grades for recommendations for developers of guidelines. Canberra, ACT: NHMRC; 2009. [Google Scholar]
- 11. National Health Medical Research Council . NHMRC guidelines for the screening, prognosis, diagnosis, management and prevention of glaucoma. Canberra, ACT: NHMRC; 2010. [Google Scholar]
- 12. Optometry Australia . Clinical practice guide for the diagnosis, treatment and management of glaucoma; 2016. [cited 2021 Nov 12]. Available from: https://www.optometry.org.au/wp‐content/uploads/Professional_support/Guidelines/optometry_australia_glaucoma_clinical_practice_guide__july_2016.pdf
- 13. Optometry Australia . Clinical practice guide: paediatric eye health and vision care. 2016. [cited 2021 Nov 12]; Available from: https://www.optometry.org.au/wp‐content/uploads/Professional_support/Guidelines/optometry_australia_paediatric_eye_health_and_vision_care_guidelines_‐_august_2016.pdf
- 14. Optometry Australia . Clinical practice guide for the diagnosis, treatment and management of anterior eye conditions. 2018. [cited 2021 Nov 12]; Available from: https://www.optometry.org.au/wp‐content/uploads/Professional_support/Guidelines/anterior_eye_clinical_practice_guide__2_.pdf
- 15. Optometry Australia . Examination and management of patients with diabetes. 2018. [cited 2021 Nov 12]; Available from: https://www.optometry.org.au/wp‐content/uploads/Professional_support/Guidelines/clinical_guideline_diabetes_revised_sept_2018_final_designed.pdf
- 16. Hart KM, Abbott C, Ly A, et al. Optometry Australia's chairside reference for the diagnosis and management of age‐related macular degeneration. Clin Exp Optom. 2020;103:254–64. [DOI] [PubMed] [Google Scholar]
- 17. Royal Australian and New Zealand College of Ophthalmologists . RANZCO referral pathway for AMD screening. Surry Hills, NSW: RANZCO; 2018. [Google Scholar]
- 18. Mitchell P, Liew G, Gopinath B, et al. Age‐related macular degeneration. Lancet. 2018;392:1147–59. [DOI] [PubMed] [Google Scholar]
- 19. Srinivasan S, Swaminathan G, Kulothungan V, et al. The association of smokeless tobacco use and pack‐years of smokeless tobacco with age‐related macular degeneration in Indian population. Cutan Ocul Toxicol. 2017;36:253–8. [DOI] [PubMed] [Google Scholar]
- 20. Michalska‐Małecka K, Kabiesz A, Nowak M, et al. Age related macular degeneration – challenge for future: pathogenesis and new perspectives for the treatment. Eur Geriatr Med. 2015;6:69–75. [Google Scholar]
- 21. Lawrenson JG, Evans JR. Advice about diet and smoking for people with or at risk of age‐related macular degeneration: a cross‐sectional survey of eye care professionals in the UK. BMC Public Health. 2013;13:564. 10.1186/1471-2458-13-564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nebbioso M, Lambiase A, Cerini A, et al. Therapeutic approaches with intravitreal injections in geographic atrophy secondary to age‐related macular degeneration: current drugs and potential molecules. Int J Mol Sci 2019;20:1693. 10.3390/ijms20071693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Downie LE, Douglass A, Guest D, et al. What do patients think about the role of optometrists in providing advice about smoking and nutrition? Ophthalmic Physiol Opt. 2017;37:202–11. [DOI] [PubMed] [Google Scholar]
- 24. O'Connor PM, Harper CA, Brunton CL, et al. Shared care for chronic eye diseases: perspectives of ophthalmologists, optometrists and patients. Med J Aust. 2012;196:646–50. [DOI] [PubMed] [Google Scholar]
- 25. Gocuk SA, Lee J‐H, Keller PR, et al. Clinical audit as an educative tool for optometrists: an intervention study in age‐related macular degeneration. Ophthalmic Physiol Opt. 2021;41:53–72. [DOI] [PubMed] [Google Scholar]
- 26. Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age‐related macular degeneration. Ophthalmology. 2013;120:844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klein ML, Francis PJ, Ferris FL 3rd, et al. Risk assessment model for development of advanced age‐related macular degeneration. Arch Ophthalmol. 2011;129:1543–50. [DOI] [PubMed] [Google Scholar]
- 28. Bright TJ, Wong A, Dhurjati R, et al. Effect of clinical decision‐support systems: a systematic review. Ann Intern Med. 2012;157:29–43. [DOI] [PubMed] [Google Scholar]
- 29. Murphy EV. Clinical decision support: effectiveness in improving quality processes and clinical outcomes and factors that may influence success. YJBM. 2014;87:187–97. [PMC free article] [PubMed] [Google Scholar]
- 30. Sutton RT, Pincock D, Baumgart DC, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3:17. 10.1038/s41746-020-0221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan JC, Thurlby DA, Shahid H, et al. Smoking and age related macular degeneration: the number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br J Ophthalmol. 2006;90:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neuner B, Komm A, Wellmann J, et al. Smoking history and the incidence of age‐related macular degeneration–results from the Muenster Aging and Retina Study (MARS) cohort and systematic review and meta‐analysis of observational longitudinal studies. Addict Behav. 2009;34:938–47. [DOI] [PubMed] [Google Scholar]
- 33. Thornton J, Edwards R, Mitchell P, et al. Smoking and age‐related macular degeneration: a review of association. Eye. 2005;19:935–44. [DOI] [PubMed] [Google Scholar]
- 34. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765. 10.1136/bmj.38398.500764.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bonner C, Fajardo MA, Doust J, et al. Implementing cardiovascular disease prevention guidelines to translate evidence‐based medicine and shared decision making into general practice: theory‐based intervention development, qualitative piloting and quantitative feasibility. Implement Sci. 2019;14:86. 10.1186/s13012-019-0927-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wilson BJ, Torrance N, Mollison J, et al. Cluster randomized trial of a multifaceted primary care decision‐support intervention for inherited breast cancer risk. Fam Pract. 2006;23:537–44. [DOI] [PubMed] [Google Scholar]
- 37. Campbell JM, Umapathysivam K, Xue Y, Lockwood C. Evidence‐based practice point‐of‐care resources: a quantitative evaluation of quality, rigor, and content. Worldviews Evid Based Nurs. 2015;12:313–27. [DOI] [PubMed] [Google Scholar]
- 38. Douthit BJ, Musser RC, Lytle KS, Richesson RL. A closer look at the "Right" format for clinical decision support: methods for evaluating a storyboard best practice advisory. J Pers Med 2020;10:142. 10.3390/jpm10040142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schnipper JL, Linder JA, Palchuk MB, et al. "Smart Forms" in an Electronic Medical Record: documentation‐based clinical decision support to improve disease management. J Am Med Inform Assoc. 2008;15:513–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thompson BT, Schoenfeld D. Usual care as the control group in clinical trials of nonpharmacologic interventions. Proc Am Thorac Soc. 2007;4:577–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Aycock DM, Clark PC, Hayat MJ. Reducing stroke risk among young adult African Americans: a feasibility study. Res Nurs Health. 2017;40:153–64. [DOI] [PubMed] [Google Scholar]
- 42. Frijling BD, Lobo CM, Hulscher MEJL, et al. Multifaceted support to improve clinical decision making in diabetes care: a randomized controlled trial in general practice. Diabet Med. 2002;19:836–42. [DOI] [PubMed] [Google Scholar]
- 43. Frijling BD, Lobo CM, Hulscher ME, et al. Intensive support to improve clinical decision making in cardiovascular care: a randomised controlled trial in general practice. BMJ Qual Saf. 2003;12:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Granger BB, Ekman I, Hernandez AF, et al. Results of the chronic heart failure intervention to improve MEdication Adherence study: a randomized intervention in high‐risk patients. Am Heart J 2015;169:539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang EK, Lee J, Choo J, et al. Randomized controlled trial of advance care planning video decision aid for the general population. J Pain Symptom Manage. 2020;59:1239–47. [DOI] [PubMed] [Google Scholar]
- 46. LaFave SE, Granbom M, Cudjoe TKM, et al. Attention control group activities and perceived benefit in a trial of a behavioral intervention for older adults. Res Nurs Health. 2019;42:476–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aycock DM, Hayat MJ, Helvig A, et al. Essential considerations in developing attention control groups in behavioral research. Res Nurs Health. 2018;41:320–8. [DOI] [PubMed] [Google Scholar]
- 48. Palmer R, Dimairo M, Latimer N, et al. Computerised speech and language therapy or attention control added to usual care for people with long‐term post‐stroke aphasia: the Big CACTUS three‐arm RCT. Health Technol Assess. 2020;24:1–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goo SJ, Frangos E, Richards EA, et al. Attitudes and perceptions toward authorized deception: a pilot comparison of healthy controls and fibromyalgia patients. Pain Med. 2020;21:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dickson WJ, Roethlisberger FJ. Counseling in an organization: A sequel to the Hawthorne researches. Boston, MA: Division of Research, Graduate School of Business Administration, Harvard; 1966. [Google Scholar]
- 51. McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Quit Victoria . Training and resources for eye health professionals. 2020 [cited 2021 Nov 12]; Available from: https://www.quit.org.au/resources/eye‐health‐professionals/training‐resources‐eye‐health‐professionals/
- 53. Sequist TD, Gandhi TK, Karson AS, et al. A randomized trial of electronic clinical reminders to improve quality of care for diabetes and coronary artery disease. J Am Med Inform Assoc. 2005;12:431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sequist TD, Zaslavsky AM, Marshall R, et al. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med. 2009;169:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mertz E, Wides C, White J. Clinician attitudes, skills, motivations and experience following the implementation of clinical decision support tools in a large dental practice. J Evid Based Dent Pract. 2017;17:1–12. [DOI] [PubMed] [Google Scholar]
- 56. Hróbjartsson A, Gøtzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J Internal Med. 2004;256:91–100. [DOI] [PubMed] [Google Scholar]
- 57. Shah R, Edgar DF, Evans BJ. How well does record abstraction quantify the content of optometric eye examinations in the UK? Ophthal Physiol Opt. 2009;29:383–96. [DOI] [PubMed] [Google Scholar]
- 58. Downie LE, Keller PR. The self‐reported clinical practice behaviors of Australian optometrists as related to smoking, diet and nutritional supplementation. PLoS One. 2015;10:e0124533. 10.1371/journal.pone.0124533 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


