Abstract
Purpose
Despite the notable increase on the prescription of antidepressants and anxiolytics during pregnancy, recommendation on maintaining the treatment during prenatal period is still controversial. We aimed to separately assess the role of effects of the antidepressants and anxiolytic and the underlying illness, controlled by potential confounding associated with miscarriage onset.
Methods
We used data from a validated pregnant cohort aged 15–49 years from 2002 to 2016 using BIFAP database. All confirmed miscarriages were used to perform a nested control analysis using conditional logistic regression. Women were classified according to use of each drug of interest into four mutually exclusive groups: nonusers, users only during prepregnancy, continuers, and initiators during first trimester. Adjusted odds ratios (aORs) for major confounders during pregnancy such as number of visits to primary care practitioners visits, obesity, smoking, HTA, diabetes with 95% confidence intervals were calculated.
Results
Compared with nonusers, antidepressants continuers had the highest increased risk of miscarriage aOR (95%) of 1.29 (1.13–1.46), being continuers of paroxetine and fluoxetine the antidepressants with the strongest association. Likewise, continuers of anxiolytics and initiators showed an increased risk of 1.19 (1.04–1.37) and 1.30 (1.13–1.50). When separating the effect between the condition itself or the treatment, women exposed during first trimester, regardless treatment duration and/or the underlying illness, had the highest risk 1.27 (1.08–1.51) for antidepressants and 1.25 (1.13–1.39) for anxiolytics.
Conclusions
Our analysis showed an association between prenatal exposure to antidepressants and anxiolytics and miscarriage onset after controlling by potential confounding adjusting for confounders and the underlying illness. This association was not supported for hypnotic medications. Further studies are warranted to evaluate the risk of miscarriage among subpopulation of pregnant women requiring these medications.
Keywords: antidepressants, anxiolytics, case control study, exposure, miscarriage
Keypoints.
Recommendations on initiating or maintaining treatment of antidepressants or anxiolytics perinatally is still controversial.
Although there is a trend to discontinue treatment, there is a high proportion of exposure during first trimester.
Risk of miscarriages were cumulated among women continuers of antidepressants, with special attention to paroxetine and fluoxetine.
Risk of miscarriages were also observed among continuers of anxiolytics, especially among users of long acting benzodiazepines.
Women exposed in early pregnancy are at increased risk of miscarriage regardless the underlying illness.
Plain language summary.
In this study, we aimed to separately assess the role of the effects of the antidepressants, anxiolytics, and hypnotics from the underlying illness as the recommendations on maintaining the treatment during prenatal period are still controversial. In this analysis, from a total of 54 210 pregnant women, 18 070 which were recorded between the years 2002 and 2016 were identified with miscarriages on the Spanish data base BIFAP. The women for this analysis were classified according to use of each drug of interest into four groups: nonusers, users only during prepregnancy, continuers, and initiators during the first trimester. Those who have been diagnosed with depression and had continued a treatment during pregnancy, had shown the highest increased risk of a miscarriage specially those who had made a continuing use of paroxetine and fluoxetine. Likewise, continuer users of anxiolytics. But the most statistical significance results are shown when separating the effect between the condition itself or the treatment, as women exposed during first trimester regardless of treatment duration and the underlying illness had showed the highest risk. On this way, there is an association between prenatal exposure to antidepressants and anxiolytics and miscarriage onset despite the underlying illness is consider a risk factor for the miscarriages.
1. INTRODUCTION
Anxiolytics and hypnotics have been experiencing an increase in prescription rates in the general population countries for many years. 1 , 2 Women are known to be at an increased risk to suffer from mental health diseases compared with men, being the childbearing age and specially the pregnancy, potential periods for its onset. The most common disorders are anxiety and depression ranging a frequency of 15%. 3 , 4 , 5 More than half of pregnancies are unintended which might prompt mental adverse conditions during pregnancy and postpartum. 6 It has been described how women with antenatal depression or anxiety are less likely to monitor and attend prenatal visits which might increase the risk of perinatal complications. 7 Summarized results coming from metaanalyses and systematic reviews have reported how antenatal anxiety is associated with several outcomes for the offspring such as preterm birth, low weight birth, and small gestational age ranging from 40% to 50% increased risk. 8 In addition, untreated anxiety and depression have been associated with poorer lifestyle factors such as smoking, obesity, substance abuse, impulsive behavior, or risk of self‐harm. 9
According to several guidelines, 10 depression and anxiety are common but remain undertreated during pregnancy and the recommendation is to encourage doctors to elaborate an individual treatment plan during pregnancy. Currently, the state of knowledge towards the safety of antidepressants during pregnancy, consider the selective serotonin reuptake inhibitors (SSRIs) as the safest option while the safety profile of benzodiazepines is still controversial. 11 , 12 , 13 Although there is a wide range of prescription rates, it has been reported how antidepressants are prescribed between 2% and 10% of all pregnancies and up to 5% for benzodiazepines. 14 , 15 , 16 , 17 Therefore its use during pregnancy might cause direct damage on the fetus, resulting on a rise of the cortisol and catecholamine levels, leading to variations in the placental function, altering uterine blood flow and furthermore uterine irritability, a deterioration on the endocrine and immune system, developing preeclampsia 18 or other comorbidities such as hypertension or diabetes that might produce obstetric complications. 19 , 20 However, in many situations maintaining the therapy is essential. There are prior studies focusing on the safety of pregnancy associated with use of these drugs, including birth defects, 21 low birth weight, preterm birth, 22 , 23 small for gestational birth and miscarriages, yet for the latter there is not a consensus in results. 3 , 4 , 24 , 25 , 26 , 27 The cornerstone still relies on the ability to distinguish between the effects of the medication, the underlying illness, and other potential confounding factors. 28 Likewise, a discontinuation of the treatment may be associated with withdrawal symptoms, depression, or anxiety relapse. 24
The unresolved questions around the safety of antidepressants and anxiolytics in early pregnancy is still a critical barrier that limits clinical decisions. We aimed to evaluate the association between use of antidepressants and anxiolytics and hypnotics in early pregnancy and the risk of miscarriages. In order to determine the treatment patterns and management of antidepressants, anxiolytic, and hypnotic medications during pregnancy in real clinical practice and its association with miscarriages, we identified and evaluated a cohort of pregnancies within BIFAP database which contains systematically recorded data on more than 10 million primary care patients in Spain.
2. METHODS
2.1. Source of information
We used data from the Spanish database BIFAP (Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria, Database for Pharmacoepidemiological Research in Primary Care). BIFAP is a nonprofit program administered and fully financed by the Spanish Agency for Medicines and Medical Devices (AEMPS) started in 2001 when primary care EMRs were fully implemented throughout Spain. BIFAP is a computerized medical longitudinal population database of anonymized electronic medical records from 10.153 primary care practitioners and pediatricians (PCP) distributed on nine participating Autonomous Regions (out of 17). BIFAP's age and sex distribution are comparable to the Spanish population, covering 8.6% of the total Spanish population at the time this study was performed, 29 , 30 containing data on sociodemographic aspects, medical diagnoses, prescriptions, medical visits, hospital admissions, laboratory results, lifestyle factors, and referrals to specialists.
According to the structure, PCPs record the information related to clinical and health problems using the International Classification of Primary Care‐Second Edition 31 or International Classification of Diseases (ICD‐9) terminologies. 32 Prescriptions issued by the PCP are coded using the Anatomical Therapeutic Chemical (ATC) codes. Data include the number of packages, intended duration, dosage, and strength. In addition, from 2011 onwards, e‐prescription is progressively being implemented in primary care centers therefore dispensation is also available. When possible, dispensing information is chosen for research if available. However, if e‐prescription is not available, paper‐based prescription information is used. The BIFAP database has been described in detail previously. 33
2.2. Source population
Our study cohort included all women of childbearing age (15–49 years) with at least 1‐year registration with their PCP between January 2002 to December 2015.On this way, we ensured information about the medical history of the subjects. For the current study, we only included one pregnancy per woman, the first pregnancy identified during the follow‐up. We used an adaptation from a valid algorithm designed by the authors and applied in other database with similar characteristics which includes the gestational age and a pregnancy outcome algorithm, both have been designed and described in detail previously. 34 , 35 The study protocol was approved by the BIFAP Scientific Committee (11_2016).
2.3. Miscarriage episodes
The cohort encompassed a total of 155 429 pregnant women, out of them 33 342 resulted in pregnancy losses. Pregnancy losses were defined as having a recorded code of ICD‐9634–639. To assign the gestational age, we subdivided the pregnancy losses according to whether or not the Last Menstrual Period (LMP) date was recorded in the database. When LMP date recorded, all pregnancy losses with a gestational age lower than 28 days or greater than 154 days (22 weeks) were excluded. When missing LMP date, we imputed the LMP date by subtracting 74 days to the recorded entry date. 35 Briefly, we subdivided all pregnancy losses in three main categories according to the code and descriptor used to entry the episode: miscarriages, terminations of pregnancy and unspecified abortions. For each group presented above, we randomly selected a sample of medical records of each subcategory and manually reviewed them. All women initially classified as miscarriages were confirmed (positive predictive value >90%). Therefore, our final sample of women suffering from miscarriages was 18 070 while remaining pregnancy losses were retained as not spontaneous. 36 The date of identification of the miscarriage was considered as the index date.
2.4. Selection of controls
Controls were randomly selected from the whole cohort of pregnancies by risk‐set sampling and individually matched to cases (3:1). To do this, for each woman suffering a miscarriage, we selected all cohort members who were still at risk within follow‐up and of the same age (+/−1 year), gestational age (in weeks) and year of LMP date. We followed a sampling method of selection of controls without replacement as controls have been part of the same cohort as the cases so, a control can be used for several cases and a case can be a control before being a case. This makes the odds ratio (OR) an unbiased estimator of the relative risk, approaching its value. 37 The matched factors minimize some source of confounding specially differences between the assorted stages of organogenesis and the disparity that could have existed on prescriptions including different pharmaceutical specialties prescribed among the years of the study. Matched controls were assigned the index date of their corresponding case.
2.5. Comorbidities and drug exposure
We collected sociodemographic characteristics (age and sex), lifestyle factors (smoking, body mass index, obesity, alcohol consumption), and the health care utilization (visits to the primary care physician and previous hospitalizations) which have also served as indicators of general health status. We used two different time frames: at any time before the LMP or between LMP date and index date (length of gestation). We collected information on maternal depression and maternal anxiety as well as other frequent conditions such as cardiovascular, metabolic, respiratory, digestive, renal, and neoplastic pathologies. Those variables were defined like having a recorded diagnosis on any of each time window mentioned above. Comorbidities were classified into antenatal condition defined as having a recorded code before LMP date regardless during pregnancy, and incident condition recorded only during pregnancy (see Supplemental Table 1).
We collected drug exposure on specific drug classes of interest such as: SSRIs, specifying some active principles, tricyclic antidepressants, and other antidepressants. Moreover, we collected anxiolytics (N05B) and hypnotics‐sedatives (N05C) and classified anxiolytics and hypnotics benzodiazepines as: short action (≤10 h) and long action (>24 h) (see Supplemental Table 2).
We defined the exposure of drugs of interest using two periods: within the 90 days prior to LMP date (prepregnancy), and during the first trimester. Use was considered when having at least one prescription in any of both periods. We classified the use of these specific drug classes as: nonusers: when there were no prescriptions in any of these periods; discontinuers: women who had at least one prescription only during the prepregnancy period; continuers: women who had at least one prescription during the prepregnancy period and also during the first trimester, and new users: women who had at least one prescription only during the first trimester.
Finally, in order to differentiate the effects of the medication from the underlying illness, we built an interaction variable. Thus, we evaluated the interaction between the underlying illness (A) for which is indicated (i.e., depression/anxiety) and antidepressants/anxiolytics (B). We created one variable with six mutually exclusive levels of exposure: Neither underlying illness (A) nonuse of treatment (B), (No A, no B), underlying illness without treatment (A and not B), underlying illness and treatment only during prepregnancy (A and B during prepregnancy), underlying illness treated during the first trimester (A and B during first trimester), no underlying illness and treatment in the prepregnancy (No A but B during prepregnancy), no underlying illness and treatment during first trimester (No A but B in the first trimester). Nonuse of treatment was defined as not having any prescription during first trimester and prepregnancy. Hypnotic medications were not considered at this point due to the small prevalence of use.
2.6. Analysis
We conducted a descriptive analysis of the characteristics among cases and controls using frequency counts and percentages for categorical variables and means with standard deviation for continuous variables. ORs with 95% confidence intervals (CIs) were calculated using conditional logistic regression adjusted for confounders. We used two models: first one adjusted by the number of PCP visits within the year prior to the index date. In addition to that, second model compiled lifestyle factors and chronic medical conditions (either antenatal or incident/during pregnancy) that could increase the risk of miscarriages such as obesity, smoking, hypertension, and diabetes. STATA version 15.0 (StataCorp LP, College Station, TX, USA) was used for all analyses.
3. RESULTS
3.1. Pregnancy cohort: Baseline characteristics and comorbidities
A total of 18 070 women with miscarriages were identified and matched to 54 210 controls by age, gestational age (in weeks), and year of LMP date. Overall, mean age of cases and controls was 31.9 years (SD:5.7). Regarding lifestyle factors, the frequency of current smoking and obesity was similar among cases and controls (corresponding percentages: 22% of current smokers and 10% obesity). The health care utilization, measured as number of PCP visits, was associated with an increased risk of miscarriage. The most prevalent conditions included hypercholesterolemia (24.5%), followed by anxiety (18.4%), depression (9.7%), and hypothyroidism (7.3%). Diabetes, depression, and anxiety were associated with a slightly increased risk of miscarriage: 1.17 (95% CI: 1.02–1.33), 1.08 (95% CI: 1.02–1.15), and 1.05 (95% CI: 1.00–1.10), respectively.
Focusing on anxiety and depression, the vast majority of women (>90%) had antenatal recorded diagnosis where the risk was observed. For example, the OR of miscarriage associated to women with antenatal depression was 1.09 (95% CI: 1.03–1.16) versus 0.75 (95% CI: 0.46–1.22) among those firstly diagnosed (incident) during pregnancy and 1.06 (95% CI: 1.01–1.11) in those with antenatal anxiety versus 0.91 (95% CI: 0.71–1.17) with incident anxiety (Table 1).
TABLE 1.
Baseline characteristics among cases and controls
| Controls N = 54 209 | Cases 18 070 | Crude OR (95% CI) | Adjusted OR* (95% CI) | |||
|---|---|---|---|---|---|---|
| Age | ||||||
| <25 years | 4160 | 7.7 | 1335 | 7.4 | NA | NA |
| 25–29 years | 8258 | 15.2 | 2678 | 14.8 | NA | NA |
| 30–34 years | 18 550 | 34.2 | 6025 | 33.3 | NA | NA |
| 35–39 years | 16 523 | 30.5 | 5610 | 31.0 | NA | NA |
| 40 and more | 6718 | 12.4 | 2422 | 13.4 | NA | NA |
| GP visits | ||||||
| 0–1 visits | 5951 | 11.0 | 784 | 4.3 | 1 (–) | 1 (–) |
| 2–4 visits | 12 323 | 22.7 | 3831 | 21.2 | 2.40 (2.21–2.61) | 2.40 (2.21–2.61) |
| 5–9 visits | 19 473 | 35.9 | 7224 | 40.0 | 2.89 (2.67–3.14) | 2.89 (2.67–3.14) |
| 10–14 visits | 9811 | 18.1 | 3724 | 20.6 | 2.97 (2.73–3.24) | 2.97 (2.73–3.24) |
| 15 visits and more | 6651 | 12.3 | 2507 | 13.9 | 2.98 (2.72–3.26) | 2.98 (2.72–3.26) |
| Obesity | 5659 | 10.4 | 1888 | 10.5 | 1.00 (0.95–1.06) | 0.92 (0.87–0.98) |
| Smoking | 12 371 | 22.8 | 4070 | 22.5 | 1.10 (1.07–1.14) | 1.02 (0.99–1.06) |
| Comorbidities | ||||||
| Depression | 4572 | 8.4 | 1744 | 9.7 | 1.16 (1.10–1.23) | 1.08 (1.01–1.15) |
| Depression prepregnancy | 4495 | 8.3 | 1723 | 9.5 | 1.17 (1.10–1.24) | 1.09 (1.02–1.15) |
| Incident depression | 77 | 0.1 | 21 | 0.1 | 0.83 (0.50–1.34) | 0.75 (0.46–1.22) |
| Anxiety | 9056 | 16.7 | 3321 | 18.4 | 1.12 (1.08–1.17) | 1.05 (1.01–1.10) |
| Antenatal anxiety | 8797 | 16.2 | 3238 | 17.9 | 1.13 (1.08–1.18) | 1.06 (1.01–1.11) |
| Incident anxiety | 259 | 0.5 | 83 | 0.5 | 0.98 (0.76–1.26) | 0.90 (0.70–1.16) |
| Migraine | 3659 | 6.7 | 1243 | 6.9 | 1.02 (0.95–1.09) | 0.96 (0.90–1.03) |
| Antenatal Migraine | 3591 | 6.6 | 1223 | 6.8 | 1.02 (0.96–1.09) | 0.96 (0.90–1.03) |
| Incident migraine | 68 | 0.1 | 20 | 0.1 | 0.88 (0.54–1.45) | 0.80 (0.49–1.33) |
| Diabetes | 839 | 1.6 | 335 | 1.9 | 1.21 (1.06–1.37) | 1.16 (1.02–1.32) |
| Antenatal Diabetes | 783 | 1.4 | 315 | 1.7 | 1.22 (1.06–1.39) | 1.17 (1.03–1.34) |
| Incident HTA Diabetes | 56 | 0.1 | 20 | 0.1 | 1.08 (0.65–1.80) | 1.01 (0.60–1.70) |
| Hypothyroidism | 3681 | 6.79 | 1326 | 7.34 | 1.09 (1.02–1.16) | 1.01 (0.95–1.08) |
| Antenatal Hypothyroidism | 3416 | 6.3 | 1254 | 6.9 | 1.11 (1.04–1.18) | 1.03 (0.96–1.10) |
| Incident Hypothyroidism | 265 | 0.5 | 72 | 0.4 | 0.82 (0.63–1.07) | 0.76 (0.58–0.99) |
| Hypercholesterolemia | 12 605 | 23.3 | 4419 | 24.5 | 1.07 (1.03–1.11) | 0.99 (0.95–1.03) |
| Antenatal Hypercholesterolemia | 12 178 | 22.5 | 4259 | 23.6 | 1.07 (1.03–1.11) | 0.99 (0.95–1.03) |
| Incident Hypercholesterolemia | 427 | 0.8 | 160 | 0.9 | 1.14 (0.95–1.37) | 1.03 (0.86–1.24) |
| Hypertension | 756 | 1.4 | 287 | 1.6 | 1.14 (1.00–1.31) | 1.05 (0.91–1.21) |
| Antenatal Hypertension | 730 | 1.3 | 275 | 1.5 | 1.13 (0.99–1.31) | 1.04 (0.91–1.20) |
| Incident Hypertension | 26 | 0.0 | 12 | 0.1 | 1.40 (0.70–2.79) | 1.26 (0.63–2.52) |
Adjusted by number of GP visits, obesity, smoking, HTA, diabetes.
3.2. Antidepressant medications and risk of miscarriage onset
We collected information on overall antidepressant medication use and by drug class: SSRIs, tricyclic antidepressants, and other antidepressants (Table 2). A total of 11.2% of the controls and 12.3% of cases received at least one prescription only during prepregnancy (i.e., discontinuers) while the proportion of continuers was 1.5% and 2.1% and less than 0.5% were initiators during pregnancy. A similar trend was observed for each specific drug class. The risk of miscarriage was concentrated among continuers, the OR was 1.29 (95% CI: 1.13–1.46) for overall antidepressants, 1.21 (95% CI: 1.04–1.40) for SSRIs and 1.52 (95% CI: 1.19–1.94) for other antidepressants. We observed a trend towards an increased risk associated with continuers of tricyclics although the imprecise results might be explained by small numbers of users (OR: 1.36 (95% CI: 0.86–2.15). Discontinuers had a very slightly increased risk of miscarriage ranging from 5% to 13%, for example, discontinuers of overall antidepressants were associated with an OR of 1.05 (95% CI: 1.00–1.11), 1.06 (95% CI: 1.00–1.12) for SSRIs and 1.13 (95% CI: 1.03–1.25) for other antidepressants.
TABLE 2.
Antidepressant medication and risk of miscarriage onset
| Controls N = 54 209 | Cases 18 070 | Crude OR (95% CI) | Adjusted OR* (95% CI) | |||
| Antidepressants | ||||||
| Nonuse | 47 230 | 87.1 | 15 428 | 85.4 | 1 (–) | 1 (–) |
| Only during prepregnancy (discontinuers) | 6060 | 11.2 | 2216 | 12.3 | 1.22 (1.07–1.18) | 1.05 (1.00–1.11) |
| Use in both periods (continuers) | 820 | 1.5 | 385 | 2.1 | 1.44 (1.27–1.63) | 1.29 (1.13–1.46) |
| Use only during pregnancy (new users) | 99 | 0.2 | 41 | 0.2 | 1.26 (0.88–1.82) | 1.12 (0.78–1.62) |
| SSRIs | ||||||
| Nonuse | 48 794 | 90.0 | 16 016 | 88.6 | 1 (–) | 1 (–) |
| Only during prepregnancy (discontinuers) | 4734 | 8.7 | 1752 | 9.7 | 1.13 (1.07–1.20) | 1.06 (1.00–1.12) |
| Use in both periods (continuers) | 597 | 1.1 | 266 | 1.5 | 1.36 (1.17–1.57) | 1.21 (1.04–1.40) |
| Use only during pregnancy (new users) | 84 | 0.2 | 36 | 0.2 | 1.30 (0.88–1.93) | 1.17 (0.79–1.73) |
| Tricyclics | ||||||
| Nonuse | 52 882 | 97.6 | 17 587 | 97.3 | 1 (–) | 1 (–) |
| Only during prepregnancy (discontinuers) | 1258 | 2.3 | 450 | 2.5 | 1.08 (0.96–1.20) | 1.00 (0.89–1.11) |
| Use in both periods (continuers) | 56 | 0.1 | 28 | 0.2 | 1.51 (0.96–2.39) | 1.36 (0.86–2.15) |
| Use only during pregnancy (new users) | 13 | 0.0 | 5 | 0.0 | 1.16 (0.41–3.25) | 1.03 (0.37–2.89) |
| Other antidepressants | ||||||
| Nonuse | 52 499 | 96.8 | 17 351 | 96.0 | 1 (–) | 1 (–) |
| Only during prepregnancy (discontinuers) | 1496 | 2.8 | 599 | 3.3 | 1.21 (1.10–1.34) | 1.13 (1.03–1.25) |
| Use in both periods (continuers) | 185 | 0.3 | 103 | 0.6 | 1.68 (1.32–2.14) | 1.52 (1.19–1.94) |
| Use only during pregnancy (new users) | 29 | 0.1 | 17 | 0.1 | 1.78 (0.98–3.23) | 1.60 (0.88–2.91) |
Note: ATC Classification: Antidepressants N06A, SSRI N06AB, Tricyclic N06AA and other antidepressants included in N06AX ATC classification.
Adjusted by number of GP visits, obesity, smoking, HTA, diabetes.
When looking at specific type of SSRIs, paroxetine, fluoxetine, and escitalopram were the most prescribed drugs. Only women continuing paroxetine and fluoxetine showed a statistically association showing an increased risk of miscarriage of 36% (Supplemental Table 3).
3.3. Anxiolytics, hypnotics, and benzodiazepines and risk of miscarriage onset
We evaluated the prescription patterns of overall anxiolytics and hypnotic medications. In addition, we also studied the most commonly used benzodiazepines divided into short‐acting (<10 h) and long‐acting (>24 h) (Table 3). Overall, a total of 2.9% of the controls and 3.3% of cases received at least one prescription of anxiolytics only during prepregnancy (i.e., discontinuers) while the proportion of continuers was 1.3% and 1.7% and 1.1% and 1.6% were initiators during pregnancy. We observed an increased risk of miscarriage associated to continuers and initiators. Corresponding estimates were 1.19 (95% CI: 1.04–1.37) for continuers and 1.30 (95% CI: 1.13–1.50) for initiators. For hypnotic medications, <0.5% of cases and controls were discontinuers, while the proportion of continuers and initiators ranged from 0.1% to 0.3%. The OR of miscarriage was 1.27 (95% CI: 0.90–1.79) and 1.10 (95% CI: 0.70–1.473) for continuers.
TABLE 3.
Use of anxiolytics, hypnotics, and benzodiazepines and risk of miscarriage onset
| Controls N = 54 209 | Cases 18 070 | Crude OR (95% CI) | Adjusted OR* (95% CI) | |||
| Anxiolytics | ||||||
| Nonuse | 51 324 | 94.7 | 16 892 | 93.5 | 1 (–) | 1 (–) |
| Only during prepregnancy (discontinuers) | 1591 | 2.9 | 588 | 3.3 | 1.12 (1.02–1.24) | 1.01 (0.91–1.11) |
| Use in both periods (continuers) | 696 | 1.3 | 309 | 1.7 | 1.35 (1.18–1.55) | 1.19 (1.04–1.37) |
| Use only during pregnancy (new users) | 598 | 1.1 | 281 | 1.6 | 1.42 (1.23–1.64) | 1.30 (1.13–1.50) |
| Short acting BDZ | ||||||
| Nonuse | 52 696 | 97.2 | 17 459 | 96.6 | 1 (–) | 1 (–) |
| Only during prepregnancy (discontinuers) | 792 | 1.5 | 281 | 1.6 | 1.07 (0.93–1.23) | 0.96 (0.84–1.10) |
| Use in both periods (continuers) | 434 | 0.8 | 191 | 1.1 | 1.33 (1.12–1.58) | 1.18 (0.99–1.40) |
| Use only during pregnancy (new users) | 287 | 0.5 | 139 | 0.8 | 1.46 (1.19–1.79) | 1.32 (1.08–1.62) |
| Long acting BDZ | ||||||
| Nonuse | 52 750 | 97.3 | 17 447 | 96.6 | 1 (–) | 1 (–) |
| Only during prepregnancy (discontinuers) | 849 | 1.6 | 339 | 1.9 | 1.21 (1.06–1.37) | 1.08 (0.95–1.23) |
| Use in both periods (continuers) | 252 | 0.5 | 122 | 0.7 | 1.46 (1.18–1.82) | 1.30 (1.04–1.61) |
| Use only during pregnancy (new users) | 358 | 0.7 | 162 | 0.9 | 1.37 (1.13–1.65) | 1.24 (1.03–1.49) |
| Hypnotics and sedatives | ||||||
| Nonuse | 53 861 | 99.4 | 17 929 | 99.2 | 1 (–) | 1 (–) |
| Only during prepregnancy (discontinuers) | 179 | 0.3 | 65 | 0.4 | 1.09 (0.82–1.45) | 0.99 (0.74–1.31) |
| Use in both periods (continuers) | 103 | 0.2 | 49 | 0.3 | 1.43 (1.02–2.01) | 1.27 (0.90–1.79) |
| Use only during pregnancy (new users) | 66 | 0.12 | 27 | 0.2 | 1.23 (0.78–1.93) | 1.10 (0.70–1.73) |
Note: ATC classification: Anxiolytics N05B. Short acting BZD: Alprazolam N05BA12, Lorazepam N05BA06, Lormetazepam N05CD06, Oxazepam N05BA04, Clotiazepam N05BA21, Triazolam N05CD05, and Midazolam N05CD08; Long acting BDZ: Flurazepam N05CD01, Clorazepate dipotassium N05BA05, Quazepam N05CD10, Diazepam N05BA01, Medazepam N05BA03, Chlordiazepoxide N05BA02, Clobazam N05BA09, Clonazepam N03AE01, Pinazepam N05BA14, Bromazepam N05BA08, and Hypnotics and sedatives N05C.
Adjusted by number of GP visits, obesity, smoking, HTA, diabetes.
For short‐acting benzodiazepines, a total of 1.5% of the controls and 1.6% of cases received at least one prescription only during prepregnancy (i.e., discontinuers); 0.8% and 1.1%, were continuers and 0.5% and 0.8% were initiators. The OR of miscarriage were greater for initiators 1.32 (95% CI: 1.08–1.62) than those how were continuers 1.18 (95% CI: 0.99–1.40). Alprazolam, bromazepam, and diazepam were the most frequently prescribed types. Initiators of bromazepam presented the highest risk of miscarriage (48%) as well as those initiating diazepam (34%) (Supplemental Table 4).
Proportion of women receiving long‐acting benzodiazepines was similar, while a 1.9% of cases and controls were discontinuers, 0.7% were continuers and 0.9% initiated treatment. Nevertheless, women continuing during pregnancy had an increased risk of 1.30 (95% CI: 1.04–1.61). Lorazepam was the most prescribed long‐acting benzodiazepine, continuers presented an OR of 1.26 (95% CI: 0.99–1.61) and 1.34 (95% CI: 1.05–1.71) for initiators. (Supplemental Table 4).
3.4. Interaction effects of medication and the underlying illness
A total of 82.1% of cases and 84.3% controls had nonrecorded depression nonrecorded prescriptions of antidepressants. A total of 1.2% of cases and 0.9% controls had depression untreated within the first trimester versus 1.1% and 0.8% with depression treated during the first trimester. In order to distinguish between the effects of the medication from the underlying illness, we built an interaction variable. First, taking as reference nondepression and nonuse of antidepressants, the risk was accumulated among those with depression and treated during first trimester (regardless they were continuers or initiators) with an OR of 1.28 (95% CI: 1.08–1.50), and OR of 1.27 (95% CI: 1.08–1.51) for those with nondepression but treated during first trimester. Of note, women with depression but not treated during first trimester had an OR of 1.14 (1.03–1.26) (Figure 1).
FIGURE 1.

Interaction between depression and prescribed antidepressants and its association with miscarriage onset
The corresponding proportion of women of cases and controls with no recorded anxiety nonrecorded prescriptions of anxiolytics were also 78.7% and 80.9%. A total of 14.8% of cases and 13.8% controls had a recorded diagnosis of anxiety but not recorded treatment within the first trimester versus 1.8% and 1.4% with anxiety treated. Using as reference group nonanxiety and nonuse of anxiolytics, those with anxiety and treated during first trimester had an OR of 1.20 (95% CI: 1.05–1.37) and 1.33 (95% CI: 1.14–1.54) for those with nonanxiety but treated during first trimester. There was no association among women with anxiety but not treated during the first trimester (Figure 2).
FIGURE 2.

Interaction between anxiety and prescribed anxiolytics and its association with miscarriage onset
When we merged both conditions and treatment, the principal results remained the same, those with anxiety/depression and treated during first trimester had an OR of 1.28 (95% CI: 1.14–1.43) and 1.22 (95% CI: 1.00–1.50) for those with nonanxiety/depression but treated during first trimester. There was no association among women with any of each condition but not treated during the first trimester [aOR: 1.04 (0.96–1.13)] (Figure 3).
FIGURE 3.
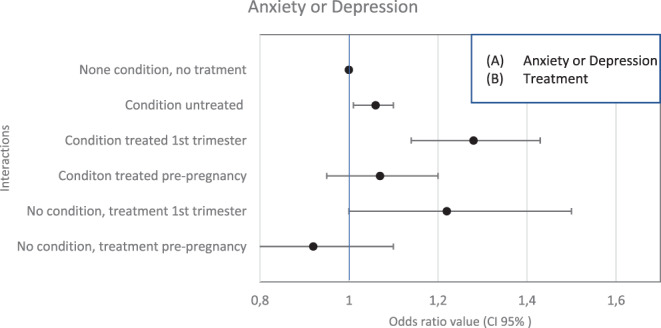
Interaction between anxiety/depression and prescribed anxiolytics/antidepressants and its association with miscarriage onset
4. DISCUSSION
In the current study of over 155 000 pregnant women living in Spain between 2005 and 2016, the overall prevalence of antidepressants and anxiolytics dramatically decreased during first trimester with respect to the antenatal exposure. Risk of miscarriages were observed among continuers of antidepressants, with special attention to paroxetine and fluoxetine. Our analysis also found an increased risk associated with use of anxiolytics for both continuers and initiators. For BZDs, those with short‐acting concentrated the risk among continuers while initiators of long‐acting had the highest risk. Interestingly, our results show how the increased risk seems to be associated with effects of the medication rather than the underlying illness.
Data of prenatal depression and anxiety is scarce in Spain. A study reported a 10.3% prevalence of depressive symptoms during first trimester. 38 In terms of drug utilization between 2000 and 2013 the daily drug dose (DDD) of antidepressants increased +200%, being this trend in line with the rest of European countries. 39 For anxiolytics, the increase expressed in DDD, was +46.1%, being lorazepam, alprazolam, and lormetazepam the most prescribed drugs. 40 The increase in consumption of these drugs seems to be superior with respect to other European countries. 41 , 42 , 43 In addition, there is a wide range of prescription rates of antidepressant among pregnant women ranging from 0.8% to 13% and approximately 2%–5% for anxiolytic–hypnotic drug 16 ; these data are in line with the proportions found in our study (1.5% for antidepressants and 2% for anxiolytics, taking controls as reference). Since these drugs are subject to potential risk effects to the mother and furthermore the unborn child, it is imperative to know the prescription trends before and during pregnancy in order to establish best clinical practice for pregnant women. In the current study we observed a high discontinuation rate of exposure during first trimester, more pronounced among those receiving antidepressants (85%–88%) compared with those receiving anxiolytics (65%–70%). In line with the observed trend, prior studies focused on assessing the prescription trends during pregnancy respective to prepregnancy have reported discontinuation rates of around 50% during first trimester for antidepressants, anxiolytics, and hypnotics. 44 , 45 Overall, the corresponding proportions of cases and controls initiating any of those medications were 0.2% for antidepressants and 1.7% for anxyolitics.
Several management options are available to treat these conditions which include psychosocial support and nonpharmacological interventions. However, when it turns to pharmacological treatment the potential harmful effects should be weighed against the risk of keep untreated during pregnancy. There is a complex matrix for clinical decisions on prescription patterns of antidepressant/anxiolytic medications during pregnancy. First, these drugs have a potential action on readily through the placenta with might be associated with adverse fetal development intra and extra‐utero 46 ; second, women who continued the treatment throughout pregnancy probably had the most severe condition which also is associated to obstetric complications 44 , 47 and third, withdrawal the treatment might also cause a relapse of any of these conditions and subsequently switching to another treatment which might be potentially harmful for the mother and child. We have observed an increased risk of miscarriage around 30% among women who continued both treatments of interest and 30% for those who initiated the treatment during first trimester compared with those who were nonexposed. When taking as reference group those exposed only during prepregnancy period, the risk remained the same: 22% for antidepressants and 18% for anxiolytics. Prior studies have also addressed the early use in pregnancy as an independent risk factor itself. 25 , 48 , 49 , 50 Yet, very few studies have considered the role of the underlying mental health conditions and further studies are warranted in order to fill major gaps in this area.
When looking to specific antidepressants paroxetine and fluoxetine showed the highest risk. As for the biological plausibility, although it remains unclear, it is thought that antidepressants, especially, SSRIs due to a mediated serotonin effect, might act putting pressure on the uterus at the early weeks of pregnancy. With regards to anxiolytics, there is scarce evidence although the mechanism might be via GABAA receptor which is modulated and enhanced by use of BZD. AN experimental study has observed how high levels of GABAA inters in both impaired uterine receptivity and embryonic development. 51 , 52 , 53
Keeping in mind all challenges, when choosing pharmacological intervention for pregnant women under mental health conditions several factors should be considered.
Decisional conflict whether to use an antidepressant or anxiolytic during pregnancy can be influenced by the primary care physicians, specialists, family, or the internet, 54 but in general, women tend to refuse to take their medication. A prior study evaluated the differences in the perception of risk of antidepressant and anxiolytics according to type of clinician (i.e., primary care, obstetrics & gynecologists and found differences in perception, confidence, and practice between clinician groups. 55 Patient decision aids (PADs) tools serve people to make difficult decisions about their healthcare choices, especially under situations where there is no unique or best option. 56 A recent systematic review evaluated the effectiveness of PADs regarding psychotropic medication use during pregnancy. Authors concluded how PDAs are useful to assist women and make decisions regarding medication use during pregnancy although evidence is still limited. 57 There is, therefore, an urgent need to improve decision making before choosing a pharmacological treatment during pregnancy which should include: adequate and complete training of healthcare professionals to fully discuss the potential risks and benefits of treatment 58 together with effective PADs in order to improve adherence.
We used data from an established and validated pregnant cohort 35 from BIFAP which owns the Spanish Agency of Medicines and Medical Devices (AEMPS) where nine regions are involved. It is representative of the Spanish population with respect to age, sex, and geographical region. 33 Likewise, countries which had universal health care systems like the UK and Spain, the PCP is the gatekeeper to the health care system. According to the National Health survey a total of 98% of all citizens visited at least once their PCPs during 2017. 59 It represents the first visit to monitor pregnancy although prenatal care is also taken by midwives, specialists, and hospitals. Following this reasoning although we might have missed some miscarriages due to visit private clinics, this should be minor since the universal healthcare system offered in Spain. However, the abortion frequencies obtained in women with recorded LMP and women who did not had it are very similar, which supports the truthfulness of our study. In addition, it is possible that women with either depression or anxiety monitored by private clinics have been overlooked; however, most PCP monitor and prescribe such drug classes in their routine daily basis. This might impact information toward severity of the maternal illness or changes in the daily dose if failing the current treatment. Nevertheless, we undertook prespecified automated computer searches for diagnoses or related conditions and this can be considered at some point compatible with indications. Thus, further studies are warranted to explore and answer this unsolved question. Nevertheless, the proportion of drug prescription rates were in line with prior studies. BIFAP includes data towards demographic data, medical diagnoses, prescriptions, laboratory results, referrals to specialists, and lifestyle factors (such as smoking, body mass index, alcohol use, and so forth). For the latter, there is a great impact on missing information for specific lifestyle factors which makes difficult to adjust by residual confounding due to these factors. Furthermore, there are free text comments associated with diagnosis which might serve to collect relevant information. Although, we have collected information on lifestyle factors, comedication, and comorbidities, we cannot discard some degree of residual confounding. Nevertheless, if some, it should be of small impact. Prior studies performed with the same database, aiming to compare the risk estimates resulting from different strategies for the handling of missing data, found not differences in risk measure. 60 , 61 In BIFAP, the prescriptions issued by the PCP are coded using ATC. However, since we used prescription or dispensing, we do not have complete information on actual drug intake, and there might have been a certain degree of drug use misclassification which is quite common to clinical studies. With such misclassification we mean the assumption of considering prescription as taking. Nevertheless, if this should happen we think it would have a nondifferential effect. Finally, drugs prescribed by physicians other than PCPs of the public sector are missing as this is a primary care database.
5. CONCLUSION
In conclusion, women with preexisting depression/anxiety tend to discontinue these agents during pregnancy although the proportion of women exposed during first trimester still remains high. Women who were exposed in early pregnancy either continued or initiated the treatment are at increased risk of miscarriage regardless the underlying illness. Further evidence concerning the treatment patterns and safety of specific drug classes in pregnant women is warranted in order to establish best clinical practice.
AUTHOR CONTRIBUTIONS
Lucía Cea Soriano, Ana Llorente‐García, and Consuelo Huerta originated and designed the study, Álvaro Kitchin and Lucía Cea Soriano contributed to the analysis of the data and to the drafting of the article. Álvaro Kitchin, Consuelo Huerta, Ana Llorente‐García, David Martínez, Paloma Ortega, and Lucía Cea Soriano collected data of the study and contributed to the interpretation of the results and to the drafting of the article. Álvaro Kitchin and Lucía Cea Soriano contributed to the analysis of the data and to the drafting of the article. All authors contributed to the final version of the article.
CONFLICT OF INTEREST
This study has not been presented or posted anywhere previously. Authors declare that there is no conflict of interest regarding the publication of this article.
ETHICS STATEMENT
The study protocol was approved by the BIFAP Scientific Committee (Reference Number 11/2016).
Supporting information
Supplemental Table1 List of codes principal comorbidities in BIFAP
Supplemental Table 2 ATC codes of principal drugs of interest
Supplemental Table 3 SSRIs and its association with miscarriage onset
Supplemental Table 4 Benzodiazepines and its association with miscarriage onset
ACKNOWLEDGMENTS
The authors would like to acknowledge the excellent collaboration of the primary care general practitioners, pediatricians, nurses, and the support of regional governments taking part in BIFAP. Author's also than the Pharmacovigilance Division and all BIFAP staff for their facilities and full access to the database. Open access funding enabled and organized by Projekt DEAL.
Kitchin Á, Huerta C, Llorente‐García A, Martínez D, Ortega P, Cea‐Soriano L. The role of prenatal exposure to antidepressants, anxiolytic, and hypnotics and its underlying illness on the risk of miscarriage using BIFAP database. Pharmacoepidemiol Drug Saf. 2022;31(8):901‐912. doi: 10.1002/pds.5488
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.
REFERENCES
- 1. Verdú ES, Fraile JS, Larrea VP, Caldentey CV. Evolución de la utilización de antidepresivos, ansiolíticos e hipnóticos en la Comunitat Valenciana. Período 2000‐2010. Aten Primaria. 2014;46(8):416‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lockhart P, Guthrie B. Trends in primary care antidepressant prescribing 1995‐2007: a longitudinal population database analysis. Br J Gen Pract. 2011;61(590):e565‐e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grigoriadis S, Graves L, Peer M, et al. Maternal anxiety during pregnancy and the association with adverse perinatal outcomes: systematic review and meta‐analysis. J Clin Psychiatry. 2018;79(5):17r12011. [DOI] [PubMed] [Google Scholar]
- 4. Eke AC, Saccone G, Berghella V. Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and risk of preterm birth: a systematic review and meta‐analysis. BJOG Int J Obstet Gynaecol. 2016;123(12):1900‐1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gavin NI, Gaynes BN, Lohr KN, Meltzer‐Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5):1071‐1083. [DOI] [PubMed] [Google Scholar]
- 6. Mercier RJ, Garrett J, Thorp J, Siega‐Riz AM. Pregnancy intention and postpartum depression: secondary data analysis from a prospective cohort. BJOG Int J Obstet Gynaecol. 2013;120(9):1116‐1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim HG, Mandell M, Crandall C, Kuskowski MA, Dieperink B, Buchberger RL. Antenatal psychiatric illness and adequacy of prenatal care in an ethnically diverse inner‐city obstetric population. Arch Womens Ment Health. 2006;9(2):103‐107. [DOI] [PubMed] [Google Scholar]
- 8. Grigoriadis S, Maziotis E, Simopoulou M, et al. The impact of thyroid autoantibodies positivity on in vitro fertilization outcome: a comprehensive review. Int Arch Clin Physiol. 2019;1:002. doi: 10.23937/IACPH-2017/1710002 [DOI] [Google Scholar]
- 9. Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. J Women's Health. 2003;12(4):373‐380. [DOI] [PubMed] [Google Scholar]
- 10. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bellantuono C, Tofani S, Di Sciascio G, Santone G. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry. 2013;35(1):3‐8. [DOI] [PubMed] [Google Scholar]
- 12. Ornoy A, Arnon J, Shechtman S, Moerman L, Lukashova I. Is benzodiazepine use during pregnancy really teratogenic? Reprod Toxicol. 1998;12(5):511‐515. [DOI] [PubMed] [Google Scholar]
- 13. Orueta Sánchez R, López Gil MJ. Manejo de fármacos durante el embarazo. Inf Ter Sist Nac Salud. 2011;35(4):107‐113. [Google Scholar]
- 14. Zoega H, Kieler H, Nørgaard M, et al. Use of SSRI and SNRI antidepressants during pregnancy: a population‐based study from Denmark, Iceland, Norway and Sweden. PLoS One. 2015;10:e0144474. doi: 10.1371/journal.pone.0144474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freeman MP, Góez‐Mogollón L, McInerney KA, et al. Obstetrical and neonatal outcomes after benzodiazepine exposure during pregnancy: results from a prospective registry of women with psychiatric disorders. Gen Hosp Psychiatry. 2018;53:73‐79. [DOI] [PubMed] [Google Scholar]
- 16. Hanley GE, Mintzes B. Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC Pregnancy Childbirth. 2014;14(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bernard N, Forest JC, Tarabulsy GM, Bujold E, Bouvier D, Giguère Y. Use of antidepressants and anxiolytics in early pregnancy and the risk of preeclampsia and gestational hypertension: a prospective study. BMC Pregnancy Childbirth. 2019;19(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95(4):487‐490. [DOI] [PubMed] [Google Scholar]
- 19. Davalos DB, Yadon CA, Tregellas HC. Untreated prenatal maternal depression and the potential risks to offspring: a review. Arch Womens Ment Health. 2012;15(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 20. Bonari L, Bennett H, Einarson A, Koren G. Risks of untreated depression during pregnancy. Can Fam Physician. 2004;50(1):37‐39. [PMC free article] [PubMed] [Google Scholar]
- 21. Engeland A, Bjørge T, Klungsøyr K, Hjellvik V, Skurtveit S, Furu K. Trends in prescription drug use during pregnancy and postpartum in Norway, 2005 to 2015. Pharmacoepidemiol Drug Saf. 2018;27(9):995‐1004. [DOI] [PubMed] [Google Scholar]
- 22. Creeley CE, Denton LK. Use of prescribed psychotropics during pregnancy: a systematic review of pregnancy, neonatal, and childhood outcomes. Brain Sci. 2019;9(9):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao X, Liu Q, Cao S, et al. A meta‐analysis of selective serotonin reuptake inhibitors (SSRIs) use during prenatal depression and risk of low birth weight and small for gestational age. J Affect Disord. 2018;241:563‐570. [DOI] [PubMed] [Google Scholar]
- 24. Ban L, Gibson JE, West J, et al. Maternal depression, antidepressant prescriptions, and congenital anomaly risk in offspring: a population‐based cohort study. BJOG Int J Obstet Gynaecol. 2014;121(12):1471‐1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ban L, Tata LJ, West J, Fiaschi L, Gibson JE. Live and non‐live pregnancy outcomes among women with depression and anxiety: a population‐based study. PLoS One. 2012;7(8):e43462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bais B, Molenaar NM, Bijma HH, et al. Prevalence of benzodiazepines and benzodiazepine‐related drugs exposure before, during and after pregnancy: a systematic review and meta‐analysis. J Affect Disord. 2020;269:18‐27. [DOI] [PubMed] [Google Scholar]
- 27. Xing D, Wu R, Chen L, Wang T. Maternal use of antidepressants during pregnancy and risks for adverse perinatal outcomes: a meta‐analysis. J Psychosom Res. 2020;137:110231. [DOI] [PubMed] [Google Scholar]
- 28. Andrade C. Offspring outcomes in studies of antidepressant‐treated pregnancies depend on the choice of control group. J Clin Psychiatry. 2017;78(3):e294‐e297. [DOI] [PubMed] [Google Scholar]
- 29. Agencia Española de Medicamentos y Productos Sanitarios . BIFAP: Base de datos para la Investigación Farmacoepidemiológica en Atención Primaria. http://bifap.aemps.es/
- 30. Antonio J, Luis J, López G. En El Sistema Sanitario Español; 2012.
- 31. Verbeke M, Schrans D, Deroose S, De Maeseneer J. The international classification of primary care (ICPC‐2): an essential tool in the EPR of the GP. Stud Health Technol Inform. 2006;124:809‐814. [PubMed] [Google Scholar]
- 32. Internacional De Enfermedades C . CIE • 9 • MC INFORMES Y ESTADÍSTICAS SANITARIAS 2013 MINISTERIO DE SANIDAD, SERVICIOS SOCIALES E IGUALDAD; 2014.
- 33. Maciá‐Martínez MA, Gil M, Huerta C, et al. Base de Datos para la Investigación Farmacoepidemiológica en Atención Primaria (BIFAP): a data resource for pharmacoepidemiology in Spain. Pharmacoepidemiol Drug Saf. 2020;29(10):1236‐1245. [DOI] [PubMed] [Google Scholar]
- 34. Cea‐Soriano L, García Rodríguez LA, Fernández Cantero O, Hernández‐Díaz S. Challenges of using primary care electronic medical records in the UKto study medications in pregnancy. Pharmacoepidemiol Drug Saf. 2013;22(9):977‐985. [DOI] [PubMed] [Google Scholar]
- 35. Sanchez Ortiz S, Llorente García A, Astasio P, Huerta C, Cea SL. An algorithm to identify pregnancies in BIFAP primary care database in Spain: results from a cohort of 155 419 pregnancies. Pharmacoepidemiol Drug Saf. 2020;29(1):57‐68. [DOI] [PubMed] [Google Scholar]
- 36. Sanchez Ortiz S, Huerta C, Llorente‐García A, Ortega P, Astasio P, Cea‐Soriano L. A validation study on the frequency and natural history of miscarriages using the Spanish primary care database BIFAP. Healthcare (Basel). 2021;9(5):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vandenbroucke JP, Pearce N. Case‐control studies: basic concepts. Int J Epidemiol. 2012;41(5):1480‐1489. [DOI] [PubMed] [Google Scholar]
- 38. Escribà‐Agüir V, Gonzalez‐Galarzo MC, Barona‐Vilar C, Artazcoz L. Factors related to depression during pregnancy: are there gender differences? J Epidemiol Community Health. 2008;62(5):410‐414. [DOI] [PubMed] [Google Scholar]
- 39. AEMPS . Utilizacion de medicamentos ansioliticose hipnoticos en España durante el periodo 2000‐2012. Mnisterio Sanid. 2014:1–4. http://tinyurl.com/pkl7zja [Google Scholar]
- 40. Sánchez MPV, Saint‐Gerons DM, De La Fuente HC, Bermejo DG, Corominas DM, Catalá‐López F. Evolución deluso de medicamentos ansiolíticos e hipnóticos en Españadurante el período 2000‐2011. Rev Esp Salud Publica. 2013;87(3):247‐255. [DOI] [PubMed] [Google Scholar]
- 41. Hurault‐Delarue C, Lacroix I, Bénard‐Laribière A, Montastruc JL, Pariente A, Damase‐Michel C. Antidepressants during pregnancy: a French drug utilisation study in EFEMERIS cohort. Eur Arch Psychiatry Clin Neurosci. 2019;269(7):841‐849. [DOI] [PubMed] [Google Scholar]
- 42. Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008;198(2):194.e1‐194.e5. [DOI] [PubMed] [Google Scholar]
- 43. Petersen I, Gilbert RE, Evans SJW, Man SL, Nazareth I. Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from the health improvement network. J Clin Psychiatry. 2011;72(7):979‐985. [DOI] [PubMed] [Google Scholar]
- 44. Cabaillot A, Bourset A, Mulliez A, et al. Trajectories of antidepressant drugs during pregnancy: a cohort study from a community‐based sample. Br J Clin Pharmacol. 2021;87(3):965‐987. [DOI] [PubMed] [Google Scholar]
- 45. Leong C, Raymond C, Château D, et al. Psychotropic drug use before, during, and after pregnancy: a population‐based study in a Canadian cohort (2001‐2013). Can J Psychiatry. 2017;62(8):543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kanto JH. Use of benzodiazepines during pregnancy, labour and lactation, with particular reference to pharmacokinetic considerations. Drugs. 1982;23(5):354‐380. [DOI] [PubMed] [Google Scholar]
- 47. Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta‐analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012‐1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sheehy O, Zhao JP, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiat. 2019;76(9):948‐957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Einarson A, Choi J, Einarson TR, Koren G. Rates of spontaneous and therapeutic abortions following use of antidepressants in pregnancy: results from a large prospective database. J Obstet Gynaecol Canada. 2009;31(5):452‐456. [DOI] [PubMed] [Google Scholar]
- 50. Hemels MEH, Einarson A, Koren G, Lanctôt KL, Einarson TR. Antidepressant use during pregnancy and the rates of spontaneous abortions: a meta‐analysis. Ann Pharmacother. 2005;39(5):803‐809. [DOI] [PubMed] [Google Scholar]
- 51. Bonnin A, Goeden N, Chen K, et al. A transient placental source of serotonin for the fetal forebrain. Nature. 2011;472(7343):347‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tian N, Liang H, Luo W, et al. GABA consumption during early pregnancy impairs endometrial receptivity and embryo development in mice. J Biochem Mol Toxicol. 2020;34(5):e22473. [DOI] [PubMed] [Google Scholar]
- 53. Piette PCM. The pharmacodynamics and safety of progesterone. Best Pract Res Clin Obstet Gynaecol. 2020;69:13‐29. [DOI] [PubMed] [Google Scholar]
- 54. Kothari A, de Laat J, Dulhunty JM, Bruxner G. Perceptions of pregnant women regarding antidepressant and anxiolytic medication use during pregnancy. Australas Psychiatry. 2019;27(2):117‐120. [DOI] [PubMed] [Google Scholar]
- 55. Williams S, Bruxner G, Ballard E, Kothari A. Prescribing antidepressants and anxiolytic medications to pregnant women: comparing perception of risk of foetal teratogenicity between Australian obstetricians and Gynaecologists, Speciality trainees and upskilled general practitioners. BMC Pregnancy Childbirth. 2020;20(1):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Connor AO, Elwyn G, Stacey D, et al. International Patient Decision Aid Standards [IPDAS] Collaboration reaches consensus on indicators for judging the quality of patient decision aids; 2005.
- 57. Broughton LC, Medlicott NJ, Smith AJ. Effectiveness of patient decision aids in women considering psychotropic medication use during pregnancy: a literature review. Arch Womens Ment Health. 2021;1:3‐578. [DOI] [PubMed] [Google Scholar]
- 58. National Institute for Health and Clinical Excellence (NICE) . Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance. NICE Guideline 192 [Published: 17 December 2014]. http://www.nice.org.uk/nicemedia/live/11004/30433/30433.pdf%5Cnguidance.nice.org.uk/cg45
- 59. Ministerio de Sanidad Consumo y Bienestar Social . Encuesta Nacional de Salud, España 2017; 2018;1–12. https://www.mscbs.gob.es/estadEstudios/estadisticas/encuestaNacional/encuestaNac2017/ENSE2017_notatecnica.pdf%0Ahttps://www.msssi.gob.es/estadEstudios/estadisticas/encuestaNacional/encuestaNac2017/ENSE2017_notatecnica.pdf
- 60. Martín‐Merino E, Calderón‐Larrañaga A, Hawley S, et al. The impact of different strategies to handle missing data on both precision and bias in a drug safety study: a multidatabase multinational population‐based cohort study. Clin Epidemiol. 2018;10:643‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. León L, Díaz Y, Puente D, Pottegård A, Montero‐Corominas D, Huerta C. Use of hydrochlorothiazide and risk of skin cancer in a large nested case‐control study in Spain. Pharmacoepidemiol Drug Saf. 2021;30(9):1269‐1278. doi: 10.1002/pds.5295 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table1 List of codes principal comorbidities in BIFAP
Supplemental Table 2 ATC codes of principal drugs of interest
Supplemental Table 3 SSRIs and its association with miscarriage onset
Supplemental Table 4 Benzodiazepines and its association with miscarriage onset
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Some data may not be made available because of privacy or ethical restrictions.


